Introduction
Prostate cancer (PC) has the second highest
incidence in malignant tumors among males (1). A study in 2017 showed that its
incidence was second only to that of lung cancer in the United
States (2). The incidence in China
is lower than that in European and American countries, but it has
significantly increased in recent years. According to 2015 cancer
statistics in China, the incidence of PC ranked 7th among male
tumors, and the disease was the only urinary system tumor in the
top 10 (3). Due to the increasing
incidence, early diagnosis and treatment of PC is essential and
needs to be improved. Currently, the main serological diagnostic
marker for PC is serum prostate specific antigen (PSA), which has
low specificity and is prone to false positives. Infection, trauma,
and prostatic hyperplasia result in an increase in the expression
of PSA, which causes patients to undergo prostate needle biopsy for
diagnosis (4). Therefore, it is
necessary for clinicians and scientific researchers to find new
serological diagnostic markers.
As a non-coding short-strand RNA with a length of
~22 nt, microRNA (miR) has been valued by increasing number of
scholars in recent years. It binds to the 3′-untranslated regions
(3′-UTR) of its downstream target gene mRNA, and inhibits the
translation and transcription of the gene, thereby changing the
gene expression (5). Studies have
proved that miRs are differentially expressed in tumors (6), cardiovascular diseases (7), and genetic diseases (8), involved in their development and
progression. miR-129 is a special miR family encoded and
synthesized through miR-129-1 and miR-129-2, and miR-129-5p is the
embodiment of miR-129 function (9).
A study has shown that miR-129 is located around fragile sites at
7q, and loss of the sites is closely related to PC (10). In the study of Catto et al
(11) miR-129 was differentially
expressed in PC and it may be a potential target for the treatment
of the disease. However, there are currently few studies on miR-129
in PC. According to a study, miR-139 located on chromosome 11q13.4,
inhibits the development and progression of malignant tumors
(12). In a study by Amemiya et
al (13), miR-139 regulates the
invasiveness of PC by targeting IGF1R, but whether it can be used
as a potential diagnostic and prognostic marker for the disease
remains unclear.
Chemotherapy is necessary for patients with
intermediate and high risks of cancer. However, there are currently
few prognostic indicators for patients with PC, and whether serum
miR can be used as a potential one remains unclear. Therefore, the
expression of miR-129 and miR-139 before treatment was observed in
this study, to determine that whether they can be used as potential
predictive indicators for the clinical efficacy on patients with
PC, so as to provide references for clinicians.
Patients and methods
Eighty-four male patients with PC undergoing
chemotherapy in The Third Affiliated Hospital of Qiqihar Medical
University (Qiqihar, China) from January 2016 to January 2017 were
enrolled as the observation group, aged 60–75 years with an average
age of 65.4±4.3 years. In this study, the patients were mostly
those with bone metastases, because bone metastasis is the
clinically common type of prostate cancer metastasis (14). Further 100 male healthy individuals
underdoing physical examination were enrolled as the control group,
aged 55–75 years with an average age of 64.2±5.1 years. The healthy
individuals had normal laboratory biochemical indices, blood
routine, immune function, PSA testing, and prostate ultrasound,
without congenital defects. This study was approved by the Medical
Ethics Committee of The Third Affiliated Hospital of Qiqihar
Medical University.
Inclusion and exclusion criteria
The inclusion criteria were as follows: Patients
were diagnosed with PC by biopsy. Patients had received endocrine
therapy. Patients met the 7th edition of TNM staging from AJCC in
the United States (15). Patients
had a pathological classification of PC with Gleason score as a
standard (16) at the time of
diagnosis. Patients who had complete clinical data were treated in
this hospital. Patients and their families were informed and they
signed an informed consent form. All patients were treated with
castration and were castration-resistant. Patients without bone
metastases were not treated.
The exclusion criteria were as follows: Patients
with congenital defects; patients complicated with other tumors;
patients who had received other chemotherapy regimens; patients
with infection, immune deficiency, neurological dysfunction, severe
cardiovascular and cerebrovascular diseases, or liver and kidney
diseases.
Detection methods
Fasting venous peripheral blood (5 ml) was extracted
from the patients before and after treatment and from the healthy
individuals in the morning of the following day. The blood was
allowed to stand for 30 min, and centrifuged at 4°C and 1509.3 × g
for 10 min to collect serum, which was subpackaged with enzyme-free
EP tubes. Part of the serum was used for this experiment, and the
rest was stored at −80°C. EasyPure miRNA Kit (TransGen Biotech;
ER601-01) was used to extract total RNA. UV spectrophotometer
(Evolution™ 201 purchased from Thermo Scientific™, Massachusetts
Institute of Technology) and agarose gel electrophoresis were used
to detect its purity, concentration, and integrity. TransScript
Green miRNA Two-Step qRT-PCR SuperMix (TransGen Biotech; AQ202-01)
was used to reverse transcribe the total RNA, with the steps
carried out according to the kit instructions. cDNA was collected
for PCR amplification. The upstream and downstream sequences of
miR-129 were 5′-GATACTCACTTTTTGCGGTCT-3′ and
5′-GTGCAGGGTCCGAGGT-3′, respectively; of miR-139 were
5′-CTCTGCTCTACAGTGCACGTGTC-3′ and 5′-TATGGTTGTTCTCGACTCCTTCAC-3′,
respectively; and of U6 were 5′-CGCTGGCAGCCACATATAC-3′ and
5′-CAGGGCATGCATATCTT-3′, respectively. The qPCR amplification
system was as follows: 1 µl of cDNA, each 0.4 µl of upstream and
downstream primers, 10 µl of 2×TransTaq® Tip Green qPCR
SuperMix, 0.4 µl of Passive Reference Dye (50X), and
ddH2O to complement to 20 µl. Conditions for the
amplification were as follows: pre-denaturation at 94°C for 30 sec,
denaturation at 94°C for 5 sec, and annealing and extension at 60°C
for 30 sec, for 40 cycles. Three identical wells were provided for
each sample, and the experiment was carried out three times. U6 was
used as an internal reference and the 2−ΔΔcq (17) method was used to analyze the
data.
Therapeutic regimen for patients in
the observation group
Patients in the observation group were treated with
docetaxel (Harbin Laiboten Pharmaceutical Co., Ltd.; SFDA, approval
no. H20153308) combined with prednisone (Fuhe Pharmaceutical Group
Co., Ltd.; SFDA, approval no. H23020385) (DP regimen) for
first-line chemotherapy. They were orally administered with
dexamethasone (4.5 mg) every 12 h at 1 day before chemotherapy, at
the day of chemotherapy, and on the 1st day after chemotherapy, so
as to prevent uroschesis and other adverse reactions. They were
intravenously dripped with docetaxel (75 mg/m2), and
orally administered with prednisone (5 mg), twice/day. A total of
21 days was 1 course of treatment. During chemotherapy, the
patients were given routine stomach protection (proton pump
inhibitors), and symptomatic and supportive treatment. The patients
in this study took a 2-week rest after the first course of
chemotherapy and then underwent the second course.
Follow-up
Patients were followed up for the overall survival
rate (OSR), once every 3 months, from the treatment with DP regimen
to the end time of the follow-up (January 1, 2019) or patient
non-survival. The patients from 2016 to 2017 were enrolled in this
study, and the OSR is the overall survival rate as of 2019.
Response evaluation criteria
The clinical efficacy was evaluated after 2 courses
of treatment. The changes of tumor size were calculated based on
MRI and CT results, and the efficacy was classified according to
Response Evaluation Criteria in Solid Tumor (RECIST) [complete
remission (CR), partial remission (PR), stable disease (SD),
progressive disease (PD)]. The detection site was the primary
tumor.
Observational indexes
Main observational indexes: expression of serum
miR-129 and miR-139 between the two groups and the expression in
the observation group between before and after treatment was
compared. The receiver operating characteristic (ROC) curves were
plotted to observe the diagnostic values of miR-129 and miR-139 in
PC. According to the clinical efficacy, patients with CR and PR
were considered as the good curative effect group, whereas those
with SD and PD were considered as the poor curative effect group.
Expression miR-129 and miR-139 before treatment between the two
groups was compared. Based on the expression before treatment, ROC
curves were plotted to observe the predictive values of miR-129 and
miR-139.
Secondary observational indexes: The survival curves
were plotted based on the survival of patients. The median
expression of miR-129 and miR-139 before treatment was used to
divide the patients into the high and low expression groups, Kaplan
Meier (K-M) survival curves were plotted and Log-rank test was used
for analysis. The clinical data of patients in the good and poor
curative effect groups were collected for univariate analysis, and
multivariate Cox regression analysis was conducted on meaningful
indicators to analyze independent prognostic factors affecting
patients.
Statistical analysis
In this study, SPSS20.0 (Cabit Information
Technology Co., Ltd.) software package was used to statistically
analyze the data, and GraphPad Prism 7 (Softhead Inc.) was used to
plot figures. Enumeration data were expressed by rate (%), tested
by Chi-square and represented by χ2. K-S test was used
to analyze data distribution. Measurement data were expressed by
mean ± standard deviation (mean ± SD). The comparison of the data
conforming to normal distribution between two groups was analyzed
by independent samples t-test, and the comparison within groups was
analyzed by paired t-test and represented by t. The comparison of
the data not conforming to normal distribution were analyzed by
non-parametric test and represented by Z. K-M survival curves were
plotted to observe the survival. Log-rank test was used to analyze
whether there was a difference in the overall survival.
Multivariate Cox regression analysis was used to compare
independent prognostic factors affecting the clinical efficacy.
P<0.05 indicated a statistically significant difference between
two groups.
Results
Comparison of clinical data
There were no statistically significant differences
in age, BMI, past medical history, history of smoking, history of
alcohol consumption, and place of residence between the observation
and control groups (P>0.05) (Table
I).
 | Table I.Clinical data of patients. |
Table I.
Clinical data of patients.
| Factors | Observation group
(n=84) | Control group
(n=100) | t/χ2/Z
value | P-value |
|---|
| Age (years) |
|
<65 | 49 (58.33) | 52 (52.00) | 0.740 | 0.390 |
| ≥65 | 35 (41.67) | 48 (48.00) |
|
|
| BMI
(kg/m2) | 22.58±1.55 | 22.84±1.84 | 1.025 | 0.307 |
| Past medical
history |
|
Hypertension | 19 (22.62) | 28 (28.00) | 0.630 | 0.427 |
|
Diabetes | 10 (11.90) | 15 (15.00) | 0.373 | 0.542 |
| History of
smoking |
|
| 0.252 | 0.616 |
| Yes | 70 (83.33) | 86 (86.00) |
|
|
| No | 14 (16.67) | 14 (14.00) |
|
|
| History of alcohol
consumption |
|
| 0.153 | 0.696 |
| Yes | 23 (27.38) | 30 (30.00) |
|
|
| No | 61 (72.62) | 70 (70.00) |
|
|
| Place of
residence |
|
| 0.393 | 0.531 |
|
Countryside | 40 (47.62) | 43 (43.00) |
|
|
| City | 44 (52.38) | 57 (57.00) |
|
|
| Gleason score |
|
<7 | 15 (17.86) | 0 (0.00) |
|
|
| 7 | 47 (55.95) | 0 (0.00) |
|
|
|
>7 | 22 (26.19) | 0 (0.00) |
|
|
| PSA (ng/ml) |
|
<10 | 13 (15.48) | 100 (10.00) | 135.552 | <0.001 |
|
10-20 | 41 (48.81) | 0 (0.00) |
|
|
|
>20 | 30 (35.68) | 0 (0.00) |
|
|
| Bone
metastasis |
|
Yes | 35 (41.67) | 0 (0.00) |
|
|
| No | 49 (58.33) | 0 (0.00) |
|
|
| TNM staging |
| Stage
III | 51 (60.71) | 0 (0.00) |
|
|
| Stage
IV | 33 (39.29) | 0 (0.00) |
|
|
Expression comparison of miR-129 and
miR-139
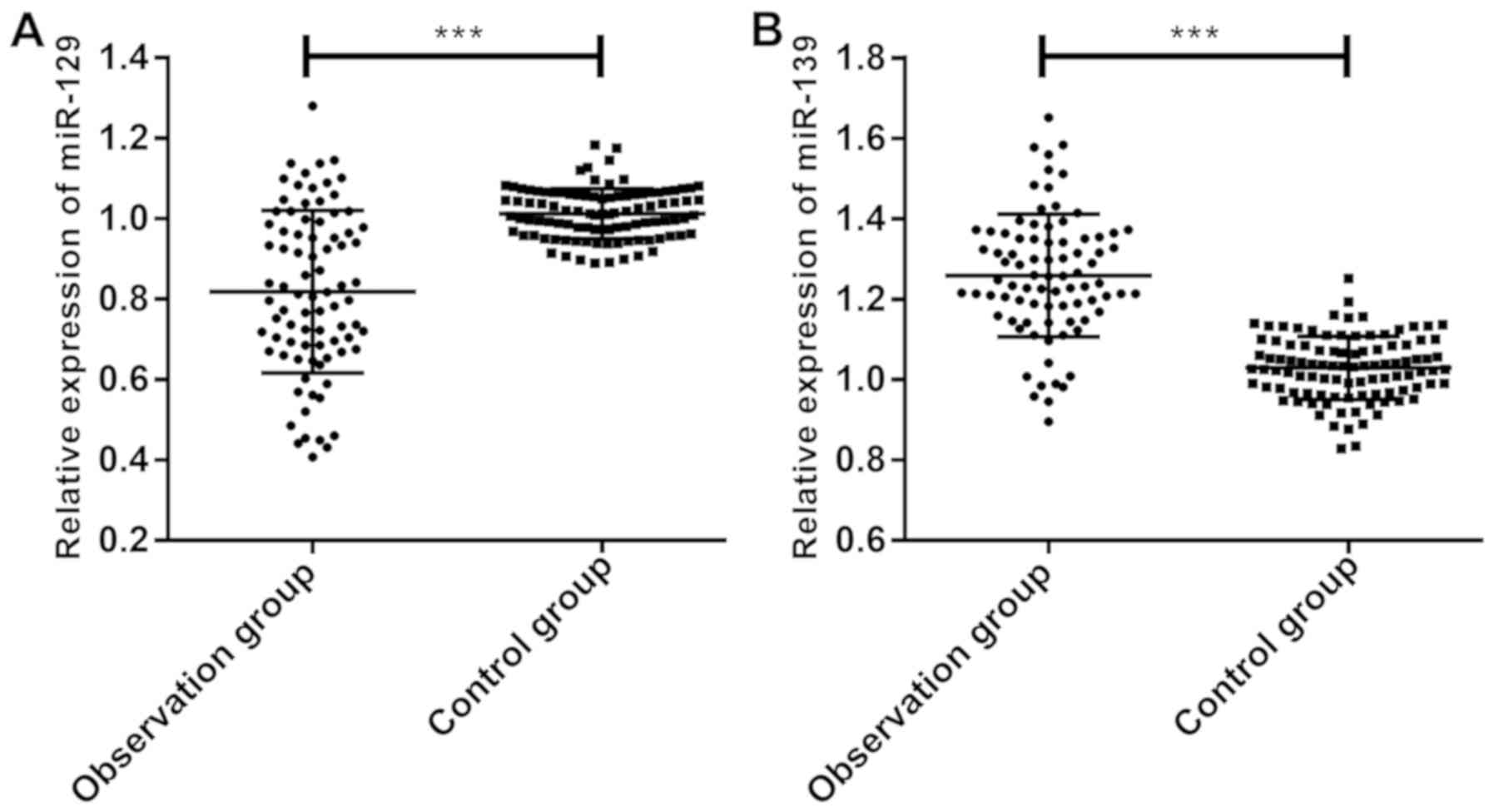
According to the comparison of miR-129 and miR-139
expression, miR-129 expression in the observation group
(0.818±0.220) was significantly lower than that in the control
group (1.013±0.062) (Fig. 1A),
whereas miR-139 expression in the observation group (1.258±0.184)
was significantly higher than that in the control group
(1.025±0.084) (P<0.05) (Fig.
1B).
Diagnostic values of miR-129 and
miR-139 in PC
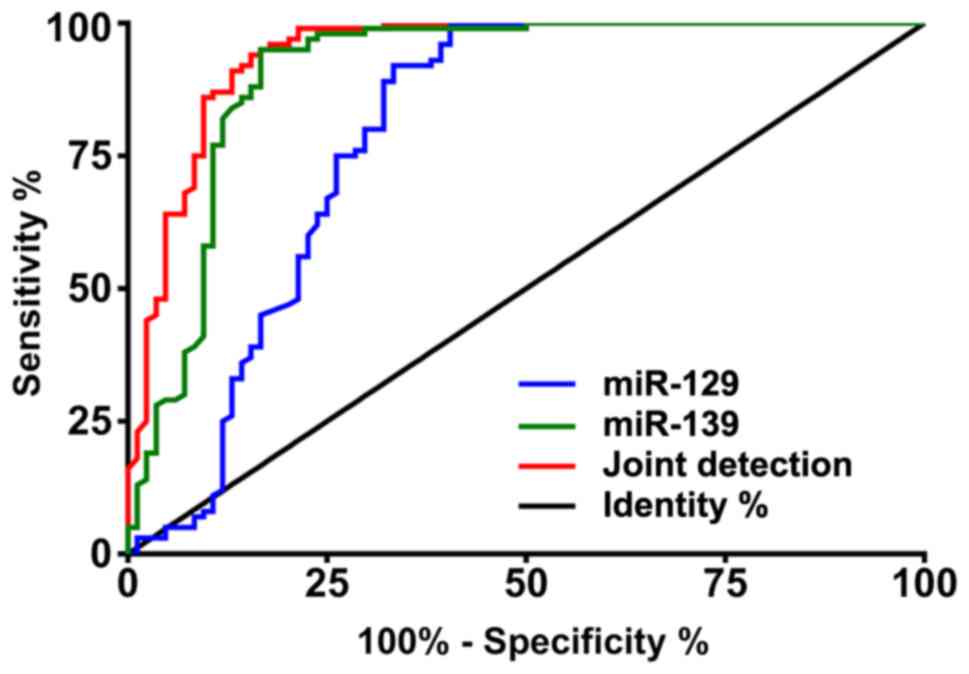
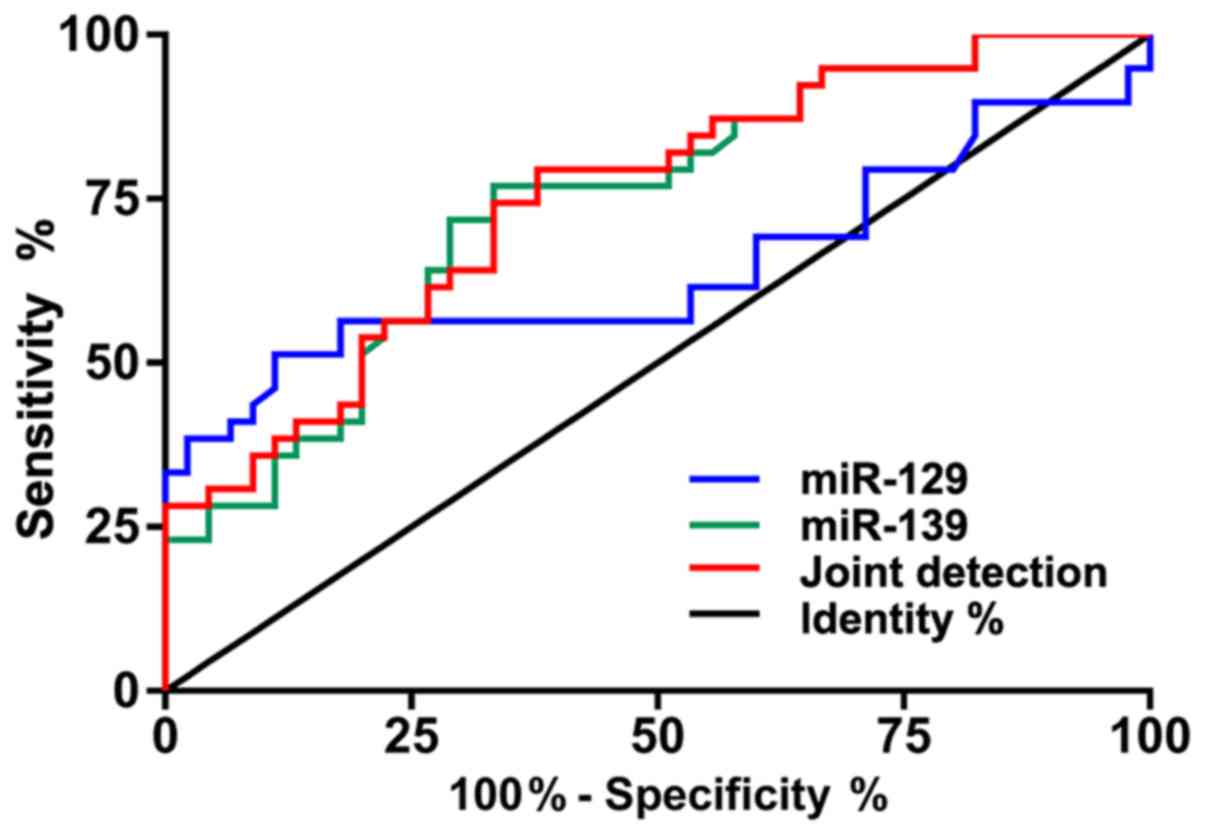
According to the ROC curves, the area under curve
(AUC) of miR-129 was 0.792, 95% CI: 0.718–0.865, that of miR-139
was 0.908, 95% CI: 0.858–0.957, and that of joint detection was
0.942, 95% CI: 0.646–0.852 (Table
II and Fig. 2).
 | Table II.ROC-related parameters. |
Table II.
ROC-related parameters.
| Indicators | AUC | 95% CI | Specificity
(%) | Sensitivity
(%) | Youden index
(%) | Cut-off value |
|---|
| miR-129 | 0.792 | 0.718–0.865 | 59.52 | 100.00 | 59.52 | >0.881 |
| miR-139 | 0.908 | 0.858–0.957 | 83.33 | 95.00 | 78.33 | <1.141 |
| Joint
detection | 0.942 | 0.906–0.978 | 84.52 | 94.00 | 78.52 | >0.480 |
Expression of miR-129 and miR-139
before and after treatment
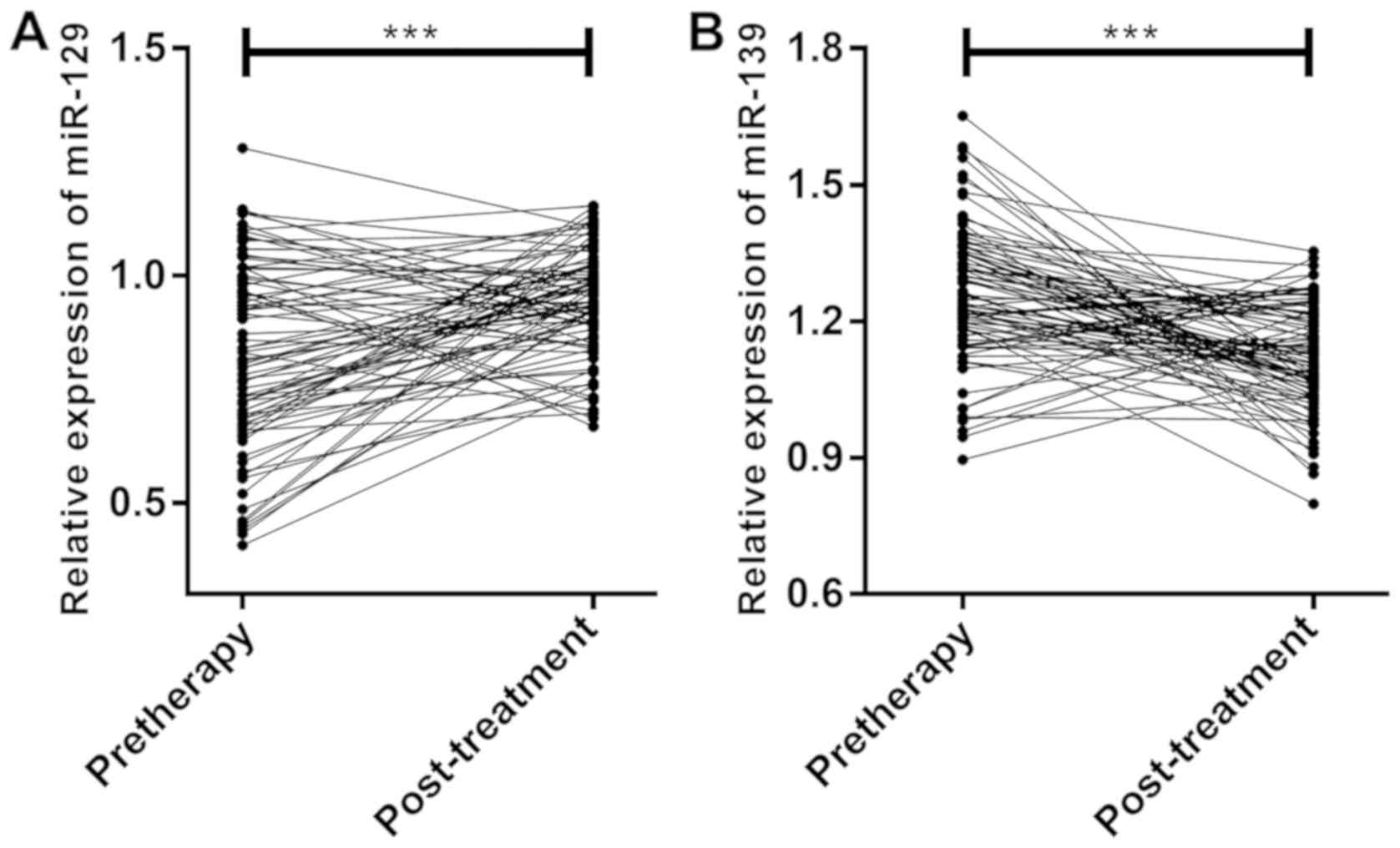
According to the comparison of miR-129 and miR-139
expression before and after treatment, miR-129 expression after
treatment (0.941±0.120) was significantly higher than that before
treatment (0.818±0.220) (P<0.001) (Fig. 3A), while miR-139 expression after
treatment (1.121±0.118) was significantly lower than that before
treatment (1.258±0.184) (P<0.001) (Fig. 3B).
Relationship between expression of
miR-129 and miR-139 before treatment and clinical efficacy
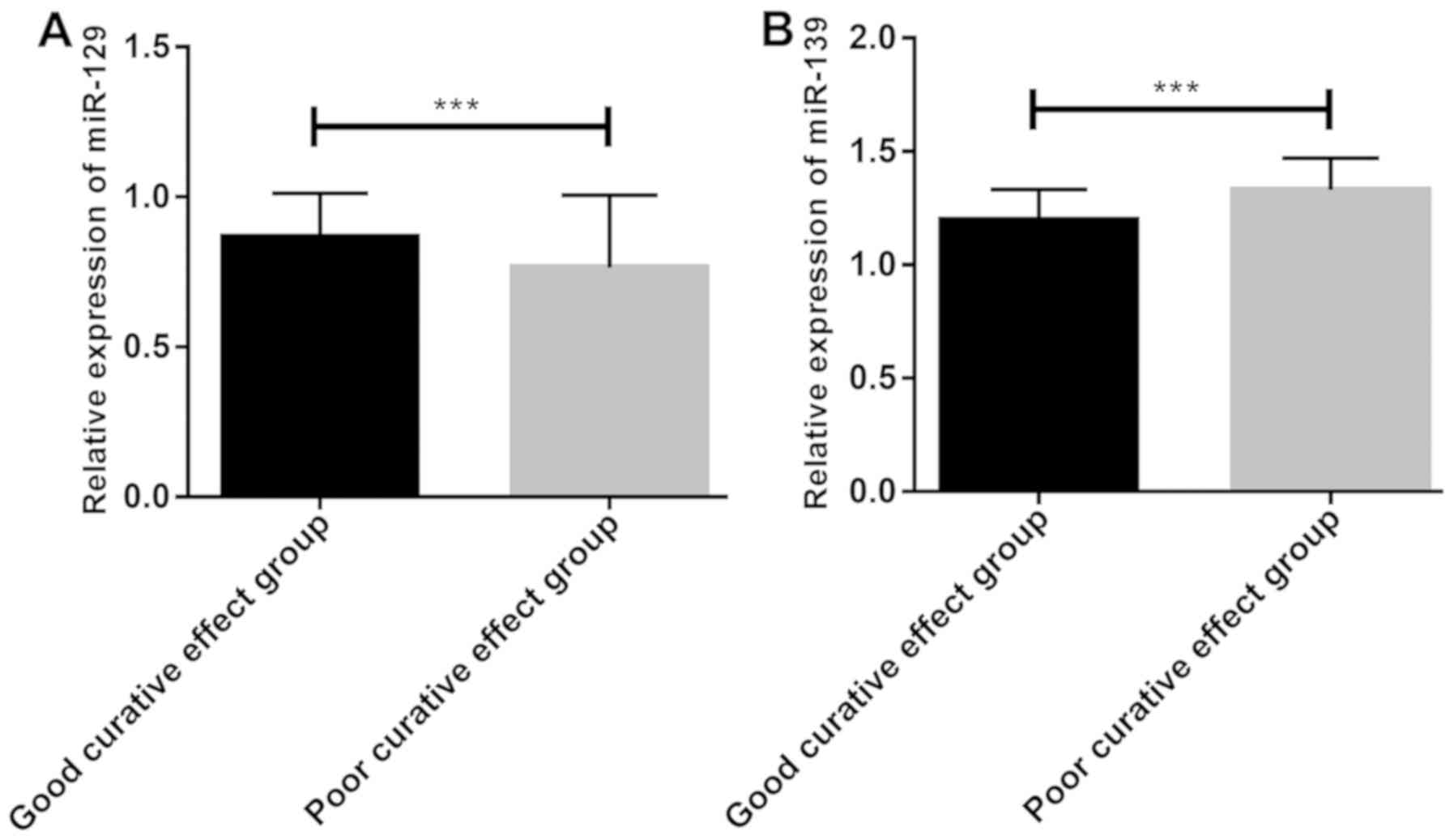
According to the evaluation of short-term clinical
efficacy based on RECIST, the observation group after treatment
consisted of 15 patients with CR, 30 with PR, 26 with SD, and 13
with PD. According to the clinical efficacy, the patients were
divided into the good curative effect group (n=45) and the poor
curative effect group (n=39). Before treatment, miR-129 expression
in the good curative effect group was significantly higher than
that in the poor curative effect group (Fig. 4A), whereas miR-139 expression was
significantly lower than that in the poor curative effect group
(P<0.05) (Fig. 4B).
According to the ROC curves based on expression of
miR-129 and miR-139 before treatment, the AUC of miR-129 was 0.646,
95% CI: 0.518–0.773, that of miR-139 was 0.741, 95% CI:
0.636–0.846, and that of joint detection was 0.749, 95% CI:
0.906–0.978 (Fig. 5 and Table III).
 | Table III.ROC-related parameters. |
Table III.
ROC-related parameters.
| Indicators | AUC | 95% CI | Specificity
(%) | Sensitivity
(%) | Youden index
(%) | Cut-off value |
|---|
| miR-129 | 0.646 | 0.518–0.773 | 88.89 | 51.28 | 40.17 | <0.701 |
| miR-139 | 0.741 | 0.636–0.846 | 66.67 | 76.92 | 43.59 | >1.233 |
| Joint
detection | 0.749 | 0.646–0.852 | 62.22 | 79.49 | 41.71 | >0.406 |
Relationship between survival and
miR-129 and miR-139
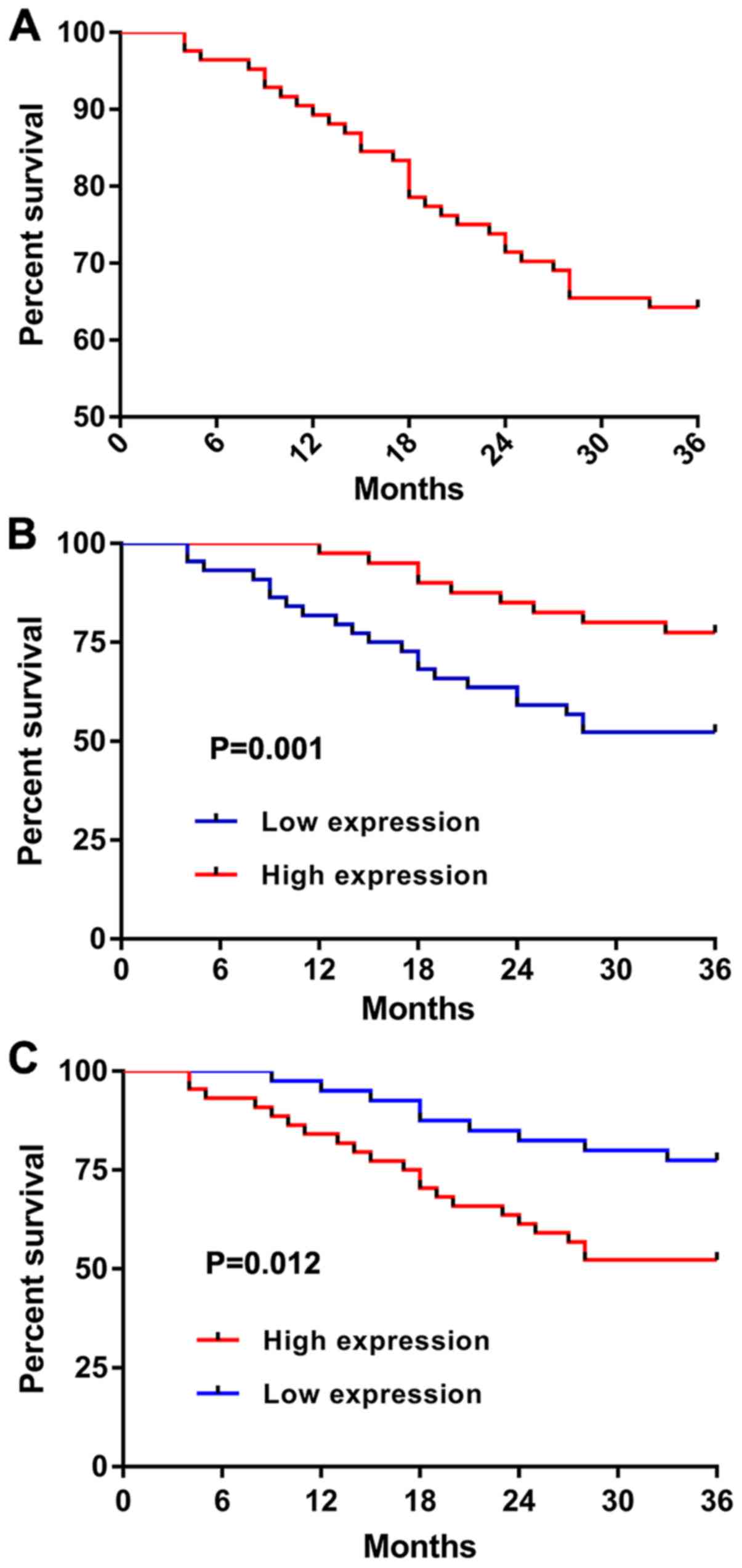
According to statistics, all patients in the
observation group were followed up, with an OSR of 64.29% (Fig. 6A). According to the median expression
of miR-129 and miR-139 before treatment, the patients were divided
into the high and low expression groups. The survival in the
miR-129 high expression group was significantly better than that in
the miR-129 low expression group (P=0.001) (Fig. 6B), whereas the survival in the
miR-139 low expression group was significantly better than that in
the miR-139 high expression group (P=0.012) (Fig. 6C).
Cox regression analysis
The assignments are shown in Table IV. According to the univariate Cox
regression analysis, Gleason score, PSA, bone metastasis, TNM
staging, miR-129, and miR-139 were prognostic risk factors
affecting patients. According to the multivariate Cox regression
analysis, these indicators were independent prognostic factors
affecting patients (Tables V and
VI).
 | Table IV.Assignment table. |
Table IV.
Assignment table.
| Factors | Assignment |
|---|
| Age | <65 years old,
1; ≥65 years old, 0 |
| BMI | A continuous
variable, analyzed with raw data |
| Hypertension | Yes, 1; no, 0 |
| Diabetes | Yes, 1; no, 0 |
| History of
smoking | Yes, 1; no, 0 |
| History of alcohol
consumption | Yes, 1; no, 0 |
| Place of
residence | Countryside, 1;
city, 0 |
| Gleason score | <7, 0; 7, 1;
>7, 2 |
| PSA | <10, 0; 10–20,
1; >20, 2 |
| Bone
metastasis | Yes, 1; no, 0 |
| TNM staging | Stage III, 1; Stage
IV, 0 |
| miR-129 | <0.818, 1;
≥0.818, 0 |
| miR-139 | <1.259, 1;
≥1.259, 0 |
 | Table V.Univariate Cox regression
analysis. |
Table V.
Univariate Cox regression
analysis.
|
|
|
|
|
|
| 95% CI for
Exp(B) |
|---|
|
|
|
|
|
|
|
|
|---|
| Factors | B | SE | Wald | Sig. | Exp(B) | Lower part | Upper part |
|---|
| Age | −0.114 | 0.369 | 0.096 | 0.757 | 0.892 | 0.433 | 1.837 |
| BMI | −0.052 | 0.108 | 0.230 | 0.631 | 0.950 | 0.769 | 1.172 |
| Hypertension | 0.695 | 0.388 | 3.205 | 0.073 | 2.004 | 0.936 | 4.291 |
| Diabetes | 0.216 | 0.537 | 0.162 | 0.688 | 1.241 | 0.433 | 3.557 |
| History of
smoking | 0.261 | 0.537 | 0.236 | 0.627 | 1.299 | 0.453 | 3.721 |
| History of alcohol
consumption | −0.819 | 0.490 | 2.787 | 0.095 | 0.441 | 0.169 | 1.153 |
| Place of
residence | −0.248 | 0.369 | 0.452 | 0.501 | 0.781 | 0.379 | 1.607 |
| Gleason score | 3.022 | 0.418 | 52.147 | 0.000 | 20.534 | 9.042 | 46.634 |
| PSA | 2.831 | 0.532 | 28.302 | 0.000 | 16.968 | 5.979 | 48.157 |
| Bone
metastasis | 1.744 | 0.414 | 17.716 | 0.000 | 5.721 | 2.539 | 12.887 |
| TNM staging | −4.645 | 1.024 | 20.564 | 0.000 | 0.010 | 0.001 | 0.072 |
| miR-129 | 1.161 | 0.414 | 7.882 | 0.005 | 3.193 | 1.420 | 7.181 |
| miR-139 | −2.488 | 0.610 | 16.610 | 0.000 | 0.083 | 0.025 | 0.275 |
 | Table VI.Multivariate Cox regression
analysis. |
Table VI.
Multivariate Cox regression
analysis.
|
|
|
|
|
|
| 95% CI for
Exp(B) |
|---|
|
|
|
|
|
|
|
|
|---|
| Factors | B | SE | Wald | Sig. | Exp(B) | Lower part | Upper part |
|---|
| Gleason score | 1.878 | 0.51 | 13.539 | 0.000 | 6.537 | 2.405 | 17.772 |
| PSA | 0.683 | 0.617 | 4.329 | 0.037 | 1.979 | 0.591 | 6.630 |
| Metastasis | 1.447 | 0.515 | 7.884 | 0.005 | 4.248 | 1.548 | 11.661 |
| TNM staging | −3.67 | 1.287 | 8.126 | 0.004 | 0.025 | 0.002 | 0.318 |
| miR-129 | 1.017 | 0.552 | 3.654 | 0.048 | 2.765 | 0.937 | 8.165 |
| miR-139 | −1.394 | 0.672 | 4.302 | 0.038 | 0.248 | 0.066 | 0.926 |
Discussion
As a malignant tumor that threatens life and health
of males, PC has an increasing incidence and mortality around the
world. According to Higano (18),
among elderly men in New Zealand, Australia, and European and
American countries, PC has a high incidence second only to lung
cancer and high mortality. The early diagnosis of PC is the main
means to improve the patients' survival rate, so it needs to be
improved urgently. Therefore, it is particularly important to find
new serological diagnostic markers.
A current study has shown that miR is closely
related to tumors and neurological diseases (19). According to studies, miR-129 and
miR-139 belong to the miR family and are differentially expressed
in tumors (20,21). Therefore, the diagnostic and
prognostic values of miR-129 and miR-139 in PC were explored in
this study to provide new serological reference indices for
clinicians. Pathological biopsy remains the gold standard of the
diagnosis of PC, but we have found that miR-129 and miR-139 have
clinical values in the diagnosis and evaluation of the disease.
In this study, serum was collected from the healthy
individuals and the patients for detection. Serum samples are
easier to collect than tumor solid samples, and they do not cause
invasive damage to patients. According to the detection, miR-129
and miR-139 expression in the observation group was significantly
lower and higher respectively than that in the control group, which
indicates that miR-129 and miR-139 are expected to be potential
diagnostic indicators for PC. According to the ROC curves, the AUC
of miR-129 was 0.792, that of miR-139 was 0.908, and that of joint
detection was 0.942, showing that the joint detection of miR-129
and miR-139 expression can well distinguish patients with PC from
healthy individuals, and that miR-129 and miR-139 can be used as
potential diagnostic indicators for patients with PC. According to
Xu et al (22), miR-129
expression in PC mononuclear cells can be used as a biomarker for
the diagnosis and prognosis of PC, with an AUC of 0.846. In a study
by Pang et al (23), the
increasing miR-139 expression in peripheral blood could be used as
a potential diagnostic indicator for patients with PC, with an AUC
of 0.936. The findings of the two studies are consistent with and
mutually verify this study. According to the above research,
miR-129 and miR-139 can be used as clinical diagnostic indicators
for PC, but whether they can be used as potential efficacy
prediction indicators for patients with advanced PC has been rarely
studied.
Clinically, there are many therapeutic regimens for
PC. However, the clinical features of early PC are not apparent, so
the disease has mostly been in its advanced stage after admission
of the patients and patients with metastatic lesions have poor
survival and prognoses (24). Such
patients can only be treated by radiotherapy and chemotherapy.
Patients treated with DP regimen, which is the first choice for the
treatment of advanced PC, have better prognoses (25). In the present study, patients in the
observation group were grouped according to the clinical efficacy
after treatment, and the relationship between expression of miR-129
and miR-139 before treatment and efficacy was compared. miR-129 and
miR-139 expression in the good curative effect group was higher and
lower respectively, than that in the poor curative effect group,
suggesting that miR-129 and miR-139 expression before treatment is
expected to be potential predictive indicator for clinical efficacy
after treatment, with high diagnostic values. According to the
median expression of miR-129 and miR-139 before treatment, the
patients were divided into the high and low expression groups. In
this study, the survival in the miR-129 high expression group was
significantly better than that in the miR-129 low expression group,
whereas the survival in the miR-139 high and low expression groups
was contrary, which indicates the predictive values of miR-129 and
miR-139 in the short-term prognosis of patients, and that miR-129
and miR-139 can be used as potential predictive indicators for
survival. According to the multivariate Cox regression analysis,
Gleason score, PSA, bone metastasis, TNM staging, miR-129, and
miR-139 were independent prognostic factors affecting patients.
This is consistent with previous findings (26,27), and
well illustrates the prognostic values of miR-129 and miR-139 in
PC.
In this study, according to the expression detection
of miR-129 and miR-139, the differential expression of miR-129 and
miR-139 can be used as potential diagnostic indicator for PC, and
miR-129 and miR-139 have predictive values for the clinical
efficacy after chemotherapy. According to the multivariate Cox
regression analysis, miR-129 and miR-139 are independent prognostic
indicators affecting patients. However, this study still has
limitations. Firstly, miR-129 and miR-139 expression in patients
with benign prostatic hyperplasia was not detected. Secondly, due
to the short duration of this study, patients were not followed up
for a long time. Finally, the cause of the differential expression
of miR-129 and miR-139 remains unclear. Therefore, the mechanism
between miR-129, miR-139 and PC as well as the expression of
miR-129 and miR-139 in patients with prostatitis or prostatic
hyperplasia detected, need to be further explored so as to verify
the results of this study.
In conclusion, miR-129 and miR-139 are expected to
be potential indicators for the diagnosis, prognosis and efficacy
prediction of PC.
Acknowledgements
Not applicable.
Funding
This study was supported by The Expression and
Significance of LEF1 in Prostate Cancer of Qiqihar Science and
Technology Planning Project (no. SFGG-201718).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZH wrote the manuscript. JG and MZ analyzed and
interpreted the patient general data. ZH and TJ performed PCR. XY
was responsible for analysis of the observation indicators. All the
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Medical Ethics
Committee of The Third Affiliated Hospital of Qiqihar Medical
University (Qiqihar, China). Patients who participated in this
study, had complete clinical data. Signed informed consents were
obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang X, Yuan T, Liang M, Du M, Xia S,
Dittmar R, Wang D, See W, Costello BA, Quevedo F, et al: Exosomal
miR-1290 and miR-375 as prognostic markers in castration-resistant
prostate cancer. Eur Urol. 67:33–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jonas S and Izaurralde E: Towards a
molecular understanding of microRNA-mediated gene silencing. Nat
Rev Genet. 16:421–433. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Romaine SP, Tomaszewski M, Condorelli G
and Samani NJ: MicroRNAs in cardiovascular disease: An introduction
for clinicians. Heart. 101:921–928. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cammaerts S, Strazisar M, De Rijk P and
Del Favero J: Genetic variants in microRNA genes: Impact on
microRNA expression, function, and disease. Front Genet. 6:1862015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu K, Huang J, Ni J, Song D, Ding M, Wang
J, Huang X and Li W: MALAT1 promotes osteosarcoma development by
regulation of HMGB1 via miR-142-3p and miR-129-5p. Cell Cycle.
16:578–587. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M,
et al: Human microRNA genes are frequently located at fragile sites
and genomic regions involved in cancers. Proc Natl Acad Sci USA.
101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Catto JW, Alcaraz A, Bjartell AS, De Vere
White R, Evans CP, Fussel S, Hamdy FC, Kallioniemi O, Mengual L,
Schlomm T, et al: MicroRNA in prostate, bladder, and kidney cancer:
A systematic review. Eur Urol. 59:671–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao G, Zhou X, Fang T, Hou Y and Hu Y:
Hyaluronic acid promotes the expression of progesterone receptor
membrane component 1 via epigenetic silencing of miR-139-5p in
human and rat granulosa cells. Biol Reprod. 91:1162014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amemiya Y, Wallis CJ, Benatar T, Kobylecky
E, Sugar L, Sherman C, Nam R and Seth AK: Abstract A079:
MicroRNA-139 regulates prostate cancer aggressiveness by targeting
IGF1R. Cancer Res 78 (16 Supplement). A079. 2018.
|
|
14
|
Conley-LaComb MK, Semaan L, Singareddy R,
Li Y, Heath EI, Kim S, Cher ML and Chinni SR: Pharmacological
targeting of CXCL12/CXCR4 signaling in prostate cancer bone
metastasis. Mol Cancer. 15:682016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chansky K, Sculier JP, Crowley JJ, Giroux
D, Van Meerbeeck J and Goldstraw P; International Staging Committee
and Participating Institutions, : The International Association for
the Study of Lung Cancer Staging Project: Prognostic factors and
pathologic TNM stage in surgically managed non-small cell lung
cancer. J Thorac Oncol. 4:792–801. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Epstein JI, Zelefsky MJ, Sjoberg DD,
Nelson JB, Egevad L, Magi-Galluzzi C, Vickers AJ, Parwani AV,
Reuter VE, Fine SW, et al: A contemporary prostate cancer grading
system: A validated alternative to the Gleason score. Eur Urol.
69:428–435. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Higano C: Androgen deprivation therapy:
Monitoring and managing the complications. Hematol Oncol Clin North
Am. 20:909–923. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Agarwal V, Subtelny AO, Thiru P, Ulitsky I
and Bartel DP: Predicting microRNA targeting efficacy in
Drosophila. Genome Biol. 19:1522018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zuo Y, Li Y, Zhou Z, Ma M and Fu K: Long
non-coding RNA MALAT1 promotes proliferation and invasion via
targeting miR-129-5p in triple-negative breast cancer. Biomed
Pharmacother. 95:922–928. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang HD, Jiang LH, Sun DW, Li J and Tang
JH: MiR-139-5p: Promising biomarker for cancer. Tumour Biol.
36:1355–1365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu S, Yi XM, Zhou WQ, Cheng W, Ge JP and
Zhang ZY: Downregulation of miR-129 in peripheral blood mononuclear
cells is a diagnostic and prognostic biomarker in prostate cancer.
Int J Clin Exp Pathol. 8:14335–14344. 2015.PubMed/NCBI
|
|
23
|
Pang C, Liu M, Fang W, Guo J, Zhang Z, Wu
P, Zhang Y and Wang J: MiR-139-5p is increased in the peripheral
blood of patients with prostate cancer. Cell Physiol Biochem.
39:1111–1117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fizazi K, Scher HI, Molina A, Logothetis
CJ, Chi KN, Jones RJ, Staffurth JN, North S, Vogelzang NJ, Saad F,
et al COU-AA-301 Investigators, : Abiraterone acetate for treatment
of metastatic castration-resistant prostate cancer: Final overall
survival analysis of the COU-AA-301 randomised, double-blind,
placebo-controlled phase 3 study. Lancet Oncol. 13:983–992. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mahammedi H, Planchat E, Pouget M, Durando
X, Curé H, Guy L, Van-Praagh I, Savareux L, Atger M, Bayet-Robert
M, et al: The new combination docetaxel, prednisone and curcumin in
patients with castration-resistant prostate cancer: A Pilot Phase
II Study. Oncology. 90:69–78. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wachter S, Gerstner N, Goldner G, Pötzi R,
Wambersie A and Pötter R: Rectal sequelae after conformal
radiotherapy of prostate cancer: Dose-volume histograms as
predictive factors. Radiother Oncol. 59:65–70. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dezhong L, Xiaoyi Z, Xianlian L, Hongyan
Z, Guohua Z, Bo S, Shenglei Z and Lian Z: miR-150 is a factor of
survival in prostate cancer patients. J BUON. 20:173–179.
2015.PubMed/NCBI
|