Introduction
Hepatitis B virus (HBV) infection is a principal
health problem worldwide. China has ~170 million individuals who
are chronic HBV carriers, 10% of whom may develop chronic hepatitis
(1). HBV is a hepatotropic and a
lymphotropic virus, and the association between HBV and hematologic
malignancies, particularly B-cell non-Hodgkin lymphoma, has been
described previously (2,3).
Multiple myeloma (MM) is the second-most common
hematologic malignancy, involving malignant plasma cells that
continuously proliferate in bone marrow (4). The treatment of MM has improved since
the emergence of novel therapeutics, including proteasome
inhibitors, immunomodulatory drugs and CD38 antibodies, which have
improved the prognosis of patients (5). However, the etiology and pathogenesis
of MM have remained unclear. MM may be associated with viral
infections. Amongst the possible candidate viruses, HBV has been
widely examined; however, the association between HBV infection and
MM is controversial. Becker et al (6) observed that HBV infection is
serologically positive in 15.8% of cases with MM and added MM to
the list of potential virus-associated lymphoma entities. Teng
et al (7) estimated that the
prevalence of chronic HBV is 11.0% in patients with MM, and chronic
hepatitis carriers exhibited poorer overall survival. However,
Marcucci and Mele (3) identified
that associations between HBV infection and MM are weak, as these
tumors are unable to form as a consequence of the chronic antigenic
stimulation of B cells.
To assess the prevalence of HBV infection and
investigate the clinicopathological characteristics and prognostic
significance, 165 patients with MM who had received at least four
cycles of chemotherapy between April 2008 and February 2017 at
Nanjing Drum Tower Hospital (Nanjing, China) were retrospectively
analyzed.
Patients and methods
Patients
The diagnosis of MM was based on the criteria
proposed by the International Myeloma Working Group (IMWG)
(8). The Standard screening tests
include total serum protein, serum and urine protein
electrophoresis (SPEP and UPEP), immunoglobulin free light chain
(FLC) in serum and bone marrow aspiration or biopsy. If a patient
had monoclonal protein detected by SPEP, UPEP, or by pathological
FLC ratio and the plasma cell count was ≥10%, a diagnosis of
multiple myeloma was clear. A total of 306 patients with MM were
diagnosed at Nanjing Drum Tower Hospital between April 2008 and
February 2017. For some patients, HBV status was unavailable or
they did not receive at least four cycles of chemotherapy, so they
were excluded from the present analysis. Therefore, the final data
file comprised 165 patients with MM to analyze the infection of HBV
and assess treatment response. The patients consisted of 88 males
(53.3%) and 77 females (46.7%). The median age of all the subjects
was 61 years (range, 40–81 years), and their median follow-up was
28.17 months (range, 5.4–90.8 months). Univariate and multivariate
analyses of overall survival (OS) and progression-free survival
(PFS) were conducted. Each patient was staged by Durie-Salmon
(9), International Staging System
(10) and Revised-ISS (11). The risk stratification of MM involved
the IMWG risk stratification and the criteria for evaluating the
therapeutic effects were in accordance with IMWG standards
(12). All the patients were
continuously followed up unless patients were lost to follow-up or
they had passed away. Variables regarding clinical manifestations,
laboratory tests and pathological reports were retrieved from the
hospital database using a medical chart review. The Ethics
Committee of Nanjing University (Nanjing, China) approved the
present study and written informed consent was obtained from all
the patients.
Detection method of HBV infection
ELISA was used to detect the HBV markers in all the
patients. HBV Casset ELISA Kit were provided by Shanghai Kehua
Biological Engineering Co., Ltd. Serum hepatitis B surface antigen
(HBsAg), hepatitis B surface antibody, hepatitis B e antigen,
hepatitis B e antibody and hepatitis B core (HBc) antibody were
detected in all the participants prior to and following treatment.
If HBsAg was serologically positive at diagnosis, the serum HBV DNA
level was measured regularly by quantitative PCR performed on an
ABI7300 Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The data of HBV markers and HBV DNA were
obtained from the Department of Clinical Laboratory, Nanjing Drum
Tower Hospital.
Detection method of liver-associated
laboratory parameters and M protein
The aspartate transaminase, bilirubin, triglyceride,
total cholesterol, high-density lipoprotein cholesterol and
low-density lipoprotein cholesterol were detected by SMT-100
automatic biochemical analyzer (Beijing Pulang New Technology Co.,
Ltd., Beijing, China). The PUN-2048A semi-automatic coagulation
analyzer (Beijing Pulang New Technology Co., Ltd.) was used to
detect the coagulation function. Immunovelocity nephelometry was
used to determine M protein by IMMAGE 800 automatic specific
protein analyzer (Beckmann Kurt Company). The data were also
obtained from the Department of Clinical Laboratory, Nanjing Drum
Tower Hospital.
Cytogenetic analysis
Bone marrow samples were subjected to chromosome
Karyotype analysis using the Giemsa-banding staining technique
(13). Chromosomal abnormalities
were described, according to the International System for Human
Cytogenetic Nomenclature 2013 (14).
Cytogenetic abnormalities included a minimum of two mitotic cells
with a gain of the same chromosome or with the same structural
abnormality and three mitotic cells with the loss of the same
chromosome. CD138-purified plasma cells were analyzed through
interphase fluorescence in situ hybridization (FISH) using
probes for chromosomes 1q21, 13q14, immunoglobulin heavy locus
(IGH) and P53 to detect the cytogenetic abnormality of MM.
According to the manufacturer's protocol, CD138+ plasma cells were
purified using anti-CD138 immunobeads and whole blood columns
(Miltenyi Biotec GmbH). A total of 5 ml bone marrow was extracted
and 50 ul of anti-CD138 immunobeads were added per ml of bone
marrow, incubated at 4°C for 15 min, centrifuged at 110 × g at 25°C
for 5 min, the supernatant was removed, and the plasma cells were
separated by column. Then, the plasma cells adsorbed on the
separation column were eluted with Elution buffer (Shanghai Qcbio
Science & Technologies Co., Ltd.), and the purified plasma
cells were hypotonicized at 25°C for 30 min, through a 0.075 m/l
potassium chloride solution, and fixed at 25°C for 30 min by
methanol/glacial acetic acid (3:1) three times and stored at ~20°C.
The probes for chromosomes 1q21, 13q14, IGH and P53 used by FISH
were purchased from Beijing Jinpujia Medical Technology Co., Ltd
(Beijing, China). ThermoBrite in situ hybridization
(NatureGene Corp, USA) was used to perform hybridization. A minimum
of 200 interphase nuclei per probe were evaluated using Olympus
BX51 fluorescence microscope. The manufacturer's protocol: cell
suspensions sorted by anti-CD138 immunobeads were dripped by
air-dry method (15). After baking
the aged slides, they were placed in proteinase K buffer (10 µg/ml)
at 45°C for 1 to 2 h, and then washed with 2 × saline-sodium
citrate solution, gradient dehydration was carried out in 70, 85
and 100% ethanol. After drying the glass slides, the prepared
probes were added to the hybridizer and denatured at 75°C for 5 min
and hybridized at 42°C for 16 h. After washing with 0.1NP-40 and
0.3-NP-40, the slides were incubated with
4,6-Diamidino-2-phenylindole (DAPI) at 25°C for 15 min in the dark
for re-staining. Then, placed the processed sample under a
fluorescence microscope (magnification, ×40; object lens) and
randomly selected a well-dispersed area and vision of cell division
to observe. The hybridization signals were observed by choosing
appropriate filters according to the fluorescein labeled on the
Fish probe. In each case, 200–500 cells were analyzed and the
percentage of positive cells with abnormal fluorescent signal was
counted. If the percentage of positive cells was greater than the
threshold, the result was positive. The threshold for 1q21
amplification, deletion of 13q14 and P53 and IGH rearrangements was
respectively 8.09, 9.01, 8.57, 9.19%. The data of cytogenetic
analysis were from Beijing Hightrust Diagnostics (Beijing,
China).
Treatment for MM
All the patients were administered at least four
cycles of a regimen of chemotherapy of either bortezomib or
dexamethasone; bortezomib, thalidomide and dexamethasone; or
dexamethasone, cyclophosphamide, etoposide and cisplatin. A total
of 16 patients received autologous stem cell transplant (ASCT)
subsequent to undergoing high-dose chemotherapy. All patients with
active HBV infection and chronic HBV infection received lamivudine
[0.1 g once a day (qd)], entecavir (0.5 mg qd) or adefovir
dipivoxil (10 mg qd) for antiviral treatment during the
therapy.
Statistical analysis
SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used for
all statistical analyses. χ2 tests and Fisher's exact
tests were performed to compare categorical variables, and a t-test
was conducted to compare numerical variables between HBV-positive
(anti-HBc positive) and HBV-negative (HBsAg negative and anti-HBc
negative) patients with MM. OS was defined as the time between
diagnosis and mortality or last documented follow-up. PFS was
calculated between the date of diagnosis and progression or
mortality. Kaplan-Meier curves for OS and PFS were analyzed with a
log-rank test for univariate analyses. Factors with P<0.05 in
the univariate analyses were included in multivariate analysis and
examined using a Cox regression model. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
Of the 165 patients with MM, 63 (38.2%) suffered
from an acute or chronic HBV infection and resolved HBV infection
(HBsAg or anti-HBc positive), and 102 (61.8%) did not exhibit HBV
infection (Table I). The number of
patients with active, chronic or resolved HBV infection was 19
(11.51%), 24 (14.54%) and 20 (12.12%), respectively (data not
shown). Furthermore, 37 patients from the whole cohort developed an
extramedullary disease (EMD) at the time of diagnosis or during
follow-up (Table I). Among the
subjects including HBV negative and positive patients, 74 had
immunoglobulin (Ig)G type M protein (44.8%), 43 had IgA type M
protein (26.1%), 36 had light chain type M protein (21.8%) and 12
had other types of M protein, including IgM, IgD and IgE (7.3%;
Table I). According to the R-ISS,
16.3% (27/165) of the patients were classified as stage 3 (Table I). According to the IMWG risk
stratification 15.2% (25/165) were described as low risk, 72.7%
(120/165) were categorized as moderate risk and 12.1% (20/165) were
recorded as high risk (Table I). All
the patients underwent at least four cycles of chemotherapy, and
49.1% (81/165) of the cases were treated with bortezomib-containing
regimens (Table I). In addition, 16
patients received ASCT following high-dosage chemotherapy.
 | Table I.Characteristics of 165 patients with
MM according to their viral hepatitis status. |
Table I.
Characteristics of 165 patients with
MM according to their viral hepatitis status.
|
| HBV status |
|
|---|
|
|
|
|
|---|
| Characteristic | Negative n (%) | Positive n (%) | P-value |
|---|
| Number of
patients | 102 | 63 |
|
| Sex |
|
| 0.083 |
|
Male | 49 (48) | 39 (61.9) |
|
|
Female | 53 (52) | 24 (38.1) |
|
| Age ≥65 years | 34 (33.3) | 22 (34.9) | 0.834 |
| Type of MM |
|
| 0.218 |
|
IgG | 40 (39.2) | 34 (54) |
|
|
IgA | 27 (26.5) | 16 (25.4) |
|
| Light
chain | 26 (25.5) | 10 (15.9) |
|
|
aOthers | 9 (8.8) | 3 (4.8) |
|
| DS stage |
|
| 0.541 |
| I | 2 (2) | 3 (4.8) |
|
| II | 22 (21.6) | 15 (23.8) |
|
|
III | 78 (76.4) | 45 (71.4) |
|
| ISS |
|
| 0.702 |
| 1 | 18 (17.6) | 14 (22.2) |
|
| 2 | 43 (42.2) | 27 (42.9) |
|
| 3 | 41 (40.2) | 22 (34.9) |
|
| R-ISS |
|
| 0.337 |
| 1 | 9 (8.8) | 10 (15.9) |
|
| 2 | 77 (75.5) | 42 (66.7) |
|
| 3 | 16 (15.7) | 11 (17.5) |
|
| IMWG risk
stratification |
|
| 0.675 |
| Low
risk | 16 (15.7) | 9 (14.3) |
|
|
Moderate risk | 72 (70.6) | 48 (76.2) |
|
| High
risk | 14 (13.7) | 6 (9.5) |
|
| Number of Bone
lesions |
|
| 0.764 |
|
None | 10 (9.8) | 6 (9.5) |
|
| 1 | 15 (14.7) | 12 (19.0) |
|
| ≥2 | 77 (75.5) | 45 (71.4) |
|
| Blast
plasma cells ≥10% | 77 (75.5) | 43 (68.3) | 0.311 |
| EMD |
|
| 0.137 |
| No | 83 (81.4) | 45 (71.4) |
|
|
Yes | 19 (18.6) | 18 (28.6) |
|
| Use of
immunomodulatory drugs | 81 (79.4) | 48 (76.2) | 0.626 |
| Use of
bortezomib | 54 (52.9) | 27 (42.9) | 0.208 |
| Stem
cell transplantation | 12 (11.8) | 4 (6.3) | 0.384 |
| Baseline laboratory
parameters |
|
|
|
|
β2-microglobulin ≥3.5
mg/l | 67 (65.7) | 39 (61.9) | 0.622 |
|
Hemoglobin <100 g/l | 64 (62.7) | 38 (60.3) | 0.755 |
|
Platelets
<100×109/l | 17 (16.7) | 14 (22.2) | 0.375 |
| Albumin
<35 g/l | 46 (45.1) | 34 (54.0) | 0.268 |
|
Alkaline phosphatase >185
IU/l | 2 (2.0) | 1 (1.6) | 1.000 |
| Lactate
dehydrogenase >245 IU/l | 12 (11.8) | 11 (17.5) | 0.305 |
| Serum
creatinine ≥177 µmol/l | 7 (6.9) | 8 (12.7) | 0.266 |
| Serum
calcium >2.65 mmol/l | 22 (21.6) | 11 (17.5) | 0.522 |
|
C-reactive protein >5
mg/l | 36 (35.3) | 23 (36.5) | 0.874 |
| Hepatic
cirrhosis | 0 (0) | 1 (1.6) | 0.382 |
| HBV status |
|
|
|
| HBsAg
negative | 102 (100) | 43 (68.3) |
|
| HBsAg
positive | 0 | 20 (31.7) |
|
| HBc
antibody negative | 102 (100) | 0 |
|
|
Anti-HBc positive | 0 | 63 (100) |
|
Comparison of the clinical parameters
between patients who were HBV-positive and HBV-negative
In the present study, patients classified as
HBV-positive included those with an acute or chronic HBV infection
and resolved HBV infection. Patients classified as HBV-negative
were those who had not been infected with HBV. In Table I, the baseline characteristics,
including sex, age, type of MM, DS stage, ISS stage, R-ISS stage,
IMWG risk stratification, number of bone lesions, ratio of blast
plasma cells, presence of EMD, therapeutic regimen and laboratory
parameters, were similar among the patients regardless of their HBV
status. Table II summarizes the
liver-associated laboratory parameters of patients with MM.
Patients classified as HBV-positive had liver dysfunction, as
indicated by the increased alanine transaminase (ALT) levels
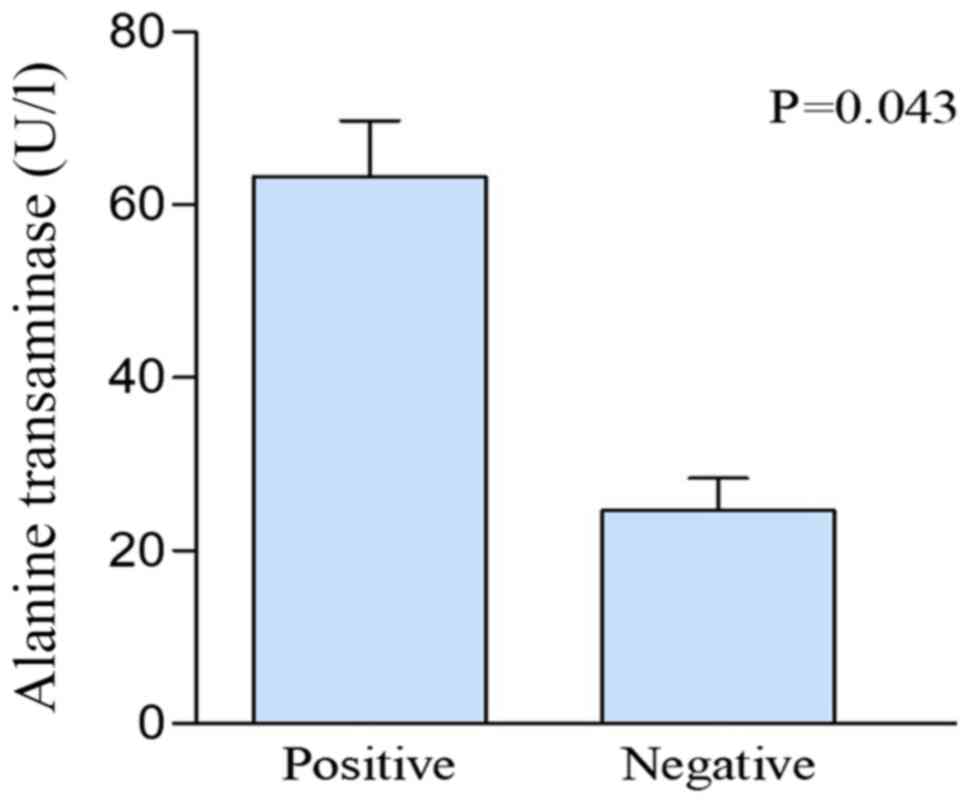
(positive vs. negative; 63.29 vs. 24.66 U/l; P=0.043; Fig. 1), compared with patients classified
as HBV-negative. The levels of aspartate transaminase, bilirubin,
triglyceride, total cholesterol, high-density lipoprotein
cholesterol, low-density lipoprotein cholesterol and coagulation
function did not significantly differ between the two groups
(P>0.05). The four chromosomal aberrations detected with FISH
were as follows: i) Gain of 1q21 (44.2%); ii) deletion of 13q14
(30.3%); iii) IGH rearrangements (31.5%); and iv) deletion of P53
(17.0%; Table III). The only
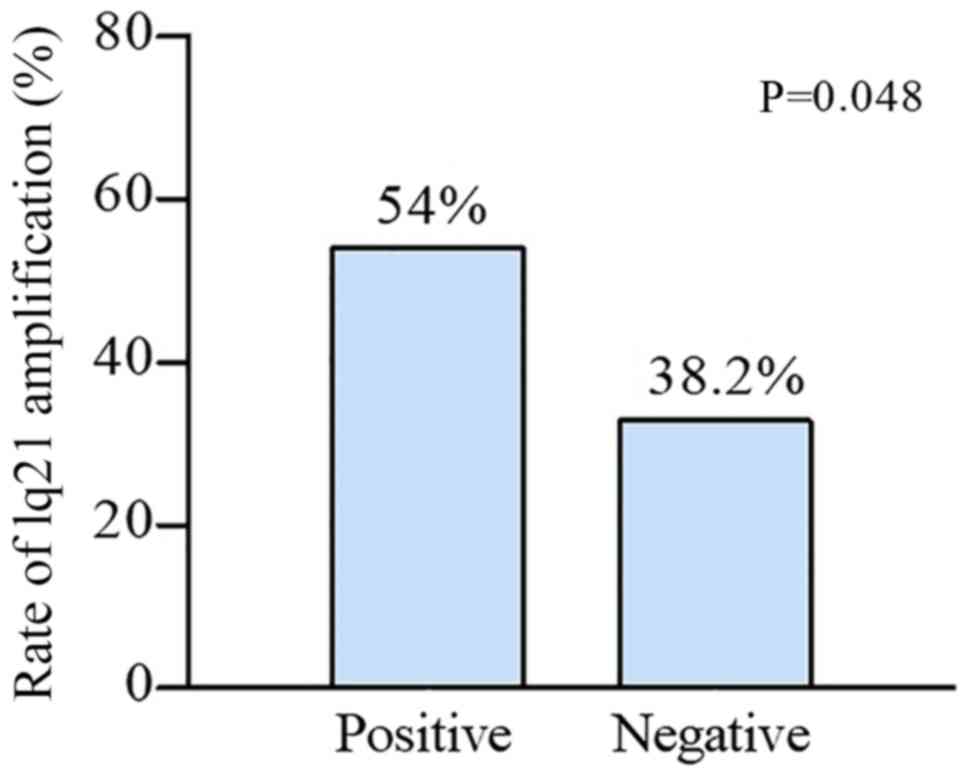
significant chromosomal aberration was gain of 1q21. In total, ~54%
of the patients who were classified as HBV-positive had this
chromosomal aberration, which was significantly higher compared
with the 38.2% of the patients who were classified as HBV-negative
with the same chromosomal aberration (P=0.048; Fig. 2). Additionally, the incidence of the
three remaining chromosomal abnormalities was higher in patients
classified as HBV-positive compared with patients that were
HBV-negative; however, these increases were not statistically
significant (P>0.05).
 | Table II.Liver-associated laboratory
parameters among patients with multiple myeloma. |
Table II.
Liver-associated laboratory
parameters among patients with multiple myeloma.
|
| HBV status |
|
|---|
|
|
|
|
|---|
| Biomarkers | Negative,
n=102 | Positive, n=63 | P-value |
|---|
| Alanine
transaminase, U/l | 24.66 | 63.29 | 0.043a |
| Aspartate
transaminase, U/l | 27.72 | 38 | 0.085 |
| Total BIL,
µmol/l | 8.66 | 12.41 | 0.092 |
| Direct BIL,
µmol/l | 3.1 | 4.93 | 0.068 |
| Triglyceride,
mmol/l | 1.52 | 1.4 | 0.377 |
| Total cholesterol,
mmol/l | 3.55 | 3.32 | 0.262 |
| High DL,
mmol/l | 0.92 | 0.84 | 0.193 |
| Low DL, mmol/l | 1.82 | 1.72 | 0.522 |
| Activated partial
thromboplastin time, sec | 32.13 | 31.74 | 0.792 |
 | Table III.Association of serological HBV status
with chromosomal aberrations. |
Table III.
Association of serological HBV status
with chromosomal aberrations.
|
| HBV status | OR |
|---|
|
|
|
|
|---|
| Aberration | Negative n (%) | Positive n (%) | OR (95% confidence
interval) | P-value |
|---|
| 1q21+ |
|
| 1.894
(1.002–3.579) | 0.048a |
| 0 | 63 (61.8) | 29 (46.0) |
|
|
| 1 | 39 (38.2) | 34 (54.0) |
|
|
| 13q14- |
|
| 1.259
(0.639–2.479) | 0.506 |
| 0 | 73 (71.6) | 42 (66.7) |
|
|
| 1 | 29 (28.4) | 21 (33.3) |
|
|
| IGH
rearrangements |
|
| 1.447
(0.741–2.827) | 0.278 |
| 0 | 73 (71.6) | 40 (63.5) |
|
|
| 1 | 29 (28.4) | 23 (36.5) |
|
|
| P53- |
|
| 1.058
(0.460–2.433) | 0.895 |
| 0 | 85 (83.3) | 52 (82.5) |
|
|
| 1 | 17 (16.7) | 11 (17.5) |
|
|
Summary of the six patients with HBV
reactivation
In the present study, all patients received
lamivudine (0.1 g qd), entecavir (0.5 mg qd) or adefovir dipivoxil
(10 mg qd) for antiviral treatment during the therapy regardless of
the quantities of HBV DNA. However, six cases progressed to active
infection from chronic HBV infection and the quantity of HBV DNA
was >500 IU/ml in two patients (cases 2 and 4; Table IV). In four cases, HBV reactivation
developed during induction therapy and the other two cases of HBV
reactivation occurred during ASCT. All patients developed the
progressive disease (Table IV).
 | Table IV.Summary of six patients with HBV
reactivation. |
Table IV.
Summary of six patients with HBV
reactivation.
| Patient | Sex | Age | Treatment | Number of times
chemotherapy was received | HBV DNA, IU/ml | Time of
reactivation | Anti-HBV
treatment | Response |
|---|
| 1 | M | 56 | VTD + CTD | 6 | <500 | During
induction | Entecavir | PD |
| 2 | F | 51 | VTD + VD +
DECP | 16 |
5.90×103 | During
induction | Lamivudine | PD |
| 3 | M | 65 | VD | 4 | <500 | During
induction | Entecavir | PD |
| 4 | F | 68 | VTD + ASCT | 4 |
1.26×103 | During ASCT | Entecavir | PD |
| 5 | F | 58 | TD + VAD + MP | 9 | <500 | During
induction | Lamivudine | PD |
| 6 | M | 43 | VTD + ASCT +
DVDT | 10 | <500 | During ASCT | Entecavir | PD |
Survival analysis
Multiple clinical parameters were assessed to
determine their association with the survival of patients with MM
(Table V). The OS of patients
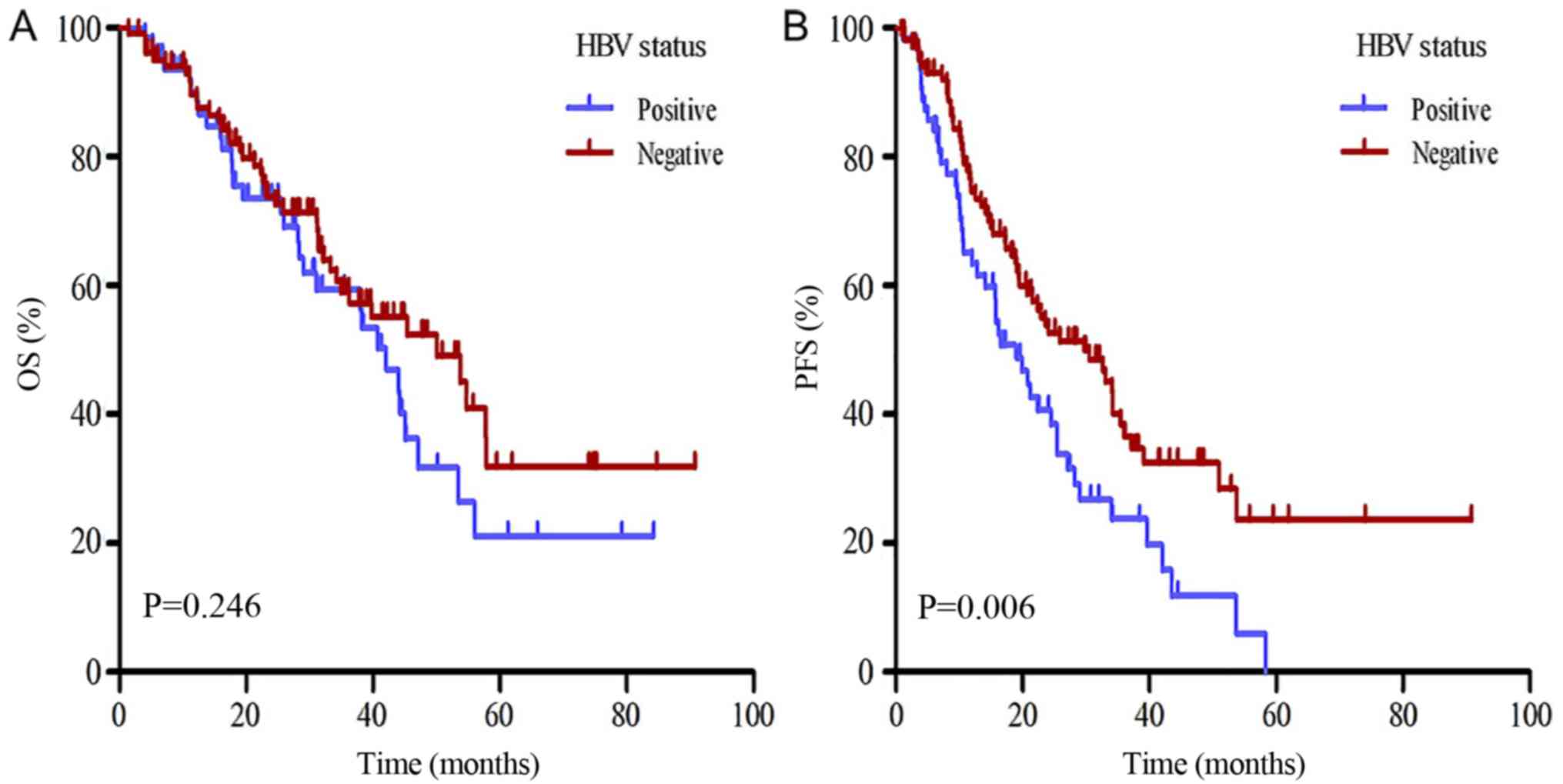
classified as HBV-positive was decreased compared with patients
classified as HBV-negative; however, this difference was not
significant (42 vs. 50 months; P>0.05; Fig. 3A). In the univariate analyses, age,
R-ISS stage, IMWG risk stratification, chromosome, ratio of blast
plasma cells, β2-microglobulin, hemoglobin, lactate
dehydrogenase (LDH), serum creatinine and serum calcium levels were
identified as significant risk factors of OS (Table V; P<0.05). In the multivariate
analysis, the LDH level was significantly >245 IU/l, the serum
creatinine level was significantly >177 µmol/l and the serum
calcium level was significantly >2.65 mmol/l (Table VI). The PFS was significantly
decreased in patients classified as HBV-positive compared with
patients classified as HBV-negative (18.97 vs. 29.67 months;
P=0.006; Fig. 3B). In the univariate
analyses, R-ISS stage, chromosome, EMD, stem cell transplantation,
hemogloblin, LDH, serum creatinine and HBV status were identified
as significant risk factors of PFS (Table V; P<0.05). The multivariate
regression analysis of the PFS-influencing factors indicated that
HBV-positive status and EMD were considered independent prognostic
factors (Table VI). The patients
classified as HBV-positive were divided into active infection,
chronic infection and resolved infection. The OS of patients with
active infection was significantly decreased compared with the
other groups (P<0.05; Fig. S1A).
The PFS of patients with active infection, chronic infection and
resolved infection was significantly decreased compared with
patients classified as HBV-negative (P<0.05; Fig. S1B).
 | Table V.Univariate analysis of survival in
patients with MM. |
Table V.
Univariate analysis of survival in
patients with MM.
| Characteristic | Median overall
survival, months | P-value | Median
progression-free survival, months | P-value |
|---|
| Sex |
| 0.688 |
| 0.887 |
|
Male | 38.20 |
| 22.33 |
|
|
Female | 47.10 |
| 22.56 |
|
| Age |
| 0.035a |
| 0.897 |
| ≥65
years | 34.93 |
| 20.80 |
|
| <65
years | 50.00 |
| 24.47 |
|
| Type of MM |
| 0.167 |
| 0.378 |
|
IgG | 50.00 |
| 25.43 |
|
|
IgA | 45.37 |
| 19.53 |
|
| Light
chain | 34.17 |
| 18.20 |
|
|
bOthers | NR |
| 34.20 |
|
| DS stage |
| 0.131 |
| 0.549 |
| I | NR |
| 12.83 |
|
| II | 42.20 |
| 27.10 |
|
|
III | 37.88 |
| 22.33 |
|
| ISS stage |
| 0.050 |
| 0.193 |
| 1 | NR |
| 35.97 |
|
| 2 | 43.97 |
| 24.10 |
|
| 3 | 37.97 |
| 20.67 |
|
| R-ISS stage |
|
<0.001a |
| 0.006a |
| 1 | NR |
| 35.97 |
|
| 2 | 53.47 |
| 25.43 |
|
| 3 | 28.37 |
| 14.50 |
|
| IMWG risk
stratification |
| 0.015a |
| 0.442 |
| Low
risk | NR |
| 32.63 |
|
|
Moderate risk | 43.97 |
| 23.57 |
|
| High
risk | 23.00 |
| 15.73 |
|
| Chromosome |
| 0.001a |
| 0.044a |
|
Normal | 54.70 |
| 24.47 |
|
|
Abnormal | 32.10 |
| 19.53 |
|
| Blast plasma
cells |
| 0.035a |
| 0.078 |
|
≥10% | 42.00 |
| 21.50 |
|
|
<10% | NR |
| 28.17 |
|
| EMD |
| 0.862 |
| 0.037a |
|
Yes | 34.93 |
| 19.40 |
|
| No | 44.17 |
| 25.43 |
|
| Use of
immunomodulatory drugs |
| 0.729 |
| 0.523 |
|
Yes | 44.17 |
| 23.57 |
|
| No | 43.97 |
| 22.87 |
|
| Use of
bortezomib |
| 0.362 |
| 0.196 |
|
Yes | 44.17 |
| 23.57 |
|
| No | 37.97 |
| 22.30 |
|
| Stem cell
transplantation |
| 0.200 |
| 0.030a |
|
Yes | NR |
| 58.37 |
|
| No | 43.97 |
| 22.30 |
|
|
β2-microglobulin |
| 0.001a |
| 0.059 |
| ≥3.5
mg/l | 36.23 |
| 21.50 |
|
| <3.5
mg/l | NR |
| 32.63 |
|
| Hemoglobin |
| 0.007a |
| 0.025a |
| ≥100
g/l | NR |
| 34.03 |
|
| <100
g/l | 39.77 |
| 20.80 |
|
| Platelets |
| 0.399 |
| 0.270 |
|
≥100×109/l | 44.17 |
| 24.10 |
|
|
<100×109/l | 42.00 |
| 18.97 |
|
| Albumin |
| 0.535 |
| 0.919 |
| ≥35
g/l | 45.37 |
| 24.47 |
|
| <35
g/l | 38.20 |
| 19.53 |
|
| Lactate
dehydrogenase |
| 0.023a |
| 0.010a |
| >245
IU/l | 23.00 |
| 10.57 |
|
| ≤245
IU/l | 45.00 |
| 25.43 |
|
| Serum
creatinine |
|
<0.001a |
| 0.003a |
| ≥177
µmol/l | 19.43 |
| 14.50 |
|
| <177
µmol/l | 47.10 |
| 24.10 |
|
| Serum calcium |
| 0.042a |
| 0.324 |
|
>2.65 mmol/l | 36.23 |
| 15.73 |
|
| ≤2.65
mmol/l | 45.00 |
| 24.10 |
|
| C-reactive
protein |
| 0.364 |
| 0.853 |
| >5
mg/l | 37.97 |
| 24.47 |
|
| ≤5
mg/l | 45.36 |
| 20.80 |
|
| HBV status |
| 0.246 |
| 0.006a |
|
Negative | 50.00 |
| 29.67 |
|
|
Positive | 42.00 |
| 18.97 |
|
 | Table VI.Multivariate analysis of survival in
patients with multiple myeloma. |
Table VI.
Multivariate analysis of survival in
patients with multiple myeloma.
| A, Overall
survival |
|---|
|
|---|
|
| Multivariate
analysisb |
|---|
|
|
|
|---|
| Parameter | HR | 95% confidence
interval | P-value |
|---|
| Age | 0.613 | 0.364–1.032 | 0.066 |
| R-ISS stage | 0.964 | 0.486–1.911 | 0.916 |
| IMWG risk
stratification | 0.914 | 0.385–2.172 | 0.839 |
| Chromosome | 0.696 | 0.398–1.217 | 0.204 |
| Blast plasma
cells | 1.041 | 0.539–2.010 | 0.905 |
|
β2-microglobulin | 1.539 | 0.782–3.029 | 0.211 |
| Hemoglobin | 1.678 | 0.879–3.204 | 0.117 |
| LDH | 2.448 | 1.082–5.537 | 0.032a |
| Serum
creatinine | 2.953 | 1.494–5.836 | 0.002a |
| Serum calcium | 2.042 | 1.118–3.728 | 0.020a |
|
| B,
Progression-free survival |
|
|
| Multivariate
analysisb |
|
|
|
|
Parameter | HR | 95% confidence
interval | P-value |
|
| R-ISS stage | 0.781 | 0.428–1.424 | 0.420 |
| Chromosome | 0.790 | 0.512–1.219 | 0.286 |
| EMD | 0.488 | 0.289–0.826 | 0.007a |
| Stem cell
transplantation | 2.267 | 0.945–5.438 | 0.067 |
| Hemoglobin | 1.493 | 0.931–2.394 | 0.096 |
| LDH | 1.810 | 0.984–3.330 | 0.056 |
| Serum
creatinine | 1.699 | 0.843–3.425 | 0.138 |
| HBV status | 0.627 | 0.417–0.943 | 0.025a |
Discussion
MM is a B-cell malignancy characterized by the
proliferation of clonal plasma cells in bone marrow. It is
frequently clinically manifested with hypercalcemia, renal
dysfunction, anemia and bone disability (16). The combination of novel induction
chemotherapy medications with ASCT is the standard method of
treatment for patients with MM. However, these patients are highly
susceptible to infections as a result of the inhibition of normal
Immunoglobulin.
HBV is a small DNA virus belonging to the
Hepadnaviridae family, which is characterized by a genome
consisting of four overlapping open reading frames: S gene, core
gene, P gene and X gene (17).
Previous studies have described lymphotropic targeting cells of
peripheral blood mononuclear cells, spleen, lymph nodes, thymus and
bone marrow (18,19). A previous retrospective case-control
trial demonstrated that the HBsAg-positive rate was significantly
increased in patients with MM compared with patients with acute
leukemia, and HBsAg positivity may be a prognostic factor for
patients with MM in HBV-endemic areas (20). China is an area with a high incidence
of HBV infection (21). In the
present study, the rates of acute and chronic HBV infection in
patients with MM were 11.51 and 14.54%, respectively, which were
increased compared with the prevalence of these infections in the
general population (22).
Rehermann et al (23) determined that HBV is unable to be
completely eradicated by the immune response, and HBsAg-negative
patients may carry traces of HBV genome in their serum for decades
after they clinically recover. HBV is able to replicate through an
RNA intermediate, integrate into its host genome, induce genomic
aberrations and instability, and remain traceable in resolved HBV
infection (HBsAg negative and anti-HBc positive) (3). The deletion of 8p chromosome is an
important factor in the development of HBV-associated tumors. In
hepatocellular carcinoma and MM, the deletion of this chromosome
has been observed in ~40% of the patients classified as
HBV-positive compared with 20–30% of the patients classified as
HBV-negative (24,25). Perhaps due to sample size, Becker
et al (25) did not identify
a significant difference between 1q21 amplification and the
pathogenesis of MM. However, the present study identified a
statistically significant gain of 1q21 between the patients
classified as HBV-positive compared with the patients classified as
HBV-negative. Chromodomain-helicase-DNA-binding protein 1-like
(CHD1L) overexpression caused by 1q21 amplification may increase
cell motility, induce filopodium formation and
epithelial-mesenchymal transition, which may contribute to tumor
cell invasion and metastasis (26).
The deregulated overexpression of B-cell lymphoma 9 protein (BCL9)
in the pre-B leukemia cell line CEMO-1, suggested that the
overexpression of BCL9 may be pathogenically essential for B-cell
malignancies with breakpoints at 1q21 (27). Therefore, it was hypothesized that
HBV infection may contribute to 1q21 amplification and cause MM
progression through the overexpression of CHD1L and BCL9; however,
this hypothesis requires further investigation with larger
cohorts.
HBV infection induces B cells to produce specific
antibodies that react with antigens on the surface of hepatocytes
and cause liver injury, thereby increasing ALT levels (28). Immunosuppressive chemotherapy for MM
frequently induces liver dysfunction in patients infected with HBV
(29). The present data demonstrated
that the level of ALT in patients with HBV infection was
significantly increased compared with the non-infected group, and
the level of transaminase increased in the majority of the
HBsAg-positive patients prior to treatment. Therefore, monitoring
the liver function of patients and timely administration of
liver-protecting drugs may improve prognoses.
Reactivation of HBV is a well-recognized
complication following systemic chemotherapy for hematological
malignancies. A previous study identified that Hhigh-dose therapy
and ASCT were significant risk factors that were positively
associated with HBV reactivation (30). Although all the patients received
lamivudine, entecavir or adefovir dipivoxil for an antiviral
treatment in the present study, there were six patients with HBV
reactivation. These cases received high-dose chemotherapy and two
of the cases received ASCT. Therefore, it is necessary to closely
monitor the HBV DNA level and antiviral therapy during high-dose
chemotherapy and ASCT of patients with MM and HBV infection.
Previous studies on the association of HBV infection
with the survival of patients with MM demonstrated that the OS of
HBsAg-positive patients who underwent ASCT was significantly
decreased compared with HBsAg-negative patients (1,31). The
present data demonstrated that the OS of the patients classified as
HBV-positive was decreased compared with the patients classified as
HBV-negative; however, this difference was not significant.
Therefore, these results differed from previous studies; however,
this difference may be attributed to the inclusion of patients with
resolved HBV infection. The LDH level was >245 IU/l, the serum
creatinine level was >177 µmol/l and the serum calcium level was
>2.65 mmol/l. These parameters were independent factors
associated with poor prognosis. These findings were consistent with
previous studies (7,32,33).
Therefore, an increase in LDH, serum creatinine and serum calcium
levels indicated a high tumor mass, suggesting poor prognosis;
however, this requires further examination. Additionally, the PFS
of the patients classified as HBV-positive and patients classified
as HBV-negative was evaluated subsequent to the patients undergoing
chemotherapy, and the results demonstrated that the PFS was
significantly shorter in the patients classified as HBV-positive.
HBV infection was considered an independent prognostic factor of
Cox analysis; however, this observation has yet to be demonstrated,
to the best of the authors' knowledge. HBV infection promotes
T-cell immunoglobulin and mucin-domain containing-3 (Tim-3)
expression on Type 1 T helper cells, and T cell dysfunction
mediated by the Tim-3/galectin-9 signaling pathway predicted poor
prognosis in patients with HBV-associated hepatocellular carcinoma
(34). Nevertheless, whether similar
mechanisms are responsible for the poor prognosis in patients
classified as HBV-positive with MM requires further
investigation.
In conclusion, HBV infection may contribute to MM
progression through 1q21 amplification and was considered to be an
independent prognostic factor among patients with MM. The close
monitoring of the level of HBV markers and the timely use of
antiviral drugs are crucial for HBV-positive patients.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Jiangsu
Provincial Medical Innovation Team (grant no. CXTDA2017046;
China).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DG and PPX contributed to the drafting the
manuscript and design of the study. CG contributed to the
acquisition, analysis and interpretation of data, YX contributed to
the collection and analysis of the data. YY, JX, RZ and BC
contributed to the conception and design of the study, and the
editing of the manuscript. All authors have read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Nanjing University approved
the present study and written informed consent was obtained from
all patients.
Patient consent for publication
All patients consented to the publication of this
research.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li J, Liu J, Huang B, Zheng D, Chen M,
Zhou Z, Xu D and Zou W: Hepatitis B virus infection status is an
independent risk factor for multiple myeloma patients after
autologous hematopoietic stem cell transplantation. Tumour Biol.
34:1723–1728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Noonan CA, Yoffe B, Mansell PW, Melnick JL
and Hollinger FB: Extrachromosomal sequences of hepatitis B virus
DNA in peripheral blood mononuclear cells of acquired immune
deficiency syndrome patients. Proc Natl Acad Sci USA. 83:5698–5702.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marcucci F and Mele A: Hepatitis viruses
and non-Hodgkin lymphoma: Epidemiology, mechanisms of
tumorigenesis, and therapeutic opportunities. Blood. 117:1792–1798.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smith L, McCourt O, Henrich M, Paton B,
Yong K, Wardle J and Fisher A: Multiple myeloma and physical
activity: A scoping review. BMJ Open. 5:e0095762015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Avet-Loiseau H, Bahlis NJ, Chng WJ, Masszi
T, Viterbo L, Pour L, Ganly P, Palumbo A, Cavo M, Langer C, et al:
Ixazomib significantly prolongs progression-free survival in
high-risk relapsed/refractory myeloma patients. Blood.
130:2610–2618. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Becker N, Schnitzler P, Boffetta P,
Brennan P, Foretova L, Maynadié M, Nieters A, Staines A, Benavente
Y, Cocco P and de Sanjose S: Hepatitis B virus infection and risk
of lymphoma: Results of a serological analysis within the European
case-control study Epilymph. J Cancer Res Clin Oncol.
138:1993–2001. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Teng CJ, Liu HT, Liu CY, Hsih CH, Pai JT,
Gau JP, Liu JH, Chiou TJ, Hsu HC, Chen PM, et al: Chronic hepatitis
virus infection in patients with multiple myeloma: Clinical
characteristics and outcomes. Clinics (Sao Paulo). 66:2055–2061.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rajkumar SV, Dimopoulos MA, Palumbo A,
Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E,
Richardson P, et al: International Myeloma Working Group updated
criteria for the diagnosis of multiple myeloma. Lancet Oncol.
15:e538–e548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Durie BG and Salmon SE: A clinical staging
system for multiple myeloma. Correlation of measured myeloma cell
mass with presenting clinical features, response to treatment, and
survival. Cancer. 36:842–854. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Greipp PR, San Miguel J, Durie BG, Crowley
JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H,
Kyle RA, et al: International staging system for multiple myeloma.
J Clin Oncol. 23:3412–3420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palumbo A, Avet-Loiseau H, Oliva S,
Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, Caltagirone S,
Lahuerta JJ, Facon T, et al: Revised international staging system
for multiple myeloma: A report from international Myeloma Working
Group. J Clin Oncol. 33:2863–2869. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kumar S, Paiva B, Anderson KC, Durie B,
Landgren O, Moreau P, Munshi N, Lonial S, Bladé J, Mateos MV, et
al: International Myeloma Working Group consensus criteria for
response and minimal residual disease assessment in multiple
myeloma. Lancet Oncol. 17:e328–e346. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang H and Chen J: Chromosome bandings.
Methods Mol Biol. 1541:59–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Simons A, Shaffer LG and Hastings RJ:
Cytogenetic nomenclature: Changes in the ISCN 2013 compared to the
2009 edition. Cytogenet Genome Res. 141:1–6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li GP, Liu Y, White KL and Bunch TD:
Cytogenetic analysis of diploidy in cloned bovine embryos using an
improved air-dry karyotyping method. Theriogenology. 63:2434–2444.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Katzel JA, Hari P and Vesole DH: Multiple
myeloma: Charging toward a bright future. CA Cancer J Clin.
57:301–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin CL and Kao JH: Natural history of
acute and chronic hepatitis B: The role of HBV genotypes and
mutants. Best Pract Res Clin Gastroenterol. 31:249–255. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Michalak TI: Occult persistence and
lymphotropism of hepadnaviral infection: Insights from the
woodchuck viral hepatitis model. Immunol Rev. 174:98–111. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sinha M, Rao CR, Premalata CS, Shafiulla
M, Lakshmaiah KC, Jacob LA, Babu GK, Viveka BK, Appaji L and
Subramanyam JR: Plasma Epstein-Barr virus and Hepatitis B virus in
non-Hodgkin lymphomas: Two lymphotropic, potentially oncogenic,
latently occurring DNA viruses. Indian J Med Paediatr Oncol.
37:146–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang B, Li J, Zhou Z, Zheng D, Liu J and
Chen M: High prevalence of hepatitis B virus infection in multiple
myeloma. Leuk Lymphoma. 53:270–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen CJ, Wang LY and Yu MW: Epidemiology
of hepatitis B virus infection in the Asia-Pacific region. J
Gastroenterol Hepatol. 15 (Suppl):E3–E6. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stasi C, Silvestri C and Voller F:
Emerging trends in epidemiology of hepatitis B virus infection. J
Clin Transl Hepatol. 5:272–276. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rehermann B, Ferrari C, Pasquinelli C and
Chisari FV: The hepatitis B virus persists for decades after
patients' recovery from acute viral hepatitis despite active
maintenance of a cytotoxic T-lymphocyte response. Nat Med.
2:1104–1108. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moinzadeh P, Breuhahn K, Stützer H and
Schirmacher P: Chromosome alterations in human hepatocellular
carcinomas correlate with aetiology and histological grade-results
of an explorative CGH meta-analysis. Br J Cancer. 92:935–941. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Becker N, Byl A, Friedrich S, Jauch A,
Schnitzler P, Egerer G, Ho AD, Goldschmidt H and Neben K: Hepatitis
B virus infection is associated with deletion of chromosome 8p in
multiple myeloma. Eur J Haematol. 90:279–285. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen L, Chan TH and Guan XY: Chromosome
1q21 amplification and oncogenes in hepatocellular carcinoma. Acta
Pharmacol Sin. 31:1165–1171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Willis TG, Zalcberg IR, Coignet LJ,
Wlodarska I, Stul M, Jadayel DM, Bastard C, Treleaven JG, Catovsky
D, Silva ML and Dyer MJ: Molecular cloning of translocation
t(1;14)(q21;q32) defines a novel gene (BCL9) at chromosome 1q21.
Blood. 91:1873–1881. 1998.PubMed/NCBI
|
|
28
|
Oh IS and Park SH: Immune-mediated liver
injury in hepatitis B virus infection. Immune Netw. 15:191–198.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bang SM, Kim SS, Park SH, Ahn JY, Cho EK,
Shin DB and Lee JH: Acute exacerbation of chronic hepatitis B
during thalidomide therapy for multiple myeloma: A case report.
Korean J Intern Med. 19:196–198. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee JY, Lim SH, Lee MY, Kim H, Sinn DH,
Gwak GY, Choi MS, Lee JH, Jung CW, Jang JH, et al: Hepatitis B
reactivation in multiple myeloma patients with resolved hepatitis B
undergoing chemotherapy. Liver Int. 35:2363–2369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li J, Huang B, Li Y, Zheng D, Zhou Z and
Liu J: Hepatitis B virus reactivation in patients with multiple
myeloma receiving bortezomib-containing regimens followed by
autologous stem cell transplant. Leuk Lymphoma. 56:1710–1717. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dimopoulos MA, Barlogie B, Smith TL and
Alexanian R: High serum lactate dehydrogenase level as a marker for
drug resistance and short survival in multiple myeloma. Ann Intern
Med. 115:931–935. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maillet D, Montiel-Cervantes L,
Padilla-González Y, Sánchez-Cortés E, Xolotl-Castillo M, Vela-Ojed
J and Reyes-Maldonado E: Serum calcium is an independent prognostic
factor of overall survival in Mexican patients with multiple
myeloma. Rev Invest Clin. 64:17–24. 2012.PubMed/NCBI
|
|
34
|
Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X,
Liu J, Shi L, Liu C, Wang G and Zou W: Tim-3/galectin-9 signaling
pathway mediates T-cell dysfunction and predicts poor prognosis in
patients with hepatitis B virus-associated hepatocellular
carcinoma. Hepatology. 56:1342–1351. 2012. View Article : Google Scholar : PubMed/NCBI
|