Introduction
Colorectal cancer is the third most common cancer in
the United States, with an estimated 1.4 million new cases
diagnosed and 0.5 million related deaths in 2017 (1). Colorectal cancer is staged based on TNM
classification (2). A correlation
between extramural extent and prognosis in local advanced rectal
cancer was reported in 1958 (3), and
the optimal cut-off points for mesorectal extension in pT3 and pT4
colorectal cancer were described in 1993 (4).
Many reports suggest that T3 subdivision might
improve the prediction of prognosis in rectal cancer (5–7). A large
study of colorectal cancer cases in 2007 reported a 3-year survival
rate of 53% among cases with poor prognosis (T3-T4 tumors showing
invasion >5 mm beyond the muscularis propria) compared to 87%
among cases with good prognosis (T1–2 tumors and T3 tumors showing
invasion of ≤5 mm beyond the muscularis propria) (8). A recent meta-analysis concluded with an
appeal to the AJCC to subdivide the T3 category with regards to
rectal cancer (9).
Although the 2003 TNM Supplement, 3rd edition,
suggests subdivision of the T3 classification into T3a-d (10), such subdivision is not yet specified
in the 2010 7th TNM classification (UICC/AJCC). The 7th edition
does include subdivision of T4 into T4a and T4b, and of Stage II
into A to C. The T3 classification is also not subdivided in the
8th Japanese Classification of Colorectal Carcinoma or in the
National Comprehensive Cancer Network guidelines.
While evidence suggests that T3 subdivision predicts
prognosis in rectal cancer, few prior reports describe a
correlation between T3 subdivision and prognosis in colon cancer.
In the present study, we aimed to validate the correlation between
the invasion distance (ID) beyond the muscularis propria (MP) and
prognosis in colorectal cancer, excluding low rectal cancer without
serosa.
Materials and methods
Study design and patient
characteristics
This retrospective study included 148 consecutive
patients with pathologically confirmed T3 colorectal cancer, who
underwent their first operation at our department between January
2008 and October 2012. We excluded patients with low rectal cancer,
which the deepest invasion part of the tumor occupied on the anal
side of the peritoneum, and patients who received pre-operative
radiotherapy and/or chemotherapy. All included patients were free
of metastatic lesions, and all operations were curative.
T staging was determined according to the 7th
version of the UICC TNM classification of colorectal carcinoma
(11). Of the 148 included patients,
118 underwent laparoscopic surgery (single-port surgery via the
TANKO approach in 24 patients), and 30 underwent open surgery.
Based on the degree of differentiation, tumors were divided into
two groups: Well and moderately differentiated tubular
adenocarcinoma, or other. All participants gave their informed
consent, and this study was approved by the Institutional Review
Board (approval number 15144).
Patient follow-up
After curative colorectal cancer resection, patient
surveillance was based on Japanese Society for Cancer of the Colon
and Rectum (JSCCR) guidelines (12).
Postoperative follow-up included serum CEA measurement every six
months, CT scanning every six months, yearly colonoscopies, and
routine outpatient visits. Recurrence was defined as radiologic and
histopathological evidence of tumor presence after surgery. Local
recurrence was defined as tumor presence at the anastomosis site,
pelvis, or peritoneum.
Adjuvant chemotherapy
Adjuvant chemotherapy was recommended for all
patients with stage III colorectal cancer, and was administered
following JSCCR guidelines (12).
For patients with high-risk colon cancer (13) and lymph node metastasis, the
attending physician decided whether to administer adjuvant
chemotherapy. Adjuvant chemotherapy was administered for a duration
of 6 months, and the regimens included UFT, UFT + UZEL, UFT + PSK
(polysaccharide-Kureha) (14),
capecitabine, TS-1, FOLFOX (5-FU + oxaliplatin), and XELOX
(capecitabine + oxaliplatin).
ID beyond the muscularis propria
Resected tumors were sliced into multiple vertical
sections at the point of maximal tumor invasion, and these sections
were embedded in paraffin. Next, 4-µm slices were cut and stained
using hematoxylin and eosin. In at least four sections from each
tumor, we measured the histological distance of the maximal tumor
invasion beyond the MP, termed the ID. These measurements were
taken without any clinical information about the patients (5).
Based on the ID, the pT3 stage was divided into
three groups: T3a, ID<1 mm; T3b, ID=1–5 mm; and T3c, ID>5 mm.
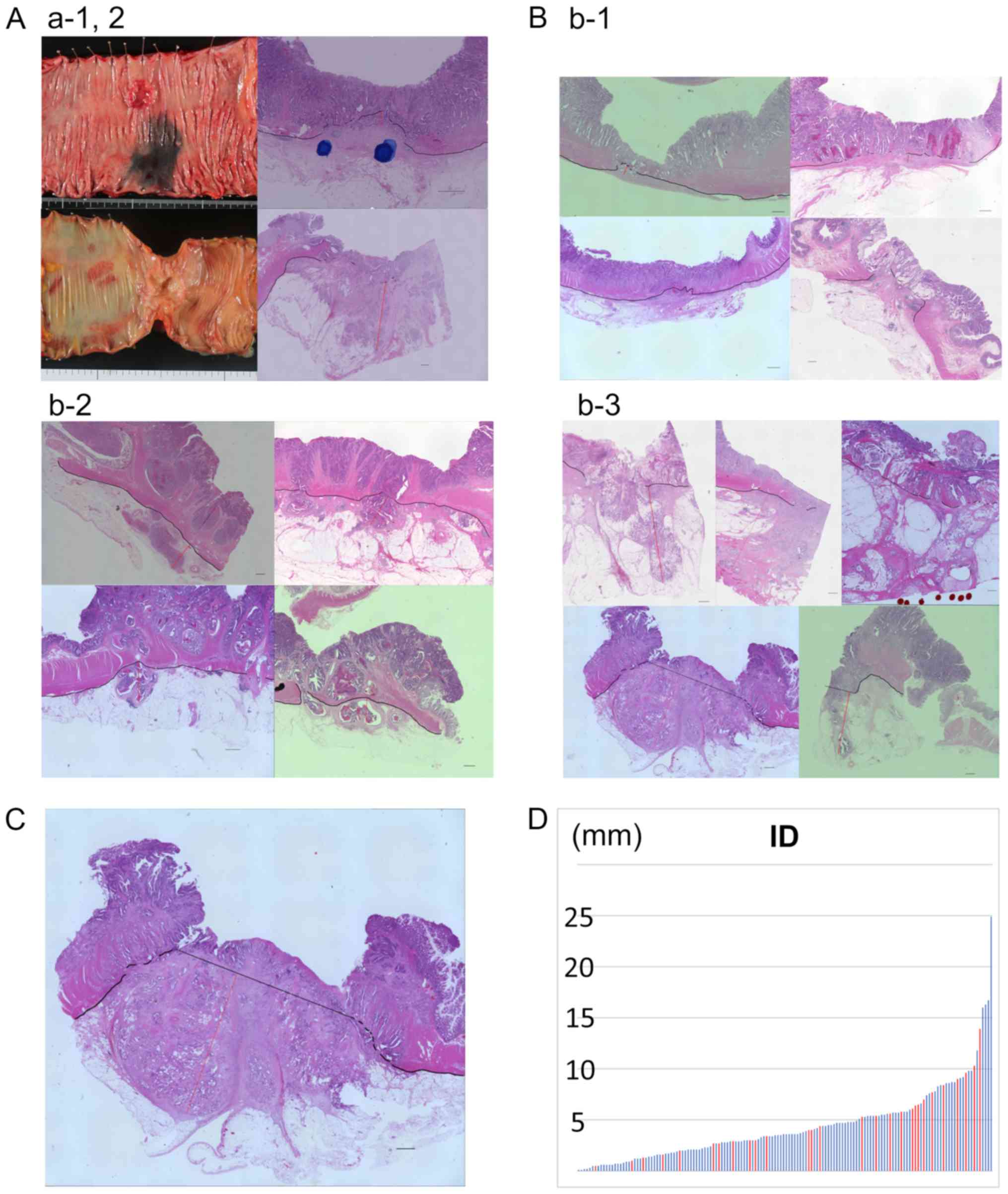
Ascending colon cancer cases categorized as T3a (ID=0.5 mm) and T3c
(ID=9.1 mm), which were both pathological stage II are shown in
Fig. 1A. The ID measurement method
in the T3a, T3b, T3c groups is shown in Fig. 1B. In cases lacking a clear MP line,
the maximal tumor ID beyond the MP was measured based on an
imaginary line drawn horizontally extending from the normal MP
lines (Fig. 1C) (5).
Statistical analysis
To assess whether the pT3 subdivision groups were
correlated with clinicopathological factors, we compared the T3a +
T3b group versus the T3c group. We compared age, BMI, tumor size,
and ID between these groups using a t test. We used Fisher's
test to investigate whether the pT3 subdivision groups were
correlated with sex, adjuvant chemotherapy, serum carcinoembryonic
antigen (CEA) levels, degree of differentiation, lymphoid
dissection number, surgical procedure, lymphoid metastasis, or
angiolymphatic invasion.
Cox regression analysis was performed to analyze the
independent prognostic factors for 3-year relapse-free survival
(RFS) and 5-year cancer-specific survival (CSS). We investigated
the correlation between pT3 group and 3-year RFS using the Wilcoxon
test, and the correlation between pT3 group and 5-year CSS using
the log-rank test. The ID values were used to construct receiver
operating characteristic (ROC) curves, from which we determined ID
cut-off values. Statistical analyses were performed using JMP Pro
13.0.0 (SAS Institute Inc., Cary, NC, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinicopathological
characteristics
Fig. 1D presents the
ID values from all cases in this study. The pT3 subdivision was T3a
in 19 patients, T3b in 81 patients, and T3c in 48 patients. The
3-year relapse rate was 5.3% among T3a patients, 11% among T3b
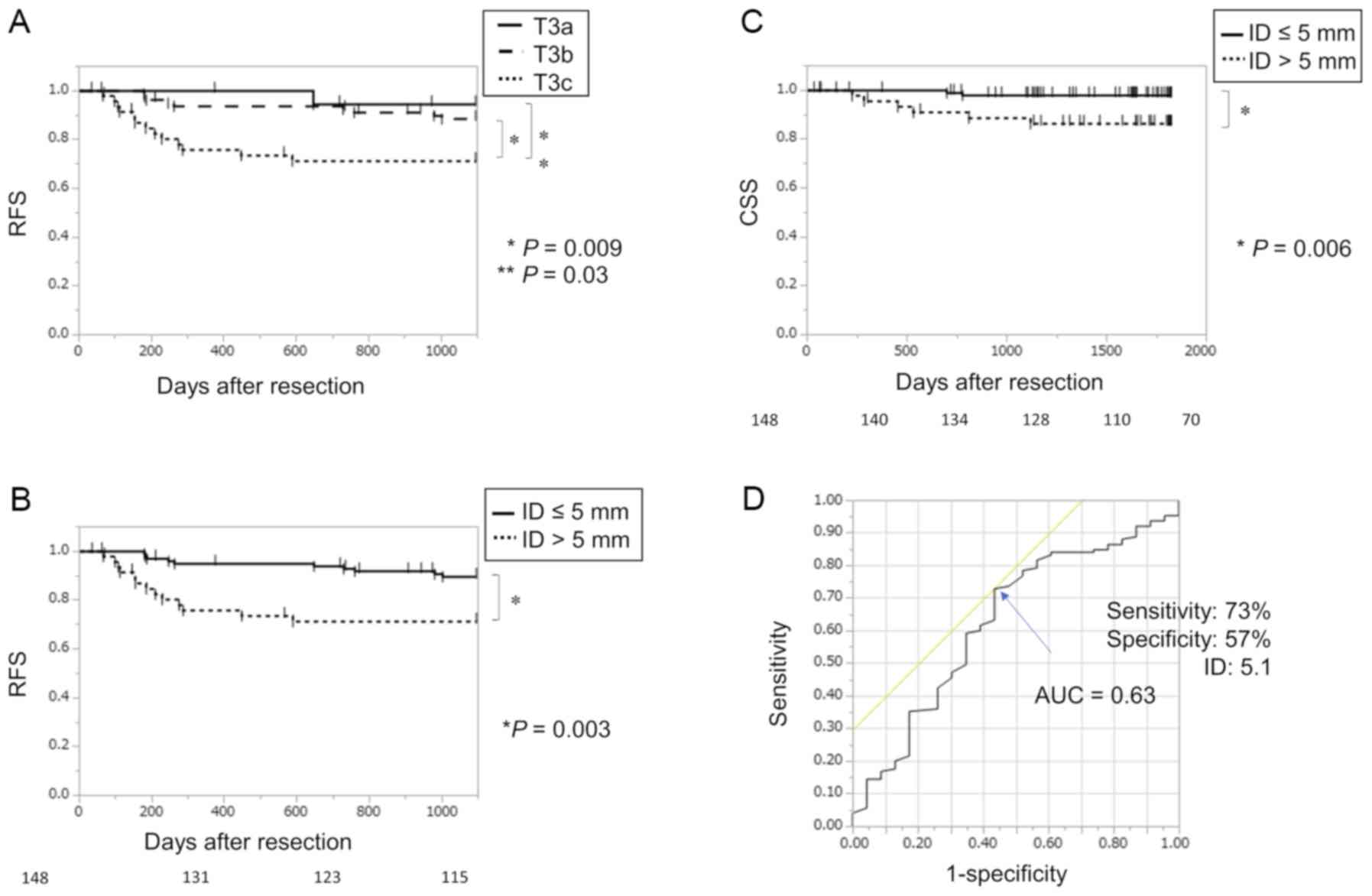
patients, and 27% among T3c patients. The 3-year RFS rate was
significantly worse in T3c patients compared to in T3a patients
(P=0.03) or T3b patients (P=0.009) (Fig.
2A). The 3-year RFS rate was worse in the T3c group compared to
in patients with an ID≤5 mm (the T3a + T3b group) (P=0.003;
Fig. 2B).
Patients' clinicopathological characteristics are
shown in Table I. Table II shows the correlations between
3-year RFS and clinicopathological characteristics, as determined
by univariate and multivariate analysis. Univariate analysis
revealed that 3-year RFS was correlated with sex (P=0.03), vascular
invasion (P=0.02), lymphoid metastasis (P=0.02), and ID with a
cut-off point of 5 mm (P=0.005). Multivariate analysis showed that
3-year RFS was independently correlated with sex (P=0.03) and ID
(P=0.02).
 | Table I.Clinicopathological characteristics of
the T3c and T3ab groups. |
Table I.
Clinicopathological characteristics of
the T3c and T3ab groups.
| Characteristics | All patients
(n=148) | T3c (>5 mm)
(n=48) | T3ab (≤5 mm)
(n=100) | P-value |
|---|
| Age (years) | 66.0±11.3 | 66.5±11.1 | 65.7±11.4 | 0.68 |
| Sex |
|
|
|
|
| Male | 87 | 29 | 58 | 0.78 |
|
Female | 61 | 19 | 42 |
|
| BMI | 22.5±4.2 | 22.3±4.9 | 22.6±3.8 | 0.77 |
| Tumor location |
|
|
|
|
| C | 12 | 8 | 4 | – |
| A | 31 | 9 | 22 | – |
| T | 15 | 8 | 7 | – |
| D | 4 | 1 | 3 | – |
| S | 44 | 9 | 35 | – |
| RS | 14 | 3 | 11 | – |
| Ra | 28 | 10 | 18 | – |
|
Right | 58 | 25 | 33 | 0.03 |
| Left | 90 | 23 | 67 |
|
| Adjuvant
chemotherapy |
|
|
|
|
| Yes | 53 | 22 | 31 | 0.08 |
| No | 95 | 26 | 69 |
|
| Serum CEA levels
(ng/ml) |
|
|
|
|
|
>5 | 62 | 19 | 43 | 0.66 |
| ≤5 | 85 | 29 | 56 |
|
| Degree of
differentiation |
|
|
|
|
| por,
sig, muc | 16 | 7 | 9 | 0.33 |
|
tub | 131 | 41 | 90 |
|
| Size (mm) | 48.2±21.0 | 59.3±26.0 | 42.9±15.6 | 0.0002 |
| Lymphnoid
dissection number |
|
|
|
|
|
≥12 | 125 | 41 | 84 | 0.82 |
|
<12 | 23 | 7 | 16 |
|
| Surgical
procedure |
|
|
|
|
|
Laparoscopy | 118 | 38 | 80 | 0.91 |
|
Open | 30 | 10 | 20 |
|
| Lymphnoid
metastasis |
|
|
|
|
|
N(+) | 64 | 28 | 36 | 0.01 |
|
N(−) | 84 | 20 | 64 |
|
| Angiolymphatic
invasion |
|
|
|
|
|
ly(+) | 130 | 46 | 84 | 0.03 |
|
ly(−) | 18 | 2 | 16 |
|
|
v(+) | 59 | 25 | 34 | 0.04 |
|
v(−) | 89 | 23 | 66 |
|
| ID (mm) | 4.4±3.6 | 8.2±3.8 | 2.5±1.4 | <0.0001 |
 | Table II.Risk factors for postoperative
recurrence in all patients using univariate and multivariate
analysis. |
Table II.
Risk factors for postoperative
recurrence in all patients using univariate and multivariate
analysis.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
|
Characteristics | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
Male/female | 2.80 | 1.12–8.50 | 0.03 | 2.76 | 1.09–8.41 | 0.03 |
| Location |
|
|
|
|
|
|
|
Left/right | 1.24 | 0.54–3.09 | 0.62 |
|
|
|
| Serum CEA levels
(ng/ml) |
|
|
|
|
|
|
|
>5/≤5 | 1.80 | 0.79–4.22 | 0.16 |
|
|
|
| Surgical
procedure |
|
|
|
|
|
|
|
Laparoscopy/open | 0.87 | 0.35–2.62 | 0.78 |
|
|
|
| Lymphoid dissection
number |
|
|
|
|
|
|
|
≥12/<12 | 0.52 | 0.22–1.44 | 0.19 |
|
|
|
| Angiolymphatic
invasion |
|
|
|
|
|
|
|
ly(+)/ly(−) | 3.42 | 0.72–61.3 | 0.14 |
|
|
|
|
v(+)/v(−) | 2.60 | 1.14–6.24 | 0.02 | 1.91 | 0.82–4.66 | 0.14 |
| Lymphoid
metastasis |
|
|
|
|
|
|
|
N(+)/N(−) | 2.63 | 1.14–6.53 | 0.02 | 1.99 | 0.84–5.05 | 0.12 |
| Degree of
differentiation |
|
|
|
|
|
|
| por,
sig, muc/tub | 1.87 | 0.54–4.97 | 0.29 |
|
|
|
| Adjuvant
chemotherapy |
|
|
|
|
|
|
|
Yes/no | 0.53 | 0.23–1.21 | 0.13 |
|
|
|
| ID (mm) |
|
|
|
|
|
|
| >5
mm/≤5 mm | 3.32 | 1.46–7.79 | 0.005 | 2.82 | 1.22–6.73 | 0.02 |
The 5-year CSS rate was worse among patients with
ID>5 mm compared to those with ID≤5 mm (P=0.006) (Fig. 2C). Univariate analysis revealed that
5-year CSS was correlated with lymphoid dissection number (P=0.02),
vascular invasion (P=0.03), and ID (P=0.009; Table III). In multivariate analysis,
5-year CSS was significantly correlated with lymphoid dissection
number (P=0.02) and ID (P=0.03).
 | Table III.Risk factors for CSS in all patients
using univariate and multivariate analysis |
Table III.
Risk factors for CSS in all patients
using univariate and multivariate analysis
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
|
Characterisitics | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
Male/female | 5.05 | 0.89–94.4 | 0.07 |
|
|
|
| Location |
|
|
|
|
|
|
|
Left/right | 1.09 | 0.27–5.31 | 0.91 |
|
|
|
| Serum CEA levels
(ng/ml) |
|
|
|
|
|
|
|
>5/≤5 | 0.84 | 0.17–3.43 | 0.81 |
|
|
|
| Surgical
procedure |
|
|
|
|
|
|
|
Open/laparoscopy | 0.24 | 0.06–1.01 | 0.05 |
|
|
|
| Lymphoid dissection
number |
|
|
|
|
|
|
|
≥12/<12 | 0.18 | 0.04–0.78 | 0.02 | 0.18 | 0.04–0.77 | 0.02 |
| Angiolymphatic
invasion |
|
|
|
|
|
|
|
ly(+)/ly(−) | – | – | 0.14 |
|
|
|
|
v(+)/v(−) | 4.86 | 1.12–33.2 | 0.03 | 3.19 | 0.71–22.2 | 0.13 |
| Lymphoid
metastasis |
|
|
|
|
|
|
|
N(+)/N(−) | 4.08 | 0.94–27.8 | 0.06 |
|
|
|
| Degree of
differentiation |
|
|
|
|
|
|
| por,
sig, muc/tub | 1.78 | 0.09–11.0 | 0.62 |
|
|
|
| Adjuvant
chemotherapy |
|
|
|
|
|
|
|
Yes/no | 0.57 | 0.08–2.48 | 0.47 |
|
|
|
| ID (mm) |
|
|
|
|
|
|
| >5
mm/≤5 mm | 4.19 | 1.03–20.4 | 0.009 | 6.19 | 1.39–43.1 | 0.03 |
Recurrence and cancer-specific
survival
Recurrence occurred in 27 patients, with 28 sites
affected during the first recurrences after surgery. Of these 27
patients, 21 exhibited distant metastases (14 liver, 8 lung) and 6
exhibited local recurrence (3 lymph node, 2 peritoneal, 1
anastomotic recurrence). Of the 148 included patients, 23 died,
including 9 deaths due to colorectal cancer.
Adjuvant chemotherapy
Adjuvant chemotherapy was administered to 53
patients. Decisions regarding adjuvant chemotherapy were made by
the physicians, with consideration of the patients' wishes. The
adjuvant therapy regimen was UFT + UZEL in 28 patients, UFT + PSK
in 6 patients, capecitabine in 5 patients, XELOX in 5 patients, and
other regimens in 9 patients.
ID cut-off value
An ROC curve was constructed using ID values and
recurrence data, and the area under the curve was 0.63 (Fig. 2D). The optimal cut-off value for
separating recurrence from non-recurrence was an ID of 5.1 mm (73%
sensitivity, 57% specificity, and 70% accuracy).
Discussion
Studies of colorectal cancer report a predominance
of the T3 stage, with the following T-stage distribution: 12% T1,
14% T2, 44% T3, 24% T4a, and 6% T4b (12). Similarly, in our department, the vast
majority of stage II and III colorectal cancer cases are
categorized as T3. The 4th TNM classification supplementary for low
rectal cancer mentions T3 subdivision as an optional
classification. However, the 7th TNM classification from 2010
recommends subdivision of T4 cases into T4a and T4b cases, while T3
cases still constitute a single category. Many reports describe
rectal cancer cases with a great mesorectal extension depth has
having a poor prognosis, and clinical trials of neoadjuvant
chemotherapy in colon cancer have used an ID of ≥5 mm to delineate
the poor prognostic group (15).
However, T3 subdivision in colon cancer is still not common, and
few reports have actually validated the prognostic influence using
pathological analysis.
In our present study, we evaluated the clinical
significance of T3 subdivision based on ID in colorectal cancer,
excluding low rectal cancer. We found that an ID of 5.1 mm was the
optimal cut-off value for separating recurrence from non-recurrence
(Fig. 2D), and thus used an ID of 5
mm as the cut-off value for analysis. This was similar to previous
studies, which have performed T3 subdivision as follows: T3a,
ID<1 mm; T3b, ID=1–5 mm; T3c, 5<ID≥15 mm; and T3d, >15 mm.
In an analysis of rectal cancer, Shin et al compared
prognosis between the T3a + T3b group and the T3c + T3d group
(6). Zinicola et al
retrospectively summarized 12 studies reporting an ID cut-off in T3
rectal cancer, and noted that the smallest standard errors were
found when using a 4-mm or 5-mm ID cut-off to analyze 5-year
survival (9). In one of the included
studies, Merkel et al used a 5-mm ID cut-off in 853 patients
from the Study Group for Colo-Rectal Carcinoma (16). Finally, in an analysis of
preoperative diagnosis using CT or MRI, a 5-mm cut-off was used as
a criterion for surgery or neoadjuvant chemotherapy in colon cancer
(15).
Standard therapy for Stage III colon cancer includes
colon resection with mesentery (17)
and oxaliplatin-based adjuvant chemotherapy (18–20). In
cases with lymph node metastasis, JSCCR guidelines recommend
adjuvant chemotherapy (12). Our
present results indicated that T3 subdivision (T3c versus T3a +
T3b) correlated with tumor size (P=0.0002), lymphoid metastasis
(P=0.01), lymphatic invasion (P=0.03), and vascular invasion
(P=0.04). Adjuvant chemotherapy was more commonly administered in
the T3c group than in the T3a + T3b group (P=0.08). In other words,
T3 subdivision was significantly correlated with prognostic
factors, and was associated with the administration of adjuvant
chemotherapy. We found that ID and sex were independent risk
factors for 3-year RFS, and that ID and lymphoid dissection number
were independent risk factors for 5-year CSS (Tables II, III). Overall, T3c cases had a worse
prognosis than T3a and T3b cases (Fig.
2A). Lymphoid metastasis, degree of differentiation, and
angiolymphatic invasion were not independent risk factors for
3-year RFS or 5-year CSS in our study, likely due to the inclusion
of patients with and without adjuvant chemotherapy. It might be
useful to use ID as a prognostic marker for T3 colorectal cancer
patients, except those with low rectal cancer, without accounting
for adjuvant chemotherapy.
Among the 64 patients in our study with lymph node
metastasis, 18 (28%) were not administered adjuvant chemotherapy.
Of these 18 patients, 6 suffered a relapse, including 4 T3c and 2
T3b cases. These data suggest that adjuvant chemotherapy should be
administered in T3c cases with lymph node metastasis. Among the 84
patients without lymph node metastasis, 7 (8%) were administered
adjuvant chemotherapy. No deaths due to colorectal cancer occurred
among the T3a cases in our study. We found that 3-year RFS was
significantly worse among T3c cases than T3b cases (P=0.009)
(Fig. 2A). Since operative stress
carries a risk of promoting tumor growth, immediate intervention is
required to eradicate micrometastases (21–25). Our
present data suggest that it might be beneficial to administer
earlier treatment, such as neoadjuvant chemotherapy, in T3c
cases.
Our present study had three limitations. First, it
was a retrospective study with a small number of cases. Second, the
adjuvant chemotherapy methods were diverse, and the administration
of adjuvant chemotherapy was decided by the attending physician.
Third, there was only a small number of events for the 5-year CSS
analysis (9 patients). The reason for the difference in gender is
unclear. We have to increase the number of the cases and to
consider again in the future, and to plan prospective study about
the problem of postoperative adjuvant chemotherapy.
Among cases of T3 colorectal cancer (excluding low
rectal cancer), ID above the muscularis propria was significantly
associated with prognosis.
Acknowledgements
Not applicable
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
MN, HT, and MF conceived and designed the study, and
acquired the data. MN, HT, MF, NM, NH, TH, CM, HY, TM, MM and YD
analyzed and interpreted the data. MN and HT drafted the article.
All authors critically revised the article for important
intellectual content, and approved the final version of the article
to be published.
Ethics approval and consent to
participate
Informed consent was obtained from all individual
participants included in the current study. The present study was
approved by the Institutional Review Board (approval no.
15144).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Mannual7th. Springer;
New York, NY: 2010
|
|
3
|
Dukes CE and Bussey HJ: The spread of
rectal cancer and its effect on prognosis. Br J Surg. 12:309–320.
1958.
|
|
4
|
Hermanek P: TNM Supplement 1993: A
commentary on uniform Use. Berlin. Springer–Verlag. 1993.
|
|
5
|
Shirouzu K, Akagi Y, Fujita S, Ueno H,
Takii Y, Komori K, Ito M and Sugihara K: Japanese Society for
Cancer of the Colon and Rectum (JSCCR) on clinical significance of
the mesorectal extension of rectal cancer: Clinical significance of
the mesorectal extension of rectal cancer: A japanese
multi-institutional study. Ann Surg. 253:704–710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shin R, Jeong SY, Yoo HY, Park KJ, Heo SC,
Kang GH, Kim WH and Park JG: Depth of mesorectal extension has
prognostic significance in patients with T3 rectal cancer. Dis
Colon Rectum. 55:1220–1228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sueda T, Ohue M, Noura S, Shingai T,
Nakanishi K and Yano M: Prognostic significance of a preoperative
magnetic resonance imaging assessment of the distance of mesorectal
extension in clinical T3 lower rectal cancer. Surg Today.
46:1249–1257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smith NJ, Bees N, Barbachano Y, Norma AR,
Swift RI and Brown G: Preoperative computed tomography staging of
nonmetastatic colon cancer predicts outcome: Implications for
clinical trials. Br J Cancer. 96:1030–1036. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zinicola R, Pedrazzi G, Haboubi N and
Nicholls RJ: The degree of extramural spread of T3 rectal cancer:
An appeal to the American joint committee on cancer. Colorectal
Dis. 19:8–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wittekind C: TNM supplement: A commentary
on uniform use. 3rd edition. Hoboken. Wiley–Liss. 2003.
|
|
11
|
Sobin LH, Gospodarowicz MK and Wittekind
C: UICC TNM Classification of Malignant Tumors7th. Wiley-Blackwell;
New York, NY: 2009
|
|
12
|
Watanabe T, Itabashi M, Shimada Y, Tanaka
S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et
al: Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2014 for the treatment of colorectal cancer. Int J Clin
Oncol. 20:207–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumar A, Knnecke HF, Renouf DJ, Lim HJ,
Gill S, Woods R, Speers C and Cheung WY: Adjuvant chemotherapy use
and outcomes of patients with high-risk versus low-risk stage II
colon cancer. Cancer. 121:527–534. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshitani S and Takashima S: Efficacy of
postoperative UFT (Tegafur/Uracil) plus PSK therapies in elderly
patients with resected colorectal cancer. Cancer Biother
Radiopharm. 24:35–40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Foxtrot Collaborative Group, : Feasibility
of preoperative chemotherapy for locally advanced, operable colon
cancer: The pilot phase of a randomised controlled trial. Lancet
Oncol. 13:1152–1160. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Merkel S, Mansmann U, Siassi M,
Papadopoulos T, Hohenberger W and Hermanek P: The prognostic
inhomogeneity in pT3 rectal carcinomas. Int J Colorectal Dis.
16:298–304. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bertelsen CA, Neuenschwander AU, Jansen
JE, Wihelmsen M, Kirkegaard-Klitbo A, Tenma JR, Bols B, Ingeholm P,
Rasmussen LA, Jepsen LV, et al: Disease-free survival after
complete mesocolic excision compared with conventional colon cancer
surgery: A retrospective, population-based study. Lancet Oncol.
16:161–168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taieb J, Tabernero J, Mini E, Subit F,
Folprecht G, Van Laethem JL, Thaler J, Bridgewater J, Petersen LN,
Blons H, et al: Oxaliplatin, fluorouracil, and leucovorin with or
without cetuximab in patients with resected stage III colon cancer
(PETACC-8): An open-label, randomized phase 3 trial. Lancet Oncol.
15:862–873. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Colvin H, Mizushima T, Eguchi H, Takiguchi
S, Doki Y and Mori M: Gastroenterological surgery in Japan: The
past, the present and the future. Ann Gastroenterol Surg. 1:5–10.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsuda T, Yamashita K, Hasegawa H,
Oshikiri T, Hosono M, Higashino N, Yamamoto M, Matsuda Y, Kanaji S,
Nakamura T, et al: Recent updates in the surgical treatment of
colorectal cancer. Ann Gastroenterol Surg. 2:129–136. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Finlay IG, Meek D, Brunton F and McArdle
CS: Growth rate of hepatic metastases in colorectal carcinoma. Br J
Surg. 75:641–644. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tanaka K, Shimada H, Miura M, Fujii Y,
Yamaguchi S, Endo I, Sekido H, Togo S and Ike H: Metastatic tumor
doubling time: Most important prehepatectomy predictor of survival
and non-recurrence of hepatic colorectal cancer metastasis. World J
Surg. 28:263–270. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeamari S, Roos E and Stewart FA: Tumour
seeding in peritoneal wound sites in relation to growth-factor
expression in early granulation tissue. Eur J Cancer. 40:1431–1440.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fahmy RG, Dass CR, Sun LQ, Chesterman CN
and Khachigian LM: Transcription factor Egr-1 supports
FGF-dependent angiogenesis during neovascularization and tumor
growth. Nat Med. 9:1026–1032. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schelfhout VR, Coene ED, Delaey B,
Waeytens AA, De Rycke L, Deleu M and De Potter CR: The role of
heregulin-alpha as a motility factor and amphiregulin as a growth
factor in wound healing. J Pathol. 198:523–533. 2002. View Article : Google Scholar : PubMed/NCBI
|