Introduction
Circulating tumor cells (CTCs) are potential
surrogates for distant metastasis and promising novel biomarkers
for malignancies (1). However, the
detection of rare tumor-derived cells among a majority of normal
hematological cells still remains technically challenging, while
diverse methods have been reported (2–4). We
recently established a novel microfluid system to capture CTCs (CTC
chip), which has the advantages of convenience, efficacy, and
flexibility (2,5).
Colorectal cancer (CRC) is the third most common
diagnosed cancer in males and the second in females; it caused
83,200 deaths globally in 2015 (6).
At the time of diagnosis, 10–30% of patients with CRC have acute
large bowel obstruction (7,8). Emergent decompression is necessary for
large bowel obstruction, and self-expandable metallic stent (SEMS)
placement is a clinical choice as a minimally invasive non-surgical
treatment. With accumulating experience and progress in equipment
development, SEMS placement has positive short-term outcomes, and
technical and clinical success rates of 91–98 and 89–92%,
respectively, have been reported (8,9).
Although the efficacy and feasibility of endoscopic
SEMS placement in obstructive CRC are well-documented, several
clinical concerns regarding SEMS placement remain (10,11). One
of the major concerns is the risk of increased cancer cells in the
bloodstream due to mechanical damage and pressure applied to the
tissues by SEMS placement, possibly leading to increased metastasis
(12,13). In fact, circulating tumor-derived DNA
levels are elevated following SEMS placement, likely due to
mechanical damage (14). Therefore,
it is hypothesized that cancer cells are also released by
mechanical damage to tissues during SEMS placement.
In this study, as a trial of clinical application of
our microfluid system to capture CTCs, we examined differences in
CTC levels before and after SEMS placement in patients with
obstructive CRC using our custom CTC chip to evaluate the risk of
increasing the quantity of cancer cells in the blood by SEMS
placement. Additionally, based on the recent evidence that cancer
stem cells released from primary lesions in the blood play a
central role in establishing metastasis in distant organs (15), we examined changes in the number of
CD133-positive CTCs, CRC stem-like cell markers (16,17), to
better show the flexibility of the custom CTC chip.
Materials and methods
Study approval
This prospective single-center observational study
was conducted at the University of Tokyo Hospital. We examined
differences in the numbers of CTCs before and after endoscopic SEMS
placement for obstructive CRC. We included thirteen patients with
primary CRC with obstruction and performed SEMS placement between
July 2017 and April 2019 at the University of Tokyo Hospital. The
study protocol was approved by the Institutional Review Board of
the University of Tokyo Hospital (approved no. 11557), and written
informed consent was obtained from all participating patients
before enrollment in this study. The study was carried out in
accordance with the relevant guidelines.
CTC chip preparation
Polymeric CTC chips consist of two types of
micropole to increase capture efficacy (2,5). To
capture CTCs, a two-step coating of CTC chip micropoles and
surfaces with antibodies was performed. First, the CTC chip was
coated with goat anti-rabbit immunoglobulin G antibodies
(#SAB3700970; Sigma-Aldrich; Merck KGaA), which were diluted 1:20
and incubated for more than 3 h at 4°C. Next, the chip was
incubated with secondary antibodies, rabbit anti-human EpCAM
antibodies (#36746; Cell Signaling Technology, Inc.), which were
diluted 1:100 and incubated for 1 h at room temperature. CTC chips
were coated with antibodies on the day before each SEMS
placement.
Sample collection and CTC chip
processing
Peripheral blood samples (5 ml) were collected into
tubes containing ethylenediaminetetraacetic acid before SEMS
placement, 24 h after SEMS placement, and 4 days after SEMS
placement. Within 12 h of collection, samples were centrifuged at
300 × g for 5 min at 4°C. After separating the plasma, we collected
1.5 ml mixed buffy coat and red blood cells as a processed sample
(5). Samples were transferred into
the prepared CTC-chip microfluidic system using an automated
syringe pump. Briefly, processed samples (1.5 ml) were applied to
the CTC chip using a syringe pump at a constant flow rate of 1.0
ml/h. The CTC chip was then rinsed with 10% bovine serum albumin in
PBS three times at the same flow rate. After CTC capture,
immunocytochemistry was performed. For preliminary experiments
using cell lines with the CTC chip, approximately 1×104 cells were
dissolved in 1.5 ml PBS and applied to the chip.
Immunocytochemistry of CTC chips
After CTC capture, immunostaining of the CTCs was
performed using human CK19 antibodies, as reported previously
(5), and, in two cases, using
anti-CD133 antibodies. When using CD133 antibodies, before fixation
with 4% paraformaldehyde in PBS, CTCs were incubated with mouse
anti-human CD133 antibodies conjugated with Alexa Fluor 615
(#130-113-671; Miltenyi Biotec GmbH), which were diluted 1:50 for
10 min at room temperature in the CTC-chip microfluidic system.
After fixation, CTCs were incubated with sheep anti-human CK19
antibodies (#sc-33119; Santa Cruz Biotechnology), which were
diluted 1:200 and incubated for 1 h. Then, donkey anti-sheep
antibodies conjugated with Alexa Fluor 488 (#1807723; Thermo Fisher
Scientific), which were diluted 1:1,000, were incubated for 30 min
as secondary antibodies to visualize CTCs. Finally, the chip was
covered with VectaShield with DAPI (Vector Laboratories). When
preliminarily testing the compatibility of our CTC chips using cell
lines, anti-CK19 antibodies and anti-CD45 antibodies were diluted
1:300, and the fluorescence was evaluated. The fluorescence signals
were detected under an Olympus AX80 microscope.
Antibodies
The antibodies tested other than the CK19 and CD133
antibodies described above were as follows: Anti-wide cytokeratin
antibodies (#ab9377, Abcam; #bs-1185R-A488, BIOSS Antibodies,
Inc.), other hosts of anti-CK19 antibodies (#sc-376126, #sc-33119,
#AF3506, Santa Cruz Biotechnology), anti-Lgr5 (#ab75732, Abcam;
#TA503316, OriGene; #130-100-876, Miltenyi Biotec GmbH;
#21833-1-AP, Proteintech Group, Inc.), anti-human EpCAM (#sc-25308,
Santa Cruz Biotechnology; #36746, Cell Signaling Technology),
anti-HaloTag (G928A, Promega Corporation), and anti-CD45
(#bs-05222R-A555, BIOSS; #14579, Cell Signaling Technology;
ab30446, Abcam; #368520, BioLegend).
Cell culture
The human colon cancer cell line HCT116 and
CD133-positive human pancreatic cancer cell line Capan-1 were
obtained from the American Type Culture Collection. HCT116 and
Capan-1 cells were maintained in McCoy's 5A media and Iscove's
modified Dulbecco's medium, respectively, with 10% fetal bovine
serum.
Plasmids and transfection
HaloTag-fused Lgr5-expressing plasmid was purchased
from Promega. HCT116 cells were transiently transfected with the
plasmid using the Effectene Transfection Reagent (Qiagen) according
to the manufacturer's instructions. At 48 h post-transfection,
cells were stained using anti-Lgr5 antibodies and anti-HaloTag
antibodies to determine the suitability of the anti-Lgr5 antibodies
for immunofluorescence cell staining.
Cell-free DNA quantitation
From separated plasmas of the patients' blood used
for counting the number of CTCs, we collected cell-free DNA.
Plasmas were centrifuged at 1,900 × g for 5 min at 4°C. The
supernatant were aspirated without disturbing the buffy coat layer.
Aspirated plasmas were centrifuged at 16,000 × g for 10 min at 4°C.
From these samples, cell-free DNAs were collected using QIAamp
Circulating Nucleic Acid Kit (Qiagen). Collected cell-free DNA
samples were quantified with NanoDrop (Thermo Fisher
Scientific).
Statistics
Statistically significant differences were
identified using ANOVA and Bonferroni post-test. P-values <0.05
were considered to indicate statistical significance.
Results
Custom CTC chip efficiently detects
CTCs in patient blood
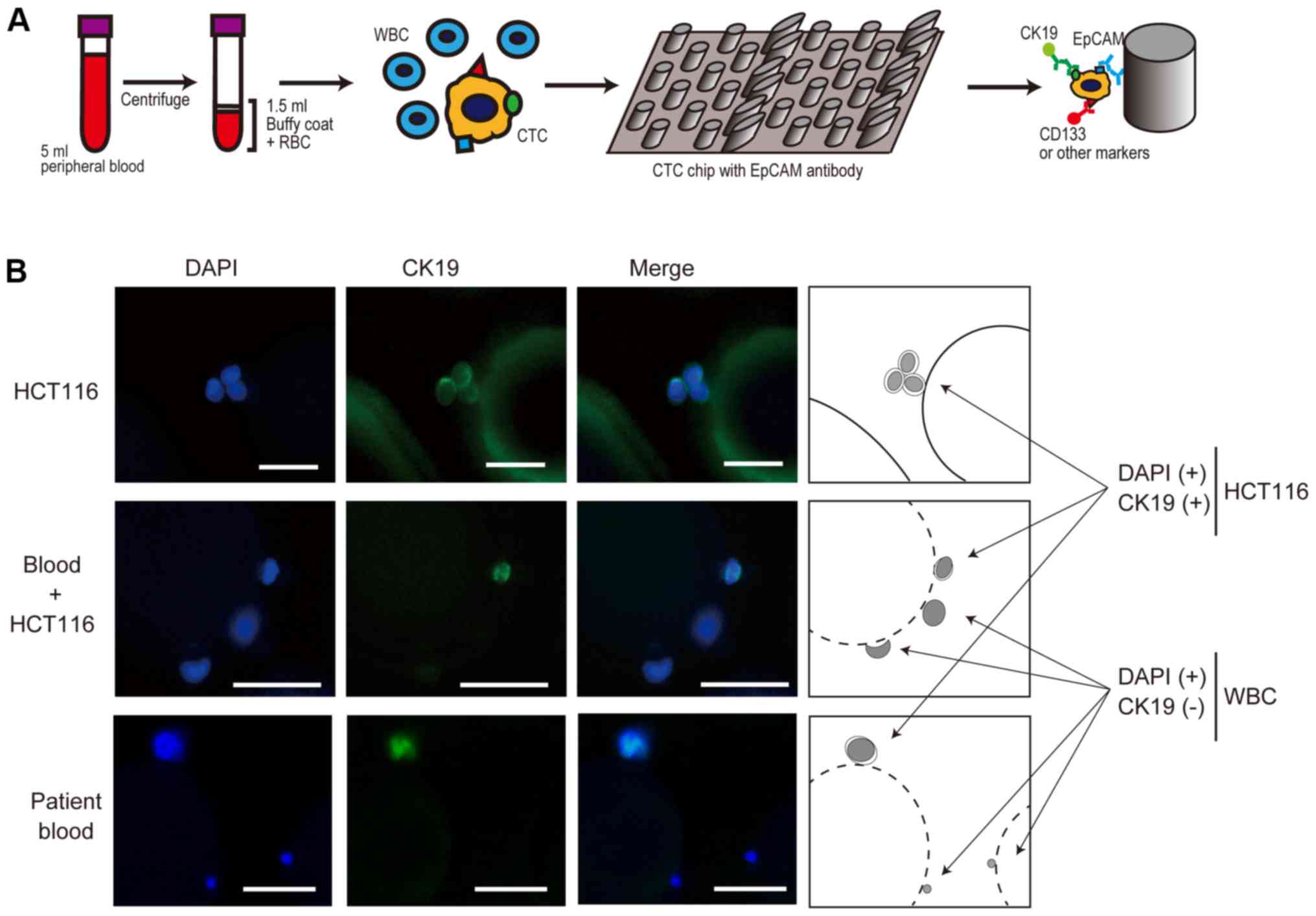
To detect CTCs in patient blood, we used a
custom-made polymeric CTC chip system, which was recently
established (18). In the first
step, the chip surface was covered with base antibodies against
capture antibodies (anti-EpCAM antibodies). In the next step,
anti-EpCAM antibodies were trapped by the base antibodies. These
antibodies are attached mainly to the micropoles on the chip
surfaces. After centrifuging 5 ml peripheral blood from a patient,
only cell components, including buffy coat and red blood cells,
were applied to the chip using an automatic syringe pump. After
washing the chip with PBS using a syringe pump, we fixed the cells
and performed immunocytochemistry using anti-CK19 antibodies to
detect epithelial cells, which were recognized as CTCs (Fig. 1A). When needed, we used other
antibodies in parallel to detect specific antigens with fluorescent
secondary antibodies (Fig. 1A).
To establish the experimental settings, we tested
several antibodies from different vendors to ensure efficient CTC
detection by examining EpCAM-positive colon cancer cell line HCT116
cells or a mixture of HCT116 cells in human blood from a healthy
volunteer. Although we used antibodies that were suitable for
immunocytochemistry, their sensitivity differed significantly
(Table I). In particular, anti-CD45
antibodies, which theoretically react with white blood cells, were
not sufficiently reactive or were non-specifically reactive in our
system (Table I). Because anti-CK19
antibodies reacted efficiently with HCT116 cells, we detected CTCs,
which were captured by anti-EpCAM antibodies, by CK19 staining.
Using this method, our CTC chip efficiently detected HCT116 cells,
HCT116 cells mixed in blood, and CTCs from patients with cancer
(Fig. 1B). The estimated capture
efficiency was approximately 80%, similar to our previous results
(2).
 | Table I.Compatibility of antibodies in the
custom circulating tumor cell chip for the detection of epithelial
cancer cell lines (HCT116; CK19 positive) and WBCs (CD45
positive). |
Table I.
Compatibility of antibodies in the
custom circulating tumor cell chip for the detection of epithelial
cancer cell lines (HCT116; CK19 positive) and WBCs (CD45
positive).
|
| WideCK-Alexa488
(BIOSS) | WideCK-Alexa488
(BIOSS) | CK-19-Alexa488 (Santa
Cruz) | WideCK-Alexa488
(Abcam) |
|---|
|
|
|
|
|
|
|---|
| Cell type | CD45-Alexa555
(BIOSS) | CD45-Alexa594
(CST) | CD45-Alexa594
(CST) | CD45-Alexa555
(Abcam) |
|---|
| HCT116 | ± | ± | + | ++ |
|
| − | − | − | ++ |
| WBC | − | − | − | Not determined |
|
| − | − | − | Not determined |
The number of CTCs increases
temporarily after endoscopic SEMS placement
As one example of clinical application of our CTC
chip, we examined changes in the number of CTCs in patients who
underwent endoscopic SEMS placement for obstructive CRC. We
collected blood just before stenting, at 24 h after stenting, and
at 4 days after stenting, and counted the CTCs. Typical CTCs from
patients showed clusters (Fig. 2A),
consistent with previous reports (19).
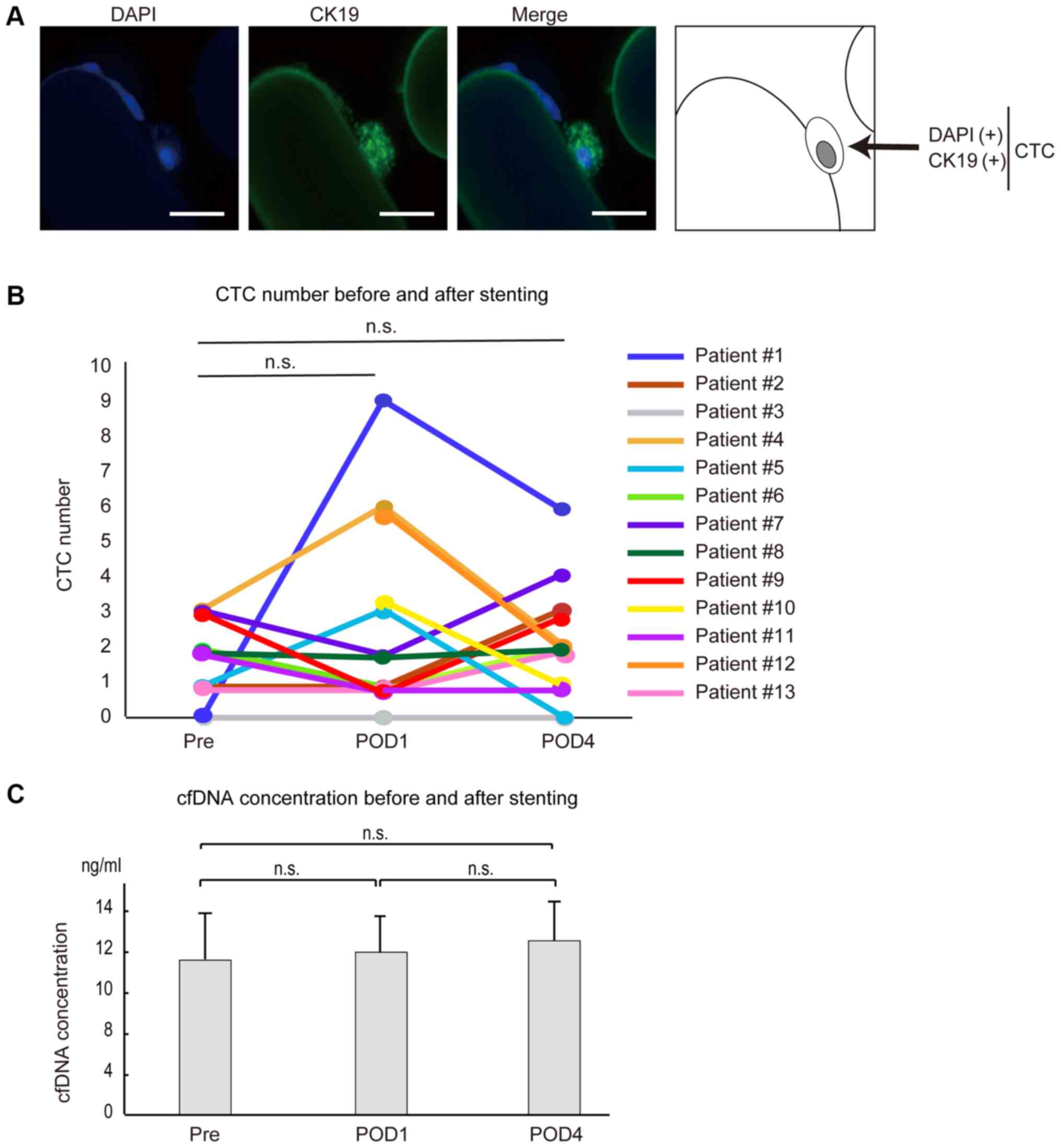 | Figure 2.CTC number, CTCs and cfDNA
concentrations following SEMS placement. (A) Fluorescence-stained
CTCs captured by the CTC chip after SEMS placement. CTCs were
stained with DAPI and CK19 (scale bar, 50 µm). (B) Differences in
the numbers of CTCs before and after SEMS placement. The number of
CTCs increased at 24 h after SEMS placement and decreased 4 days
after SEMS placement in three cases (patient 1, 4 and 5). The
number of CTCs was slightly increased at 4 days after SEMS
placement compared with those before SEMS placement in three cases
(patient 2, 7 and 13). The number of CTCs before SEMS placement was
not tested in two cases (patient 10 and 12). In the remaining five
cases (patient 3, 6, 8, 9 and 11), CTC numbers remained unchanged
or decreased. (C) cfDNA concentrations in plasma were determined in
patients 1–6 before and after SEMS placement. Pre, before SEMS
placement; POD1 and POD4, 24 h and 4 days after SEMS placement,
respectively; n.s., no significance; CTC, circulating tumor cells;
cfDNA, cell-free DNA; SEMS, self-expandable metallic stents. |
Among the 13 cases examined (Table II), CTCs were detected at some time
point except in one patient (patient #3). No obvious correlation
between detectability and tumor stage, tumor differentiation grade,
or tumor marker level was detected (Table II). At 24 h after stenting, three
(patients #1, #4 and #5) of the 11 patients in whom CTCs were
measured at pre-stenting showed increased numbers of CTCs compared
with those at the pre-stenting stage (Fig. 2B and Table II). However, the number of CTCs
decreased at 4 days after stenting in all of these patients
(Fig. 2B and Table II), suggesting that the increased
number of CTCs after SEMS placement was temporary. Although three
cases (patients #2, #7 and #13) showed the largest numbers of CTCs
at day 4 after SEMS placement, the degree of increase was only
marginal (one or two CTCs), and CTC numbers did not differ
significantly throughout the time course, as in the cases of
patients #6 and #8, who had equal numbers of CTCs in their blood
samples before and 4 days after stenting (Fig. 2B and Table II). The number of CTCs remained
unchanged or decreased in two cases (patients #9 and #11) at 4 days
after stenting compared with those at pre-stenting stage (Fig. 2B and Table II). The overall changes in CTC
numbers at around the SEMS placement were not statistically
significant. These results suggest that the number of CTCs may
increase, but only temporarily, or may not significantly change,
even after endoscopic SEMS placement.
 | Table II.Clinical and pathological
characteristics of patients who underwent SEMS placement for
obstructive colorectal cancer. |
Table II.
Clinical and pathological
characteristics of patients who underwent SEMS placement for
obstructive colorectal cancer.
| Patient no. | Age | Sex | Location | TNM | Pathology | CEA (ng/ml) | CA19-9 (U/ml) | CTC Pre | CTC POD1 (CD133
positive) | CTC POD4 (CD133
positive) |
|---|
| 1 | 42 | F | Sigmoid | T3N0H0P0M1a pStage
IVA | Tub1 | 575.2 | 361 | 0 | 9 | 6 |
| 2 | 69 | M | Rectum | T4aN3H2P0M1b pStage
IVB | Por | 28.3 | 97 | 1 | 1 | 3 |
| 3 | 61 | M | Sigmoid | T4aN1H0P1M1b pStage
IVB | Tub1 | 2.6 | 11 | 0 | 0 | 0 |
| 4 | 64 | M | Rectum | T4bN1H0P0M0 pStage
IIIC | Tub2 | 3.9 | 27 | 3 | 6 | 2 |
| 5 | 67 | F | Rectum | T4aN1H1P0M1a pStage
IVA | Tub1 | 4.0 | 13 | 1 | 3 | 0 |
| 6 | 81 | F | Sigmoid | T3N0H0P0M0 cStage
IIA | Tub1 | 12.6 | 30 | 2 | 1 | 2 |
| 7 | 73 | F | Ascending | T4aN0H0P2M1b cStage
IVB | Tub2 | 49.5 | 5,320 | 3 (3) | 2 (2) | 4 (3) |
| 8 | 83 | M | Descending | T4aN2H0P0M0 cStage
IIIC | Tub1 | 7.7 | 23 | 2 (2) | 2 (0) | 2 (2) |
| 9 | 84 | F | Sigmoid | T3N0H0P0M0 cStage
IIA | Tub1 | 9 | 20 | 3 (0) | 1 (1) | 2 (1) |
| 10 | 67 | F | Sigmoid | T4bN2H0P0M0 pStage
IIIC | Tub1 | 177.7 | 17 | n.d. | 3 (2) | 1 (1) |
| 11 | 72 | M | Transverse | T3N0H0P0M0 pStage
IIA | Muc | 1.5 | 7 | 2 (2) | 1 (1) | 1 (1) |
| 12 | 80 | M | Descending | T4aN0H0P0M0 pStage
IIB | Tub2 | 5.1 | 8 | n.d. | 6 (5) | 2 (2) |
| 13 | 88 | F | Sigmoid | T4aN1H0P0M0 pStage
IIIB | Tub1 | 69.8 | 269 | 1 (0) | 1 (0) | 2 (1) |
CTC numbers and cell-free DNA
concentration were not correlated
Cell-free DNA (cfDNA) is released from cancerous
lesions and is a promising biomarker in the liquid biopsy field. To
determine whether changes in CTC numbers and cfDNA were correlated,
we determined the cfDNA concentration and the number of CTCs in six
cases (patients #1-6). Total cfDNA concentration, at around 10
ng/ml, was not significantly changed by SEMS placement in this
study (Fig. 2C and Table SI). Although the number of CTCs
increased at 24 h after SEMS placement in patients #1 and #4, cfDNA
concentration did not change significantly (Table SI), and the trend was not always
correlated with changes in the number of CTCs (Table SI). From these results, it appears
that CTCs and cfDNAs are released into the blood by different
molecular mechanisms from cancerous lesions, and that SEMS
placement does not have a significant impact on these
phenomena.
Detection of CD133-positive cancer
stem-like cells
Although CTCs are recognized as surrogates of
distant metastasis, research has shown that not all CTCs have the
potential to colonize distant organs. Because a recent report
indicated that cancer stem-cells expressing Lgr5 are critical for
the colonization of distant organs and the establishment of distant
metastases (15), we attempted to
stain for Lgr5 by immunocytochemistry, but were unsuccessful due to
the lack of antibodies suitable for Lgr5 immunohistochemistry
(Fig. S1). Alternatively, we
searched for CD133-positive cancer stem-like cells among the CTCs
detected in seven patients (Table
II). Before examining the patient samples, we examined Capan-1
pancreatic cancer cell lines, some of which showed CD133
positivity. In fact, we detected some CD133-positive Capan-1 cells,
and efficiently captured them (Fig.
3A).
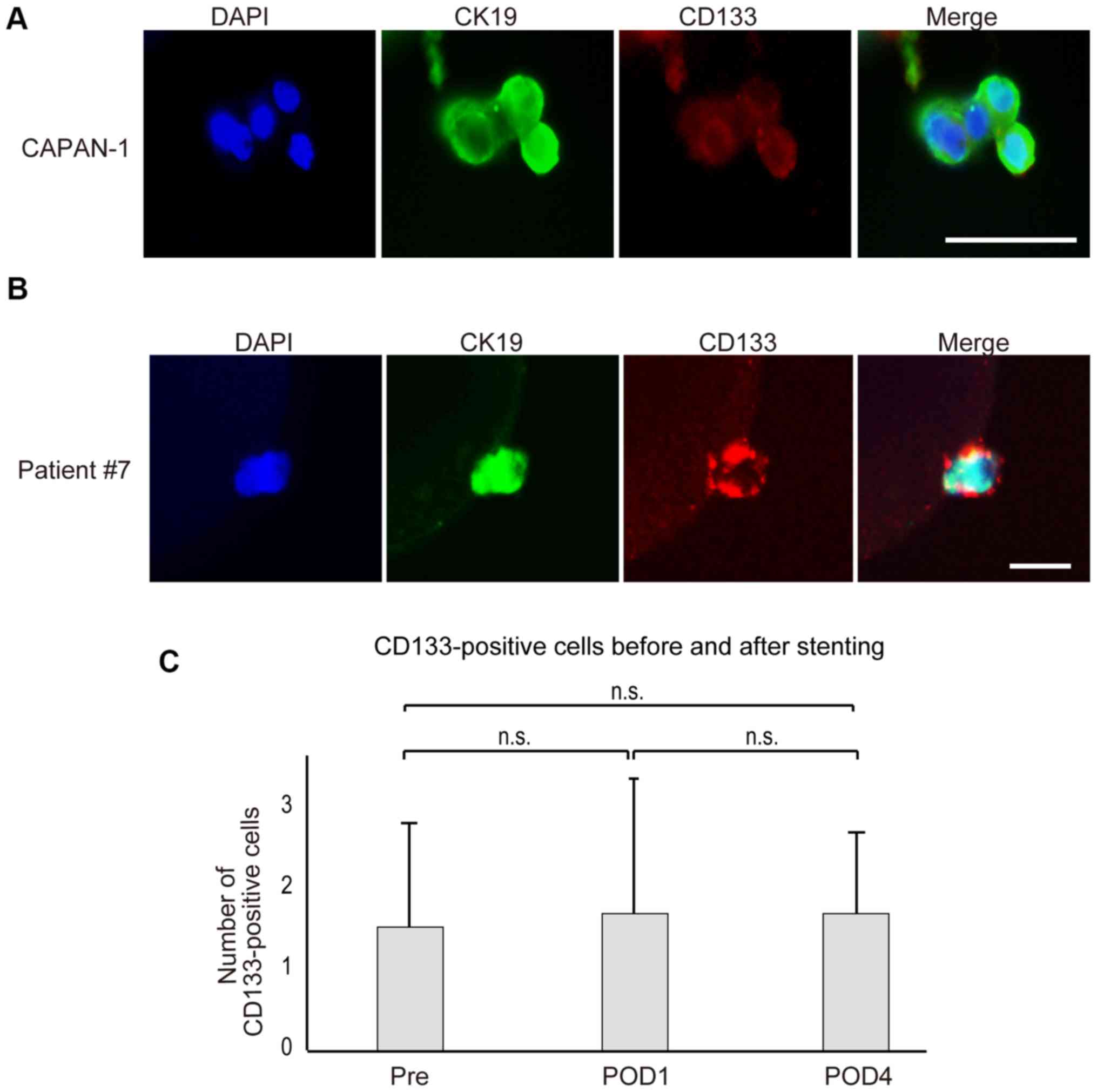 | Figure 3.Images of circulating cancer stem-like
cells stained with CD133. (A) CD133-positive pancreatic cancer
Capan-1 cells were captured and stained with DAPI (blue), CK19
(green) and CD133 (red). A number of cells were CD133 positive
(scale bar, 50 µm). (B) Representative images of the captured
circulating tumor cells stained with DAPI, CK19 and CD133 in a
sample obtained from patient 7 (scale bar, 50 µm). (C) The number
of CD133-positive cells in the blood did not change significantly
before and after SEMS placement. In seven cases (patients 7–13),
CD133-positive cells were determined around stenting. The number of
CD133-positive cells did not change significantly in response to
stenting. SEMS, self-expandable stents; Pre, before SEMS placement;
POD1 and POD4, 24 h and 4 days after SEMS placement, respectively;
n.s., no significance. |
Although the number of CTCs in patient #7 was
increased slightly at day 4 after SEMS placement, the number of
CD133-positive cells was not increased (3, 2, and 3 before, 24 h
after, and 4 days after SEMS placement, respectively; Fig. 3B and Table II). In patient #8, the total number
of CTCs did not change, and the number of CD133-positive cells did
not increase (2, 0, and 2 before, 24 h after, and 4 days after SEMS
placement, respectively). In other five cases except one case
(patient #13) in which one CD133-positive cell was detected only at
4 days after SEMS placement, CD133-positive cells did not increase
at 4 days after stenting (Table
II). Overall, the changes of the number of CD133-positive cells
by SEMS placement were not statistically significant (Fig. 3C). Although the number of the cases
tested is small, these results suggest that even in cases in which
the number of CTCs increased after SEMS placement, cancer stem-like
cells barely increased following stent placement, and such analyses
could be done by the custom CTC chip.
Discussion
Because CTCs may reflect characteristics of primary
cancer lesions, CTC detection will be crucial for personalized
medicine, which will be implemented in the very near future.
However, their detection remains challenging because convenient
methods for CTC detection have not yet been established. In this
study, we applied a novel microfluid system to determine CTC
numbers around endoscopic SEMS placement, as a trial.
Although endoscopic decompression with an SEMS for
obstructive malignant bowel obstruction is clinically effective,
without severe invasiveness, and improves short-term outcomes
(20,21), its potential oncological safety is a
concern due to the related risks of tumor dissemination by direct
effects of physical forces on carcinoma tissue. However, we
observed that endoscopic SEMS placement may temporarily increase
CTC numbers, but does not drastically increase the number of cancer
cells or significantly increase the release of cancer stem cells
into the bloodstream. We found that the increase in the number of
CTCs just after SEMS placement was temporary, as CTC levels tended
to return rapidly to their former state, by day 4 after placement.
These results suggest that the release of cancer cells into the
blood by SEMS placement is due mainly to temporary compression
related to aspects of the placement technique, such as the air
supply and the initiation of physical force on the tissues, and the
potential increased release of cancer cells into the blood does not
significantly continue, due to SEMS placement itself.
The mechanism of the decrease in CTC numbers after
the temporary increase caused by SEMS placement can be explained by
two main hypotheses: That those cells cannot survive because they
become trapped by immune surveillance or reticuloendothelial
systems, or that those cells colonize distant organs. Recent
reports have shown that not all CTCs have the potential for
micrometastasis by the colonization of distant organs, but some
specific CTCs have such features, which may be cancer stem-cell
like features (22). In this study,
we initially attempted to determine the existence of Lgr5-positive
cancer cells, which were recently reported to be colonic stem cells
(23), but because no antibody
against Lgr5 was suitable for immunocytochemistry, despite testing
of several antibodies, we alternatively tested for the presence of
CD133, which has also been recognized as a cancer stem-cell marker
in colon cancer (23), among CTCs
after SEMS placement. Because we did not observe an increase in
CD133-positive CTCs after SEMS placement, even in the cases who
showed significant increases in the numbers of CTCs after SEMS
placement, we speculate that the temporary increase in CTCs just
after the SEMS placement is not strongly related to micrometastasis
potency, and most of these cells may be subject to clearance via
immune surveillance, resulting in a decrease after several days.
These findings may reflect the small initial number of cancer stem
cells and the potential that physiological force alone does not
lead to the release of cancer stem cells into the bloodstream. In
fact, by our follow up data, while patient #7 had metastatic
lesions already even before stenting, patient #8, who showed the
CD133-positive cells at around SEMS placement, did not show any
macroscopic metastatic lesions for one year even without any
adjuvant chemotherapy after stenting. Full testing of these
hypotheses requires long-term observation of the occurrence of
distant metastasis after SEMS placement and the numbers of
circulating cancer stem cells, examining a larger number of
cases.
Currently, the only CTC detection system approved
for clinical use in the United States is the automated ‘CellSearch’
system (24), which uses an antibody
against EpCAM. Although this system has long been used as the gold
standard for the detection of CTCs, it is relatively inflexible. In
our study, we used our custom-made CTC detection chip, which is
more flexible, as we performed immunostaining for CD133 after the
capture of CTCs by antibodies against EpCAM. As aforementioned, the
biological significance of CTCs may differ among cells, and we may
identify cells with more malignant potential, such as cancer stem
cells, by using specific antibodies against those cells. Because we
can change the antibodies for cell capture in this system as
needed, we can focus on biologically significant antigen-expressing
cells other than EpCAM. Therefore, in the future, the capture of
specific CTCs by our system and examination of relationships
between such CTC features and clinical courses may provide novel
insights in the field of clinical oncology.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Sayaka Ito
(Department of Gastroenterology, University of Tokyo, Japan) for
technical assistance.
Funding
The current study was supported by Grants-in-Aid
from the Ministry of Education, Culture, Sports, Science and
Technology of Japan (grant nos. 19H03430, 18H05024 and 17K15923),
Scientific Research on Innovative Areas (grant no. 18H05024), The
Yasuda Medical Foundation, the Japan Coffee Association, the Japan
Foundation for Applied Enzymology and the Project for Cancer
Research And Therapeutic Evolution from Japan Agency for Medical
Research and Development (grant no. JP18cm0106602).
Availability of data and materials
The datasets used and/or analyzed are available from
the corresponding author on reasonable request.
Authors' contributions
RI, TK and MO designed the current study and wrote
the manuscript. RI, NO, TK, ET, KS and TS performed the majority of
the experiments. RI, SY, RK, AN, RH and NT collected clinical
samples. TO created the CTC-chip. KK designed and supervised the
entire project. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Institutional
Review Board of the University of Tokyo Hospital (approval no.
11557). Written informed consent was obtained from all
participating patients prior to enrollment.
Patient consent for publication
Written informed consent for publication was
obtained from all participating patients.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
CTC
|
circulating tumor cell
|
|
SEMS
|
self-expandable metallic stent
|
|
cfDNA
|
cell-free DNA
|
References
|
1
|
de Albuquerque A, Kubisch I, Stölzel U,
Ernst D, Boese-Landgraf J, Breier G, Stamminger G, Fersis N and
Kaul S: Prognostic and predictive value of circulating tumor cell
analysis in colorectal cancer patients. J Transl Med. 10:2222012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohnaga T, Shimada Y, Takata K, Obata T,
Okumura T, Nagata T, Kishi H, Muraguchi A and Tsukada K: Capture of
esophageal and breast cancer cells with polymeric microfluidic
devices for CTC isolation. Mol Clin Oncol. 4:599–602. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Su DW and Nieva J: Biophysical
technologies for understanding circulating tumor cell biology and
metastasis. Transl Lung Cancer Res. 6:473–485. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gabriel MT, Calleja LR, Chalopin A, Ory B
and Heymann D: Circulating tumor cells: A review of non-EpCAM-based
approaches for cell enrichment and isolation. Clin Chem.
62:571–581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chikaishi Y, Yoneda K, Ohnaga T and Tanaka
F: EpCAM-independent capture of circulating tumor cells with a
‘universal CTC-chip’. Oncol Rep. 37:77–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T,
Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E, et
al: Global, regional, and national cancer incidence, mortality,
years of life lost, years lived with disability, and
disability-adjusted life-years for 29 cancer groups, 1990 to 2016:
A systematic analysis for the global burden of disease study. JAMA
Oncol. 4:1553–1568. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Little MW, Oakley T, Briggs JH, Sutcliffe
JA, Allouni AK, Makris G, Bratby MJ, Tapping CR, Patel R, Wigham A,
et al: Technical and clinical outcomes following colonic stenting:
A seven-year analysis of 268 procedures. Cardiovasc Intervent
Radiol. 39:1471–1478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saito S, Yoshida S, Isayama H, Matsuzawa
T, Kuwai T, Maetani I, Shimada M, Yamada T, Tomita M, Koizumi K, et
al: A prospective multicenter study on self-expandable metallic
stents as a bridge to surgery for malignant colorectal obstruction
in Japan: Efficacy and safety in 312 patients. Surg Endosc.
30:3976–3986. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saida Y, Enomoto T, Takabayashi K, Otsuji
A, Nakamura Y, Nagao J and Kusachi S: Outcome of 141 cases of
self-expandable metallic stent placements for malignant and benign
colorectal strictures in a single center. Surg Endosc.
25:1748–1752. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maruthachalam K, Lash GE, Shenton BK and
Horgan AF: Tumour cell dissemination following endoscopic stent
insertion. Br J Surg. 94:1151–1154. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sabbagh C, Browet F, Diouf M, Cosse C,
Brehant O, Bartoli E, Mauvais F, Chauffert B, Dupas JL, Nguyen-Khac
E and Regimbeau JM: Is stenting as ‘a bridge to surgery’ an
oncologically safe strategy for the management of acute,
left-sided, malignant, colonic obstruction? A comparative study
with a propensity score analysis. Ann Surg. 258:107–115. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van den Berg MW, Sloothaak DA, Dijkgraaf
MG, van der Zaag ES, Bemelman WA, Tanis PJ, Bosker RJ, Fockens P,
ter Borg F and van Hooft JE: Bridge-to-surgery stent placement
versus emergency surgery for acute malignant colonic obstruction.
Br J Surg. 101:867–873. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamashita S, Tanemura M, Sawada G, Moon J,
Shimizu Y, Yamaguchi T, Kuwai T, Urata Y, Kuraoka K, Hatanaka N, et
al: Impact of endoscopic stent insertion on detection of viable
circulating tumor cells from obstructive colorectal cancer. Oncol
Lett. 15:400–406. 2018.PubMed/NCBI
|
|
14
|
Takahashi G, Yamada T, Iwai T, Takeda K,
Koizumi M, Shinji S and Uchida E: Oncological assessment of stent
placement for obstructive colorectal cancer from circulating
cell-free DNA and circulating tumor DNA dynamics. Ann Surg Oncol.
25:737–744. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Sousa e Melo F, Kurtova AV, Harnoss JM,
Kljavin N, Hoeck JD, Hung J, Anderson JE, Storm EE, Modrusan Z,
Koeppen H, et al: A distinct role for Lgr5+ stem cells in primary
and metastatic colon cancer. Nature. 543:676–680. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen S, Song X, Chen Z, Li X, Li M, Liu H
and Li J: CD133 expression and the prognosis of colorectal cancer:
A systematic review and meta-analysis. PLoS One. 8:e563802013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagata H, Ishihara S, Kishikawa J, Sonoda
H, Murono K, Emoto S, Kaneko M, Sasaki K, Otani K, Nishikawa T, et
al: CD133 expression predicts post-operative recurrence in patients
with colon cancer with peritoneal metastasis. Int J Oncol.
52:721–732. 2018.PubMed/NCBI
|
|
18
|
Ohnaga T, Shimada Y, Moriyama M, Kishi H,
Obata T, Takata K, Okumura T, Nagata T, Muraguchi A and Tsukada K:
Polymeric microfluidic devices exhibiting sufficient capture of
cancer cell line for isolation of circulating tumor cells. Biomed
Microdevices. 15:611–616. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cima I, Kong SL, Sengupta D, Tan IB, Phyo
WM, Lee D, Hu M, Iliescu C, Alexander I, Goh WL, et al:
Tumor-derived circulating endothelial cell clusters in colorectal
cancer. Sci Transl Med. 8:345ra892016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tilney HS, Lovegrove RE, Purkayastha S,
Sains PS, Weston-Petrides GK, Darzi AW, Tekkis PP and Heriot AG:
Comparison of colonic stenting and open surgery for malignant large
bowel obstruction. Surg Endosc. 21:225–233. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arezzo A, Balague C, Targarona E, Borghi
F, Giraudo G, Ghezzo L, Arroyo A, Sola-Vera J, De Paolis P,
Bossotti M, et al: Colonic stenting as a bridge to surgery versus
emergency surgery for malignant colonic obstruction: Results of a
multicentre randomised controlled trial (ESCO trial). Surg Endosc.
31:3297–3305. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schild T, Low V, Blenis J and Gomes AP:
Unique metabolic adaptations dictate distal organ-specific
metastatic colonization. Cancer Cell. 33:347–354. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeki SS, Graham TA and Wright NA: Stem
cells and their implications for colorectal cancer. Nat Rev
Gastroenterol Hepatol. 8:90–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Riethdorf S, Fritsche H, Müller V, Rau T,
Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Jänicke F, et al:
Detection of circulating tumor cells in peripheral blood of
patients with metastatic breast cancer: A validation study of the
CellSearch system. Clin Cancer Res. 13:920–928. 2007. View Article : Google Scholar : PubMed/NCBI
|