Introduction
Hepatocellular carcinoma (HCC) is a severe form of
cancer with extensive global morbidity and mortality. It is the
fifth most common tumor type and the third leading source of
cancer-related mortality, causing 500,000 deaths annually (1,2).
Notably, more than 50% of global HCC morbidity arises in China
(3,4). As it imposes a serious social and
economic burden on society, it is vital that doctors identify liver
cancer at an early stage, when treatment is less complicated and
more likely to succeed. Identification of meaningful tumor
biomarkers is a valuable approach to improving early diagnostic
efforts.
Dendritic cell-specific intercellular adhesion
molecule-grabbing non-integrin-related protein (DC-SIGNR) is a type
II transmembrane protein receptor that has been linked to the
innate immune response against pathogens and tumors, which makes it
a relevant target for cancer biomarker-related studies (5,6).
DC-SIGNR is mainly expressed in liver sinuses, lymphoid tissues and
placental capillaries (7,8). DC-SIGNR may therefore serve a
specialized role in these tissues.
Previous studies on DC-SIGNR in the context of
oncology have yielded new insights. For example, DC-SIGNR
expression levels are altered in certain tumor types or blood
samples from cancer patients; Jiang et al reported that
secreted DC-SIGNR levels in patient's serum samples with colon
cancer were significantly higher compared with healthy controls;
however no detectable upregulation was observed in DC-SIGNR
expression in tumor cells relative to normal tissue (9). By contrast, Liu et al reported
that serum DC-SIGNR levels in patients with lung cancer were lower
compared with healthy controls, whereas in a subset of patients
with brain metastases, serum DC-SIGNR levels were higher compared
with patients without metastases (10). Another study identified significant
increases in serum DC-SIGNR in patients with gastric cancer
relative to healthy controls (11).
Considering the limited number of studies assessing
DC-SIGNR expression in HCC tissues, the aim of the present study
was to compare the levels of this protein in tumor tissue samples
and in adjacent non-cancerous tissues from patients with HCC. A
combination of immunohistochemical and bioinformatics analyses was
used to evaluate DC-SIGNR expression in HCC and characterize its
potential functions.
Materials and methods
Patients and samples
A total of 267 HCC samples and 166 adjacent
non-tumor liver tissue samples were collected from patients who
underwent surgery at Zhejiang Provincial People's Hospital
(Hangzhou, China) between January 2010 and December 2017. The
tissues were confirmed to be cancerous or non-cancerous by hospital
pathologists, fixed with 4% formalin for 24 h at room temperature
and embedded in paraffin. Information on patient sex, age, tumor
size, number, location, Edmondson grade and site of tumor
metastasis was collected during patient hospitalization and
treatment. Because some clinical data were missing or unavailable,
the total number of some clinical indicators was <267. Overall
survival (OS) was determined using either the date of patients'
death or the last follow-up time point. This study was approved by
the Review Board of the Hospital Ethics Committee, and written
informed consent was obtained from each participant prior to data
collection.
Immunohistochemical staining
Paraffin-embedded specimens were used to design
three microarrays with the help of the Shanghai BioChip Co., Ltd.
(Shanghai, China). Immunohistochemistry (IHC) was performed using
the following protocol: Three tissue section microarrays (5 µm)
were heated at 70°C for 2 h, washed three times in a xylene
solution to remove paraffin, rehydrated in decreasing
concentrations of ethanol (100, 95, 85 and 75%; each for 5 min),
and boiled in Tris-EDTA (TE) buffer (Tris, 1.21 g/l; EDTA, 0.37
g/l; Tween-20, 0.5 ml/l) under high pressure (103 kPa) for 3 min to
facilitate antigen retrieval. The samples were incubated with 3%
hydrogen peroxide for 15 min to prevent endogenous peroxidase
activity, blocked with 10% goat non-immune serum (reagent A;
Histostain®-plus Bulk kit; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) for 20 min to reduce non-specific binding
and incubated with anti-DC-SIGNR antibody (1:400, cat. no.
ab169783; Human CD299 Antibody; Abcam, Cambridge, UK) overnight at
4°C. Sections were washed and incubated with a biotinylated
secondary antibody (reagent B; Histostain®-plus Bulk
kit; Thermo Fisher Scientific, Inc.) for 15 min. Samples were
exposed to streptavidin-peroxidase (reagent C;
Histostain®-plus Bulk kit; Thermo Fisher Scientific,
Inc.) for an additional 15 min and a chromogenic reaction was
performed using the 3,3′-diaminobenzidine color substrate solution
(OriGene Technologies, Inc.; Beijing, China) according to the
manufacturer's protocol. Color development was terminated when a
brown signal indicative of staining was evident in the sample.
Hematoxylin (cat. no. C0107; Beyotime Institute of Biotechnology,
Haimen, China) staining was performed for 3–5 min. To finalize the
staining process, samples were dehydrated in increasing
concentrations of ethyl alcohol (75, 85, 95, and 100%) for 5 min
each, soaked three times in xylene for 10 min and mounted with a
gelatin resin. All procedures were performed at room temperature
unless otherwise specified.
Immunohistochemical staining
evaluation
Immunohistochemically stained samples were
independently evaluated by two pathologists from the Department of
Pathology of the Zhejiang Provincial People's Hospital based on the
intensity and proportion of stained cells. The intensity of stained
cells was scored from 0–3 as follows: 0, no staining; 1, weak
staining; 2, medium staining; 3, strong staining. The proportion of
stained cells were scored from 0–4: 0, no cells stained; 1, 1–25%
cells stained; 2, 26–50% cells stained; 3, 51–75% cells stained; 4,
>75% cells stained. The intensity and frequency scores were
multiplied to produce an overall score used to confirm the level of
DC-SIGNR expression in HCC tissues; tissues scoring <6 were
defined as having low expression, whereas those with scores ≥6 were
defined as having high expression.
Bioinformatics analysis of online
databases
Using the Oncomine databases (www.oncomine.org), DC-SIGNR mRNA levels in HCC and
normal tissues were compared. The analysis was performed under the
following filtering conditions: Gene, DC-SIGNR; analysis type,
cancer versus normal analysis; cancer type, hepatocellular
carcinoma; data type, mRNA. Co-expression was also analyzed in the
top four datasets using the screening conditions: Gene, DC-SIGNR;
analysis type, co-expression analysis; cancer type, liver cancer.
Ten-year survival analyses of patients with specific DC-SIGNR
levels were performed using the Kaplan-Meier Plotter (http://kmplot.com). Biological process and signaling
pathway analysis and annotation for DC-SIGNR were performed using
the STRING data platform (https://string-db.org).
Statistical analysis
SPSS 16.0 software (SPSS, Inc.) was used for all
statistical analyses. χ2 test was performed to assess
the association between DC-SIGNR expression and different
clinicopathological parameters. OS curves were generated using the
Kaplan-Meier method, with the P-value derived from the log-rank
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical characteristics of
patients
HCC samples from 267 patients, including 219 male
and 48 female patients between 25 and 90 years old (mean age,
58±11.5 years) were analyzed. At the time of the last follow-up
visit, 103 patients remained alive and 65 were deceased; the status
of the remaining patients was unavailable (Table I).
 | Table I.Expression of DC-SIGNR in
hepatocellular carcinoma tissue samples. |
Table I.
Expression of DC-SIGNR in
hepatocellular carcinoma tissue samples.
|
|
| DC-SIGNR
expression |
|
|---|
|
|
|
|
|
|---|
| Clinical
parameters | Number | Low | High | P-value |
|---|
| Sex |
|
|
| 0.454 |
| Male | 219 | 164 | 55 |
|
|
Female | 48 | 37 | 11 |
|
| Age (years) |
|
|
| 0.338 |
|
<55 | 105 | 81 | 24 |
|
| ≥55 | 162 | 120 | 42 |
|
| Location |
|
|
| 0.255 |
| Left | 51 | 34 | 17 |
|
|
Right | 153 | 119 | 34 |
|
| Left +
right | 15 | 12 | 3 |
|
| Tumor size (cm) |
|
|
| 0.012 |
|
<5 | 144 | 100 | 44 |
|
| ≥5 | 118 | 97 | 21 |
|
| Tumor number |
|
|
| 0.384 |
|
Single | 217 | 162 | 55 |
|
|
Multiple | 50 | 39 | 11 |
|
| Edmondson
grade |
|
|
| 0.001 |
|
I+II | 157 | 106 | 51 |
|
|
III | 109 | 94 | 15 |
|
| Metastasis |
|
|
| 0.074 |
| M0 | 241 | 179 | 62 |
|
| M1 | 21 | 19 | 2 |
|
| Microvascular
invasion |
|
|
| 0.523 |
|
Absent | 99 | 77 | 22 |
|
|
Present | 102 | 80 | 22 |
|
| Cirrhosis |
|
|
| 0.246 |
|
Negative | 84 | 66 | 18 |
|
|
Positive | 183 | 135 | 48 |
|
| Hepatitis B
antigen |
|
|
| 0.479 |
|
Negative | 51 | 39 | 12 |
|
|
Positive | 210 | 157 | 53 |
|
| AFP |
|
|
| 0.280 |
|
<50 | 115 | 82 | 33 |
|
|
≥50 | 103 | 78 | 25 |
|
| Status |
|
|
| 0.031 |
|
Deceased | 65 | 56 | 9 |
|
|
Alive | 103 | 75 | 28 |
|
DC-SIGNR expression in HCC
tissues
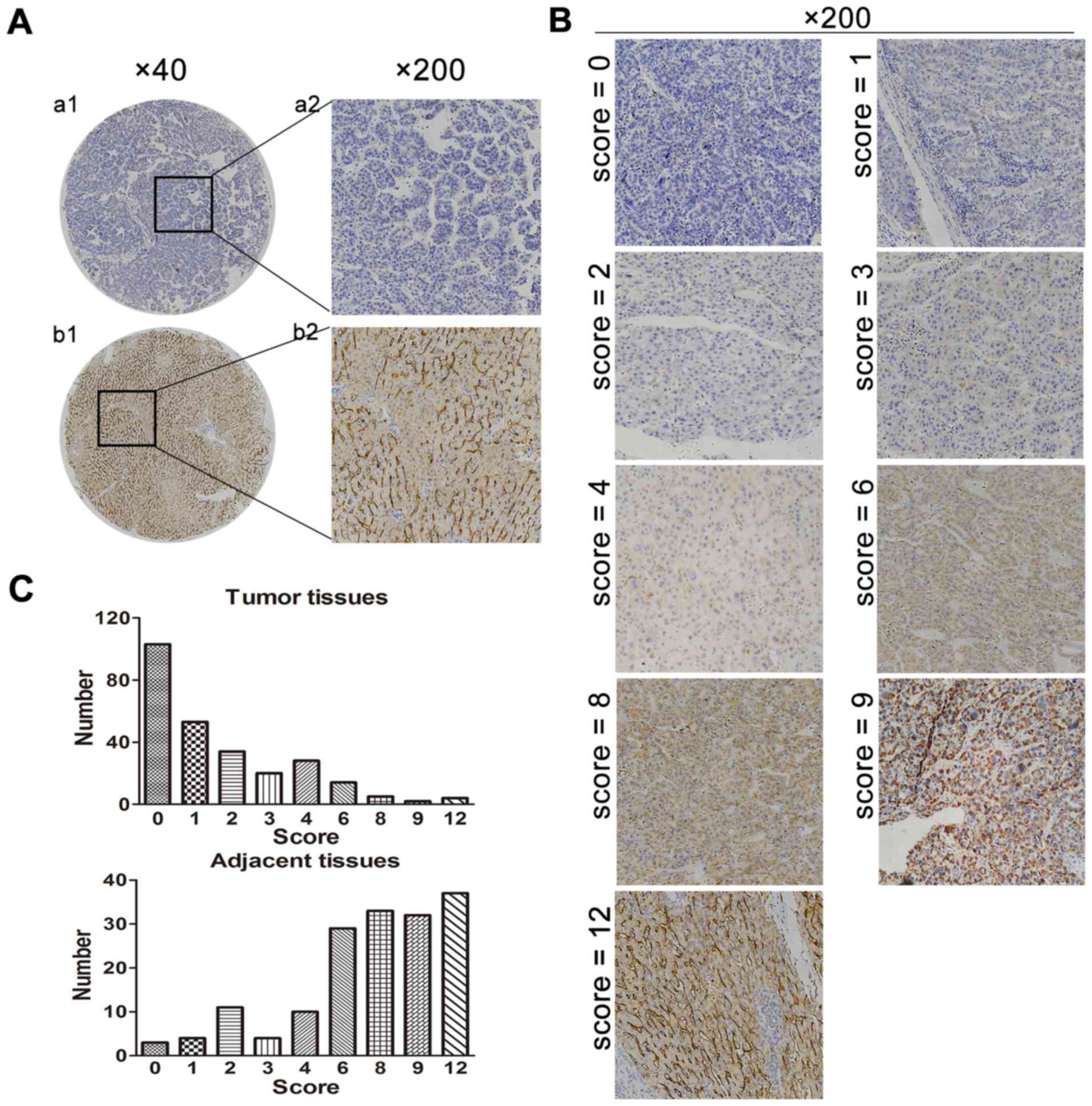
DC-SIGNR expression was analyzed by IHC in 267 HCC
specimens and 166 adjacent non-cancerous tissues. There were
201/267 (75.3%) HCC tissue samples and 31/166 (18.7%) adjacent
non-cancerous samples with low expression of DC-SIGNR. DC-SIGNR
expression in HCC tissues was significantly lower compared with
expression in adjacent non-cancerous tissue (P<0.001; Table II; Fig.
1A and B). The distribution of DC-SIGNR staining scores in the
samples demonstrated a higher incidence of low scores in tumor
tissues compared with adjacent non-cancerous tissues (Fig. 1C).
 | Table II.Low-expression of DC-SIGNR in
HCC. |
Table II.
Low-expression of DC-SIGNR in
HCC.
|
|
| DC-SIGNR
expression |
|
|---|
|
|
|
|
|
|---|
| Tissue type | Number | Low | High | P-value |
|---|
| HCC | 267 | 201 | 66 |
<0.001 |
| Adjacent
tissue | 166 | 31 | 135 |
|
| Average survival
months |
| 26.79 | 36.70 |
|
Association between DC-SIGNR
expression and clinicopathological features
Tumor size was identified as significantly
associated with low levels of DC-SIGNR expression (P=0.012;
Table I); low DC-SIGNR expression
was also associated with Edmondson grade (P=0.001; Table I). Sex, age, tumor location,
metastasis, microvascular invasion, cirrhosis, α-fetoprotein (AFP)
concentration, and hepatitis B infection were not associated with
DC-SIGNR expression (Table I).
Association between DC-SIGNR
expression and clinical outcome in patients
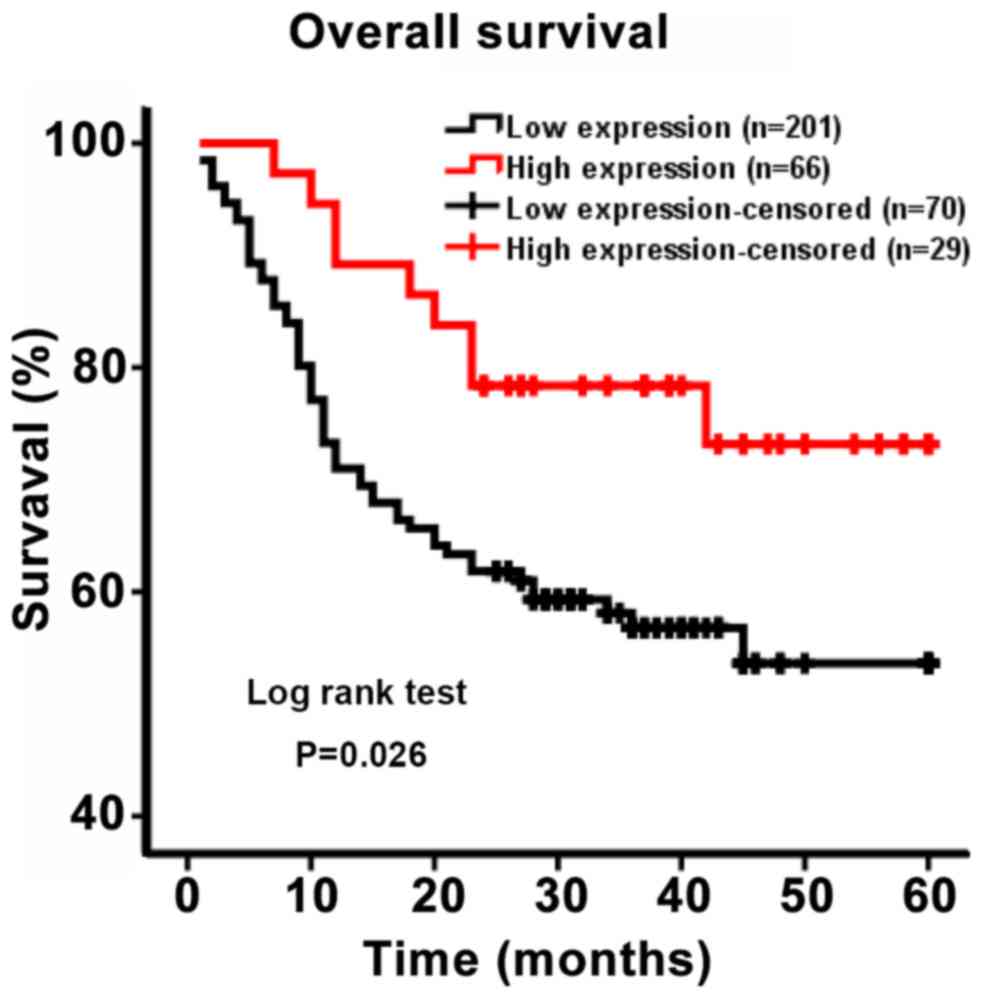
To evaluate the association between DC-SIGNR
expression and patient survival, a Kaplan-Meier curve of OS was
generated. The five-year survival rate differed significantly
between patients with high and low DC-SIGNR expression (P=0.026;
Fig. 2). Patients with low DC-SIGNR
expression survived for an average of 26.79 months, whereas those
with high expression survived for an average of 36.70 months
(Table II). Therefore, the data
suggested that patients with HCC who exhibited low levels of
DC-SIGNR expression were likely to survive for a shorter period of
time.
Bioinformatics analysis for DC-SIGNR
expression in patients with HCC
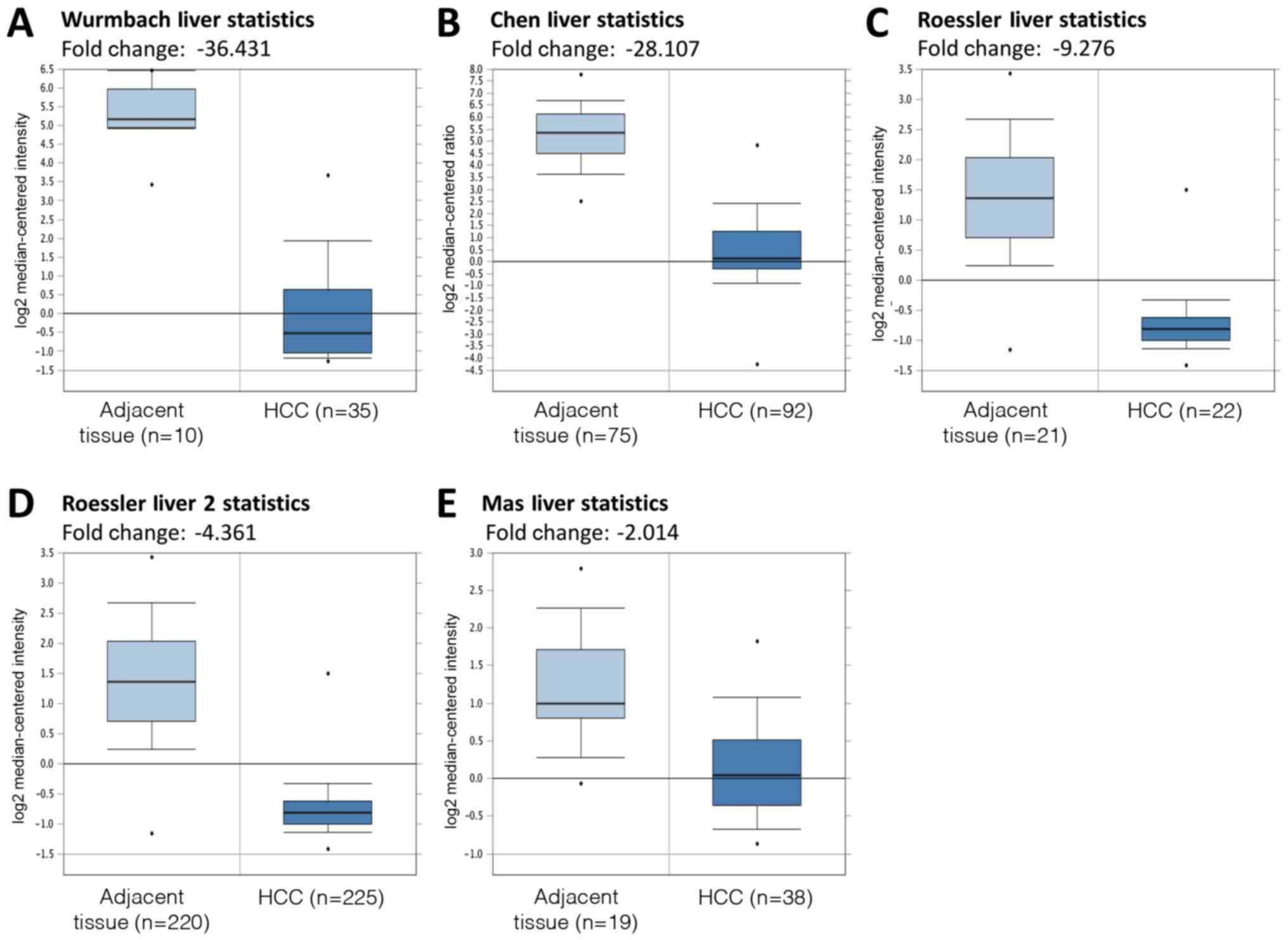
DC-SIGNR mRNA expression levels in HCC and adjacent
non-cancerous tissues were compared using the Oncomine databases.
Five datasets were selected for analysis: Wurmbach Liver Statistics
(12), Chen Liver Statistics
(13), Roessler Liver Statistics,
Roessler Liver 2 Statistics (14)
and Mas Liver Statistics (15). The
results of this analysis revealed that DC-SIGNR mRNA expression was
significantly lower in HCC tissues compared with adjacent tissues
(P<0.05; Fig. 3).
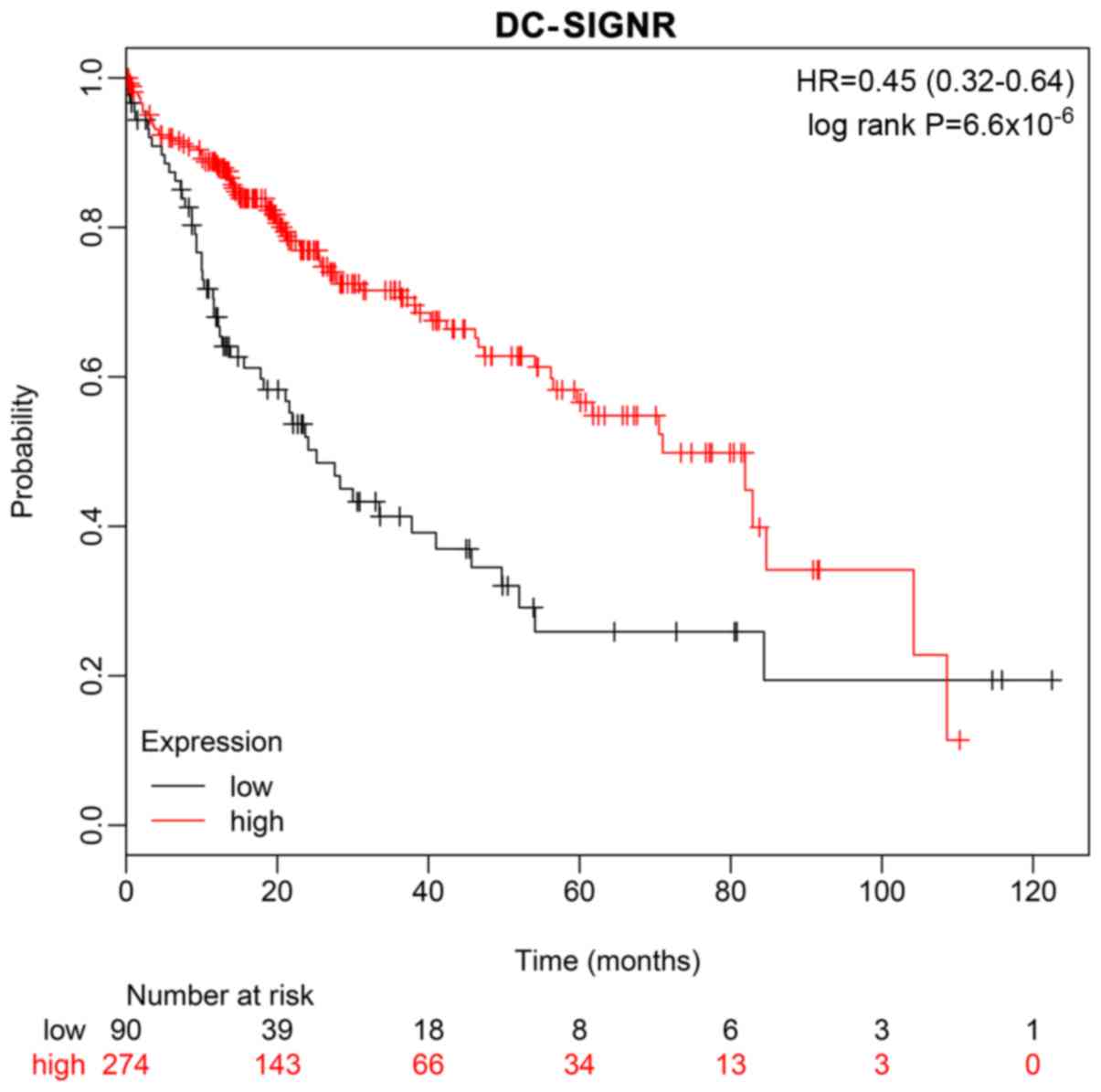
A ten-year survival rate prediction analysis was
performed using the Kaplan-Meier Plotter database, which confirmed
that low expression of DC-SIGNR was associated with shorter
survival (Fig. 4).
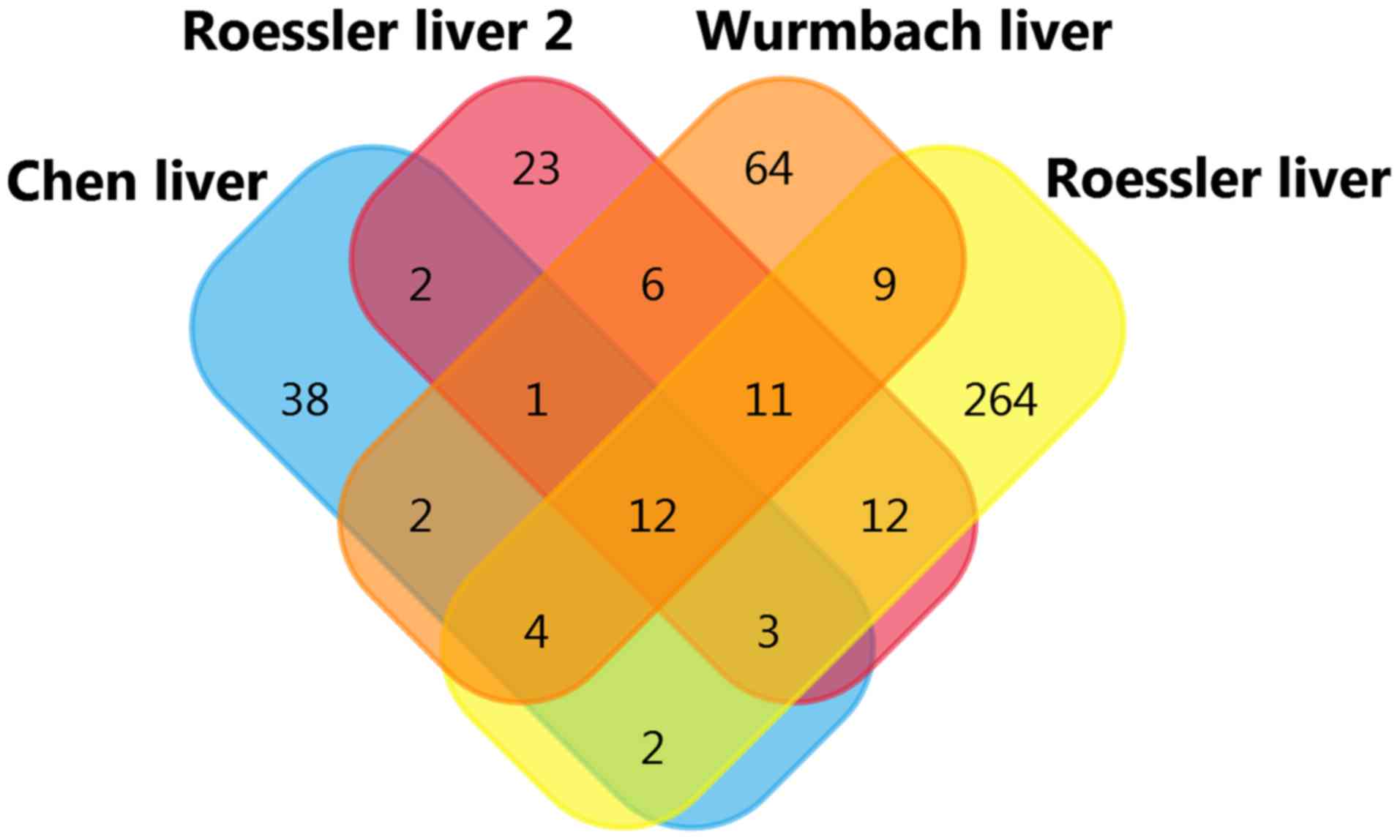
DC-SIGNR co-expression analysis was conducted using
the Oncomine databases. The top four datasets were selected for
analysis: Wurmbach Liver statistics (12), Roessler Liver Statistics (14), Chen Liver Statistics (13), and Roessler Liver 2 Statistics
(14), with gene correlation values
>0.50. A total of twelve co-expressed genes were found (Table III; Fig.
5). This indicates that the function or product of DC-SIGNR may
be associated with these genes.
 | Table III.DC-SIGNR co-expression genes with the
cut-off for selection defined as an appearance in four
datasets. |
Table III.
DC-SIGNR co-expression genes with the
cut-off for selection defined as an appearance in four
datasets.
| Gene | Gene name | Number of
appearances |
|---|
| CETP | Cholesteryl ester
transfer protein | 4 |
| CLEC1B | C-type lectin
domain family 1 member B | 4 |
| CRHBP |
Corticotropin-releasing hormone-binding
protein | 4 |
| DNASE1L3 | Deoxyribonuclease 1
like 3 | 4 |
| ECM1 | Extracellular
matrix protein 1 | 4 |
| GPM6A | Glycoprotein
M6A | 4 |
| MARCO | Macrophage receptor
with collagenous structure | 4 |
| MT1E | Metallothionein
1E | 4 |
| MT1F | Metallothionein
1F | 4 |
| MT1H | Metallothionein
1H | 4 |
| MT1X | Metallothionein
1X | 4 |
| VIPR1 | Vasoactive
intestinal peptide receptor 1 | 4 |
Biological process and signaling pathway enrichment
analyses related to DC-SIGNR were conducted using STRING. The
results of these analyses indicated that DC-SIGNR expression was
strongly associated with the immune response, as indicated by
pathway ID, GO.0002376, GO.0006955, GO.0050776, GO.0002682,
GO.0002684 GO.0045087, 4660, 4650 and 4662 (Tables IV and V).
 | Table IV.Top 10 statistically meaningful
biological processes associated with DC-SIGNR. |
Table IV.
Top 10 statistically meaningful
biological processes associated with DC-SIGNR.
| Rank | Pathway ID | Pathway
description | Matching
proteins |
|---|
| 1 | GO.0002223 | Stimulatory C-type
lectin receptor signaling pathway | DC-SIGN, HRAS,
ICAM2, ICAM3, KRAS, NRAS, RAF1 |
| 2 | GO.0031347 | Regulation of
defense response | DC-SIGN, HRAS,
ICAM2, ICAM3, IL10, IL4, KRAS, NRAS, RAF1 |
| 3 | GO.0006952 | Defense
response | DC-SIGN, CD83,
HRAS, ICAM2, ICAM3, IL10, ITIH4, KRAS, NRAS, RAF1 |
| 4 | GO.0002376 | Immune system
process | DC-SIGN, CD83,
HRAS, ICAM2, ICAM3, IL10, IL4, KRAS, NRAS, RAF1 |
| 5 | GO.0006955 | Immune
response | DC-SIGN, CD83,
HRAS, ICAM2, ICAM3, IL10, KRAS, NRAS, RAF1 |
| 6 | GO.0050776 | Regulation of
immune response | DC-SIGN, HRAS,
ICAM2, ICAM3, IL10, KRAS, NRAS, RAF1 |
| 7 | GO.0002682 | Regulation of
immune system processes | DC-SIGN, CD83,
HRAS, ICAM2, ICAM3, IL10, KRAS, NRAS, RAF1 |
| 8 | GO.0002684 | Positive regulation
of immune system processes | DC-SIGN, CD83,
HRAS, ICAM2, ICAM3, KRAS, NRAS, RAF1 |
| 9 | GO.0045087 | Innate immune
response | DC-SIGN, HRAS,
ICAM2, ICAM3, IL4, KRAS, NRAS, RAF1 |
| 10 | GO.0000186 | Activation of MAPKK
activity | HRAS, KRAS, NRAS,
RAF1 |
 | Table V.Top 10 statistically meaningful
signaling pathways associated with DC-SIGNR. |
Table V.
Top 10 statistically meaningful
signaling pathways associated with DC-SIGNR.
| Rank | Pathway ID | Pathway
description | Matching
proteins |
|---|
| 1 | 4660 | T cell receptor
signaling pathway | HRAS, IL10, IL4,
NRAS, RAF1 |
| 2 | 4664 | Fc ε receptor
antibody signaling pathway | HRAS, IL4, NRAS,
RAF1 |
| 3 | 4068 | FoxO signaling
pathway | HRAS, IL10, NRAS,
RAF1 |
| 4 | 4650 | Natural killer cell
mediated cytotoxicity | HRAS, ICAM2, NRAS,
RAF1 |
| 5 | 5219 | Bladder cancer | HRAS, NRAS,
RAF1 |
| 6 | 4370 | Vascular
endothelial growth factor signaling pathway | HRAS, NRAS,
RAF1 |
| 7 | 4662 | B cell receptor
signaling pathway | HRAS, NRAS,
RAF1 |
| 8 | 4720 | Long-term
potentiation | HRAS, NRAS,
RAF1 |
| 9 | 4730 | Long-term
depression | HRAS, NRAS,
RAF1 |
| 10 | 4917 | Prolactin signaling
pathway | HRAS, NRAS,
RAF1 |
Discussion
Liver tumor development is a complex process, with
many distinct factors participating in tumorigenesis and
progression, often through key changes in gene expression (16,17). To
the best of our knowledge, DC-SIGNR expression in this context has
not been extensively studied, and whether it is expressed in HCC
tissues has not been clarified to date. To resolve this question,
267 HCC specimens were collected and the association between
DC-SIGNR expression and clinical outcomes was evaluated.
Patients with low DC-SIGNR expression in tumor
tissue were more likely to have larger tumors and a higher
Edmondson grade. These results were similar to those reported by
Liu et al, which revealed low DC-SIGNR expression in serum
samples from patients with lung cancer (10). Similarly, Jiang et al reported
that DC-SIGNR expression was not detectable in colon cancer foci or
in matched normal tissues (9).
Lymphoid tissues from patients with non-Hodgkin's lymphoma have
also been demonstrated to be DC-SIGNR-negative by IHC (18). In contrast to these findings, other
studies have demonstrated that DC-SIGNR is upregulated in gastric
cancer and colon cancer liver metastases (11,19).
These distinct findings indicated that DC-SIGNR expression may vary
between tumor types, tissues samples, or at different stages of the
disease.
DC-SIGN and DC-SIGNR are homologous genes involved
in immune response (20,21). DC-SIGN is expressed on dendritic
cells; it binds to the T cell receptor to mediate T cell activation
(22). DC-SIGN is also expressed on
macrophages and is involved in phagocytosis (23). Therefore, it may suggest that normal
expression of DC-SIGNR on the surface of liver cells may be a
required gene product for the removal of abnormal cells by
signaling the mutation status of hepatocytes to immune cells
including T cells, NK cells or macrophages; downregulation of this
protein may facilitate tumor formation.
Based on the IHC and bioinformatics analyses of
DC-SIGNR, DC-SIGNR downregulation in liver cells may not be
essential for tumor physiology, as tumor location, number,
metastasis, microvascular invasion and AFP were not associated with
DC-SIGNR expression. Instead, DC-SIGNR downregulation may act by
suppressing the local immune response to the tumor.
In conclusion, DC-SIGNR is an important marker of
immunity in HCC tissues, and its downregulation is associated with
reduced patient survival. However, multiple conclusions have been
drawn on the role and significance of DC-SIGNR. Therefore, further
study is required to examine its function in HCC, to guide HCC
treatment and to facilitate predictions for long-term survival in
patients with HCC.
Acknowledgements
Not applicable.
Funding
This work was supported by the grants from The
National Science Foundation of China (grant. no. 81672474 to DSH),
Funds of Science Technology Department of Zhejiang Province (grant
no. 2015C0303 to DSH; grant no. LGF18H160024 to ZMH; grant no.
LGF18H160025 to XLH) and Zhejiang Province Bureau of Health (grant
nos. WKJ-ZJ-1812 and 2018ZZ002 to ZMH).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
HBX and HJW designed and conducted the experiments.
SSS and JGZ were in charge of sample processing. XLH, ZMH and CWZ
performed data analysis. DSH and XZM revised the manuscript
critically for important intellectual content, participated in
online data analysis and gave final approval of the version to be
published. HBX reviewed and wrote the manuscript. All authors have
read and approved of the final version of the manuscript.
Ethics approval and consent to
participate
The research was approved by the Review Board of
Hospital Ethics Committee, Zhejiang Provincial People's Hospital
(Hangzhou, China), and written informed consent from was obtained
each participant before data collection.
Patient consent for publication
Patients agreed that the researchers would use their
clinical specimens and that the results would be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zuo T, Zheng R, Zeng H, Zhang S and Chen
W: Analysis of liver cancer incidence and trends in China. Zhonghua
Zhong Liu Za Zhi. 37:691–696. 2015.(In Chinese). PubMed/NCBI
|
|
4
|
Zhu Q, Qiao GL, Zeng XC, Li Y, Yan JJ,
Duan R and Du ZY: Elevated expression of eukaryotic translation
initiation factor 3H is associated with proliferation, invasion and
tumorigenicity in human hepatocellular carcinoma. Oncotarget.
7:49888–49901. 2016.PubMed/NCBI
|
|
5
|
van Kooyk Y and Rabinovich GA:
Protein-glycan interactions in the control of innate and adaptive
immune responses. Nat Immunol. 9:593–601. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chaudhary O, Kumar S, Bala M, Singh J,
Hazarika A and Luthra K: Association of DC-SIGNR expression in
peripheral blood mononuclear cells with DC-SIGNR genotypes in HIV-1
infection. Viral Immunol. 28:472–475. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lalor PF, Lai WK, Curbishley SM, Shetty S
and Adams DH: Human hepatic sinusoidal endothelial cells can be
distinguished by expression of phenotypic markers related to their
specialised functions in vivo. World J Gastroenterol. 12:5429–5439.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Engering A, van Vliet SJ, Hebeda K,
Jackson DG, Prevo R, Singh SK, Geijtenbeek TB, van Krieken H and
van Kooyk Y: Dynamic populations of dendritic cell-specific ICAM-3
grabbing nonintegrin-positive immature dendritic cells and
liver/lymph node-specific ICAM-3 grabbing nonintegrin-positive
endothelial cells in the outer zones of the paracortex of human
lymph nodes. Am J Pathol. 164:1587–1595. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang Y, Zhang C, Chen K, Chen Z, Sun Z,
Zhang Z, Ding D, Ren S and Zuo Y: The clinical significance of
DC-SIGN and DC-SIGNR, which are novel markers expressed in human
colon cancer. PLoS One. 9:e1147482014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu X, Zhang H, Su L, Yang P, Xin Z, Zou
J, Ren S and Zuo Y: Low expression of dendritic cell-specific
intercellular adhesion molecule-grabbing nonintegrin-related
protein in lung cancer and significant correlations with brain
metastasis and natural killer cells. Mol Cell Biochem. 407:151–160.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Zhang Q, Zhang M, Yuan M, Wang Z,
Zhang J, Zhou X, Zhang Y, Lin F, Na H, et al: DC-SIGNR by
influencing the lncRNA HNRNPKP2 upregulates the expression of CXCR4
in gastric cancer liver metastasis. Mol Cancer. 16:782017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wurmbach E, Chen YB, Khitrov G, Zhang W,
Roayaie S, Schwartz M, Fiel I, Thung S, Mazzaferro V, Bruix J, et
al: Genome-wide molecular profiles of HCV-induced dysplasia and
hepatocellular carcinoma. Hepatology. 45:938–947. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen X, Cheung ST, So S, Fan ST, Barry C,
Higgins J, Lai KM, Ji J, Dudoit S, Ng IO, et al: Gene expression
patterns in human liver cancers. Mol Biol Cell. 13:1929–1939. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roessler S, Jia HL, Budhu A, Forgues M, Ye
QH, Lee JS, Thorgeirsson SS, Sun Z, Tang ZY, Qin LX and Wang XW: A
unique metastasis gene signature enables prediction of tumor
relapse in early-stage hepatocellular carcinoma patients. Cancer
Res. 70:10202–10212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mas VR, Maluf DG, Archer KJ, Yanek K, Kong
X, Kulik L, Freise CE, Olthoff KM, Ghobrial RM, McIver P and Fisher
R: Genes involved in viral carcinogenesis and tumor initiation in
hepatitis C virus-induced hepatocellular carcinoma. Mol Med.
15:85–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Basu AK: DNA damage, mutagenesis and
cancer. Int J Mol Sci. 19:9702018. View Article : Google Scholar
|
|
17
|
Falco M, Palma G, Rea D, De Biase D, Scala
S, D'Aiuto M, Facchini G, Perdonà S, Barbieri A and Arra C: Tumour
biomarkers: Homeostasis as a novel prognostic indicator. Open Biol.
6:1602542016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Z, Chen K, Yan L, Yang Z, Zhu Z,
Chen C, Zeng J, Wei W, Qi X, Ren S and Zuo Y: Low expression of
dendritic cell-specific intercellular adhesion molecule-grabbing
nonintegrin-related protein in non-Hodgkin lymphoma and significant
correlations with lactic acid dehydrogenase and β2-microglobulin.
Biochem Cell Biol. 91:214–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Na H, Liu X, Li X, Zhang X, Wang Y, Wang
Z, Yuan M, Zhang Y, Ren S and Zuo Y: Novel roles of DC-SIGNR in
colon cancer cell adhesion, migration, invasion, and liver
metastasis. J Hematol Oncol. 10:282017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cox N, Pilling D and Gomer RH: DC-SIGN
activation mediates the differential effects of SAP and CRP on the
innate immune system and inhibits fibrosis in mice. Proc Natl Acad
Sci USA. 112:8385–8390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Soilleux EJ: DC-SIGN (dendritic
cell-specific ICAM-grabbing non-integrin) and DC-SIGN-related
(DC-SIGNR): Friend or foe? Clin Sci (Lond). 104:437–446. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gringhuis SI, Kaptein TM, Wevers BA,
Mesman AW and Geijtenbeek TB: Fucose-specific DC-SIGN signalling
directs T helper cell type-2 responses via IKKe- and CYLD-dependent
Bcl3 activation. Nat Commun. 5:38982014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang K, Liu X, Liu Y, Wang X, Cao L, Zhang
X, Xu C, Shen W and Zhou T: DC-SIGN and Toll-like receptor 4
mediate oxidized low-density lipoprotein-induced inflammatory
responses in macrophages. Sci Rep. 7:32962017. View Article : Google Scholar : PubMed/NCBI
|