Introduction
First line therapy for locally advanced and
metastasized pancreatic cancer comprises the chemotherapeutic
compound gemcitabine, which belongs to the class of nucleoside
analogues (1,2). However, resistance towards gemcitabine
is common and gives one explanation for the poor prognosis being
associated with its monotherapeutic usage. Add-ons of other drugs
such as the tyrosinekinase-inhibitor (TKI) erlotinib (EGFR-TKI) or
nab-paclitaxel improve survival only slightly and are affiliated
with additional side effects (3).
Oncolytic viruses (OVs) constitute
replication-competent particles, which are mostly non-cytotoxic for
normal tissues exhibiting intact anti-viral immune defense
signaling. In contrast, these OVs are able to selectively infect,
replicate within and lyse tumor cells mostly displaying impaired
anti-viral interferon pathways (4).
Newly released viral particles then can infect hitherto uninfected
neighboring tumor cells which lead to subsequent waves of oncolytic
tumor cell death and induction of a profound systemic anti-tumoral
immune response (5–7).
Numerous OVs are currently under investigation for
pancreatic cancer therapy and many clinical trials are being
performed (8–11). We here employed measles vaccine virus
(MeV)-a negative-stranded RNA virus, belonging to the paramyxovirus
group. MeV has an excellent safety record as it has been used as a
vaccine for more than 50 years. With regard to its oncolytic
efficiency, a first case of durable complete remission after a
single systemic MeV treatment was reported recently for a patient
suffering from therapy-refractory multiple myeloma (12). In preclinical models, pancreatic
cancer was shown to be susceptible to MeV as well (13–16).
Importantly, MeV recently also was found to infect and lyse
gemcitabine-resistant pancreatic carcinoma cells (14).
In the present study, we further investigated the
influence of gemcitabine on the oncolytic efficiency of MeV in more
human pancreatic cancer cell lines. We also characterized the
influence of gemcitabine on replication of MeV, as viral
replication is postulated to be a prerequisite for effectiveness of
oncolysis and subsequent rounds of infection. In a previous study
we could show that senescent tumor cells, such as the human
hepatoma cell line HepG2, the human mammary gland cancer cell line
MCF7 and the pancreatic cancer cell line MIA PaCa-2, can be
infected and lysed by MeV more efficiently than their non-senescent
counterparts (17). Accordingly,
viral replication was even more efficient in senescent cells.
Finally, we wanted to determine whether gemcitabine-induced tumor
cell senescence could be impaired by previous infection with MeV or
not.
Materials and methods
Cell culture/viral stocks
MIA PaCa-2, PANC-1, and BxPC-3 (all human pancreatic
carcinoma) and Vero (African green monkey kidney) cells were
obtained from the German Collection of Microorganisms and Cell
Cultures (DSMZ, Braunschweig, Germany) and cultured in Dulbecco's
modified Eagle's medium (Biochrom, Berlin, Germany) with 10% fetal
calf serum (PAA, Pasching, Austria) in a humidified incubator
(37°C, 5% CO2). Our prototypic suicide gene-armed vector
MeV-SCD was generated from a commercially available original
monovalent vaccine batch of MeV strain Mérieux (Sanofi-Pasteur,
Leimen, Germany), as described recently (18). Recombinant MeV carrying a green
fluorescent marker protein (MeV-GFP) was generated by real
time-polymerase chain reaction cloning from an original vaccine
batch of the MeV Schwarz strain (Mérieux, Sanofi-Pasteur, Lyon,
France).
Substance
Gemcitabine was obtained from LC Laboratories
(Woburn, MA, USA) and solved in dimethyl sulfoxide.
Cell viability assays
Cells were plated in 24-well plates
(4×104 cells per well) and allowed to adhere for 24 h.
Infection with MeV was performed after washing cells once with
phosphate buffered saline (PBS) in Opti-MEM serum-reduced medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 3 h
at 37°C. Virus was allowed to infect tumor cells for 3 h. Then the
medium was changed with the addition of the chemotherapeutic
compound (i.e., gemcitabine) (Fig.
1A). Cell viabilities were determined after an incubation time
of 72 h, employing the Suforhodamine B assay (SRB, measuring
remaining cell mass) (19) as well
as the MTT assay (measuring cellular enzyme activity, a
yellow-colored tetrazole
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide,
which is reduced to formazan (purple color) by living cells)
(20). For SRB assay, cells were
fixed with cold 10% trichloroacetic acid (TCA) and dried at 40°C.
Cells were stained with SRB staining solution (0.4% Sulforhodamine
B in 1% acetic acid), and after drying, stain was solubilized in 10
mM Tris base, pH 10.5. Optical density was measured at a wavelength
of 550 nm in a microtiter plate reader. For MTT assay, cells were
washed with warm PBS and stained with MTT staining solution (MTT
2.5 mg/ml solubilized in DMEM without phenol red). Then plates were
incubated at 37°C for 2 h. After incubation time, the staining
solution was removed and plates were frozen at −20°C. For
measurement, stained cells were solubilized in MTT solvent (3.7%
HCl in isopropanol). Optical density was measured at a wavelength
of 570 nm (reference wave length 650 nm).
Virus growth curves
To measure viral replication comparing MeV-SCD alone
with the combination of MeV-SCD and gemcitabine, virus growth
curves were generated. Infection was performed in analogy to the
cell viability assays with the same multiplicities of infection
(MOIs; ratios of infectious viral particles/tumor cells) of MeV-SCD
and concentrations of gemcitabine using six-well plates. Three
hours post infection (hpi) the inoculum was removed and cells were
washed three times with PBS. Then, 1 ml of medium with or without
gemcitabine was added. Supernatants and cells were harvested at
indicated time points (Fig. 2).
Viral titers were determined according to the method of Kärber and
Spearman (21,22). Samples of either cell suspensions
undergoing extractions of the cellular contents (graphs to the left
in Fig. 3: Represented by ‘cell
associated virus’) or cell culture medium supernatants (graphs to
the right in Fig. 3: ‘Released
virus’) were used to re-infect Vero cells with dilution series.
After 96 h, Vero cells were fixed with 4% formaldehyde and stained
with fluorescent antibodies (primary antibody: Anti-MeV-NP, clone
120; no. 95040312; ECACC, Salisbury, UK), secondary antibody: Goat
anti-mouse (Alexa Fluor 546; Invitrogen; Thermo Fisher Scientific,
Inc.), both diluted 1:1,000 in TBS-Tween). Cells were analyzed via
fluorescence microscopy.
 | Figure 2.Chemovirotherapy employing Gem
together with the oncolytic measles vaccine virus in three
pancreatic cancer cell lines. (A) Setting: Tumor cells were
infected with MeV-SCD (here denoted as MeV) at 24 h after plating.
Add-on of Gem was performed at 3 hpi when medium was changed. The
total incubation time of virus was 72 h. (B) Cell viability was
measured using two different assays [SRB (black bars) and MTT
(white bars) assays, respectively] and normalized to an uninfected
(MOCK-treated) control (set to 100% cell viability). MOIs of MeV
and Gem concentrations were chosen at low enough levels to reduce
tumor cell masses <50% when used as a single compound. When used
in combination, the remaining tumor cell mass was found to be
<5% in all three tumor cell lines. For MeV, MOIs of 0.4 (MIA
PaCa-2), 0.075 (PANC-1) and 0.125 (BxPC-3) were chosen,
respectively. For Gem, concentrations of 0.03 µM (MIA PaCa-2),
0.075 µM (PANC-1) and 0.02 µM (BxPC-3) were used, respectively.
Data are presented as the mean ± standard deviation of three
independent experiments. GEM, gemcitabine; hpi, hours post
infection; MOIs, multiplicities of infection; MeV, measles vaccine
virus; SRB, Sulforhodamin B. |
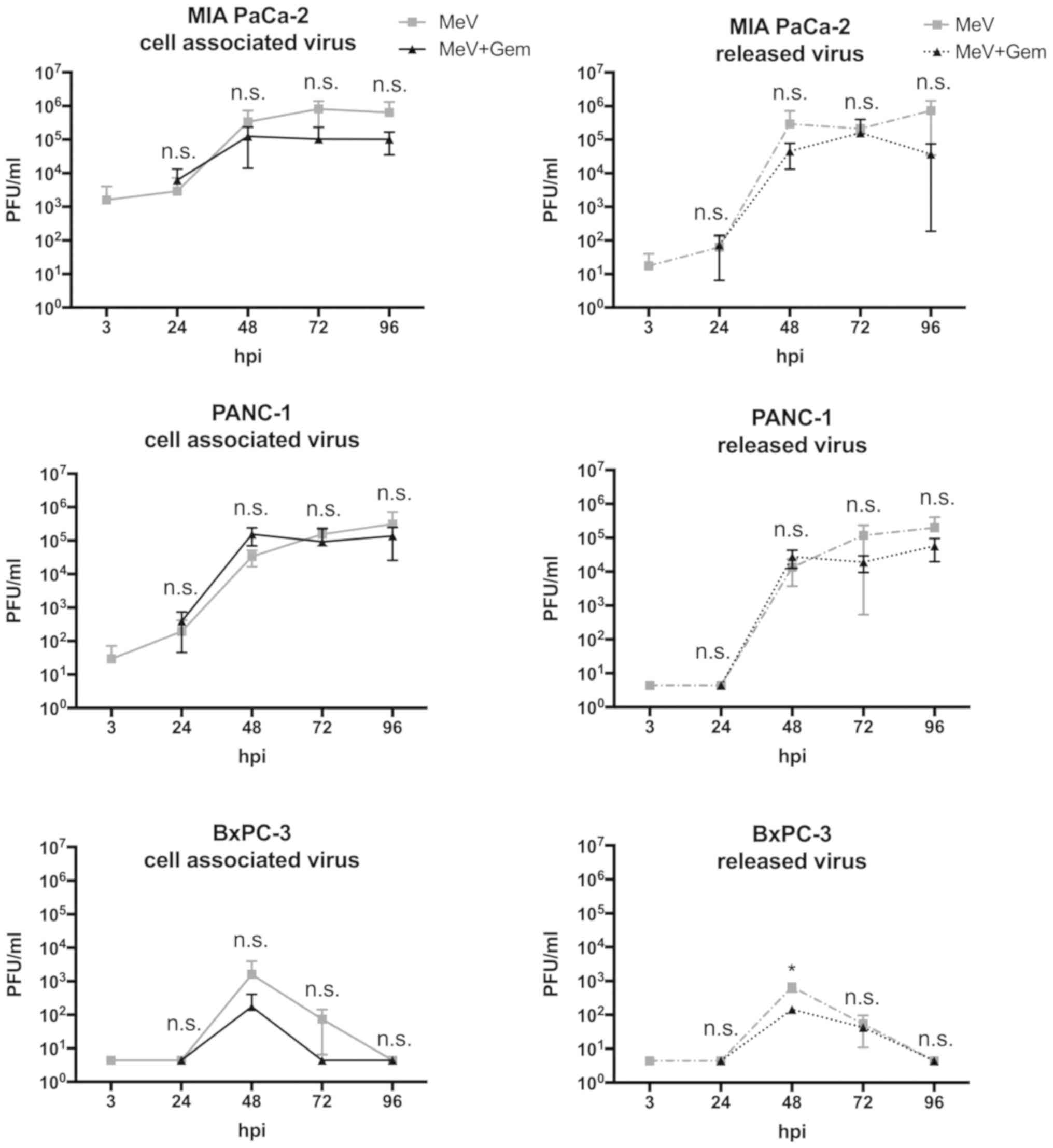 | Figure 3.Virus growth curves illustrating the
course of viral replication in pancreatic cancer cells infected
with oncolytic MeV. Virus growth of MeV-SCD (here denoted as MeV)
was determined both in cell suspensions (continuous line graphs,
cell associated virus) as well as in tumor cell culture
supernatants (dotted line graphs, released virus). Viral
replication was compared between: i) Single treated, i.e., ‘only’
infected tumor cells (MeV; mono-virotherapy, grey graphs); and ii)
chemovirotherapeutic treated tumor cells, being infected first and
then treated additionally with gemcitabine at 3 hpi (MeV+Gem, black
graphs). Notably, tumor-cell specific multiplicities of infection
of MeV and concentrations of Gem were used equal to the
concentrations employed before in the viability assays
(Sulforhodamin B and MTT). Except for the 48-h-value of released
virus in the cell line BXPC-3, there was no statistically
significant difference between the values of viral growth with or
without Gem. All statistical analyses were conducted with
Bonferroni's multiple comparison test. *P<0.05 vs. MeV-Gem. PFU,
plaque forming units; n.s., not significant; hpi, hours post
infection; GEM, gemcitabine; MeV, measles vaccine virus. |
Senescence assay
For senescence assay, cells were seeded in 6-well
plates (2×104 cells per well). For fixation and
staining, the Senescence Cells Histochemical Staining kit
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used. Fixed
cells were treated with staining solution containing 2.5% X-Gal
(5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) and incubated
for 12 to 16 h at 37°C in a carbon dioxide depleted atmosphere.
Senescence-associated-β-galactosidase (SA-β-Gal) positive cells
appearing light blue in bright field microscopy were calculated as
percentage of total cell counts using an Olympus IX50 inverted
microscope (Olympus, Center Valley, PA, USA).
Detection of GFP expression
To illustrate viral infection microscopically,
infection was performed using a MeV encoding a green fluorescent
marker protein (MeV-GFP). To detect fluorescence, an Olympus IX50
microscope was used.
Statistical analyses
Results in the figures are expressed as mean values
with their standard errors. One-way analysis of variance with
Bonferroni's multiple comparison test was used to determine the
statistical differences when comparing multiple groups. An unpaired
t-test, confidence interval 95% and two-tailed, was used to
determine statistical differences between two groups compared once.
All analyses were performed using GraphPad Prism v.4.03 (GraphPad
Software, Inc., La Jolla, CA, USA). According to this analyses, a
P>0.05 was marked as not significant (n.s.) and a P<0.05 was
considered to indicate a statistically significant difference.
Results
Efficiency of chemovirotherapy
To investigate a combinatorial effect of MeV and
gemcitabine, drug concentrations of both agents were chosen low
enough to aim for a remaining cell viability of more than 50% in
comparison to a non-treated MOCK-control when applied as single
agents (Fig. 2B). Two different cell
viability assays were performed: i) Sulforhodamin B (SRB) assays,
measuring remaining cell masses, and ii) MTT assays, measuring
cellular enzyme activity as a second option.
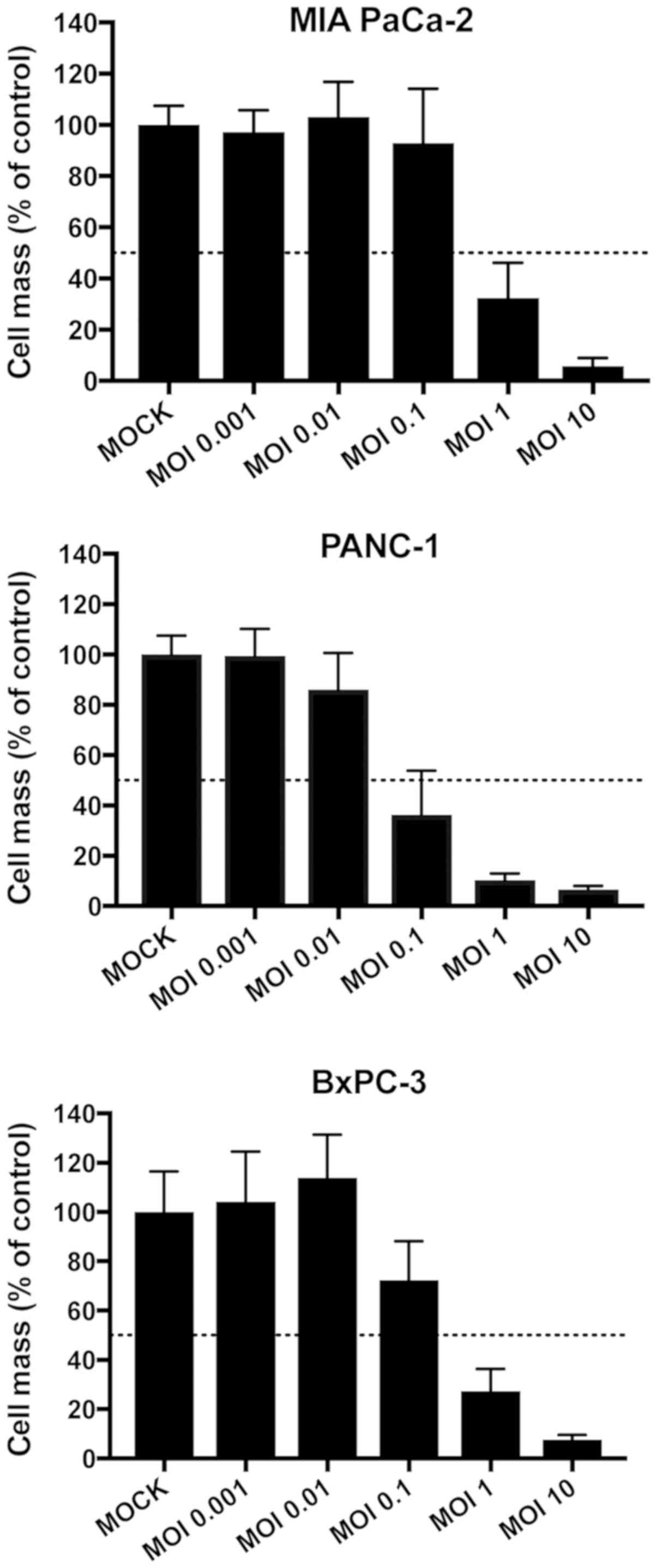
To first determine the adequate dosages for both
chemotherapy and virotherapy, tumor cells were infected with
increasing multiplicities of infection (MOIs) of MeV-SCD ranging
from 0.001 to 10 or treated with different concentrations of
gemcitabine in monotherapy. The objective was to find dosages
leading to reductions of tumor cell masses between 15 and 45% in
monotherapy. The preliminary finding experiments of MeV are shown
in Fig. 1. The ultimate dosages of
both MeV and gemcitabine are depicted within Fig. 2. Beneath the preliminary experiments
depicted in Fig. 1, further
experiments were performed to determine the optimal MOIs for the
combinatorial approach with steps in between the initially chosen
MOIs (Fig. 2B). When comparing these
two sets of experiments (Fig. 1 and
Fig. 2B) slight differences in tumor
cell viabilities at 72 h post infection were detected which could
be explained by biological variations. As a result of both SRB and
MTT assays, cell viabilities were found to undercut the 50%
threshold in all tumor cell lines when MeV-SCD and gemcitabine were
combined (Fig. 2B), which
constitutes a considerable cytotoxic effect in contrast to the
rather low efficiencies of the therapeutics alone
(‘monotherapeutic’ results).
In MIA PaCa-2 (Fig.
2B, upper panel), cell viabilities of the MeV-SCD plus
gemcitabine combination therapy even were found to be reduced to
less than 30% of the controls, whereas cell viabilities did not
undercut 70% for MeV-SCD and 55% for gemcitabine [when always
applying the same amounts of MeV-SCD (MOI 0.4) and gemcitabine
(0.03 µM)]. In PANC-1 (Fig. 2B,
middle panel), single agent treatments led to cell viabilities
higher than 65% (MeV-SCD, MOI 0.075) and higher than 75% (0.075 µM
gemcitabine), whereas cell viabilities in dual agent treatments
were found to drop down to a range of 40–45% only. In BxPC-3
(Fig. 2B, lower panel), cell
viabilities for MeV-SCD (MOI 0.125) alone were about 80% and for
gemcitabine (0.02 µM) alone about 65%; combination of both
compounds resulted in cell viabilities of less than 50%.
Taken together, combination therapies significantly
reduced cell viabilities were found in all three human pancreatic
carcinoma cell lines for the combinatorial approach in comparison
to single-agent therapies. Consequently, it became obvious that
both agents did not negatively influence the cytotoxic potency of
each other in pancreatic cancer cell lines. To confirm this, we
investigated replication of MeV-SCD as well as therapy-induced
senescence (TIS) caused by gemcitabine in our combinatorial
approach.
Chemotherapeutic influence on viral
replication
Viral replication within infected tumor cells
constitutes one of the most important modes of action of oncolytic
virotherapy, as it is indispensable for tumor cell lysis, release
of progeny virus particles and further rounds of tumor cell
infections. Therefore, we set out to quantify viral replication in
absence and presence of gemcitabine. For this purpose, virus growth
curves were generated for: i) viral replication within tumor cells
(Fig. 3, ‘cell associated virus’,
continuous graphs) and, ii) viral particles being released into
supernatants (Fig. 3, ‘released
virus’, dotted graphs). To compare viral replication of MeV-SCD
alone with the combination of MeV-SCD and gemcitabine, virus growth
curves for MeV alone and for the combination therapy were generated
under the same conditions, i.e., MOIs of MeV-SCD and concentrations
of gemcitabine were the same as applied for SRB and MTT assays
before.
As a result, viral replication was found to be quite
similar for both conditions: Mono-virotherapy or combined
chemovirotherapy. For two human pancreatic carcinoma cell lines
(MIA PaCa-2 and BxPC-3) viral replication was slightly inhibited in
the course of an additional treatment with gemcitabine (Fig. 3): In MIA PaCa-2, cell associated
viral titers at 72 h post infection (hpi) were 8×106
plaque forming units (PFU) for MeV-SCD alone and 1×106
PFU for the chemovirotherapeutic combination. In BxPC-3, cell
associated viral titers at 48 hpi were 1.6×103 PFU for
MeV-SCD and 0.17×103 PFU for the chemovirotherapeutic
combination. It must be pointed out that cell viabilities very
likely were reduced due to addition of the chemotherapeutic
compound gemcitabine (MOIs of MeV-SCD were the same for both
conditions). Furthermore, viral replication in BxPC-3 was rather
low compared to the other two cell lines and did not exceed
104 PFU. To further investigate this phenomenon in more
detail, cell numbers seeded per well were raised to
2×105 cells and 3×105 per well, respectively
(data not shown). However, the extent of virus replication was not
altered significantly by merely elevating the cell numbers.
Therapy-induced senescence (TIS) in
MeV-infected tumor cells
To investigate whether the effect of gemcitabine
comprises foremost cytotoxicity or additionally senescence in MIA
PaCa-2, human pancreatic carcinoma cells infected with MeV-SCD, a
senescence-associated β-galactosidase (SA-β-Gal) assay was
performed (Fig. 4). Interestingly,
gemcitabine was found to induce senescence both in uninfected and
in infected MIA PaCa-2 cells with the same potency (Fig. 4A; 70–80% senescent cells when
normalized to the total cell count), when gemcitabine was applied
in concentrations being identical to the ones used for the
chemovirotherapeutic combination experiments before (0.03 µM).
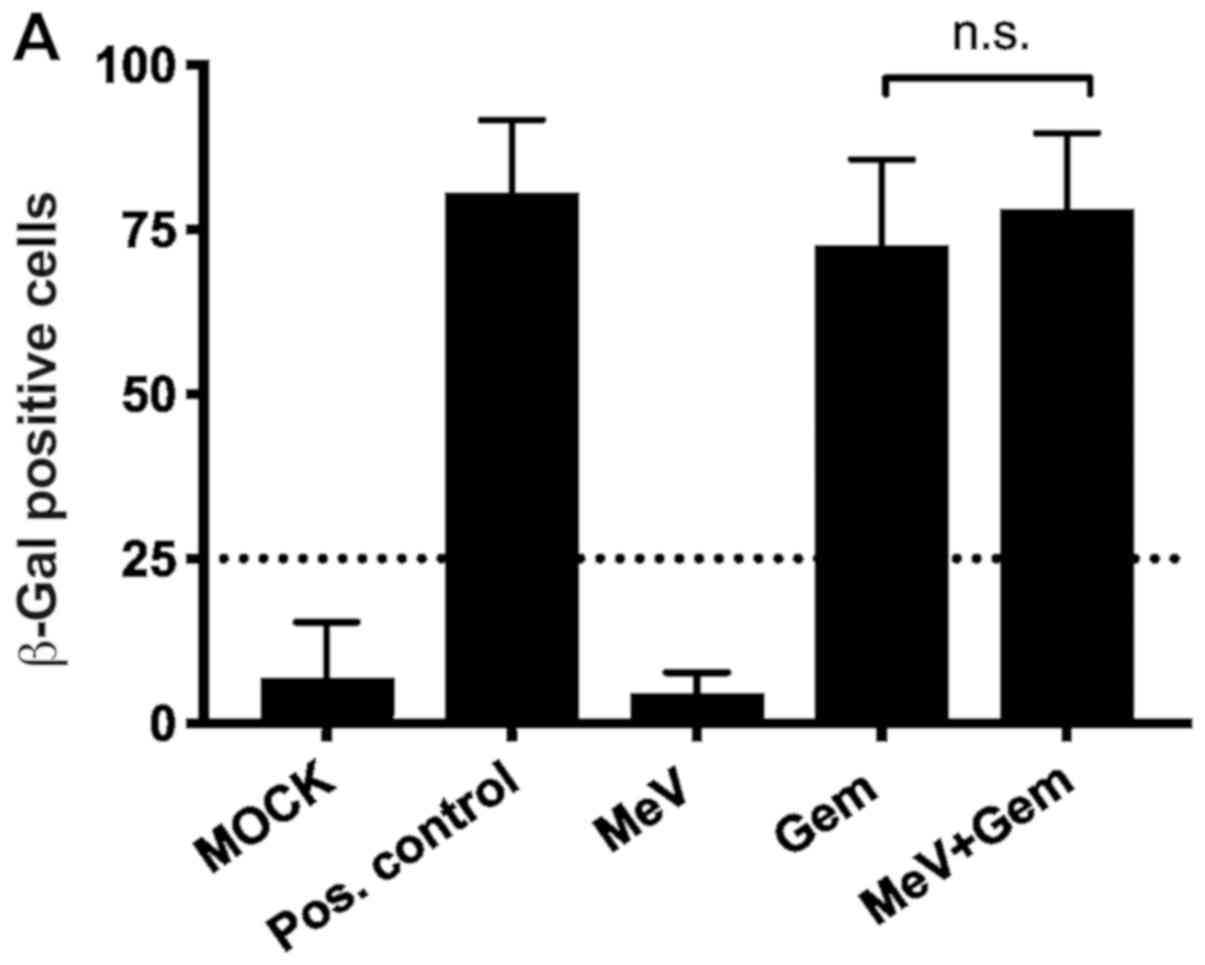 | Figure 4.SA-β-Gal assay illustrating
therapy-induced senescence in MIA PaCa-2 cells. (A) Tumor cells
were either infected with the oncolytic MeV-SCD (here denoted as
MeV) or treated with Gem at 3 hpi, and underwent combined
chemovirotherapeutic treatment (MeV+Gem) or were left untreated
(MOCK); pos. control: Gem 100 µM; then, expression of SA-β-gal was
determined 72 h later. Statistical analyses were conducted with an
unpaired t-test, confidence interval 95% and two-tailed). (B) Time
dependency of senescence induction. Gem (0.03 µM) was added either
at 3, 24 or 48 hpi. Expression of SA-β-gal was determined again at
72 hpi. There was no statistically significant impairment of
induction of senescence by the presence of virus. Statistical
analyses were conducted with Bonferroni's multiple comparison test.
hpi, hours post infection; SA-β-Gal, senescence-associated
β-galactosidase; Gem, gemcitabine; hpi, hours post infection; pos.
control, positive control; n.s., not significant; MeV, measles
vaccine virus. |
Compared to the positive control (gemcitabine 100
µM), it revealed the same effectiveness in inducing senescence in
the remaining cells.
Tumor cell mass was not only significantly reduced
by combination of MeV-SCD and gemcitabine as shown in Fig. 2B but also consisted mostly of
senescent cells, which are in a permanent cell cycle arrest.
To determine whether the time point of the addition
of gemcitabine after the infection with MeV-SCD was crucial for the
induction of senescence another setting for the SA-β-Gal assay was
chosen (Fig. 4B). The add-on of
gemcitabine was delayed either 24 or 48 h after infection with
MeV-SCD to allow the virus to infect and replicate efficiently
before the onset of TIS. As a result, the percentage of senescent
cells was found to range between 70 and 90% independently of the
time span between infection and additional treatment with
gemcitabine. Accordingly, no significant differences in the time
courses of TIS-induction were observed.
Visualization of a contemporaneous
presence of senescence and MeV-infection in the same tumor
cells
To reassure that senescence and infection with MeV
did not only occur as side-by-side phenomena but simultaneously in
the same, identical cells, we microscopically investigated whether
MeV-infected MIA-PaCa-2 tumor cells were also positive for SA-β-Gal
staining. For this purpose tumor cells were infected with MeV-GFP
(MOI 0.4), a MeV encoding a green fluorescent marker protein (GFP),
which helps to detect infected tumor cells easily by fluorescence
microscopy, and then again treated with gemcitabine (0.03 µM) at 3
hpi. As a result, senescence became clearly visualized by the light
blue color being detected in the respective SA-β-Gal assay
(Fig. 5A) as well as by an enlarged
and flattened cellular phenotype (Fig.
5C), whereas infection with MeV-GFP was visualized by
fluorescence microscopy (Fig. 5B).
Furthermore, MeV-induced multinucleated syncytia (GFP-positive;
Fig. 5E) were also found to undergo
senescence (Fig. 5D and E) in the
course of addition of gemcitabine at 3 hpi.
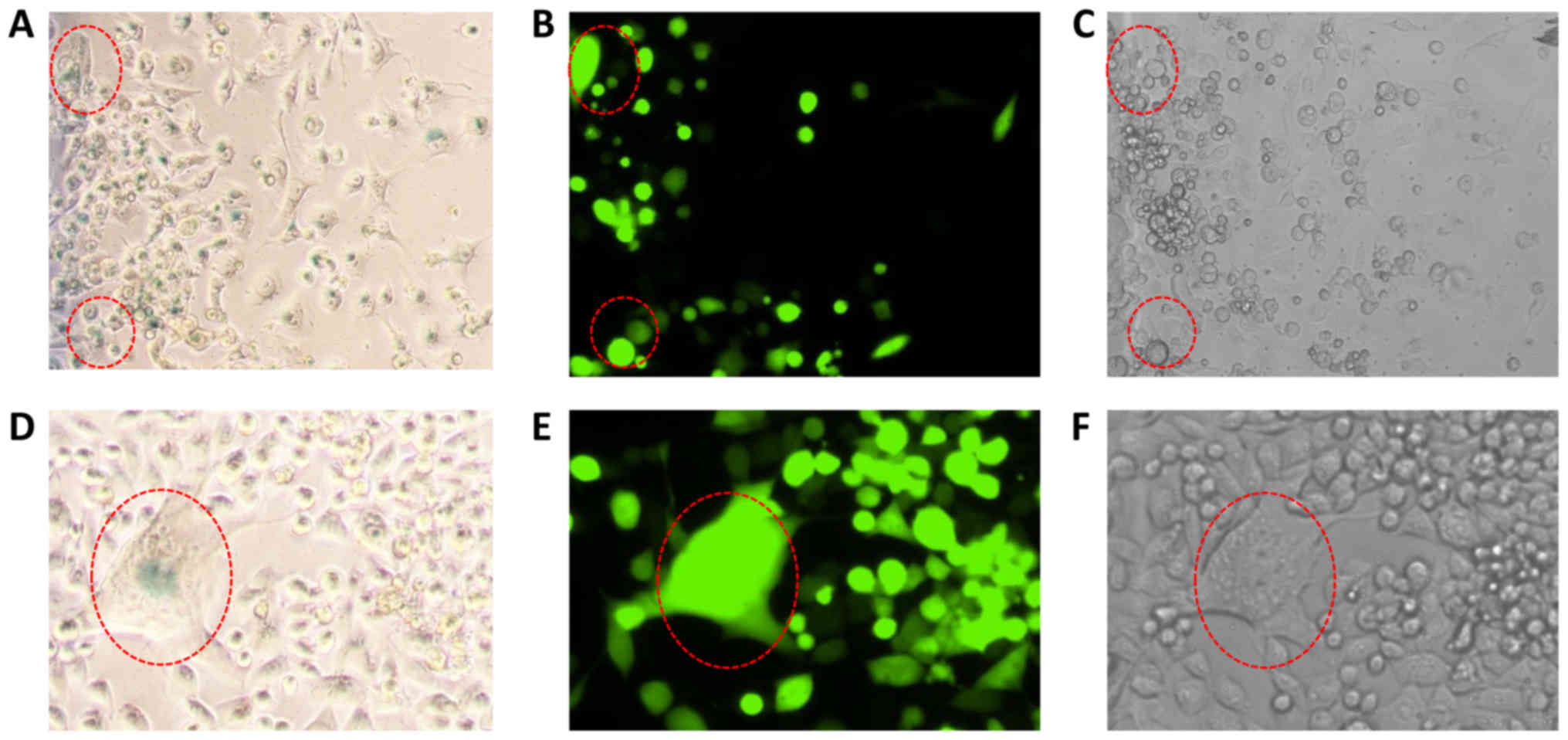 | Figure 5.Senescence patterns induced by
gemcitabine in pancreatic cancer cells infected with oncolytic MeV.
Upper panel (all magnification, ×4): (A) MIA PaCa-2 tumor cells
infected with the GFP marker gene encoding oncolytic MeV (MeV-GFP)
exhibited blue staining of senescence-associated β-galactosidase,
detected by bright field microscopy. (B) Visualization of MeV-GFP
infected tumor cells by fluorescence microscopy. (C) Light
microscopy of the same sector exhibiting an enlarged and flattened
phenotype of tumor cells, being characteristic for the induction of
therapy-induced senescence. Red dotted circles indicate examples of
MeV-GFP infected senescent tumor cells. Lower panel (all
magnification, ×10): (D) Higher magnification of a MeV (MeV-GFP)
induced syncytium of MIA-PaCa-2 tumor cells exhibiting a blue
colored (β-galactosidase positive) senescent phenotype. (E) Proving
infection with MeV-GFP, this syncytium (encircled in red) exhibited
a strong GFP-mediated fluorescence signal. (F) Light microscopy
depicted the multinucleated phenotype being typical for MeV-induced
syncytia. Tumor cells (MIA PaCa-2) were treated with the respective
concentrations used in the combination experiments (MOI of MeV:
0.4; concentration Gem: 0.03 µM), pictures were taken at 72 hpi.
Gem, gemcitabine; hpi, hours post infection; MeV, measles vaccine
virus; GFP, green fluorescent protein. |
Discussion
MeV constitutes a novel biological compound to
overcome therapeutic resistance of pancreatic cancer, despite the
fact that anti-viral resistances (primary/secondary) do exist or
arise (23). Therefore, novel
combination strategies to treat pancreatic cancer have to be
developed. As gemcitabine constitutes a first-line
therapeutic for the treatment of pancreatic cancer (e.g., in
combination therapy with the tyrosine kinase inhibitor EGFR-TKI),
we investigated whether the combination of MeV with that very agent
showed superior effects when compared to either agent alone.
Cell viability assays showed a superior cytotoxic
effect for the combination of MeV and gemcitabine when compared to
both therapeutics administered as single agents alone. These
results are also interesting for a possible transfer to clinical
scenarios considering that lower drug concentrations lead to lower
side-effects, as already shown in combination with another
oncolytic virus, i.e., Myxoma virus (24). Two different assays were performed,
SRB assay measuring cell mass and MTT assay measuring metabolic
activity of cells. For the tumor cell lines MIA PaCa-2 and BxPC-3
results of both assays were found to be quite similar. In contrast,
for PANC-1 tumor cells, when treated solely with gemcitabine, we
observed a significant difference between the results of the SRB
and the MTT assay (Fig. 2B; third
pair of black and white bars from the left): Cell viability in the
MTT assay was about 100%, whereas cell mass in the SRB assay was
measured less than 80% in comparison to the control. A likely
explanation for that difference is the senescence-inducing potency
of gemcitabine. Cell metabolism in senescent cells remains active,
which is measured by the MTT assay. Interestingly, cell viability
of the combination of both MeV and gemcitabine was not found to
differ between both assays.
The phenomenon of senescence is of high interest for
the field of cancer therapy and is considered as one of the major
stress responses to cancer therapies, e.g., to chemotherapeutic
compounds (25,26). However, senescent cells can escape
the senescence-associated cell cycle arrest or secrete soluble
factors that might eventually exert pro-tumorigenic effects on
neighboring cells. Accordingly, it is required to develop senolytic
regimens which are instrumental in ablating such senescent cells
before any of these escape or tumor-inducing events could occur. In
this way, OVs should be tested thoroughly in combination with
chemotherapeutic compounds for their senolytic potentials,
respectively. Several drugs-including gemcitabine-are able to
induce senescence in tumor cells resulting in a permanent cell
cycle arrest and consequently maintaining cells in a less
malignant/less proliferative state (17). In a previous study we could show that
MeV can infect, replicate in and lyse senescent cells including
pancreatic cancer cells even more efficiently than non-senescent
cells (17). In this context, we
investigated whether senescence can be induced in ‘already’
MeV-infected tumor cells.
For this purpose, we first infected pancreatic
cancer cells with MeV and then added gemcitabine at several
different time points up to 48 hpi with MeV. The results indicate
senescence-induction is not altered by infection with MeV,
independently of the time point of the addition of gemcitabine. In
summary these findings suggest that senescence and MeV infection
are not inconsistent cellular mechanisms when cytotoxicity is the
target. No matter which mode of application was chosen concerning
the sequence of administration, induction of senescence led to an
increased oncolytic cell death when compared to MeV infection
alone.
Similar results already have been obtained for the
combination of the oncolytic Coxsackievirus A21 in combination with
the senescence inducing agent doxorubicine. Simultaneous
application of virus and drug as well as infection and addition of
the chemotherapeutic compound 24 h later showed synergistic effects
concerning cytotoxicity. No influence of doxorubicine on viral
replication was observed (27).
Gemcitabine has already been co-administered with different OVs. In
one study pancreatic carcinoma cell lines were treated with
gemcitabine in combination with myxoma virus (MYXV). The drug was
found to inhibit viral gene expression upon simultaneous
administration. Sequential treatment, however, resulted in a
striking decrease in tumor cell viability when compared to the
respective monotherapies. Interestingly, the optimal sequence (drug
first or virus first) was found to be dependent on the tumor cell
line to be addressed (24).
Gemcitabine also was shown to increase the oncolytic efficiency of
parvovirus H-1PV in pancreatic carcinoma cells when administered 24
h before the virus (28).
Viral replication is a crucial mechanism for the
efficiency of OV as it ensures a multiplication of the oncolytic
potency as well as further spreading of viral particles. Thus, the
influence of gemcitabine on viral replication was an important
aspect to investigate. In our setting, we observed a slight
decrease in viral replication when gemcitabine was administered
additionally (Fig. 2). In another
study, oncolytic herpes simplex virus type 1 (HSV-1) was shown to
replicate better in the presence of gemcitabine (29). However, a different application
scheme was used with gemcitabine being added 6 h prior to
infection. The reduced tumor cell number due to the cytotoxic
effect of gemcitabine might have caused the reduced viral
replication in our work, as the presence of vital cells is crucial
for the replication of MeV. Direct alteration of viral replication
by gemcitabine is also very likely as gemcitabine was originally
developed as anti-viral therapy and shows anti-viral activity
against numerous RNA and DNA viruses (30). Of note, viral replication was only
decreased but not suppressed and cytotoxicity was significantly
higher with MeV than with gemcitabine alone. Thus, we can deduce
that an anti-viral activity of gemcitabine is very likely but the
efficiency is not high enough to suppress the cytotoxic mechanism
of action of oncolytic MeVs.
Combination of gemcitabine and MeV constitutes a
reasonable new approach to overcome therapeutic resistance of
pancreatic cancer cells in vitro. We were able to show that
a combination of suboptimal concentrations of both therapeutics led
to a significant increase in cytotoxicity in three tumor cell lines
when compared to single agent treatment. It remains to be
determined, whether there is a distinct molecular pathway that
leads to the observed combinatorial effect. However, both
therapeutics were shown to work well together and did not alter
significantly efficacy of each other. For further research, it is
indispensable to transfer our findings to in vivo scenarios
in order to learn more on possible obstacles and the most
advantageous way to apply these therapeutics. It is very important
to optimize delivery of therapeutics especially in pancreatic
cancer, as it is hard to reach pancreatic tumor tissues via
systemic approaches due to its hypoxia as described earlier.
Therefore, alternative modes of application should be tested in
vivo, e.g., intratumoral or intraperitoneal (i.p.) routes
(31).
Furthermore, new findings concerning tumor biology
should be taken into consideration searching for other therapeutic
options. In that context, it is very important to analyze different
therapeutic regimens (32), and the
influence of the immune system on virotherapy-considering both
‘negative’ aspects as it weakens viral infection and ‘positive’
aspects as the activation of the immune system leads to anti-tumor
immune defense (7,18). In a recently published review the
necessity of a very profound investigation and understanding of
tumor biology was pointed out as well (33). Moreover, it was accented that
identification of synergistically working chemovirotherapy is
urgently needed-aiming not only for an exploitation of cytopathic
effects but also for an alteration of the immunosuppressive
microenvironment of pancreatic cancer (34,35).
Acknowledgements
The authors would like to thank Mrs. Irina Smirnow
[Department of Internal Medicine VIII (Medical Oncology and
Pneumology), University Hospital Tuebingen, Tuebingen, Germany],
Mrs. Andrea Schenk [Department of Vegetative and Clinical
Physiology, University Hospital Tuebingen, Tuebingen, Germany],
Mrs. Christine Geisler [Department of Internal Medicine I
(Gastroenterology, Gastroenterologic Oncology, Hepatology,
Infectiology and Geriatric Medicine), University Hospital
Tuebingen, Tuebingen, Germany], Dr Julia Beil [Department of
Internal Medicine VIII (Medical Oncology and Pneumology),
University Hospital Tuebingen], Dr. Martina Schell (Hain
Lifescience GmbH, Nehren, Germany) and Dr Frank Essmann
(Interfaculty Institute of Biochemistry, Tuebingen, Germany) for
their support of this work.
Funding
The present study was supported by the intramural
IZKF-scholarship of the Faculty of Medicine (University of
Tuebingen) awarded to VM, PASCOE pharmazeutische Praeparate GmbH, a
grant from the Else-Uebelmesser-Stiftung (grant no. D3021947), the
German Research Council (Deutsche Forschungsgemeinschaft) and the
Open Access Publishing Fund of University of Tuebingen.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
UML conceived and designed the study, interpreted
the data and reviewed the manuscript. VM performed the experiments
and was a major contributor in writing the manuscript. SB
contributed to the experiments and the interpretation of the data
as well as writing the manuscript. AB, SV and CL assisted with the
senescence experiments and the interpretation of the SRB viability
assay. MB and SV analyzed and interpreted the data. NPM interpreted
the data and reviewed the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
hpi
|
hours post infection
|
|
MeV
|
measles vaccine virus
|
|
MOI
|
multiplicity of infection (ratio
infectious particles/cell)
|
|
OV
|
oncolytic virus
|
|
PFU
|
plaque forming unit
|
References
|
1
|
Stathis A and Moore MJ: Advanced
pancreatic carcinoma: Current treatment and future challenges. Nat
Rev Clin Oncol. 7:163–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang P, Chubb S, Hertel LW, Grindey GB
and Plunkett W: Action of 2′,2′-difluorodeoxycytidine on DNA
synthesis. Cancer Res. 51:6110–6117. 1991.PubMed/NCBI
|
|
3
|
Gresham GK, Wells GA, Gill S, Cameron C
and Jonker DJ: Chemotherapy regimens for advanced pancreatic
cancer: A systematic review and network meta-analysis. BMC Cancer.
14:4712014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vacchelli E, Eggermont A, Sautès-Fridman
C, Galon J, Zitvogel L, Kroemer G and Galluzzi L: Trial watch:
Oncolytic viruses for cancer therapy. Oncoimmunology. 2:e246122013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sze DY, Reid TR and Rose SC: Oncolytic
virotherapy. J Vasc Interv Radiol. 24:1115–1122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Forbes NE, Krishnan R and Diallo JS:
Pharmacological modulation of anti-tumor immunity induced by
oncolytic viruses. Front Oncol. 4:1912014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chiocca EA and Rabkin SD: Oncolytic
viruses and their application to cancer immunotherapy. Cancer
Immunol Res. 2:295–300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakao A, Kasuya H, Sahin TT, Nomura N,
Kanzaki A, Misawa M, Shirota T, Yamada S, Fujii T, Sugimoto H, et
al: A phase I dose-escalation clinical trial of intraoperative
direct intratumoral injection of HF10 oncolytic virus in
non-resectable patients with advanced pancreatic cancer. Cancer
Gene Ther. 18:167–175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hecht JR, Bedford R, Abbruzzese JL, Lahoti
S, Reid TR, Soetikno RM, Kirn DH and Freeman SM: A phase I/II trial
of intratumoral endoscopic ultrasound injection of ONYX-015 with
intravenous gemcitabine in unresectable pancreatic carcinoma. Clin
Cancer Res. 9:555–561. 2003.PubMed/NCBI
|
|
10
|
Mulvihill S, Warren R, Venook A, Adler A,
Randlev B, Heise C and Kirn D: Safety and feasibility of injection
with an E1B-55 kDa gene-deleted, replication-selective adenovirus
(ONYX-015) into primary carcinomas of the pancreas: A phase I
trial. Gene Ther. 8:308–315. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wennier S, Li S and McFadden G: Oncolytic
virotherapy for pancreatic cancer. Expert Rev Mol Med. 13:e182011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Russell SJ, Federspiel MJ, Peng KW, Tong
C, Dingli D, Morice WG, Lowe V, O'Connor MK, Kyle RA, Leung N, et
al: Remission of disseminated cancer after systemic oncolytic
virotherapy. Mayo Clin Proc. 89:926–933. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carlson SK, Classic KL, Hadac EM, Dingli
D, Bender CE, Kemp BJ and Russell SJ: Quantitative molecular
imaging of viral therapy for pancreatic cancer using an engineered
measles virus expressing the sodium-iodide symporter reporter gene.
AJR Am J Roentgenol. 192:279–287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bossow S, Grossardt C, Temme A, Leber MF,
Sawall S, Rieber EP, Cattaneo R, von Kalle C and Ungerechts G:
Armed and targeted measles virus for chemovirotherapy of pancreatic
cancer. Cancer Gene Ther. 18:598–608. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ellerhoff TP, Berchtold S, Venturelli S,
Burkard M, Smirnow I, Wulff T and Lauer UM: Novel
epi-virotherapeutic treatment of pancreatic cancer combining the
oral histone deacetylase inhibitor resminostat with oncolytic
measles vaccine virus. Int J Oncol. 49:1931–1944. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Awano M, Fujiyuki T, Shoji K, Amagai Y,
Murakami Y, Furukawa Y, Sato H, Yoneda M and Kai C: Measles virus
selectively blind to signaling lymphocyte activity molecule has
oncolytic efficacy against nectin-4-expressing pancreatic cancer
cells. Cancer Sci. 107:1647–1652. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weiland T, Lampe J, Essmann F, Venturelli
S, Berger A, Bossow S, Berchtold S, Schulze-Osthoff K, Lauer UM and
Bitzer M: Enhanced killing of therapy-induced senescent tumor cells
by oncolytic measles vaccine viruses. Int J Cancer. 134:235–243.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berchtold S, Lampe J, Weiland T, Smirnow
I, Schleicher S, Handgretinger R, Kopp HG, Reiser J, Stubenrauch F,
Mayer N, et al: Innate immune defense defines susceptibility of
sarcoma cells to measles vaccine virus-based oncolysis. J Virol.
87:3484–3501. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Skehan P, Storeng R, Scudiero D, Monks A,
McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S and Boyd MR:
New colorimetric cytotoxicity assay for anticancer-drug screening.
J Natl Cancer Inst. 82:1107–1112. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kärber G: Beitrag zur kollektiven
Behandlung pharmakologischer Reihenversuche. Archiv für Experiment
Pathol und Pharmakol. 162:480–483. 1931. View Article : Google Scholar
|
|
22
|
Spearman C: The method of ‘Right and Wrong
Cases’ (Constant Stimuli) without Gauss's formula. Br J Psychol.
2:227–242. 1908.
|
|
23
|
Noll M, Berchtold S, Lampe J, Malek NP,
Bitzer M and Lauer UM: Primary resistance phenomena to oncolytic
measles vaccine viruses. Int J Oncol. 43:103–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wennier ST, Liu J, Li S, Rahman MM, Mona M
and McFadden G: Myxoma virus sensitizes cancer cells to gemcitabine
and is an effective oncolytic virotherapeutic in models of
disseminated pancreatic cancer. Mol Ther. 20:759–768. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krizhanovsky V, Xue W, Zender L, Yon M,
Hernando E and Lowe SW: Implications of cellular senescence in
tissue damage response, tumor suppression, and stem cell biology.
Cold Spring Harb Symp Quant Biol. 73:513–522. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rodier F and Campisi J: Four faces of
cellular senescence. J Cell Biol. 192:547–556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Skelding KA, Barry RD and Shafren DR:
Enhanced oncolysis mediated by Coxsackievirus A21 in combination
with doxorubicin hydrochloride. Invest New Drugs. 30:568–581. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Angelova AL, Aprahamian M, Grekova SP,
Hajri A, Leuchs B, Giese NA, Dinsart C, Herrmann A, Balboni G,
Rommelaere J and Raykov Z: Improvement of gemcitabine-based therapy
of pancreatic carcinoma by means of oncolytic parvovirus H-1PV.
Clin Cancer Res. 15:511–519. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eisenberg DP, Adusumilli PS, Hendershott
KJ, Yu Z, Mullerad M, Chan MK, Chou TC and Fong Y: 5-fluorouracil
and gemcitabine potentiate the efficacy of oncolytic herpes viral
gene therapy in the treatment of pancreatic cancer. J Gastrointest
Surg. 9:1068–1079. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hertel LW, Boder GB, Kroin JS, Rinzel SM,
Poore GA, Todd GC and Grindey GB: Evaluation of the antitumor
activity of gemcitabine (2′,2′-difluoro-2′-deoxycytidine). Cancer
Res. 50:4417–4422. 1990.PubMed/NCBI
|
|
31
|
Wang H, Chen NG, Minev BR, Zimmermann M,
Aguilar RJ, Zhang Q, Sturm JB, Fend F, Yu YA, Cappello J, et al:
Optical detection and virotherapy of live metastatic tumor cells in
body fluids with vaccinia strains. PLoS One. 8:e711052013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yurttas C, Berchtold S, Malek NP, Bitzer M
and Lauer UM: Pulsed versus continuous application of the prodrug
5-fluorocytosine to enhance the oncolytic effectiveness of a
measles vaccine virus armed with a suicide gene. Hum Gene Ther Clin
Dev. 25:85–96. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Singh HM, Ungerechts G and Tsimberidou AM:
Gene and cell therapy for pancreatic cancer. Expert Opin Biol Ther.
15:505–516. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zamarin D, Holmgaard RB, Subudhi SK, Park
JS, Mansour M, Palese P, Merghoub T, Wolchok JD and Allison JP:
Localized oncolytic virotherapy overcomes systemic tumor resistance
to immune checkpoint blockade immunotherapy. Sci Transl Med.
6:226ra322014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Engeland CE, Grossardt C, Veinalde R,
Bossow S, Lutz D, Kaufmann JK, Shevchenko I, Umansky V, Nettelbeck
DM, Weichert W, et al: CTLA-4 and PD-L1 checkpoint blockade
enhances oncolytic measles virus therapy. Mol Ther. 22:1949–1959.
2014. View Article : Google Scholar : PubMed/NCBI
|