Introduction
Colonoscopy is the criterion standard technique for
identification of colonic neoplasia, and resection of colorectal
adenomas is the most effective method for preventing colorectal
cancer (1). Improved endoscopic
procedures with higher rates of adenoma detection may improve the
efficacy of colonoscopy for preventing cancer (1–3).
Chromoendoscopy, which was implemented in the 1980s, has improved
identification of small and flat colorectal lesions, and several
randomized controlled trials have demonstrated significantly better
rates of small neoplastic lesion detection by pan-colonic
chromoendoscopy compared with conventional colonoscopy (4,5).
However, performing total colonic dye-spraying endoscopy requires a
certain degree of endoscopic skills and longer extubation compared
with conventional colonoscopy (6).
The use of narrow-band imaging (NBI), which applies
short-wavelength light to enabling detection of the absorbance
spectrum of hemoglobin, during colonoscopy is expected to enhance
adenoma detection without prolonging the extubation period
(7). However, the ability of
first-generation NBI systems to increase adenoma detection has not
been demonstrated, considering that intestinal fluids such as bile
appear red in color, like blood, and that the luminal brightness is
lower in NBI than in conventional colonoscopy (8). To offset these constraints, blue laser
imaging (BLI), which utilizes narrow-band laser light combined with
white laser light, was developed. However, the brightness of BLI is
still not adequate for detecting distant lesions (9).
Linked color imaging (LCI) is a novel endoscopic
system developed by Fujifilm Co. that increases color contrast by
utilizing short-wavelength narrow-band laser light combined with
white laser light on the basis of BLI technology (9). Unlike BLI and NBI, the luminal
brightness of LCI is not impaired compared with that of
conventional colonoscopy, even at a distant view; consequently, LCI
may improve the visibility of colonic neoplasms (10,11).
However, thus far, the extent to which LCI improves the visibility
of colonic polyps has not been clarified quantitatively. Therefore,
in this study, we evaluated the color differences between colonic
polyps and their surrounding mucosa and compared the color
differences among conventional colonoscopy, BLI, LCI, and
chromoendoscopy.
Patients and methods
Endoscopic procedure
This was a retrospective single-center study.
Between December 2016 and May 2017, all patients who received a
total colonoscopy at the Inoue Gastroenterology and Endoscopy
Clinic were recruited. The Ethics Committee of Osaka Medical
College approved this study, and written informed consent was
waived because of the retrospective design. For the endoscopic
procedures, signed informed consent was obtained from the patients.
The inclusion criteria were as follows: i) surveillance colonoscopy
of known colorectal adenomas or cancer; ii) screening colonoscopy
in patients with positive fecal occult blood; and iii) diagnostic
colonoscopy in patients with symptoms, such as rectal bleeding,
abdominal pain, and change in bowel habits. Patients with
inadequate bowel preparation, colitis, and melanosis did not enter
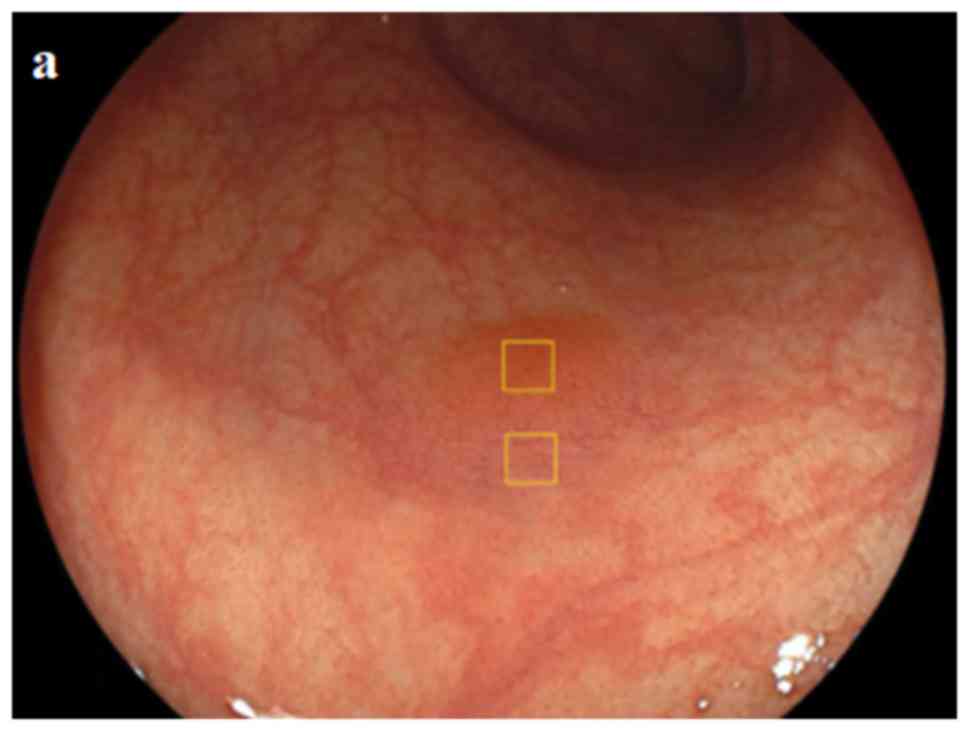
the study. For each polyp identified, pictures with three different
color images (WL, BLI, and LCI) for the same lesion and from the
same point of view were selected for evaluation. Among the selected
pictures, those of chromoendoscopy for the same lesion and from the
same point of view were also used for evaluation. In the case of
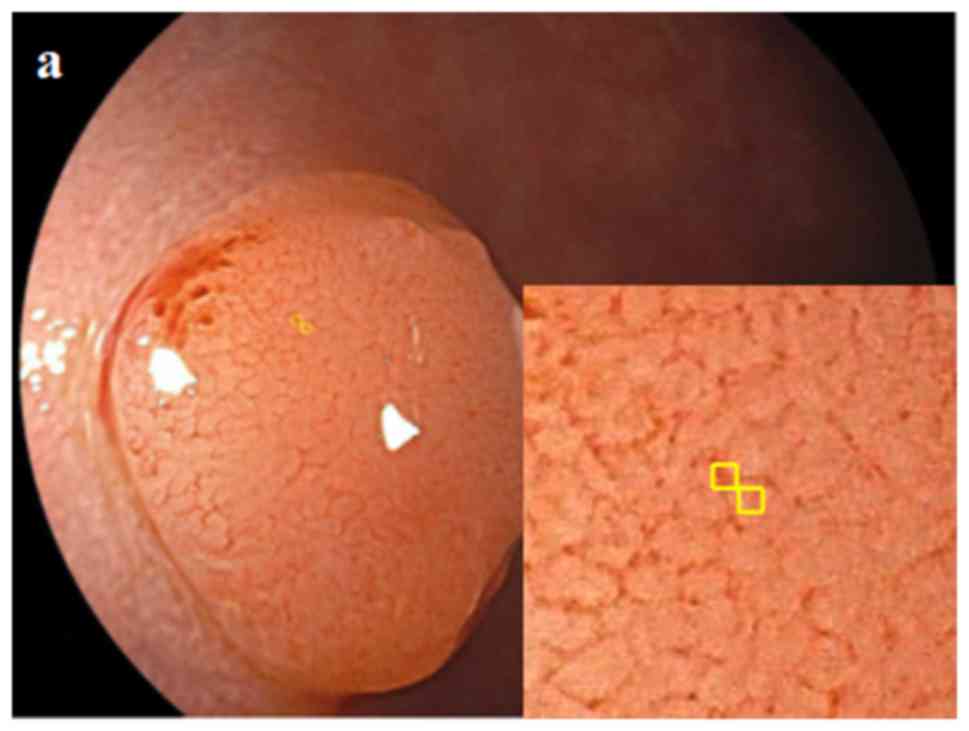
magnified colonoscopic images, high magnification (×80) pictures
with three different color images for the same lesion and from the
same point of view were selected for evaluation. Colonoscopy was
conducted using the LASEREO system (FUJIFILM) with an EC-L600ZP
endoscope. Endoscopy was performed using the WL, BLI, and LCI
modalities. All endoscopic images were stored in JPEG format. An
experienced endoscopist (T.I.) performed the endoscopic procedures
and selection of the images, and an experienced pathologist (Y.E.)
examined all resected tissues.
Calculation of the color
difference
The color difference (ΔE) was calculated using the
CIELAB color space, a three-dimensional color space that comprises
a black-white axis (L*), a red-green axis (a*), and a
yellow-blue axis (b*) and that associates color perception
with colorimetric values. L* is defined as lightness,
a* as the red-green component, and b* as the
yellow-blue component (12). ΔE
between a polyp (p) and the surrounding mucosa (b) was calculated
according to the following formula: ΔEpb=(Lp-Lb)2+(ap-ab)2+(bp-bb)2.
According to Sato et al, we determined the
L*a*b* values (13). Briefly, this was done as follows: i)
the corresponding regions [for non-magnified images, the polyp and
surrounding mucosa, 64 pixels each (Fig.
1), and for magnified images, the vessel and non-vessel areas,
9 pixels each (Fig. 2)] were
selected on WL, BLI, and LCI images using Adobe Photoshop Elements
15; ii) the median RGB value was determined; and iii) the
L*a*b* value was calculated from the average
of the RGB values.
Statistical analysis
Statistical analyses were conducted using Stat View
software, version 5.0 (SAS Institute, Inc.). All data are expressed
as means ± standard deviation. One-way analysis of variance was
performed for multiple comparisons, followed by Fisher's exact
test. P-values of less than 0.05 were considered to be
statistically significant.
Results
Color differences in non-magnified
images
From December 2016 to May 2017, 64 patients with 114
polyps were enrolled in this study. Among these polyps, 113 (64
patients) and 95 (53 patients) were assessed for color differences
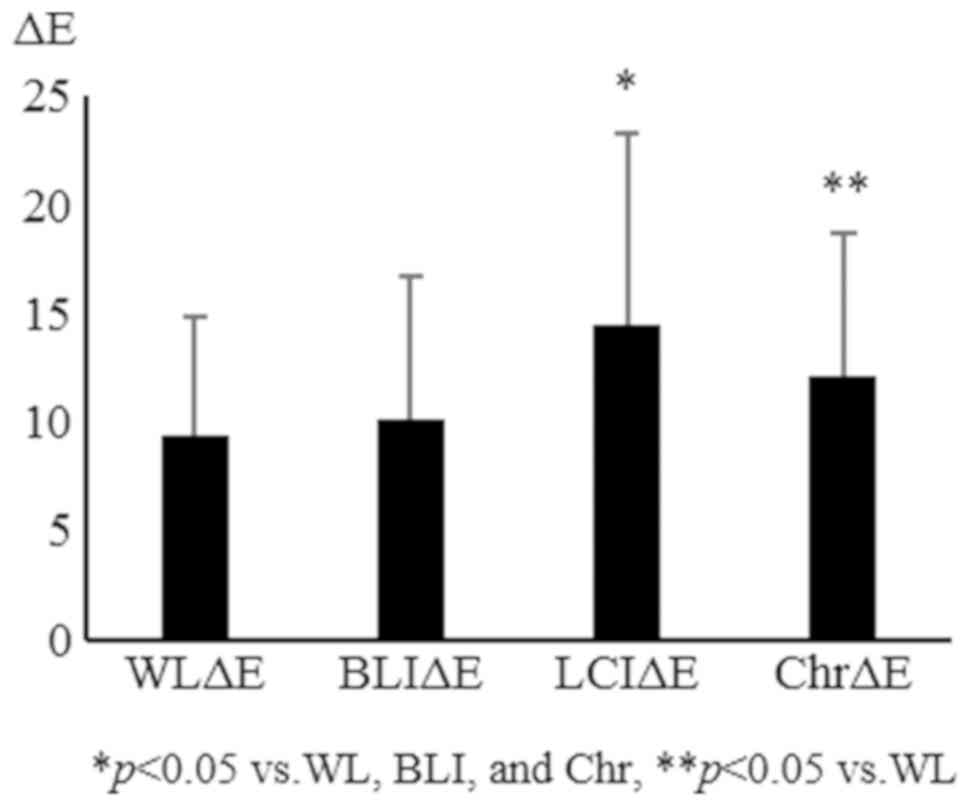
in non-magnified and magnified (×80) images, respectively (Table I). The overall ΔE was significantly
increased with LCI and chromoendoscopy compared with WL and BLI.
The ΔE values were 11.0±6.6, 10.7±7.0, 15.1±9.3, and 14.8±7.2 for
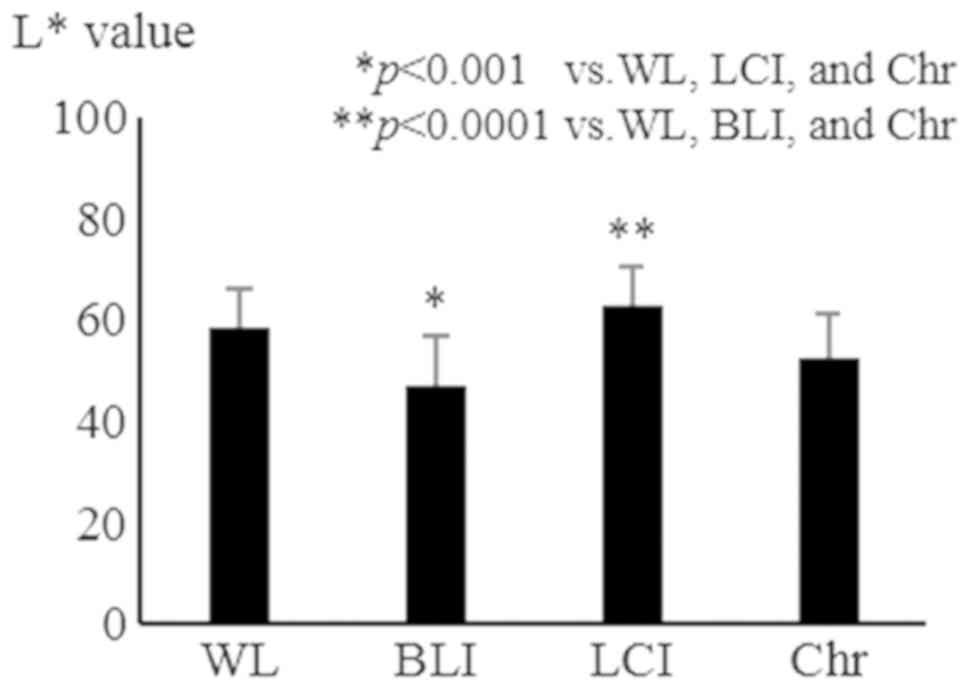
WL, BLI, LCI, and chromoendoscopy, respectively (Fig. 3). L*, which represents the
lightness of a color, was significantly reduced by BLI compared
with WL, LCI, and chromoendoscopy (54.8±8.4, 47.7±9.7, 58.5±8.8,
and 51.7±9.4 for WL, BLI, LCI, and chromoendoscopy, respectively)
(Fig. 4). The ΔE values of all
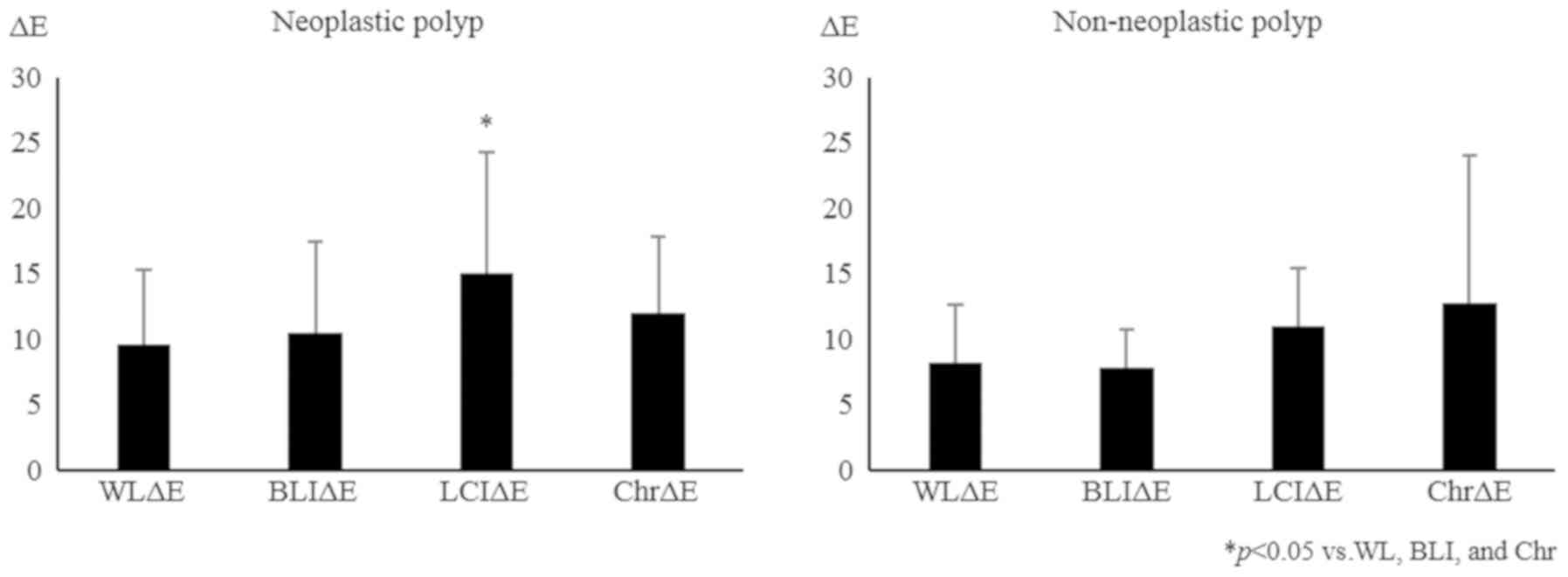
neoplastic lesions (adenoma and cancer) were increased by LCI
compared with WL, BLI, and chromoendoscopy. On the other hand, the
ΔE values of the non-neoplastic lesions were enhanced by
chromoendoscopy compared with WL, BLI, and LCI, although the
differences were not significant (Fig.
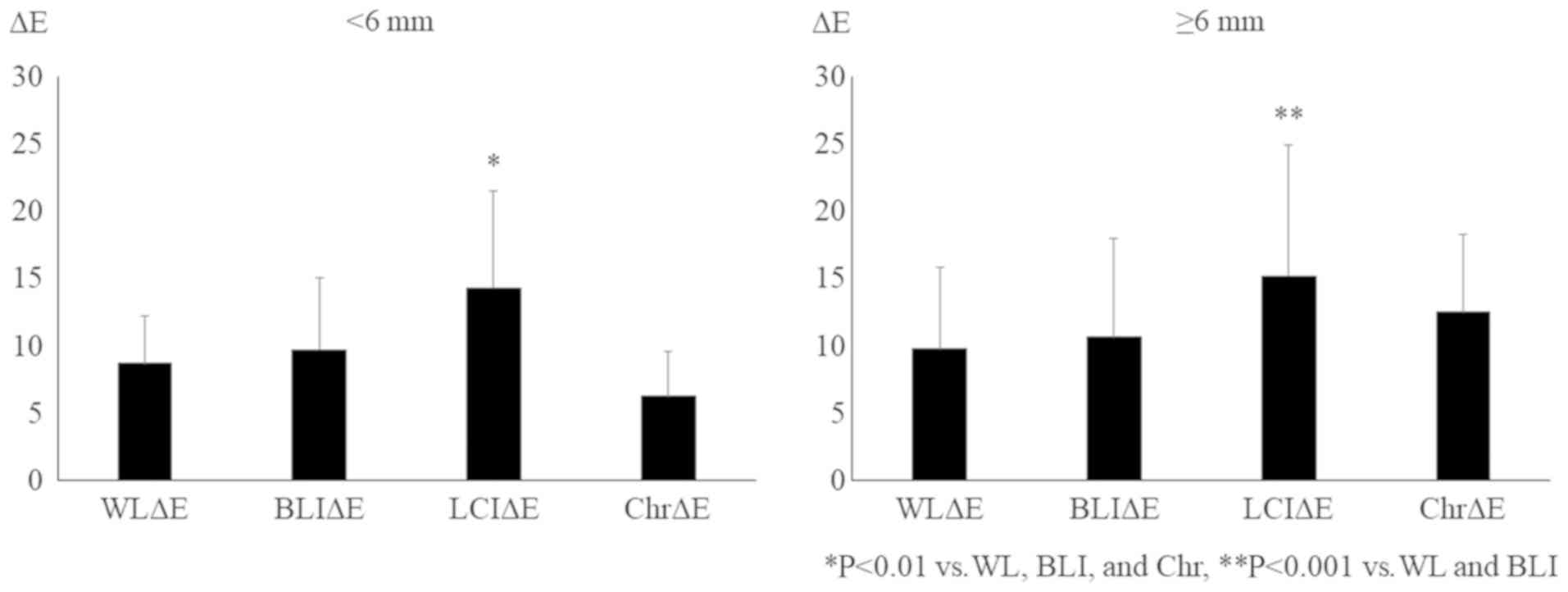
5). Regarding the effect of lesion size, LCI increased the ΔE
of neoplastic polyps in not only large (more than 6 mm in diameter)
but also small (less than 6 mm) lesions (Fig. 6). These findings suggest that LCI
enhances the visibility of polyps, especially neoplastic polyps,
even though they are small lesions, without impairing the lightness
of the color.
 | Table I.Baselines characteristics of the
patients and polyps. |
Table I.
Baselines characteristics of the
patients and polyps.
| Characteristic | Value |
|---|
| Number of
patients | 64 |
| Sex, male/female | 39/25 |
| Age, years, mean ±
SD | 59.8±13.5 |
| Number of polyps | 114 |
| Polyp size, mm, mean
± SD | 7.6±5.0 |
| Number of
non-magnified images, (WL:BLI:LCI:Chr), n | 113: 113: 113:
53 |
| Number of magnified
images, WL:BLI:LCI, n | 95: 95: 95 |
| Polyp location,
right-sided:left-sided: | 52: 40: 22, |
| rectum, n (%) | (45.6: 35.1:
19.3) |
| Pathological
diagnosis, neoplastic: non-neoplastic, n (%) | 99: 15, (86.8:
13.2) |
Color differences in magnified
images
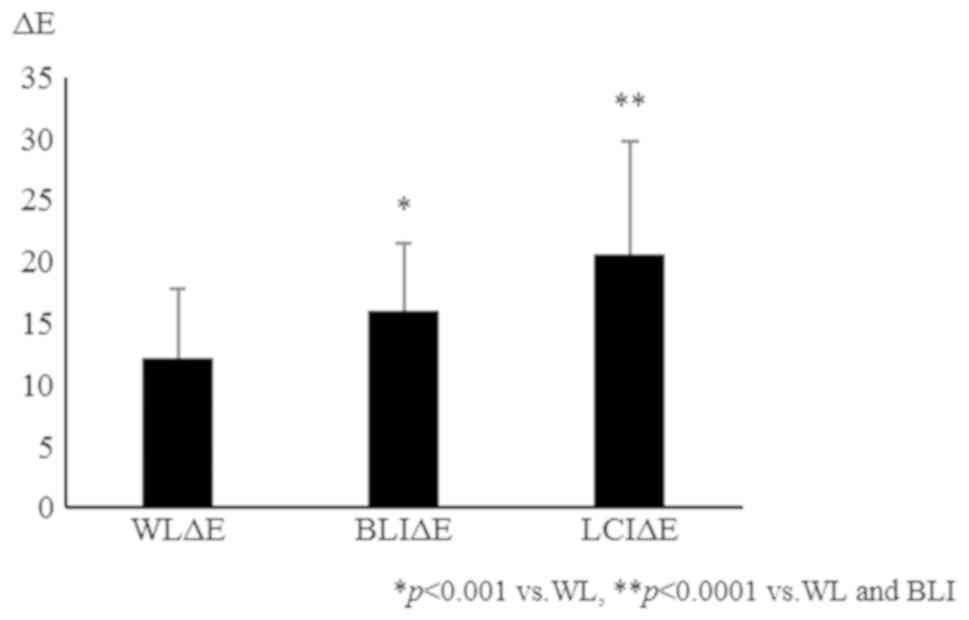
Using magnified pictures of polyps, we calculated
color differences between the vessel and non-vessel areas in the
WL, BLI, and LCI images. The ΔE was 12.2±5.6, 16.1±5.5, and
20.6±9.2 for WL, BLI, and LCI, respectively. Overall, ΔE was
significantly increased in BLI and LCI images compared with that in
WL images. BLI and LCI also had a significantly increased ΔE in
magnified images of neoplastic polyps (Fig. 7). These results indicate that BLI and
LCI are useful in magnifying colonoscopy for diagnosis of colonic
polyps.
Discussion
To our knowledge, this is the first reported
quantitative evaluation of the degree to which LCI enhances colonic
polyp visibility. The results of this study revealed that LCI,
compared with WL and BLI, significantly increased the color
difference between polyps and their corresponding surrounding
mucosa, without impairing luminal brightness. In particular, LCI
significantly enhanced the visibility of neoplastic lesions,
regardless of their size, but not non-neoplastic lesions, such as
hyperplastic polyps and sessile serrated adenoma/polyps
(SSA/P).
Considering that resection of polyps using
colonoscopy has been reported to prevent the occurrence of
colorectal cancer dramatically, by 76–90%, colonoscopy is
considered to be the most effective tool for colon cancer
prevention (2). However, 17–24% of
adenomas may be missed during colonoscopic examination (13,14).
Therefore, improved endoscopic methods that can accurately detect
adenomas have been sought. Thus far, pan-colonic chromoendoscopy
has been reported to increase the detection of small neoplastic
lesions significantly, with significantly prolonged extubation
times (6,15,16).
Since the NBI system was proposed for use during colonoscopy,
several studies have been conducted to investigate the effect of
NBI on improving the detection of colonic polyps (8). However, in these studies using
first-generation NBI, it was not shown that NBI improves the
adenoma detection rate compared with WL endoscopy (8). The reasons why these studies failed are
considered to be related to the characteristics of NBI. For
example, on NBI colonoscopy, intestinal fluids such as bile appear
reddish, like blood, and the luminal brightness is reduced compared
with that of conventional colonoscopy (7). Recently, a new-generation NBI system,
which produces a twofold brighter image than the previous system,
was reported to improve colonic polyp visibility and detection
(17). Another novel image enhanced
endoscopy (IEE) system, BLI, which enables one to obtain brighter
images similar to new-generation NBI via the use of two different
lasers creating white light illumination and short wavelength
narrow-band light observation, was also revealed to improve the
visibility of colorectal polyps (18,19).
However, thus far, the extent to which these IEE systems improve
the visibility of colonic polyps has not been evaluated
quantitatively.
Interestingly, in this study, LCI, but not BLI,
quantitatively improved the visibility of colonic polyps; the
corresponding surrounding mucosa was darker using BLI compared with
that using WL and LCI. These results suggest that BLI still cannot
provide enough luminal brightness to improve the color difference
between colonic polyps and their corresponding surrounding mucosa,
even though BLI creates brighter images than the old-generation IEE
system. Therefore, we suggest that LCI is most suitable for routine
colonoscopic examination for colonic polyp detection.
In the case of magnifying observation, BLI and LCI
provided a significant color difference between the vessel and
non-vessel (surface) area. It is well known that pit pattern
analysis via magnifying chromoendoscopy is an accurate diagnostic
method for the differentiation of colorectal lesions. Recently,
several studies found that BLI magnification is accurate enough to
diagnose most colorectal polyps (20,21).
Thus, the results regarding magnified images using BLI in this
study confirm the results of those previous reports. We suggest
that LCI is also useful for magnifying observation.
This study has some limitations. First, obtaining
exact same timing of the three different color images (WL, BLI, and
LCI) for the same lesion is impossible because of time lags.
Second, this was a single-center retrospective study, and the
number of samples was small. Moreover, all endoscopic procedures
and selection of the images were performed by the same endoscopist.
Third, because BLI uses a narrow wavelength range to provide
information about microvessels, the color difference calculation
using the CIELAB color space might not reflect the visibility of
colonic polyps exactly. Therefore, a larger sample size may be
required to determine the efficacy of LCI in improving the
visibility of non-neoplastic polyps including SSA/P.
In conclusion, LCI significantly improved the
visibility of colonic polyps irrespective of the size of the
lesion, without impairing the brightness of the color, and LCI and
BLI significantly improved the color differences in the magnified
images of neoplastic polyps. These outcomes support the routine use
of LCI for colonic polyp detection and of BLI for improving
magnifying observations of the colonic polyps detected by LCI. In
this study, LCI did not influence color differences in
non-neoplastic lesions, such as hyperplastic polyps and SSA/P.
Considering that SSA/P was recently recognized to have a similar
malignant potential as that of traditional adenoma, further
investigation is needed.
Acknowledgements
This abstract was presented at the 2018 Digestive
Disease Week in Washington, DC, USA and was published in
Gastrointestinal Endoscopy 2018; 87 (6S): AB485.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YT performed the data collection and procedures; TI
and KaK wrote the manuscript and analyzed the data. TI also
contributed to drafting conception and design; KN, HT, YH, TO, SN,
KeK and TT participated in collecting the data; YE and KH
contributed to the interpretation of the data and supervised the
study. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Committee of Osaka Medical College (approval no. 2429). Due to the
retrospective design of the current study and patient
anonymization, the review board determined that informed consent
was not required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Winawer SJ and Zauber AG: Colonoscopic
polypectomy and the incidence of colorectal cancer. Gut.
48:753–754. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Winawer SJ, Zauber AG, Ho MN, O'Brien MJ,
Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF,
et al: Prevention of colorectal cancer by colonoscopic polypectomy.
The national polyp study workgroup. N Engl J Med. 329:1977–1981.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaminski MF, Wieszczy P, Rupinski M,
Wojciechowska U, Didkowska J, Kraszewska E, Kobiela J, Franczyk R,
Rupinska M, Kocot B, et al: Increased rate of adenoma detection
associates with reduced risk of colorectal cancer and death.
Gastroenterology. 153:98–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tada M, Katoh S, Kohli Y and Kawai K: On
the dye spraying method in colonofiberscopy. Endoscopy. 8:70–74.
1977. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tada M and Kawai K: Research with the
endoscope: New techniques using magnification and chromoscopy. Clin
Gastroenterol. 15:417–437. 1986.PubMed/NCBI
|
|
6
|
Brooker JC, Saunders BP, Shah SG, Thapar
CJ, Thomas HJ, Atkin WS, Cardwell CR and Williams CB: Total colonic
dye-spray increases the detection of diminutive adenomas during
routine colonoscopy: A randomized controlled trial. Gastrointest
Endosc. 56:333–338. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Inoue T, Murano M, Murano N, Kuramoto T,
Kawakami K, Abe Y, Morita E, Toshina K, Hoshiro H, Egashira Y, et
al: Comparative study of conventional colonoscopy and pan-colonic
narrow-band imaging system in the detection of neoplastic colonic
polyps: A randomized, controlled trial. J Gastroenterol. 43:45–50.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ng SC and Lau JY: Narrow-band imaging in
the colon: Limitations and potentials. J Gastroenterol Hepatol.
26:1589–1596. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okada M, Sakamoto H, Takezawa T, Hayashi
Y, Sunada K, Lefor AK and Yamamoto H: Laterally spreading tumor of
the rectum delineated with linked color imaging technology. Clin
Endosc. 49:207–208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshida N, Naito Y, Murakami T, Hirose R,
Ogiso K, Inada Y, Dohi O, Kamada K, Uchiyama K, Handa O, et al:
Linked color imaging improves the visibility of colorectal polyps:
A video study. Endosc Int Open. 5:E518–E525. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Min M, Deng P, Zhang W, Sun X, Liu Y and
Nong B: Comparison of linked color imaging and white-light
colonoscopy for detection of colorectal polyps: A multicenter,
randomized, crossover trial. Gastrointest Endosc. 86:724–730. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sato Y, Sagawa T, Hirakawa M, Ohnuma H,
Osuga T, Okagawa Y, Tamura F, Horiguchi H, Takada K, Hayashi T, et
al: Clinical utility of capsule endoscopy with flexible spectral
imaging color enhancement for diagnosis of small bowel lesions.
Endosc Int Open. 2:E80–E87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rex DK, Cutler CS, Lemmel GT, Rahmani EY,
Clark DW, Helper DJ, Lehman GA and Mark DG: Colonoscopic miss rates
of adenomas determined by back-to-back colonoscopies.
Gastroenterology. 112:24–28. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bensen S, Mott LA, Dain B, Rothstein R and
Baron J: The colonoscopic miss rate and true one-year recurrence of
colorectal neoplastic polyps. Polyp prevention study group. Am J
Gastroenterol. 94:194–199. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hurlstone DP, Cross SS, Slater R, Sanders
DS and Brown S: Detecting diminutive colorectal lesions at
colonoscopy: A randomised controlled trial of pan-colonic versus
targeted chromoscopy. Gut. 53:376–380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Le Rhun M, Coron E, Parlier D, Nguyen JM,
Canard JM, Alamdari A, Sautereau D, Chaussade S and Galmiche JP:
High resolution colonoscopy with chromoscopy versus standard
colonoscopy for the detection of colonic neoplasia: A randomized
study. Clin Gastroenterol Hepatol. 4:349–354. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ogiso K, Yoshida N, Siah KT, Kitae H,
Murakami T, Hirose R, Inada Y, Dohi O, Okayama T, Kamada K, et al:
New-generation narrow band imaging improves visibility of polyps: A
colonoscopy video evaluation study. J Gastroenterol. 51:883–890.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshida N, Hisabe T, Hirose R, Ogiso K,
Inada Y, Konishi H, Yagi N, Naito Y, Aomi Y, Ninomiya K, et al:
Improvement in the visibility of colorectal polyps by using blue
laser imaging (with video). Gastrointest Endosc. 82:542–549. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Togashi K, Nemoto D, Utano K, Isohata N,
Kumamoto K, Endo S and Lefor AK: Blue laser imaging endoscopy
system for the early detection and characterization of colorectal
lesions: A guide for the endoscopist. Therap Adv Gastroenterol.
9:50–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakano A, Hirooka Y, Yamamura T, Watanabe
O, Nakamura M, Funasaka K, Ohno E, Kawashima H, Miyahara R and Goto
H: Comparison of the diagnostic ability of blue laser imaging
magnification versus pit pattern analysis for colorectal polyps.
Endosc Int Open. 5:E224–E231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshida N, Yagi N, Inada Y, Kugai M,
Okayama T, Kamada K, Katada K, Uchiyama K, Ishikawa T, Handa O, et
al: Ability of a novel blue laser imaging system for the diagnosis
of colorectal polyps. Dig Endosc. 26:250–258. 2014. View Article : Google Scholar : PubMed/NCBI
|