Introduction
Hepatocellular carcinoma (HCC) is one of the most
prevalent primary malignancies of the liver (1). It is the fourth leading cause of
cancer-associated mortality worldwide (2), and the third leading cause in China
(3), where chronic hepatitis B virus
infection and aflatoxin exposure are major risk factors (2). Surgical resection is the primary method
of treating HCC, but due to rapid disease progression, most
patients exhibit extensive metastasis before the time of surgery.
Moreover, only ~20% of HCC patients undergo radical surgical
resection (4); following resection,
the 2-year HCC recurrence rate is as high as 55%, and most patients
develop unresectable metastatic disease (5). The 5-year survival rate of HCC patients
is <50% (6). Therefore,
identification of early indicator molecules for postoperative
recurrence and survival, and the improvement of long-term survival,
are urgently required.
Interleukins (ILs)-6, IL-8, IL-17 and IL-10, as well
as other inflammation- or immunity-related cytokines, have received
attention for their association with tumors. These cytokines can
either promote or inhibit the development of tumors (7), and are critical for assessing the risk
of postoperative recurrence and long-term survival. IL-8, also
known as CXC chemokine 8, has received increasing interest as a
tumor inflammatory factor. IL-8 is a member of the chemokine family
(8) and plays an important role in
the tumor microenvironment, influencing tumor progression and
regulating neovascularization, tumor cell growth, apoptosis and
cell migration (9,10). It is expressed in a variety of tumors
such as lung (11), breast (12) and colon cancer (13); a study by Wang et al (14) demonstrated that IL-8 expression is
elevated in HCC. IL-8 promotes tumor cellular proliferation and
neovascularization, either directly or indirectly, through tumor
vascular endothelial cell receptors, thereby promoting tumor growth
and metastasis (14,15).
IL-8 also induces the activation of the classical
mitogen-activated protein kinase (MAPK) signaling cascade, and
subsequent downstream phosphorylation of both extracellular
signal-regulated kinase (ERK)1 and ERK2 in neutrophils and tumor
cells (16). ERK1/2 are key members
of the MAPK family, whose activation is closely associated with the
occurrence and development of various tumors (17,18).
Furthermore, Schmitz et al (19) detected high expression levels of
ERK1/2 in HCC tissues, and ERK1/2 activation in HCC also
constitutes an independent prognostic factor. Although these data
reveal a possible role for IL-8 and ERK1/2 in tumor progression,
the relationship between IL-8 and/or ERK2 expression in HCC
tissues, and postoperative recurrence and survival, remains
unclear.
To further study the relationship between IL-8
and/or ERK2 levels in HCC, and recurrence and survival after
hepatectomy, the expression levels of IL-8 and ERK2 in non-tumor
liver tissues and HCC tissues were determined using the Oncomine™
database and immunohistochemistry (IHC). Subsequently, reverse
transcription-quantitative (RT-q) PCR was used to quantify IL-8 and
ERK2 expression in the tumor tissues of 67 patients with HCC, and
their relationship with HCC clinical pathological features was then
determined. This in-depth study of the risk factors of HCC
recurrence and survival provides a theoretical basis for improving
the long-term survival of patients with HCC.
Materials and methods
Data retrieval from the Oncomine™
database
To investigate the clinical importance of IL-8 and
ERK2 in HCC, Oncomine™ [https://www.oncomine.org/resource/main.html; GSE14323
(GPL571) and GSE14520 (GPL3921)] was searched for published data to
analyze the mRNA expression levels of IL-8 and ERK2 in HCC tissues
and non-tumor liver tissues (20,21).
Patient information
The use of patient samples in the present study was
approved by The Hunan Normal University Medical Ethics Committee,
and accords with the provisions stated in The Declaration of
Helsinki, as revised in 2013. A total of 67 frozen HCC specimens
were collected from patients who underwent surgery at the
Department of General Surgery, Affiliated Changsha Hospital, Hunan
Normal University between January 2002 and December 2012. An
additional 60 paraffin-embedded HCC tissues and adjacent non-tumor
liver tissues were collected. The inclusion criteria were: i) R0
tumor resection and ii) postoperative pathology confirmed as HCC.
The exclusion criteria were: i) Administration of any anti-cancer
treatment before surgery; ii) serious complications or death within
30 days post-surgery; iii) non-tumor related mortality; and iv)
incomplete clinical, pathological or surgical data. The 67 HCC
patients were aged between 36 and 83 years, with a median age of
55.0 years. There was a total of 55 men and 12 women, with a
male-to-female ratio of 4.58:1. According to the TNM staging
detailed in the eighth edition of the American Joint Committee on
Cancer (AJCC) Cancer Staging Manual (22), 42 patients had stage I tumors, 18 had
stage II tumors, and 7 had stage III tumors.
IHC
All patient specimens were fixed in 10% neutral
formalin, embedded in paraffin, cut into 4-µm-thick serial sections
and stained as previously described (23). The primary antibody against IL-8 was
purchased from R&D Systems, Inc., (1:500; cat. no. AF-208-NA),
and the primary antibody against ERK2 was purchased from Santa Cruz
Biotechnology, Inc., (1:200, cat. no. SC-1647). Expression levels
were scored as the proportion of the immuno-positive staining area
(0, 0%; 1, 1–25%; 2, 26–50%; and 3, 51–100%) multiplied by the
staining intensity (0, negative; 1, low; 2, medium; 3, high), and
ranged from 0 to 9. The scores were independently evaluated by two
pathologists.
RT-qPCR
Total RNA was extracted from the frozen HCC tissues
using TRIzol® (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Subsequently,
reverse transcription was performed using the PrimeScript™ RT kit
(Takara Bio, Inc.) according to the manufacturer's protocols. SYBR
Premix EX Taq™ (Takara Bio, Inc.) was used for qPCR (according to
the manufacturer's protocol) on an ABI 7900 Prism HT (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Relative gene
expression was quantified using the 2−ΔΔCq method
(24), and the patients were divided
into high- and low-IL-8 and -ERK2 expression groups using the
median expression value as the cut-off point. The PCR primers were
as follows: IL-8 forward, 5′-AAGAAACCACCGGAAGGAAC-3′, and reverse,
5′-ACTCCTTGGCAAAACTGCAC-3′; ERK2 forward,
5′-GAAGGTGCCTACGGGATGG-3′, and reverse, 5′-GGTCAATGGTTGGTGTGCGG-3′;
and GAPDH forward, 5′-AACAGCCTCAAGATCATCAGCA-3′, and reverse,
5′-CATGAGTCCTTCCACGATACCA-3′. The thermocycling conditions were as
follows: Initial denaturation for 30 sec at 98°C, followed by 24
cycles of 98°C for 15 sec and 72°C for 30 sec, and lastly 72°C for
5 min to allow final extension before cooling to 4°C.
Case follow-up
Follow-ups in the form of outpatient visits or
telephone calls were conducted for all cases that met the study
criteria. The regular follow-up plan was as follows: i) Review
every 3 months within 2 years after surgery; ii) review every 6
months after 2–5 years; and iii) review every year after 5 years.
The review included an analysis of liver function, an abdominal
ultrasound and a chest radiograph. If required, enhanced computed
tomography, magnetic resonance imaging and needle biopsy were
performed. Recurrence was defined as confirmation of the presence
of new lesions in or outside of the liver via imaging studies or
biopsy. Overall survival (OS) was defined as the time between the
date of surgery to death or follow-up, and disease-free survival
(DFS) was defined as the date of surgery to relapse or follow-up.
Both OS and DFS were calculated on a monthly basis, and the
follow-up deadline was December 2018.
Statistical analysis
Statistical processing was performed using SPSS
software v.19.0 (IBM Corp.). Spearman's correlation analysis was
used to calculate the correlation between IL-8 and ERK2 expression
levels. The χ2 test and Fisher's exact probability test
were used to analyze the correlation between IL-8 and ERK2
expression in HCC tissues, and patient clinicopathological
features. Survival analysis was performed using Kaplan-Meier
curves, and the relationship between IL-8 and/or ERK2 expression
and postoperative recurrence, and survival in patients with HCC was
determined using the log-rank test. Univariate and multivariate
analyses of HCC recurrence and survival were performed using the
Cox proportional hazard model to screen for variables. The data are
presented as the mean ± standard deviation, and P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of IL-8 and ERK2 in
non-tumor liver tissues and HCC tissues
The mRNA expression levels of IL-8 and ERK2 were
retrieved from two published HCC datasets published in the
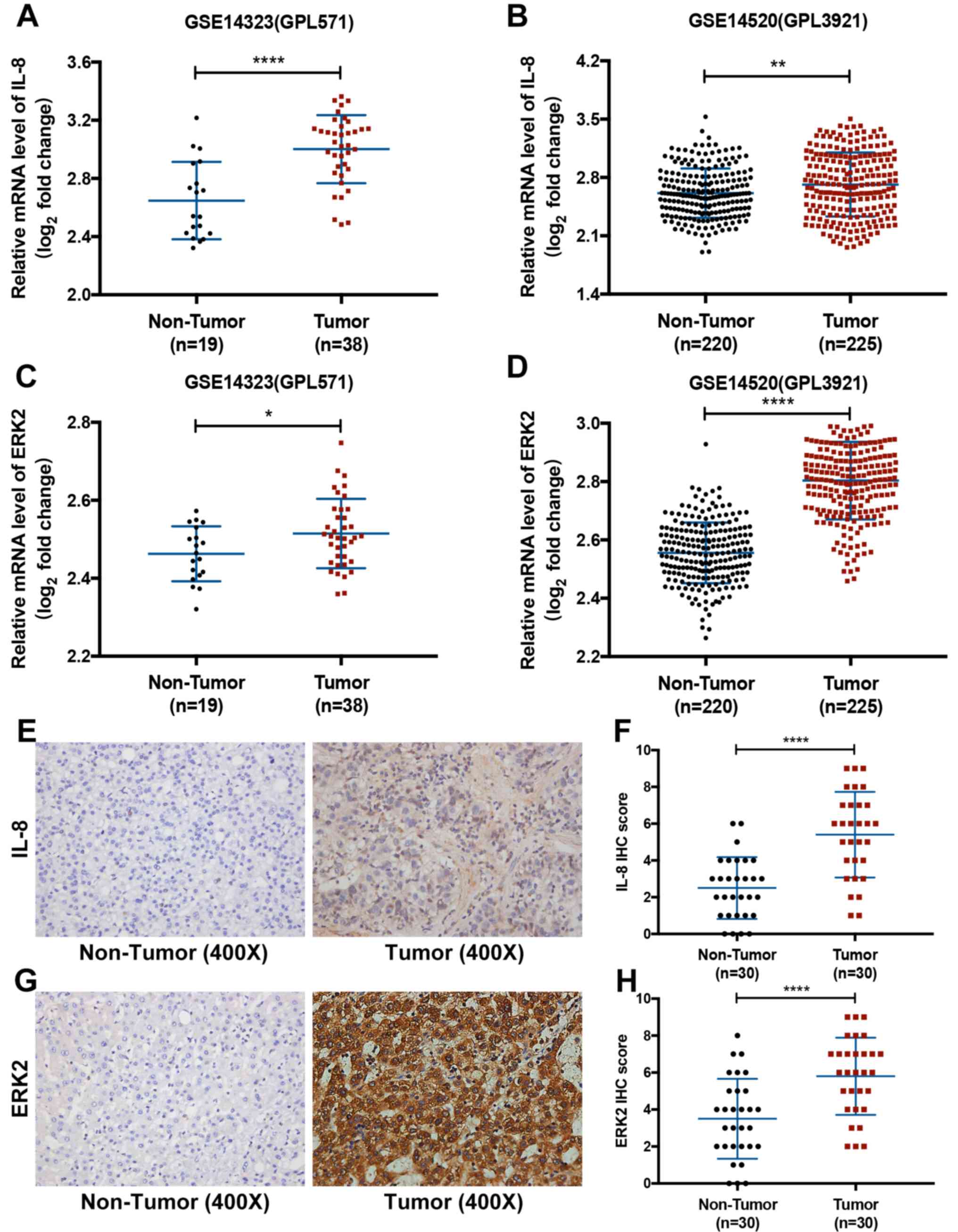
Oncomine™ database. It was observed that the mRNA levels of both
IL-8 and ERK2 were significantly higher in HCC tissues compared
with non-tumor liver tissues from both datasets (P<0.05;
Fig. 1A-D). Subsequently, this
result was validated in patient samples using IHC. The protein
expression levels of IL-8 and ERK2 were significantly higher in HCC
tissues compared with non-tumor liver tissues (P<0.05; Fig. 1E-H).
Relationship between IL-8 and/or ERK2
expression and the clinicopathological features of HCC
IL-8 and ERK2 exhibited high expression rates in
43.28 (29/67) and 34.33% (23/67) of the HCC tissues, respectively.
Additionally, Pearson's correlation analysis indicated a positive
correlation between IL-8 and ERK2 expression (r=0.764; P<0.001;
Table I). Although no significant
correlation was observed between IL-8 expression and
clinicopathological features in HCC tissues, ERK2 expression was
significantly associated with both tumor size and differentiation
(P<0.05; Table II). Moreover,
IL-8 and ERK2 co-expression was also significantly associated with
tumor size and differentiation (P<0.05; Table II).
 | Table I.Association between mRNA expression
levels of IL-8 and ERK2. |
Table I.
Association between mRNA expression
levels of IL-8 and ERK2.
|
| IL-8 expression |
|
|
|---|
|
|
|
|
|
|---|
| ERK2 expression | High | Low | Pearson's contingency
coefficient | P-value |
|---|
| High | 22 | 1 | 0.764 |
<0.001a |
| Low | 7 | 37 |
|
|
 | Table II.Correlations of IL-8 and ERK2 mRNA
expression with clinicopathological characteristics. |
Table II.
Correlations of IL-8 and ERK2 mRNA
expression with clinicopathological characteristics.
|
| IL-8 |
| ERK2 |
| IL-8 and ERK2 |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Parameter | High | Low | P-value | High | Low | P-value | Both high | Others | P-value |
|---|
| Sex |
|
| 0.158 |
|
| 0.678 |
|
| 0.765 |
|
Male | 26 | 29 |
| 20 | 35 |
| 19 | 36 |
|
|
Female | 3 | 9 |
| 3 | 9 |
| 3 | 9 |
|
| Age |
|
| 0.372 |
|
| 0.940 |
|
| 0.747 |
| <50,
years | 7 | 13 |
| 7 | 13 |
| 6 | 14 |
|
| ≥50,
years | 22 | 25 |
| 16 | 31 |
| 16 | 31 |
|
| Alcoholism |
|
| 0.438 |
|
| 0.180 |
|
| 0.325 |
|
Absence | 18 | 27 |
| 13 | 32 |
| 13 | 32 |
|
|
Presence | 11 | 11 |
| 10 | 12 |
| 9 | 13 |
|
| HBV |
|
| 0.623 |
|
| 1.000 |
|
| 1.000 |
|
Positive | 23 | 33 |
| 19 | 37 |
| 18 | 38 |
|
|
Negative | 6 | 5 |
| 4 | 7 |
| 4 | 7 |
|
| Cirrhosis |
|
| 0.502 |
|
| 1.000 |
|
| 1.000 |
|
Absence | 0 | 2 |
| 1 | 1 |
| 0 | 2 |
|
|
Presence | 29 | 36 |
| 22 | 43 |
| 22 | 43 |
|
| AFP |
|
| 0.660 |
|
| 0.634 |
|
| 0.958 |
| ≤400
µg/l | 22 | 27 |
| 16 | 33 |
| 16 | 33 |
|
| >400
µg/l | 7 | 11 |
| 7 | 11 |
| 6 | 12 |
|
| Albumin |
|
| 0.669 |
|
| 1.000 |
|
| 1.000 |
| <35
g/l | 2 | 5 |
| 2 | 5 |
| 2 | 5 |
|
| ≥35
g/l | 27 | 33 |
| 21 | 39 |
| 20 | 40 |
|
| ALT |
|
| 0.401 |
|
| 0.848 |
|
| 0.435 |
| ≤60
U/l | 26 | 30 |
| 20 | 36 |
| 20 | 36 |
|
| >60
U/l | 3 | 8 |
| 3 | 8 |
| 2 | 9 |
|
| AST |
|
| 0.839 |
|
| 0.923 |
|
| 0.728 |
| ≤42
U/l | 22 | 28 |
| 17 | 33 |
| 17 | 33 |
|
| >42
U/l | 7 | 10 |
| 6 | 11 |
| 5 | 12 |
|
| PLT |
|
| 0.352 |
|
| 0.923 |
|
| 0.803 |
|
<100×109/l | 9 | 8 |
| 6 | 11 |
| 6 | 11 |
|
|
≥100×109/l | 20 | 30 |
| 17 | 33 |
| 16 | 34 |
|
| Bilirubin |
|
| 0.623 |
|
| 1.000 |
|
| 1.000 |
| ≤22
µmol/l | 23 | 33 |
| 19 | 37 |
| 18 | 38 |
|
| >22
µmol/l | 6 | 5 |
| 4 | 7 |
| 4 | 7 |
|
| Tumor number |
|
| 0.662 |
|
| 1.000 |
|
| 1.000 |
|
Single | 24 | 34 |
| 20 | 38 |
| 19 | 39 |
|
|
Multiple | 5 | 4 |
| 3 | 6 |
| 3 | 6 |
|
| Tumor size |
|
| 0.117 |
|
| 0.013a |
|
| 0.019a |
| ≤5
cm | 19 | 32 |
| 13 | 38 |
| 12 | 39 |
|
| >5
cm | 9 | 6 |
| 9 | 6 |
| 9 | 6 |
|
| Tumor margin |
|
| 0.981 |
|
| 0.627 |
|
| 0.762 |
| ≤2
cm | 10 | 13 |
| 7 | 16 |
| 7 | 16 |
|
| >2
cm | 19 | 25 |
| 16 | 28 |
| 15 | 29 |
|
| Pathological
differentiation |
|
| 0.134 |
|
| 0.014a |
|
| 0.008a |
|
High | 19 | 31 |
| 13 | 37 |
| 12 | 38 |
|
| Middle
and low | 10 | 7 |
| 10 | 7 |
| 10 | 7 |
|
| Microvascular tumor
thrombus |
|
| 0.469 |
|
| 0.524 |
|
| 0.415 |
|
Yes | 10 | 10 |
| 8 | 12 |
| 8 | 12 |
|
| No | 19 | 28 |
| 15 | 32 |
| 14 | 33 |
|
| Capsule
invasion |
|
| 0.971 |
|
| 0.846 |
|
| 0.951 |
|
Yes | 23 | 30 |
| 19 | 34 |
| 18 | 35 |
|
| No | 6 | 8 |
| 4 | 10 |
| 4 | 10 |
|
| TNM stage |
|
| 0.236 |
|
| 0.078 |
|
| 0.061 |
|
I+II | 24 | 36 |
| 18 | 42 |
| 17 | 43 |
|
|
III | 5 | 2 |
| 5 | 2 |
| 5 | 2 |
|
Relationship between IL-8 and/or ERK2
expression and postoperative prognosis
In the data from the 67 HCC samples, the median
follow-up time was 49.56±25.79 months (range, 1.8–78.1 months). The
OS rates of patients at 1, 3 and 5 years were 85.08, 65.67 and
58.21%, respectively, while DFS rates were 67.16, 49.25 and 40.30%,
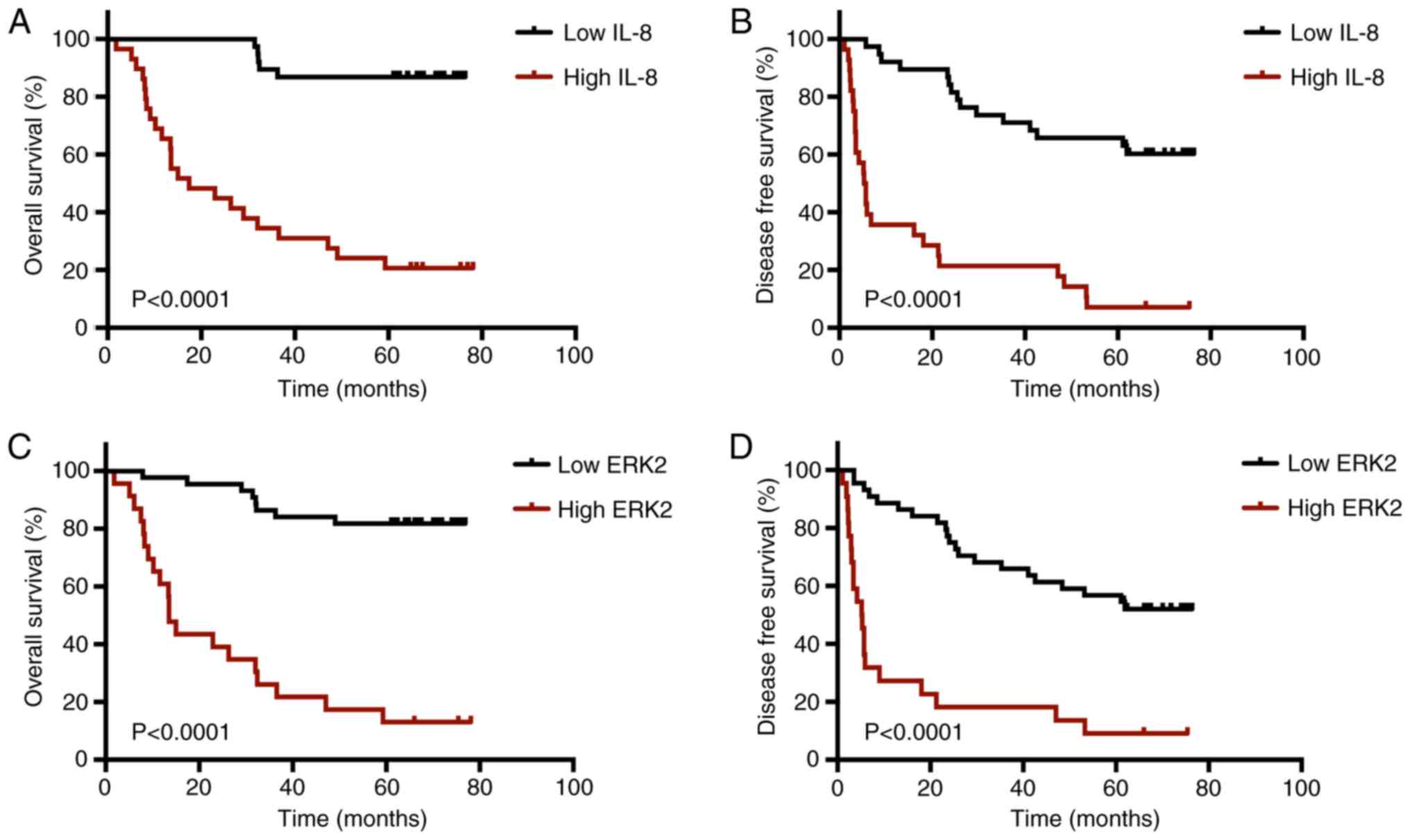
respectively (Data not shown). Kaplan-Meier survival analysis of 67
patients with HCC exhibited significantly shorter OS (P<0.0001;
Fig. 2A) and DFS (P<0.0001;
Fig. 2B) times in the IL-8
high-expression group compared with the low-expression group.
Furthermore, the ERK2 high-expression group had shorter OS
(P<0.0001; Fig. 2C) and DFS
(P<0.0001; Fig. 2D) times than
the corresponding low-expression group. Based on these results, the
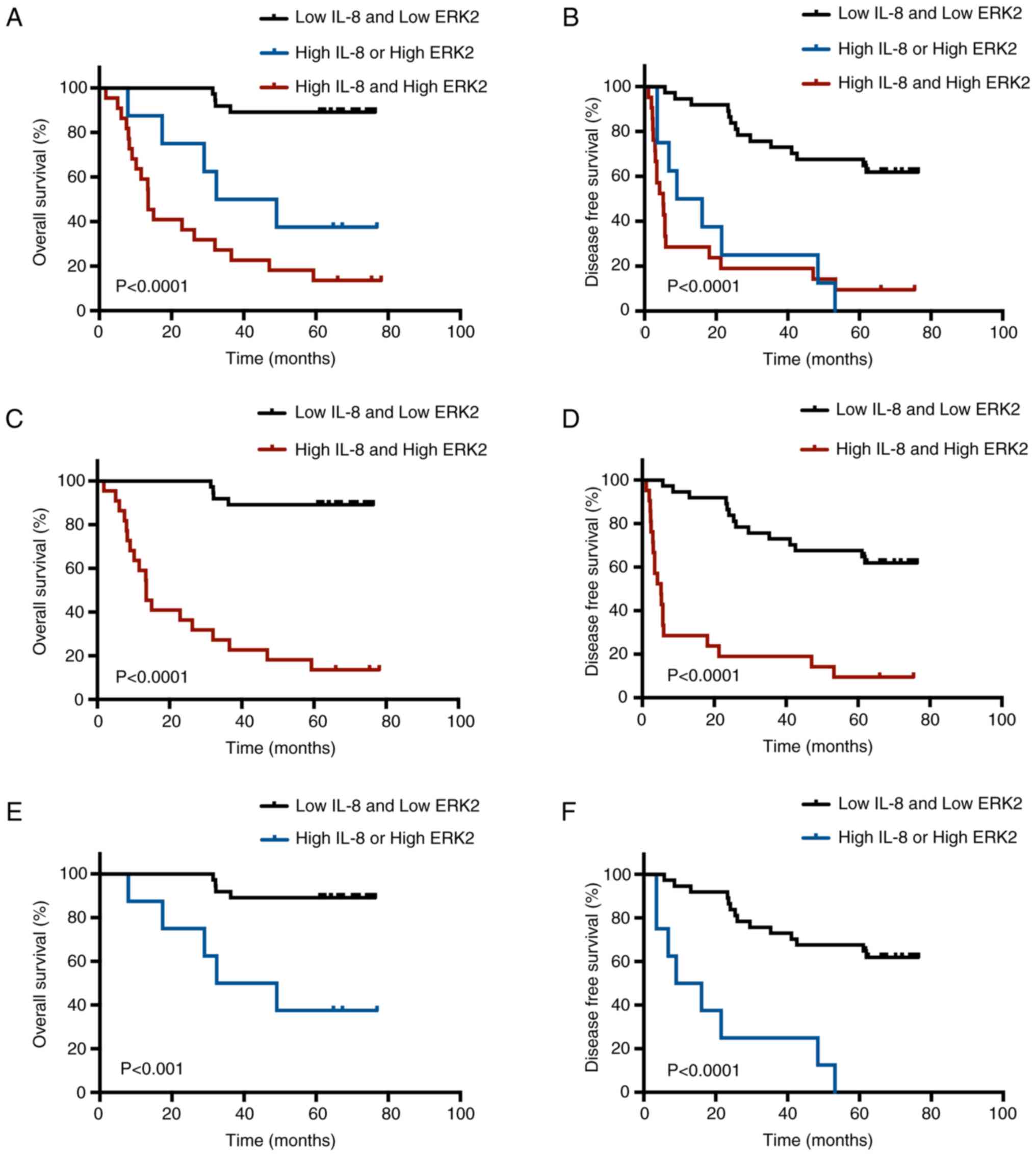
67 patients with HCC were divided into three groups for further
Kaplan-Meier analysis (low IL-8 and low ERK2 group, high IL-8 or
high ERK2 group, and high IL-8 and high ERK2 group). OS
(P<0.001; Fig. 3A, C and E) and
DFS (P<0.0001; Fig. 3B, D and F)
were significantly shorter in the IL-8 and/or ERK2 high-expression
group than in the low-expression group.
Given the significant correlation between IL-8 and
ERK2 expression, univariate and multivariate Cox proportional
hazard analyses were performed on the IL-8 expression group, the
ERK2 expression group and the IL-8 and ERK2 expression group.
Further multivariate Cox regression analysis was then performed on
the significant factors identified from univariate analysis
(Tables III and IV). The multivariate Cox regression
analysis of the IL-8 expression group showed that TNM stage III
[hazard ratio (HR)=6.246; 95% confidence interval (CI),
2.233–17.471; P<0.001] and high IL-8 expression (HR=12.369; 95%
CI, 4.589–33.341; P<0.001) were independent risk factors for OS
(Table V), while a platelet count
(PLT) <100×109/l (HR=2.106; 95% CI, 1.071–4.141;
P=0.031), TNM stage III (HR=3.477; 95% CI, 1.367–8.847; P=0.009)
and high-IL-8 expression (HR=6.620; 95% CI, 3.350–13.084;
P<0.001) were all independent risk factors for DFS (Table VI). Multivariate Cox regression
analysis of the ERK2 expression group showed that TNM stage III
(HR=4.832; 95% CI, 1.760–13.269; P=0.002) and high ERK2 expression
(HR=10.011; 95% CI, 4.268–23.479; P<0.001) were independent risk
factors for OS (Table V), while
PLT<100 109/l (HR=2.171; 95% CI, 1.115–4.226;
P=0.023), TNM stage III (HR=2.873; 95% CI, 1.153–7.156; P=0.023)
and high ERK2 expression (HR=5.263; 95% CI, 2.760–10.036;
P<0.001) were independent risk factors for DFS (Table VI). Furthermore, multivariate Cox
regression models of the IL-8 and ERK2 expression groups showed
that TNM stage III (OS: HR=4.595; 95% CI, 1.682–12.549; P=0.003.
DFS: HR=2.821; 95% CI, 1.134–7.017; P=0.026) with IL-8 and ERK2
co-expression (OS: HR=9.082; 95% CI, 3.974–20.757; P<0.001. DFS:
HR=4.918; 95% CI, 2.586–9.355; P<0.001) was an independent risk
factor for both OS and DFS (Tables V
and VI). PLT<100
109/l (HR=2.101; 95% CI, 1.080–4.086; P=0.029) was also
shown to be an independent risk factor for DFS (Table VI).
 | Table III.Univariate analysis of variables with
patient overall survival (Cox regression model). |
Table III.
Univariate analysis of variables with
patient overall survival (Cox regression model).
|
| Univariate
analysis |
|---|
|
|
|
|---|
| Variable | Hazard ratio | 95% CI | P-value |
|---|
| Sex (male vs.
female) | 2.143 | 0.647–7.101 | 0.212 |
| Age (≥50 vs.
<50, years) | 1.046 | 0.460–2.375 | 0.915 |
| HBV (positive vs.
negative) | 2.110 | 0.895–4.975 | 0.088 |
| Cirrhosis (present
vs. absent) | 1.053 | 0.143–7.756 | 0.960 |
| AFP (>400 µg/l
vs. ≤400 µg/l) | 1.205 | 0.531–2.737 | 0.655 |
| Albumin (>35 g/l
vs. ≥35 g/l) | 1.159 | 0.350–3.840 | 0.809 |
| ALT (>60 U/l vs.
≤60 U/l) | 1.352 | 0.547–3.340 | 0.513 |
| AST (>42 U/l vs.
≤42 U/l) | 1.859 | 0.856–4.035 | 0.117 |
| PLT
(<100×109/l vs. ≥100×109/l) | 1.992 | 0.918–4.323 | 0.081 |
| Bilirubin (>20
µmol/l vs. ≤20 µmol/l) | 1.740 | 0.704–4.302 | 0.231 |
| Tumor number
(multiple vs. single) | 1.799 | 0.683–4.740 | 0.235 |
| Tumor size (>5
cm vs. ≤5 cm) | 2.580 | 1.187–5.606 | 0.017a |
| Tumor margin (≤2 cm
vs. >2 cm) | 1.052 | 0.476–2.326 | 0.900 |
| Pathological
differentiation (middle and low vs. high) | 1.800 | 0.813–3.987 | 0.147 |
| Microvascular tumor
thrombus (yes vs. no) | 1.347 | 0.609–2.979 | 0.462 |
| Capsule invasion
(yes vs. no) | 1.063 | 0.431–2.623 | 0.894 |
| TNM stage (III vs.
I+II) | 5.364 | 2.123–13.554 |
<0.001a |
| IL-8 (positive vs.
negative) | 11.618 | 4.373–30.863 |
<0.001a |
| ERK2 (positive vs.
negative) | 10.090 | 4.366–23.317 |
<0.001a |
| IL-8 and ERK2 (both
vs. others) | 3.938 | 2.424–6.397 |
<0.001a |
 | Table IV.Univariate analysis of variables with
patient disease-free survival (Cox regression model). |
Table IV.
Univariate analysis of variables with
patient disease-free survival (Cox regression model).
|
| Univariate
analysis |
|---|
|
|
|
|---|
| Variable | Hazard ratio | 95% CI | P-value |
|---|
| Sex (male vs.
female) | 1.567 | 0.660–3.719 | 0.309 |
| Age (≥50 vs.
<50, years) | 1.631 | 0.867–3.069 | 0.129 |
| HBV (positive vs.
negative) | 1.500 | 0.693–3.244 | 0.304 |
| Cirrhosis (present
vs. absent) | 1.380 | 0.190–10.042 | 0.750 |
| AFP (>400 µg/l
vs. ≤400 µg/l) | 1.537 | 0.798–2.960 | 0.198 |
| Albumin (<35 g/l
vs. ≥35 g/l) | 1.008 | 0.360–2.826 | 0.988 |
| ALT (>60 U/l vs.
≤60 U/l) | 1.064 | 0.472–2.398 | 0.882 |
| AST (>42 U/l vs.
≤42 U/l) | 1.209 | 0.607–2.411 | 0.589 |
| PLT
(<100×109/l vs. ≥100×109/l) | 1.940 | 1.015–3.708 | 0.045a |
| Bilirubin (>20
µmol/l vs. ≤20 µmol/l) | 1.400 | 0.648–3.028 | 0.392 |
| Tumor number
(multiple vs. single) | 1.766 | 0.783–3.983 | 0.171 |
| Tumor size (>5
cm vs. ≤5 cm) | 2.070 | 1.075–3.987 | 0.030a |
| Tumor margin (≤2 cm
vs. >2 cm) | 1.157 | 0.601–2.226 | 0.663 |
| Pathological
differentiation (middle and low vs. high) | 1.085 | 0.532–2.212 | 0.822 |
| Microvascular tumor
thrombus (yes vs. no) | 1.064 | 0.544–2.079 | 0.856 |
| Capsule invasion
(yes vs. no) | 1.088 | 0.520–2.276 | 0.822 |
| TNM stage (III vs.
I+II) | 3.071 | 1.284–7.342 | 0.012a |
| IL-8 (positive vs.
negative) | 6.098 | 3.172–11.720 |
<0.001a |
| ERK2 (positive vs.
negative) | 5.048 | 2.701–9.433 |
<0.001a |
| IL-8 and ERK2 (both
vs. others) | 2.607 | 1.868–3.639 |
<0.001a |
 | Table V.Multivariate analysis of variables
with patient overall survival (Cox regression model). |
Table V.
Multivariate analysis of variables
with patient overall survival (Cox regression model).
| Variable | Hazard ratio | 95% CI | P-value |
|---|
| IL-8 |
|
|
|
| TNM
stage (III vs. I+II) | 6.246 | 2.233–17.471 |
<0.001a |
| IL-8
(high vs. low) | 12.369 | 4.589–33.341 |
<0.001a |
| ERK2 |
|
|
|
| TNM
stage (III vs. I+II) | 4.832 | 1.760–13.269 | 0.002a |
| ERK2
(high vs. low) | 10.011 | 4.268–23.479 |
<0.001a |
| IL-8 and ERK2 |
|
|
|
| TNM
stage (III vs. I+II) | 4.595 | 1.682–12.549 | 0.003a |
| IL-8
and ERK2 (both vs. others) | 9.082 | 3.974–20.757 |
<0.001a |
 | Table VI.Multivariate analysis of variables
with patient disease-free survival (Cox regression model). |
Table VI.
Multivariate analysis of variables
with patient disease-free survival (Cox regression model).
| Variable | Hazard ratio | 95% CI | P-value |
|---|
| IL-8 |
|
|
|
| PLT
(<100×109/l vs. | 2.106 | 1.071–4.141 | 0.031a |
|
≥100×109/l) |
|
|
|
| TNM
stage (III vs. I+II) | 3.477 | 1.367–8.847 | 0.009a |
| IL-8
(high vs. low) | 6.620 | 3.350–13.084 |
<0.001a |
| ERK2 |
|
|
|
| PLT
(<100×109/l vs. | 2.171 | 1.115–4.226 | 0.023a |
|
≥100×109/l) |
|
|
|
| TNM
stage (III vs. I+II) | 2.873 | 1.153–7.156 | 0.023a |
| ERK2
(high vs. low) | 5.263 | 2.760–10.036 |
<0.001a |
| IL-8 and ERK2 |
|
|
|
| PLT
(<100×109/l vs. | 2.101 | 1.080–4.086 | 0.029a |
|
≥100×109/l) |
|
|
|
| TNM
stage (III vs. I+II) | 2.821 | 1.134–7.017 | 0.026a |
| IL-8
and ERK2 (both vs. others) | 4.918 | 2.586–9.355 |
<0.001a |
Discussion
The present study demonstrated that HCC patients
with high IL-8 and/or ERK2 expression had significantly higher
risks of recurrence and death than those with low expression. The
Oncomine™ database and IHC were used to show that IL-8 and ERK2
expression were significantly higher in HCC tissues compared with
non-tumor liver tissues. A positive correlation was also found
between IL-8 and ERK2 expression in tissues from HCC patients using
RT-qPCR. Moreover, IL-8 and/or ERK2 expression was significantly
associated with tumor size and differentiation, and patients with
high IL-8 and/or ERK2 expression had a poorer prognosis than the
low expression group. Multivariate survival analysis further
supported the high expression of IL-8 and/or ERK2 in HCC as an
independent risk factor for OS and DFS.
The important role played by inflammation and the
immune response in the occurrence and development of tumors has
gradually become more recognized. A previous study reported that
IL-8 is closely associated with the occurrence and development of
HCC (25). In the present study, it
was observed that IL-8 was expressed to a significantly higher
level in HCC tissues, compared with non-tumor liver tissues,
consistent with the results of a previous study (14). Schmitz et al (19) detected high expression levels of
ERK1/2 in patient HCC tissues, and this was also detected in the
HCC tissues examined in the present study. Waugh and Wilson
(16) reported that IL-8 in tumor
cells was able to promote ERK1/2 phosphorylation, thereby promoting
tumor growth and metastasis. A significant positive correlation
between IL-8 and ERK2 in HCC tissues was also detected, indicating
that high expression of IL-8 influences disease progression by
activating ERK2.
There is increasing evidence that serum IL-8 levels
are an effective predictor of prognosis in patients with HCC
(26), pancreatic cancer (27) and lymphoma (28), and that high nuclear ERK2 expression
is an indicator of poor prognosis in patients with invasive breast
cancer (29). However, these studies
have been limited to the use of serum IL-8, and the relationship
between IL-8 and/or ERK2 expression in HCC tissues and patient
prognosis is poorly understood. The present study found that
patients with high expression levels of IL-8 and/or ERK2 in HCC
tissues had a worse prognosis than those in the low expression
group. In addition, high expression levels of IL-8 and/or ERK2 in
HCC tissues were shown to be an independent risk factor for OS and
DFS in HCC. These data provide new insights for research into HCC
progression, which may offer novel targets for the development of
anti-tumor drugs.
The cytokine network is a complex system, with
cytokine gene expression affected by other cytokines and the
existence of synergistic restrictions and inhibition (30,31). As
well as elevated IL-8 levels, patients with HCC also exhibit
characteristic changes in expression of various cytokines, such as
decreased IL-2 or elevated IL-6 and tumor necrosis factor-α
(32–34). Therefore, in addition to the
application of IL-8 and ERK2 as early indicator molecules, the
deregulation of other cytokines in HCC should be examined to
advance disease understanding, diagnosis and treatment. Moreover,
because the present study is a single-center study of a small
sample size, future studies of larger sample populations should be
investigated to confirm these findings.
In summary, a significant positive correlation
between the expression of IL-8 and ERK2 in HCC tissues was
reported. It was also found that high expression levels of IL-8
and/or ERK2 in HCC tissues were an independent risk factor for OS
and DFS, and that HCC patients with high IL-8 and/or ERK2
expression had worse prognoses than those with low expression. The
results of the present study support the hypothesis that patients
with high expression of IL-8 and/or ERK2 should undergo more
frequent follow-ups. Additionally, IL-8 and ERK2 are potential
predictors of postoperative prognosis in patients with HCC, and
therefore, may be used as therapeutic targets for the development
of drugs that prevent HCC recurrence, thereby improving the
long-term survival of patients with HCC.
Acknowledgements
The authors would like to thank Dr Sarah Williams
(University of Oxford, Oxford, UK) for her assistance in revising
this manuscript.
Funding
The present study was supported by the Hunan
Provincial Natural & Science Foundation (grant no.
2018JJ6126).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
Study conception and design were conducted by GH,
who also provided administrative support. YD, QY and BH provided
the study materials. YD, QY, BH and ZH collected and assembled the
data, and data analysis and interpretation were undertaken by YD
and ZN. The manuscript was written and approved by all of the
authors.
Ethics approval and consent to
participate
The use of materials in the present study was
approved by the Hunan Normal University Medical Ethics Committee,
and all patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gao J, Xie L, Yang WS, Zhang W, Gao S,
Wang J and Xiang YB: Risk factors of hepatocellular
carcinoma-Current status and perspectives. Asian Pac J Cancer Prev.
13:743–752. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hong J, Hu K, Yuan Y, Sang Y, Bu Q, Chen
G, Yang L, Li B, Huang P, Chen D, et al: CHK1 targets spleen
tyrosine kinase (L) for proteolysis in hepatocellular carcinoma. J
Clin Inves. 122:2165–2175. 2012. View
Article : Google Scholar
|
|
5
|
Cha C, Fong Y, Jarnagin WR, Blumgart LH
and DeMatteo RP: Predictors and patterns of recurrence after
resection of hepatocellular carcinoma. J Am Coll Surg. 197:753–758.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bosch FX, Ribes J, Diaz M and Cleries R:
Primary liver cancer: Worldwide incidence and trends.
Gastroenterology. 127:S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin WW and Karin M: A cytokine-mediated
link between innate immunity, inflammation, and cancer. J Clin
Invest. 117:1175–1183. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zlotnik A and Yoshie O: Chemokines: A new
classification system and their role in immunity. Immunity.
12:121–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan A, Chen JJ, Yao PL and Yang PC: The
role of interleukin-8 in cancer cells and microenvironment
interaction. Front Biosci. 10:853–865. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lazennec G and Richmond A: Chemokines and
chemokine receptors: New insights into cancer-related inflammation.
Trends Mol Med. 16:133–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Wang L, Zhang M, Jin M, Bai C and
Wang X: Potential mechanism of interleukin-8 production from lung
cancer cells: An involvement of EGF-EGFR-PI3K-Akt-Erk pathway. J
Cell Physiol. 227:35–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mohamed MM: Monocytes conditioned media
stimulate fibronectin expression and spreading of inflammatory
breast cancer cells in three-dimensional culture: A mechanism
mediated by IL-8 signaling pathway. Cell Commun Signal. 10:32012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ning Y, Labonte MJ, Zhang W, Bohanes PO,
Gerger A, Yang D, Benhaim L, Paez D, Rosenberg DO, Nagulapalli
Venkata KC, et al: The CXCR2 antagonist, SCH-527123, shows
antitumor activity and sensitizes cells to oxaliplatin in
preclinical colon cancer models. Mol Cancer Ther. 11:1353–1364.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Wang W, Wang L, Wang X and Xia J:
Regulatory mechanisms of interleukin-8 production induced by tumour
necrosis factor-α in human hepatocellular carcinoma cells. J Cell
Mol Med. 16:496–506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harimoto N, Shirabe K, Abe T, Kajiyama K,
Nagaie T, Gion T, Kuroda Y and Maehara Y: Interleukin-8 producing
hepatocellular carcinoma with pyrexia. HPB Surg. 2009:4614922009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Waugh DJ and Wilson C: The interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cowley S, Paterson H, Kemp P and Marshall
CJ: Activation of MAP kinase kinase is necessary and sufficient for
PC12 differentiation and for transformation of NIH 3T3 cells. Cell.
77:841–852. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mansour SJ, Matten WT, Hermann AS, Candia
JM, Rong S, Fukasawa K, Vande Woude GF and Ahn NG: Transformation
of mammalian cells by constitutively active MAP kinase kinase.
Science. 265:966–970. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmitz KJ, Wohlschlaeger J, Lang H,
Sotiropoulos GC, Malago M, Steveling K, Reis H, Cicinnati VR,
Schmid KW and Baba HA: Activation of the ERK and AKT signalling
pathway predicts poor prognosis in hepatocellular carcinoma and ERK
activation in cancer tissue is associated with hepatitis C virus
infection. J Hepatol. 48:83–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mas VR, Maluf DG, Archer KJ, Yanek K, Kong
X, Kulik L, Freise CE, Olthoff KM, Ghobrial RM, McIver P and Fisher
R: Genes involved in viral carcinogenesis and tumor initiation in
hepatitis C virus-induced hepatocellular carcinoma. Mol Med.
15:85–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roessler S, Jia HL, Budhu A, Forgues M, Ye
QH, Lee JS, Thorgeirsson SS, Sun Z, Tang ZY, Qin LX and Wang XW: A
unique metastasis gene signature enables prediction of tumor
relapse in early-stage hepatocellular carcinoma patients. Cancer
Res. 70:10202–10212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kamarajah SK, Frankel TL, Sonnenday C, Cho
CS and Nathan H: Critical evaluation of the American Joint
Commission on Cancer (AJCC) 8th edition staging system for patients
with Hepatocellular Carcinoma (HCC): A surveillance, epidemiology,
end results (SEER) analysis. J Surg Onco. 117:644–650. 2018.
View Article : Google Scholar
|
|
23
|
He L, Fan X, Li Y, Chen M, Cui B, Chen G,
Dai Y, Zhou D, Hu X and Lin H: Overexpression of zinc finger
protein 384 (ZNF 384), a poor prognostic predictor, promotes cell
growth by upregulating the expression of Cyclin D1 in
Hepatocellular carcinoma. Cell Death Dis. 10:4442019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ren Y, Poon RT, Tsui HT, Chen WH, Li Z,
Lau C, Yu WC and Fan ST: Interleukin-8 serum levels in patients
with hepatocellular carcinoma: Correlations with
clinicopathological features and prognosis. Clin Cancer Res.
9:5996–6001. 2003.PubMed/NCBI
|
|
26
|
Loosen SH, Schulze-Hagen M, Leyh C, Benz
F, Vucur M, Kuhl C, Trautwein C, Tacke F, Bruners P, Roderburg C
and Luedde T: IL-6 and IL-8 serum levels predict tumor response and
overall survival after TACE for primary and secondary hepatic
malignancies. Int J Mol Sci. 19(pii): E17662018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feng L, Qi Q, Wang P, Chen H, Chen Z, Meng
Z and Liu L: Serum levels of IL-6, IL-8, and IL-10 are indicators
of prognosis in pancreatic cancer. J Int Med Res. 46:5228–5236.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nacinovic-Duletic A, Stifter S, Dvornik S,
Skunca Z and Jonjic N: Correlation of serum IL-6, IL-8 and IL-10
levels with clinicopathological features and prognosis in patients
with diffuse large B-cell lymphoma. Int J Lab Hematol. 30:230–239.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakopoulou L, Mylona E, Rafailidis P,
Alexandrou P, Giannopoulou I and Keramopoulos A: Effect of
different ERK2 protein localizations on prognosis of patients with
invasive breast carcinoma. APMIS. 113:693–701. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Becher B, Spath S and Goverman J: Cytokine
networks in neuroinflammation. Nature reviews. Immunology.
17:49–59. 2017.PubMed/NCBI
|
|
31
|
Morel PA, Lee REC and Faeder JR:
Demystifying the cytokine network: Mathematical models point the
way. Cytokine. 98:115–123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu XL, Li FQ, Liu LX, Li B and Zhou ZP:
TNF-α, HGF and macrophage in peritumoural liver tissue relate to
major risk factors of HCC Recurrence. Hepatogastroenterology.
60:1121–1126. 2013.PubMed/NCBI
|
|
33
|
Peng Q, Li H, Lao X, Deng Y, Chen Z, Qin X
and Li S: Association of IL-2 polymorphisms and IL-2 serum levels
with susceptibility to HBV-related hepatocellular carcinoma in a
Chinese Zhuang population. Infect Genet Evol. 27:375–381. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pine SR, Mechanic LE, Enewold L,
Chaturvedi AK, Katki HA, Zheng YL, Bowman ED, Engels EA, Caporaso
NE and Harris CC: Increased levels of circulating interleukin 6,
interleukin 8, C-reactive protein, and risk of lung cancer. J Natl
Cancer Inst. 103:1112–1122. 2011. View Article : Google Scholar : PubMed/NCBI
|