Introduction
The most common type of thyroid cancer is papillary
thyroid cancer (PTC), which has a good overall prognosis with a
10-year survival rate >90% (1).
Studies have increasingly focused on the overdiagnosis and
overtreatment of thyroid disease worldwide (2,3). A study
published in New Zealand in 2016 showed that thyroid cancer is
overdiagnosed worldwide, suggesting that these tumors do not result
in symptoms or death (4). However, a
small percentage of patients experience a more aggressive disease,
including extrathyroidal extension, clinical lymph node metastasis
and distant metastasis. The Thyroid Imaging Reporting and Data
System (TIRADS) is helpful in differentiating thyroid nodules by
offering a risk stratification model (5). Depending on a constellation or number
of suspicious ultrasound (US) features, fine-needle aspiration
cytology (FNAC) is recommended (6,7).
However, the non-diagnostic rate of thyroid FNAC ranges from 5–20%
(8). The risk of malignancy
following a non-diagnostic FNAC result is estimated to be from
1.7–6.6% (9). Repeated FNAC is
recommended for clinical management in cases of non-diagnostic
aspirates and can produce satisfactory results in most cases
(10,11). However, non-diagnostic results are
obtained again in as many as 50% of second-repeated FNAC analyses
(12). A thyroid nodule with
non-diagnostic FNAC results raises the controversial question of
whether a diagnostic thyroidectomy should be performed.
According to TIRADS, various sonographic features of
a thyroid nodule, including the presence of a solid nodule,
hypoechogenicity, irregular margins, microcalcifications, a
taller-than-wide shape and cervical lymph node metastasis, were
associated with an increased likelihood of malignancy. However, no
single sonographic feature or combination of features is adequately
sensitive or specific for identifying all malignant nodules.
Contrast-enhanced ultrasound (CEUS) and strain elastography (SE)
are adjunct US imaging techniques that are used to differentiate
benign from malignant thyroid nodules in addition to TIRADS.
Recently, some prospective studies have confirmed the high
predictive value of CEUS and elastography in identifying malignancy
(13,14). Furthermore, a study demonstrated that
CEUS combined with real-time elastography (RTE) could significantly
increase the diagnostic performance for the differential diagnosis
of malignant and benign thyroid nodules compared with that of CEUS
or RTE alone (15). However, no
reports have evaluated thyroid nodules with non-diagnostic FNAC
results using a combination of these two technologies. Therefore,
the present study investigated the effects of combining CEUS and SE
on the diagnosis of thyroid nodules with non-diagnostic FNAC
results by comparing imaging findings with postoperative
histological results.
Materials and methods
Patients
The present study was conducted between October 2013
and March 2017. Based on the number of suspicious US features that
were associated with the nodule (solid composition without cystic
components, hypoechogenicity/marked hypoechogenicity,
microcalcifications, lobulated or ill-defined margins, and a
taller-than-wide shape), each nodule was classified according to
the TIRADS as: TIRADS 3, no suspicious features; TIRADS 4A, one
suspicious feature; TIRADS 4B, two suspicious features; TIRADS 4C,
three or four suspicious features; or TIRADS 5, five suspicious
features (16). Thyroid nodule
diagnostic FNAC was recommended for nodules with one or more
suspicious sonographic features. All nodules subjected to FNAC were
assessed. The ethics committee of The Second Affiliated Hospital
Zhejiang University School of Medicine approved this study, and all
subjects provided written informed consent prior to their
examinations.
A total of 247 patients with 260 nodules and
non-diagnostic FNAC results were recruited in the present study.
Patients (n=21; 24 nodules) who did not undergo thyroidectomy were
excluded from the data analysis. Finally, 236 nodules from 226
patients were analyzed. The sizes of the thyroid nodules ranged
from 10–28 mm. The patients' ages ranged between 18 and 71 years
(mean ± standard deviation, 55.9±14.7 years), and 38.5% (n=87) of
the patients were male.
Equipment and contrast agent
Conventional US and SE examinations were performed
using a 5–13 MHz transducer (Esaote MyLab 90; The Esaote Group).
For CEUS, another 3–9 MHz transducer (Esaote MyLab 90; Esaote
Group) equipped with contrast-specific, continuous-mode software
was used. Sulfur hexafluoride (SonoVue®; Bracco Imaging
SpA) was used as the US contrast agent.
Performance of conventional US, SE and
CEUS
Conventional US, SE and CEUS of the thyroid nodules
were performed by two radiologists (Dr. PH and Dr. QW both with
>15, 10 and 4 years of experience in US, SE, and CEUS,
respectively). Each patient lay in the supine position with the
neck exposed for the US examination. The nodules were all
classified according to the TIRADS, based on the number of
suspicious features present in each nodule.
SE was performed with light pressure while
maintaining the probe perpendicular to the skin surface and
stationery for several seconds to allow the acquisition of good
quality elastic images with green marks on the screen. Images were
displayed in a split-screen mode, with the conventional US images
of the nodule with the region of interest including the nodule and
sufficient surrounding thyroid tissue (B-mode images) on the right
and the elastography images superimposed on the corresponding
gray-scale US image on the left, and the tissue stiffness was
displayed by a continuum of colors from green (soft tissue) to red
(hard tissue).
CEUS was performed following the SE examination on
the same or following day. An L522 (3–9 MHz) linear array
transducer with an acoustic pressure of 60 kPa was used in each
patient. The mechanical index (MI; 0.05–0.07) was automatically
selected by the system according to the beam-focus depth. The
contrast agent SonoVue® was reconstituted by adding 0.9%
saline (5 ml) and gently shaking the vial by hand to form a
homogeneous microbubble suspension. A 19-gauge catheter was
inserted into the antecubital fossa vein, and SonoVue®
was administered as a bolus injection (1.2 ml), followed
immediately by a 10 ml of saline flush via a three-way port in each
contrast study. The entire movie sequence (at least 3 min) was
stored on magnetic optical disks for analysis.
Image interpretation
The SE images were evaluated by two radiologists and
classified into five different patterns according to the 5-point
Rago scoring system (17) as
follows: a score of 1 indicated even elasticity throughout the
whole nodule; a score of 2 indicated elasticity in a large part of
the nodule; a score of 3 indicated elasticity only at the periphery
of the nodule; a score of 4 indicated no elasticity in the nodule;
and a score of 5 indicated no elasticity in the nodule or the area
with posterior shadowing.
The two radiologists also analyzed the video clips
from CEUS. The CEUS features at peak enhancement were summarized as
follows: i) A shape enhancement was classified as regular or
irregular based on the shapes observed following the contrast agent
injection; ii) a margin enhancement was defined as clear or unclear
based on the clarity of the margins between the lesion and
peripheral tissue; iii) an area enhancement was defined as <50
or ≥50% based on the area of the enhanced part/lesion section at
the peak enhancement; iv) the type of enhancement included
homogeneous enhancement (relative homogeneous diffuse enhancement
of the lesions) and heterogeneous enhancement (diffuse enhancement
with non-homogeneous or regional microvesicle distribution); and v)
the degree of enhancement was characterized as low, equal or high
intensity compared with the surrounding thyroid parenchyma
(18,19). The definitions of the SE and CEUS
patterns are summarized in Table
I.
 | Table I.SE scores and CEUS scores. |
Table I.
SE scores and CEUS scores.
| Score | SE patterns | CEUS patterns |
|---|
| 1 | Elasticity in the
whole nodule: Homogeneously | Shape enhancement:
Regular or irregular based on the shapes |
|
| green | observed following
injection of contrast agent |
| 2 | Elasticity in a large
part of the nodule: Predominantly in green with a few blue areas or
spots | Margin enhancement:
Clear or unclear based on the clarity of the margin between the
lesion and peripheral tissue |
| 3 | Elasticity only at
the peripheral part of the nodule: Predominantly red with few green
areas or spots | Area enhancement:
<50 or ≥50% based on the area of the enhancement part/the lesion
section at the peak enhancement |
| 4 | No elasticity in the
nodule: Completely red | Type of enhancement:
Homogeneous (relative homogeneous diffuse enhancement in lesions),
and heterogeneous (diffuse enhancement presenting non-homogeneous
or regional microvesicle distribution) |
| 5 | No elasticity in the
nodule and in the posterior shadowing: Red area is larger than the
nodule on conventional ultrasound | Enhancement degree:
Low, equal or high intensity when compared with the surrounding
thyroid parenchyma based on time-intensity curves |
On CEUS, based on the results of previous studies
(20,21), nodules with more than one suspicious
feature (including low enhancement; heterogeneous and homogeneous)
were considered as malignant thyroid nodules. On SE, elasticity
scores from 3–5 were considered as the diagnostic criterion for
malignant nodules. The patients were diagnosed using the following
methods with the combined CEUS and SE: CEUS- and SE-positive or
either CEUS- or SE-positive results indicated malignancy, whereas
CEUS- and SE-negative results indicated that the lesion was
benign.
Pathological examination
All cytological diagnoses were recorded using the
Bethesda System for Reporting Thyroid Cytopathology (22) by the pathologist at the Department of
Pathology (The Second Affiliated Hospital, Zhejiang University
School of Medicine, Hangzhou, China) with >10 years of
experience in thyroid cytology. The specimens were smeared onto
glass slides (3–4 µm), fixed with 95% ethyl alcohol (for 4 h at
room temperature), and stained with Diff-Quik and Papanicolaou at
room temperature in the following steps: i) The smear was immersed
in 75% alcohol for 1 sec; ii) stained with hematoxylin for 3–5 min;
iii) immersed in 1.5% hydrochloric acid for 5–10 sec; iv) washed
with water; v) immersed in 75% then 95% alcohol for 10 sec each
time; iv) immersed in orange green/eosin azure solution for 3–5
min; vii) immersed in 95% alcohol and anhydrous alcohol; and viii)
immersed in xylene solution, wet-sealed and observed with 10× and
20× objective light microscope. The remainder of the material was
rinsed in saline for processing as a cell block, to aid in DNA
testing if required (data not shown). All the 236 nodules were
classified as Bethesda III–IV and were included in the present
study. The final result was based on histology.
Statistical analysis
The SPSS software package (version 13.0; SPSS Inc.)
was used for the statistical analysis of data. The agreement
regarding the nodule natures between the two pathologists (PH and
QW) was quantified using the kappa statistic. A kappa statistic
>0.6 was considered indicative of moderate agreement (23). Disagreements were resolved by
consensus or arbitration by a third observer (DJR or DMR). The
diagnostic sensitivity, specificity, positive predictive value
(PPV) and negative predictive value (NPV) of SE, CEUS, and the
combination of SE and CEUS were also assessed by receiver operating
characteristic (ROC) curves. P<0.05 was considered to indicate a
statistically significant difference.
Results
The US features
In patients with non-diagnostic FNAC results, the US
features of irregular shape, aspect ratio ≥1 (taller-than-wide),
and ill-defined margin significantly differed between the malignant
and benign nodules. In addition, the features of heterogeneous
appearance, near capsular location and a higher elastic score,
which are not recommended by TIRADS, also significantly differed
between the malignant and benign nodules (Table II). These positive features might
explain the high rate of non-diagnostic FNAC results. Based on the
TIRADS, 12 (5.0%), 51 (21.6%), 137 (58.1%) and 36 (15.3%) nodules
were classified in categories 4A, 4B, 4C and 5, respectively. The
rates of malignancy according to TIRADS were 8.3% (n=1/12), 9.8%
(n=5/51), 27.0% (n=37/137) and 52.8% (n=19/36). The rate of
malignancy in patients with initially non-diagnostic FNAC results
was 26.3%. The thyroid malignancies with initially non-diagnostic
FNAC results included 52 conventional papillary carcinomas, 3
medullary carcinomas and 7 follicular papillary carcinomas. No
significant score differences were found between the conventional
papillary carcinomas and other carcinomas (data not shown).
 | Table II.Ultrasound features. |
Table II.
Ultrasound features.
|
| Pathological results,
n (%) |
|
|---|
|
|
|
|
|---|
| Ultrasound
features | Malignant | Benign | P-value |
|---|
| Echogenicity |
|
| 0.362 |
|
Hyperechogenicity | 9 (16.1) | 47 (83.9) |
|
|
Hypoechogenicity | 39 (31.2) | 86 (68.8) |
|
|
Isoechogenicity | 14 (25.4) | 41 (74.6) |
|
| Calcification |
|
| 0.603 |
|
With | 33 (27.0) | 89 (73.0) |
|
|
Without | 29 (25.4) | 85 (74.6) |
|
| Shape |
|
| 0.001 |
|
Regular | 23 (16.0) | 121 (84.0) |
|
|
Irregular | 39 (42.4) | 53 (57.6) |
|
| Elasticity
score |
|
| <0.001 |
|
>3 | 40 (83.3) | 8 (16.7) |
|
| ≤3 | 22 (11.7) | 166 (88.3) |
|
| Aspect ratio |
|
| <0.001 |
|
<1 | 30 (18.1) | 136 (81.9) |
|
| ≥1 | 32 (45.7) | 38 (54.3) |
|
| Margin |
|
| <0.001 |
|
Well-defined | 21 (14.2) | 127 (85.8) |
|
|
Ill-defined | 41 (46.6) | 47 (53.4) |
|
| Size,
10–28 mm | 62 (26.3) | 174 (73.7) |
|
| Echotexture |
|
| <0.001 |
|
Heterogeneous | 28 (49.1) | 29 (50.9) |
|
|
Homogeneous | 34 (18.9) | 145 (80.9) |
|
| Location |
|
| <0.001 |
| Near
capsular | 21 (30.9) | 47 (69.1) |
|
| Far
from capsular | 41 (24.4) | 127 (75.6) |
|
The SE and CEUS assessment
The findings from the SE and CEUS showed
statistically significant differences in the enhancement margins,
shape, enhancement area, intensity and type of enhancement between
the benign and malignant thyroid nodules (all P<0.01; data not
shown). A total of 53 thyroid carcinoma cases (85.5%) had a high
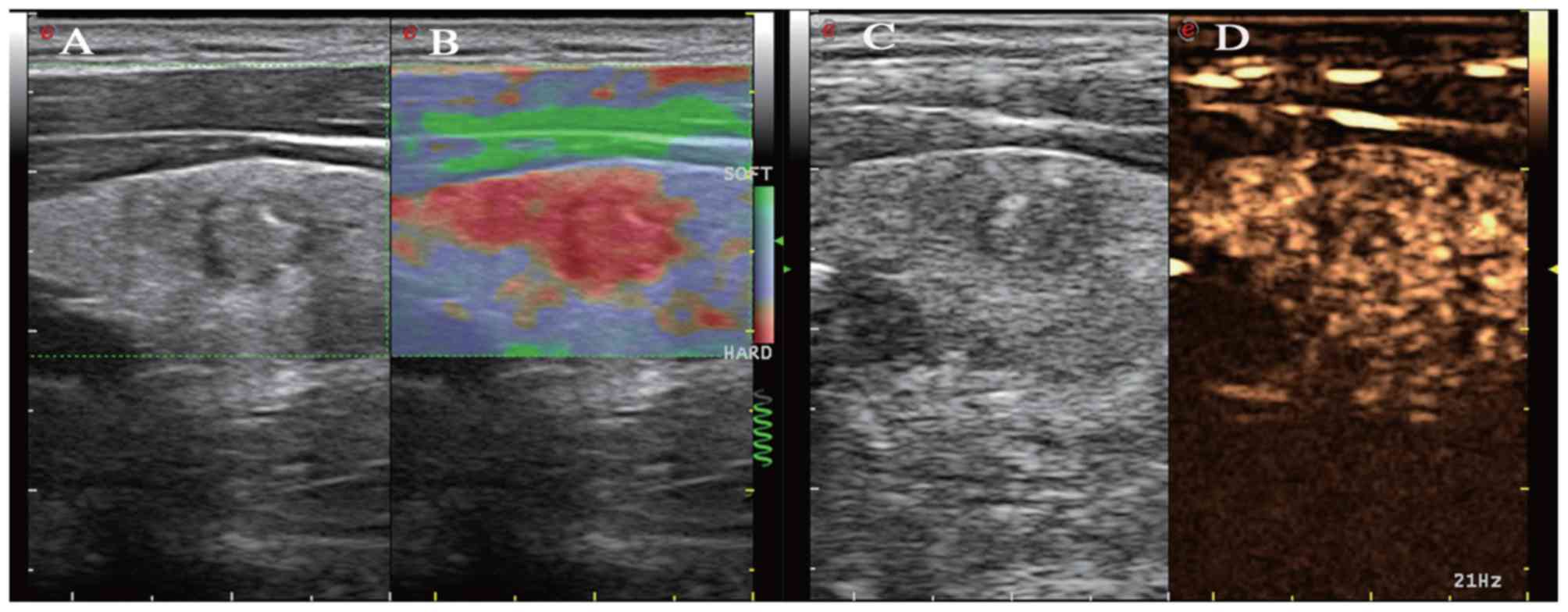
elastic score and/or exhibited hypoenhancement. Fig. 1 shows a non-diagnostic nodule with a
5 SE score and hypoenhancement CEUS pattern. The results also
indicated that there were significant differences between the
malignant and benign nodules with an elasticity score of 1–2 and
those with a score of 3–5 (P<0.001; Table III). Three medullary carcinomas and
one follicular carcinoma had a low SE score and were iso- or
hyper-enhanced in the present study. The interobserver agreement
calculated by the kappa statistic was 0.91 (95% confidence
interval, 0.82–1), indicating high degree of agreement (data not
shown).
 | Table III.Comparison of the sensitivity,
specificity, PPV, NPV and accuracy of CEUS alone, SE alone and
combination of CEUS + SE in the non-diagnostic FNAC nodules. |
Table III.
Comparison of the sensitivity,
specificity, PPV, NPV and accuracy of CEUS alone, SE alone and
combination of CEUS + SE in the non-diagnostic FNAC nodules.
|
| Pathological
results, n |
|
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Ultrasound
features | Malignant | Benign | Sensitivity, % | Specificity, % | PPV, % | NPV, % | Accuracy, % | P-value |
|---|
| Elastography |
|
| 80.6 | 85.6 | 66.7 | 92.5 | 84.3 | <0.001 |
|
Positivea | 50 | 25 |
|
|
|
|
|
|
|
Negativeb | 12 | 149 |
|
|
|
|
|
|
| CEUS |
|
| 59.7 | 95.9 | 84.1 | 86.9 | 86.4 | <0.001 |
|
Positivea | 37 | 7 |
|
|
|
|
|
|
|
Negativeb | 25 | 167 |
|
|
|
|
|
|
| SE + CEUS |
|
| 85.5 | 89.1 | 73.6 | 94.5 | 88.1 | <0.001 |
|
Positivea | 53 | 19 |
|
|
|
|
|
|
|
Negativeb | 9 | 155 |
|
|
|
|
|
|
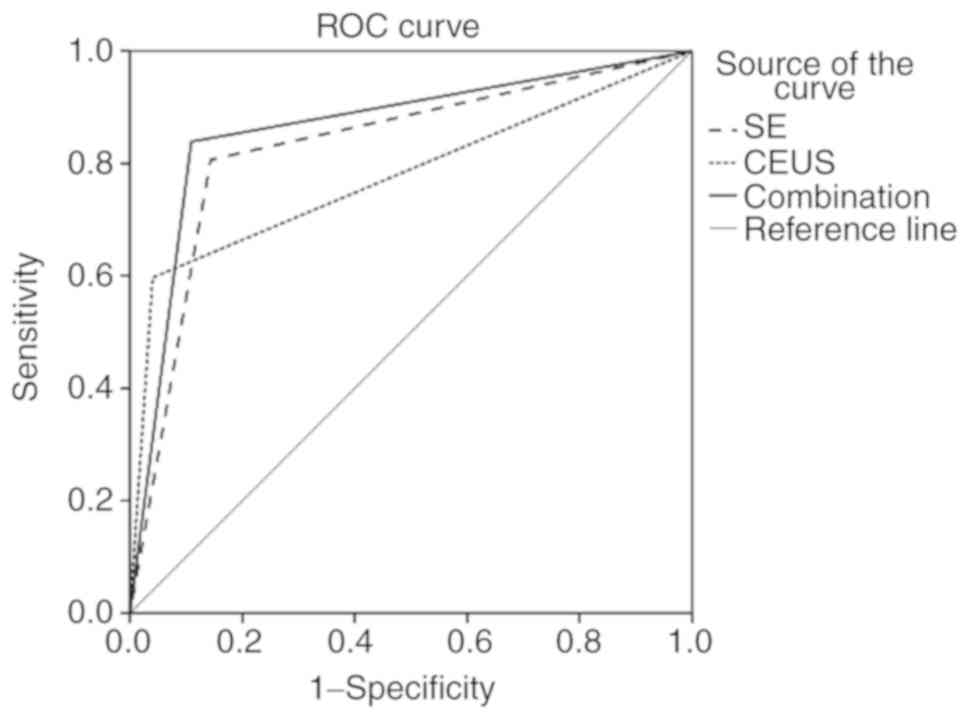
The ROC analysis
ROC analysis was used to determine whether the
findings from the SE and CEUS could help differentiate thyroid
carcinoma from benign nodules with non-diagnostic FNAC results. The
cut-off value was chosen by the Youden index (specificity +
sensitivity −1). The ROC curves of CEUS alone, SE alone and the
combination of CEUS and SE (SE + CEUS) in the diagnosis of benign
and malignant thyroid nodules are shown in Fig. 2. Statistically significant
differences in the AUC values were detected among CEUS, SE and CEUS
+ SE (all P<0.001). The comparisons of the sensitivity,
specificity, PPV, NPV and accuracy of CEUS alone, SE alone and CEUS
+ SE for thyroid nodules with non-diagnostic FNAC results are shown
in Table III. The sensitivity,
specificity, PPV, NPV, accuracy and AUC in predicting malignant
thyroid nodules were 80.6, 85.6, 66.7, 92.5, 84.3% and 0.831,
respectively, for SE alone; 59.7, 95.9, 84.1, 86.9, 86.4% and
0.778, respectively, for CEUS alone; and 83.9, 89.1, 73.6, 94.5,
88.1% and 0.865, respectively, for CEUS + SE. The combined SE and
CEUS approach had a higher sensitivity, NPV and accuracy than SE or
CEUS alone in predicting benign and malignant thyroid nodules with
non-diagnostic FNAC results (P<0.05).
Discussion
Despite the important progress achieved in the field
of diagnostic imaging, the preoperative detection of thyroid cancer
remains challenging (22). FNAC
plays a pivotal role in the diagnosis of thyroid cancer and
prevents unnecessary surgery (18).
The overall incidence of thyroid cancer is 5.3–28% in patients with
thyroid nodules who undergo FNAC (24,25).
Previous studies have shown that the non-diagnostic rate of FNAC
for thyroid nodules was between 5 and 19% (9,26).
Although many studies have focused on the overtreatment of thyroid
nodules, patients with non-diagnostic FNAC results could benefit
from an accurate diagnosis, which could prevent additional stress
to the patient caused by the knowledge of the possibility of
malignancy.
In the present study, the incidence rate of thyroid
tumors in patients with non-diagnostic FNAC results was 26.3%
(n=62/236); this rate was higher than that reported in a previous
study (27). The possible reasons
causing this discrepancy may include but are not limited to several
reasons. Firstly, fewer patients were included in these studies
compared with the present study. Secondly, some studies did not
employ the TIRADS, which is considered the most useful tool for
thyroid nodule screening. Most of the patients in the present study
(73.4%) were assigned as categories 4C and 5 of the TIRADS, which
might have caused bias. Thirdly, as shown in the present study,
several US features, including calcifications, cystic components, a
higher SE score, and near capsular location, may be associated with
the increased rate of non-diagnostic FNAC results (Table III); these factors will be
considered in future studies. A recent study showed that the
prevalence of malignancy in thyroid nodules with indeterminate
cytology was 27% (28). The
differences in proportions of these sonographic patterns are
thought to explain the observed interinstitutional variability in
the risk of malignancy of the indeterminate categories of thyroid
cytology. Additionally, only one pathologist reviewed these FNAC
slides, and FNAC was performed by multiple radiologists over a long
period (between 2013 and 2017). Despite the fact that the present
study included patients with pathological confirmation of disease,
further attention should be paid to nodules with non-diagnostic
FNAC results in future studies.
Several studies suggested that SE and CEUS could
differentiate malignant from benign nodules both qualitatively and
quantitatively (29–31). However, neither of these two imaging
technologies is recommended by the TIRADS, due to concerns
regarding operator dependency and reproducibility. Considering the
designs of previous studies and the value of SE and CEUS in the
present study, the performance of the combination of these two
technologies were evaluated in thyroid nodules with non-diagnostic
FNAC results.
The use of SE and CEUS, alone or in combination,
were investigated in the present study. SE and CEUS are simple and
fast to perform, do not require offline strain image reconstruction
and may be more practical than other technologies for clinical use.
The SE and CEUS findings revealed statistically significant
differences in enhancement and elastic features between the benign
and malignant nodules. The reason for these different findings may
be that malignant tissues are usually harder than benign tissues
and that anomalous vascular distribution may be present in
malignant nodules. However, not all tumor tissues had increased
elastic scores. In total, 9 malignant nodules showed false negative
results, and 19 benign nodules were misdiagnosed as malignant
tumors. The gross anatomy and cellular patterns of follicular
carcinoma overlap with those of benign follicular adenoma, which
explains why this type of thyroid malignancy can only be
differentiated from benign follicular adenoma when capsular or
vascular invasion is observed histologically (32). These findings are consistent with the
results reported by Rubaltelli et al (33) and Cantisani et al (34). Another 5 PTCs showed CEUS negative
results, which is likely due to the size of the nodule. These PTCs
were >2 cm, and a previous study revealed an association between
the nodule size and CEUS enhancement pattern (35).
Recently, Sui et al (15) found that CEUS combined with
elastography could significantly increase the diagnostic
performance in the differential diagnosis of malignant and benign
thyroid nodules compared with CEUS or elastography alone. Rago
et al (17) evaluated 195
consecutive thyroid nodules in 176 patients with indeterminate or
non-diagnostic FNAC results with SE and found a sensitivity of
96.8% and a specificity of 91.8% in distinguishing benign and
malignant nodules. Similarly, the findings of the present study
indicated that the diagnostic power (including the sensitivity, NPV
and accuracy) of SE + CEUS proved to be higher than that of either
modality alone. The combination of SE and CEUS provides noninvasive
imaging of tissue characteristics and an indirect characterization
of intranodular vascularization. Most patients of the present study
declined repeated FNAC due to anxiety, as the patients wanted to
avoid the additional stress caused by the possibility of
malignancy. The combination of SE and CEUS might be helpful to
patients with non-diagnostic results. If the combination of CEUS
and SE shows negative results, a nodule with a suspicious feature
could be followed up in a short time, rather than subjecting the
patient to biopsy or resection. Therefore, the addition of the
combination with a higher negative predictive value could be
appropriate for benign non-diagnostic nodules.
One of the limitations of the present study include
that the SE and CEUS were performed in patients who were known to
be candidates for thyroid FNAC, which might potentially impact the
scoring of the nature of the thyroid nodules. Future studies that
are based on a completely blinded evaluation are necessary to
provide conclusive evidence regarding the roles of SE and CEUS in
the diagnosis of thyroid nodules. Secondly, papillary carcinoma and
follicular carcinoma differ in clinical aspects and the cytological
structure. Although no significant difference was found between
conventional papillary carcinomas and follicular papillary
carcinomas in the present study, the guidelines for the
differentiation of malignancy according to the pathological type
should be further studied. Thirdly, only one pathologist reviewed
the FNAC slides and the intra- and inter-observer reliability in
the interpretation of the cytopathology of thyroid FNA was not
determined.
In conclusion, the combination of CEUS and SE has
high sensitivity, NPV and accuracy in determining malignant thyroid
nodules with cytologically non-diagnostic results compared with
CEUS or SE alone.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81527803 and
81420108018).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PTH and ZML provided administrative support and
designed the study. QW, CY, MQP, JC and ZML recruited patients and
acquired consumables. MQP, CXY, GM and ZML collected and organized
the data. ZML, QW and JC analyzed and interpreted the data. All
authors were involved in the conception and design of the study,
and wrote and provided final approval of the manuscript for
publication.
Ethics approval and consent to
participate
The Ethics Committee of The Second Affiliated
Hospital Zhejiang University School of Medicine approved the
present study, and all subjects provided written informed consent
prior to their examinations.
Patient consent for publication
All subjects provided written informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Caron NR and Clark OH: Papillary thyroid
cancer. Curr Treat Options Oncol. 7:309–319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cronan JJ: Thyroid nodules: Is it time to
turn off the us machines? Radiology. 247:602–604. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jung M: Breast, prostate, and thyroid
cancer screening tests and overdiagnosis. Curr Probl Cancer.
41:71–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vaccarella S, Franceschi S, Bray F, Wild
CP, Plummer M and Dal Maso L: Worldwide thyroid-cancer epidemic?
The increasing impact of overdiagnosis. N Engl J Med. 375:614–617.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tessler FN, Middleton WD, Grant EG, Hoang
JK, Berland LL, Teefey SA, Cronan JJ, Beland MD, Desser TS, Frates
MC, et al: ACR thyroid imaging, reporting and data system
(TI-RADS): White paper of the ACR TI-RADS committee. J Am Coll
Radiol. 14:587–595. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mittendorf EA, Tamarkin SW and McHenry CR:
The results of ultrasound-guided fine-needle aspiration biopsy for
evaluation of nodular thyroid disease. Surgery. 132:648–653. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gharib H, Papini E, Paschke R, Duick DS,
Valcavi R, Hegedüs L, Vitti P and AACE/AME/ETA Task Force on
Thyroid Nodules: American association of clinical endocrinologists,
associazione medici endocrinologi, and european thyroid association
medical guidelines for clinical practice for the diagnosis and
management of thyroid nodules: Executive summary of
recommendations. J Endocrinol Invest. 33:51–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alexander EK, Heering JP, Benson CB,
Frates MC, Doubilet PM, Cibas ES and Marqusee E: Assessment of
nondiagnostic ultrasound-guided fine needle aspirations of thyroid
nodules. J Clin Endocrinol Metab. 87:4924–4927. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al Maqbali T, Tedla M, Weickert MO and
Mehanna H: Malignancy risk analysis in patients with inadequate
fine needle aspiration cytology (FNAC) of the thyroid. PLoS One.
7:e490782012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ali SZ: Thyroid cytopathology: Bethesda
and beyond. Acta Cytol. 55:4–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baloch Z, LiVolsi VA, Jain P, Jain R,
Aljada I, Mandel S, Langer JE and Gupta PK: Role of repeat
fine-needle aspiration biopsy (FNAB) in the management of thyroid
nodules. Diagn Cytopathol. 29:203–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Orija IB, Pineyro M, Biscotti C, Reddy SS
and Hamrahian AH: Value of repeating a nondiagnostic thyroid
fine-needle aspiration biopsy. Endocr Pract. 13:735–742. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Zhang MB, Luo YK, Li J, Wang ZL
and Tang: The value of peripheral enhancement pattern for
diagnosing thyroid cancer using contrast-enhanced ultrasound. Int J
Endocrinol. 2018:16259582018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bojunga J and Mondorf A: Thyroid
elastography. Laryngo-Rhino-Otologie. 98:150–156. 2019.PubMed/NCBI
|
|
15
|
Sui X, Liu HJ, Jia HL and Fang QM:
Contrast-enhanced ultrasound and real-time elastography in the
differential diagnosis of malignant and benign thyroid nodules. Exp
Ther Med. 12:783–791. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ,
Park SH, Jung HK, Choi JS, Kim BM and Kim EK: Thyroid imaging
reporting and data system for US features of nodules: A step in
establishing better stratification of cancer risk. Radiology.
260:892–899. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rago T, Santini F, Scutari M, Pinchera A
and Vitti P: Elastography: New developments in ultrasound for
predicting malignancy in thyroid nodules. J Clin Endocrinol Metab.
92:2917–2922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang B, Jiang YX, Liu JB, Yang M, Dai Q,
Zhu QL and Gao P: Utility of contrast-enhanced ultrasound for
evaluation of thyroid nodules. Thyroid. 20:51–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Q, Xu YB, Jiang J, Ma WQ, Wang H, Li
M and Lei XY: Differential diagnostic value of contrast-enhanced
ultrasound in calcified thyroid nodules. Zhonghua Er Bi Yan Hou Tou
Jing Wai Ke Za Zhi (Chinese). 48:726–729. 2013.
|
|
20
|
Wang Y, Nie F, Liu T, Yang D, Li Q, Li J
and Song A: Revised value of contrast-enhanced ultrasound for solid
hypo-echoic thyroid nodules graded with the thyroid imaging
reporting and data system. Ultrasound Med Biol. 44:930–940. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu Q, Li Y and Wang Y: Diagnostic value of
‘absent’ pattern in contrast-enhanced ultrasound for the
differentiation of thyroid nodules. Clin Hemorheol Microcirc.
63:325–334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cibas ES and Ali SZ: The 2017 bethesda
system for reporting thyroid cytopathology. Thyroid. 27:1341–1346.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Landis JR and Koch GG: An application of
hierarchical kappa-type statistics in the assessment of majority
agreement among multiple observers. Biometrics. 33:363–374. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim EK, Park CS, Chung WY, Oh KK, Kim DI,
Lee JT and Yoo HS: New sonographic criteria for recommending
fine-needle aspiration biopsy of nonpalpable solid nodules of the
thyroid. AJR Am J Roentgenol. 178:687–691. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bessey LJ, Lai NB, Coorough NE, Chen H and
Sippel RS: The incidence of thyroid cancer by fine needle
aspiration varies by age and gender. J Surg Res. 184:761–765. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Williams BA, Bullock MJ, Trites JR, Taylor
SM and Hart RD: Rates of thyroid malignancy by FNA diagnostic
category. J Otolaryngol Head Neck Surg. 42:612013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akgul O, Ocak S, Keskek M, Koc M and Tez
M: Risk of malignancy in non-diagnostic thyroid fine-needle
aspiration biopsy in multinodular goitre patients. Endocr Regul.
45:9–12. 2011.PubMed/NCBI
|
|
28
|
Valderrabano P, McGettigan MJ, Lam CA,
Khazai L, Thompson ZJ, Chung CH, Centeno B and McIver B: Thyroid
nodules with indeterminate cytology: Utility of the American
thyroid association sonographic patterns for cancer risk
stratification. Thyroid. 28:1004–1012. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rago T, Scutari M, Santini F, Loiacono V,
Piaggi P, Di Coscio G, Basolo F, Berti P, Pinchera A and Vitti P:
Real-time elastosonography: Useful tool for refining the
presurgical diagnosis in thyroid nodules with indeterminate or
nondiagnostic cytology. J Clin Endocrinol Metab. 95:5274–5280.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schleder S, Janke M, Agha A, Schacherer D,
Hornung M, Schlitt HJ, Stroszczynski C, Schreyer AG and Jung EM:
Preoperative differentiation of thyroid adenomas and thyroid
carcinomas using high resolution contrast-enhanced ultrasound
(CEUS). Clin Hemorheol Microcirc. 61:13–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma JJ, Ding H, Xu BH, Xu C, Song LJ, Huang
BJ and Wang WP: Diagnostic performances of various gray-scale,
color doppler, and contrast-enhanced ultrasonography findings in
predicting malignant thyroid nodules. Thyroid. 24:355–363. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Trimboli P and Crescenzi A: Thyroid core
needle biopsy: Taking stock of the situation. Endocrine.
48:779–785. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rubaltelli L, Corradin S, Dorigo A,
Stabilito M, Tregnaghi A, Borsato S and Stramare R: Differential
diagnosis of benign and malignant thyroid nodules at
elastosonography. Ultraschall Med. 30:175–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cantisani V, D'Andrea V, Mancuso E,
Maggini E, Di Segni M, Olive M, Lodise P, Palermo S, De Antoni S,
Redler A, et al: Prospective evaluation in 123 patients of strain
ratio as provided by quantitative elastosonography and
multiparametric ultrasound evaluation (ultrasound score) for the
characterisation of thyroid nodules. Radiol Med. 118:1011–1021.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yuan Z, Quan J, Yunxiao Z, Jian C and Zhu
H: Association between real-time contrast-enhanced ultrasound
characteristics and thyroid carcinoma size. Mol Clin Oncol.
3:743–746. 2015. View Article : Google Scholar : PubMed/NCBI
|