Introduction
One of the leading causes of cancer death worldwide
is lung cancer (1). There are 1.8
million new cases of lung cancer each year, and 1.6 million people
succumb to lung cancer (2). Nearly
70% of patients with lung cancer are in an advanced stage with
localized spread and/or distant metastasis (3).
Among the types of diagnosed lung cancers, non-small
cell lung cancer (NSCLC) accounts for ~80% (4). Despite advances in treatment, the
5-year survival rate of patients with lung cancer is still <15%
(5). The presence of local
recurrence of tumors and distant metastasis may be the most common
cause of death (6). The
carcinogenesis and progression of NSCLC involve both environmental
and genetic factors and are multistep processes (7–9).
Although chemotherapy, radiotherapy, surgery, molecular-targeted
therapy and immunotherapy have greatly improved the survival and
prognosis of patients with NSCLC to date, the underlying molecular
mechanisms remain unclear and require further study (10). Therefore, it is important to study
the genes involved in the metastasis, tumor progression and
invasion, as well as to determine the molecular mechanisms
underlying the effects of these genes in the treatment and
prediction of NSCLC prognosis.
The basic leucine zipper ATF-like transcription
factor (BATF) family comprises three members (BATF, BATF2 and
BATF3) and belongs to the group of activator protein 1 (AP-1)
transcription factors (11). BATF is
located on human chromosome 14q24.3; there is only one transcript
(NM_006399) of BATF (12). The
protein encoded by this gene is a nuclear alkaline leucine zipper
protein, which belongs to the AP-1/activating transcription factor
(ATF) superfamily. The BATF protein is a negative regulator of
AP-1/ATF transcription events, and AP-1 complexes activate or
inhibit their target genes (13).
Regulation of the AP-1 gene effects cell survival, differentiation
and proliferation (14,15). An increasing number of studies
suggest that BATF may affect cancer development (16–18).
However, the functional mechanisms of BATF in NSCLC are poorly
understood.
The present study used The Cancer Genome Atlas
(TCGA) data to analyze BATF expression in NSCLC. Through a series
of experiments, the role of BATF in NSCLC was explored. The results
could provide important information to explain the pathogenesis of
NSCLC and reveal that BATF may be a potential novel target for the
treatment of non-small cell lung cancer.
Materials and methods
BATF expression analysis in TCGA
database
TCGA is a publicly funded project and contains RNA
expression data for various types of cancer, including NSCLC
(http://cancergenome.nih.gov/). The mRNA
expression profiling data of NSCLC and matched adjacent mucosa was
downloaded from TCGA (datasets TCGA-38, TCGA-49 and TCGA-50)
(19).
Cell lines and culture conditions
The human NSCLC cell line A549 was purchased from
the Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences and cultured in RPMI-1640 medium (Thermo Fisher
Scientific, Inc) with 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.), 100 IU/ml penicillin (Sigma-Aldrich; Merck KGaA)
and 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA), at 37°C in
a humidified incubator containing 5% CO2.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from tissue samples and
cultured cells using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. cDNA was synthesized from total RNA (500 ng) in a 10
µl reaction volume using a PrimeScript RT-PCR kit (Takara Bio,
Inc.) using the following conditions: 5 min at 25°C, 25 min at 52°C
and 10 min at 80°C. Then, qPCR was performed using the power SYBR
Green master mix (Thermo Fisher Scientific, Inc.). GAPDH was used
as an internal control. The primers used were: BATF forward,
5′-AGAAGAGTTCAGAGGAGGGA-3′ and reverse, 5′-CGTTCTGTTTCTCCAGGTCT-3′;
GAPDH forward, 5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′. qPCR was performed using SYBR Premix
Ex Taq II (Takara Biotechnology Co., Ltd.), and the thermocycling
conditions used were: 95°C for 10 min, followed by 39 cycles of
95°C for 1 min, 55°C for 30 sec, and 72°C for 45 sec. The
2−ΔΔCq method was used to determine relative
(BATF/GAPDH) mRNA expression (20).
Lentiviral shRNA vector construction
and infection
For knockdown of BATF, a lentiviral shRNA vector
targeting the human BATF gene was constructed by Shanghai
GeneChem Co., Ltd. The targeting sequence of BATF was synthesized
and cloned into the lentiviral vector pGVX115-GFP (Shanghai
GeneChem Co., Ltd.) to produce pGV115-shBATF at 37°C for 2 h. For
control cells, transfection was performed using an empty vector
carrying GFP. To establish stable cell lines, 2 µg/ml puromycin was
added to the medium for 1 week following transfection with the
lentiviral vectors. Knockdown efficiency was verified using RT-qPCR
and western blot analysis.
Western blot analysis
Cell lines were harvested 3 days post transfection
and washed twice with cold PBS, and then cell lysates were prepared
by adding radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology) containing 1 mM phenylmethanesulfonyl.
The lysate was stored at −20°C until further experimentation.
Proteins (~30 µg each lane) were separated with 12% SDS-PAGE, and
transferred onto PVDF membranes (EMD Millipore; Merck KGaA).
Membranes were incubated with primary antibodies to BATF (1:500;
cat. no. ab236876; Abcam), and β-actin (1:500; cat. no. sc-47778;
Santa Cruz Biotechnology) overnight at 4°C. Next, the membrane was
washed and incubated with the corresponding secondary antibodies
[horseradish peroxidase-conjugated (HRP) goat anti-rabbit; 1:5,000;
cat. no. P044801-2; Dako; Agilent Technologies or HRP goat
anti-mouse IgG H&L; 1:1,000; cat. no. ab6708; Abcam or goat
anti-rabbit IgG; 1:1,500; cat. no. bs-0295G; BIOSS] at room
temperature for 1 h. Protein bands were quantified using a
bio-imaging system (DNR Bio-Imaging Systems, Ltd.).
Cell proliferation and viability
assays
Cell proliferation and viability were analyzed by
the Celigo imaging cytometry system and MTT assay, respectively.
The fluorescence intensity of entire wells, of transfected A549
cells was detected, and the number of cells was automatically
calculated by the Celigo imaging cytometry system (Nexcelom
Bioscience LLC). Cell viability was measured using a Cell Viability
kit (MTT; Roche Diagnostics) solubilized in 150 µl dimethyl
sulfoxide according to the manufacturer's instructions. The
absorption of the solution was measured at 570 nm at various time
points.
Apoptosis assay
A total of 1×105 cells/well were incubated in 6-well
plates for 24 h and treated with shBATF and shCtrl for 48 h, both
at 37°C. The cells were washed, and the Annexin V-APC Apoptosis
Detection kit (eBioscience, Inc.) was used according to the
manufacturer's instructions. Cells were analyzed by flow cytometry
(FACScalibur; BD Biosciences), and the percentage of apoptotic
cells was determined using ModFit LT v5.0 software (Verity Software
House, Inc.). Each experiment was performed three times.
Caspase 3/7 activity was measured using a
homogeneous luminescence-based assay with a Caspase 3/7 Glo Assay
kit (Promega Corporation) according to the manufacturer's
instructions. Following transfection with shCtrl and shBATF, A549
cells were treated with 0, 0.01, 0.1 and 1.0 µM cetuximab for 24 h.
For luminosity, the cells were analyzed by an Infinite F500
multifunction microplate reader (Tecan Trading AG).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5 software (GraphPad Software, Inc.). Statistical differences
were analyzed and calculated by unpaired two-tailed Student's
t-test or one-way analysis of variance (ANOVA) followed by
Bonferroni's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
The expression levels of BATF in TCGA
dataset
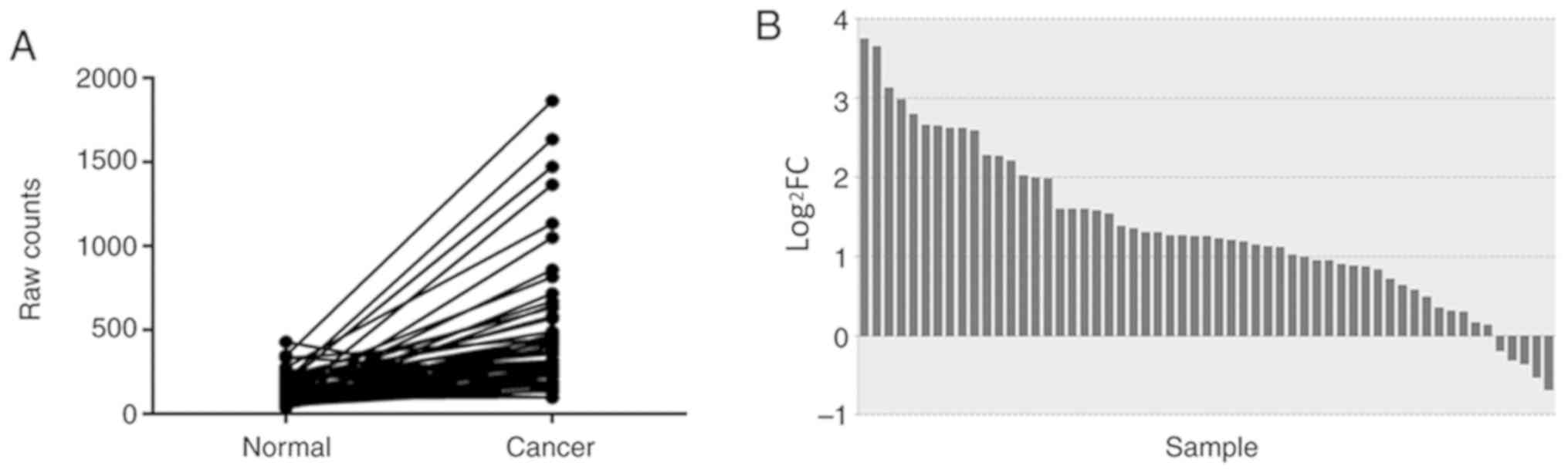
In TCGA database, a total of 57 pairs of mRNA
expression profiles of NSCLC tissues and adjacent non-tumor tissues
were screened. The differences in the expression levels of NSCLC
and adjacent non-tumor tissues were analyzed and described using a
paired samples linear graph (Fig.
1A) and a bar graph (Fig. 1B).
Compared with that in adjacent non-tumor tissues, BATF expression
was significantly upregulated in NSCLC
(P=6.56×10−6).
Knockdown efficiency of BATF by shRNA
lentivirus infection in A549 cells
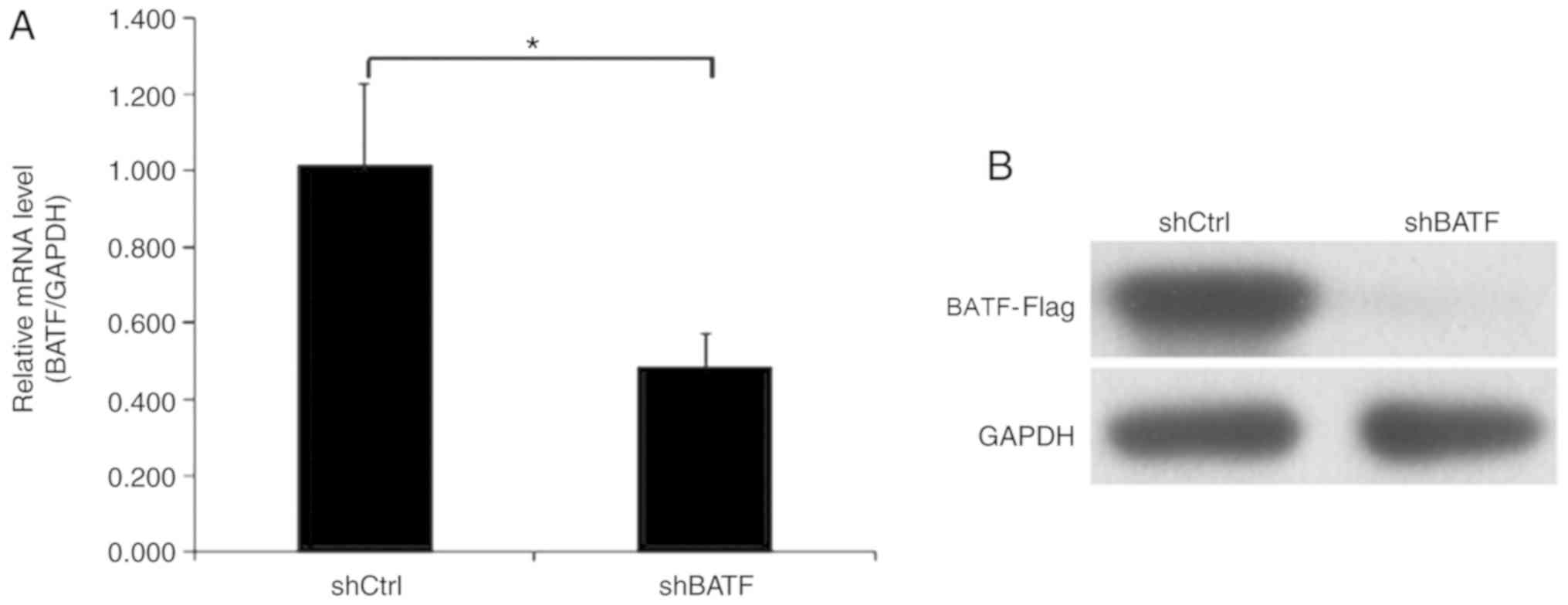
Endogenous BATF expression in human NSCLC A549 cells
was detected by RT-qPCR. In the A549 cell line, BATF was moderately
expressed, which indicated that A549 cells were suitable for BATF
knockdown analysis. To investigate the role of BATF in A549 cells,
lentiviruses carrying shBATF and shCtrl were used to infect A549
cells; the transfection efficiency was evaluated using RT-qPCR, and
the results revealed that BATF mRNA levels were reduced by 50% in
the shBATF group compared with the shCtrl group (Fig. 2A). Western blot analysis demonstrated
similar results (Fig. 2B).
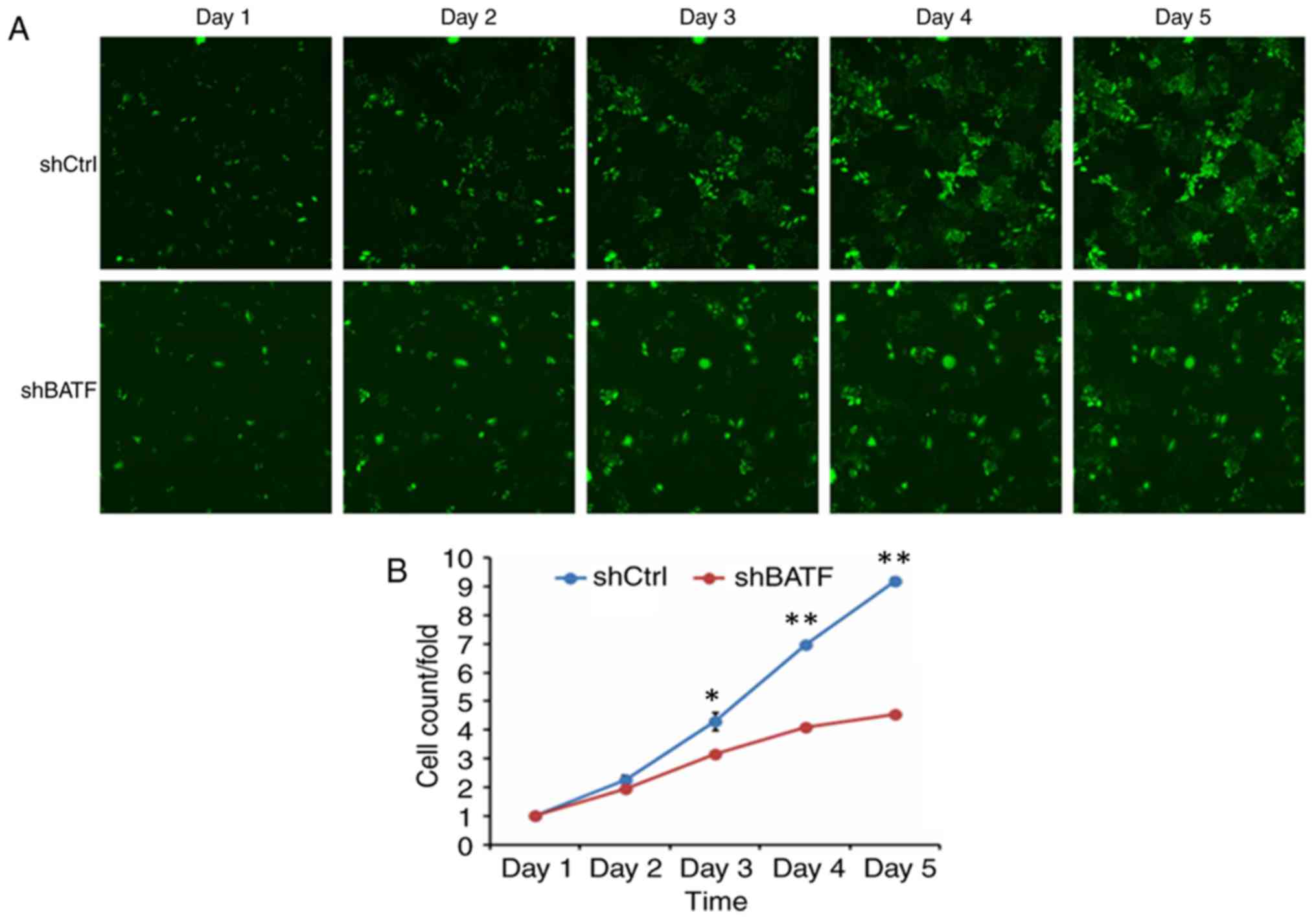
Knockdown of BATF inhibits the
proliferation of A549 cells
To generate the cell growth curve, a cell imaging
system and an MTT assay were used. As shown in Fig. 3, the proliferation in the shBATF
group was significantly inhibited compared with that in the shCtrl
group according to the Celigo imaging cytometry system
(P<0.01).
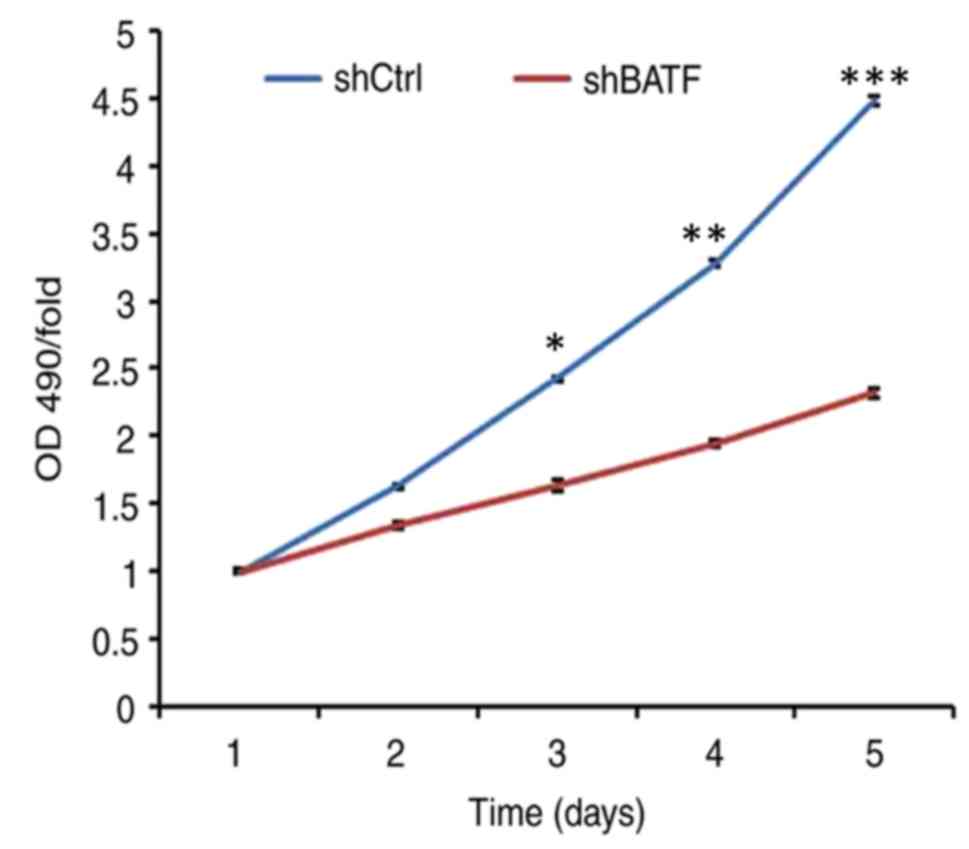
Cell viability was determined by MTT assay. The
results demonstrated that in the A549 cell line, the cell viability
of the shBATF group was significantly lower (P<0.001) between
days 3 and 5 compared with that of the shCtrl group (Fig. 4).
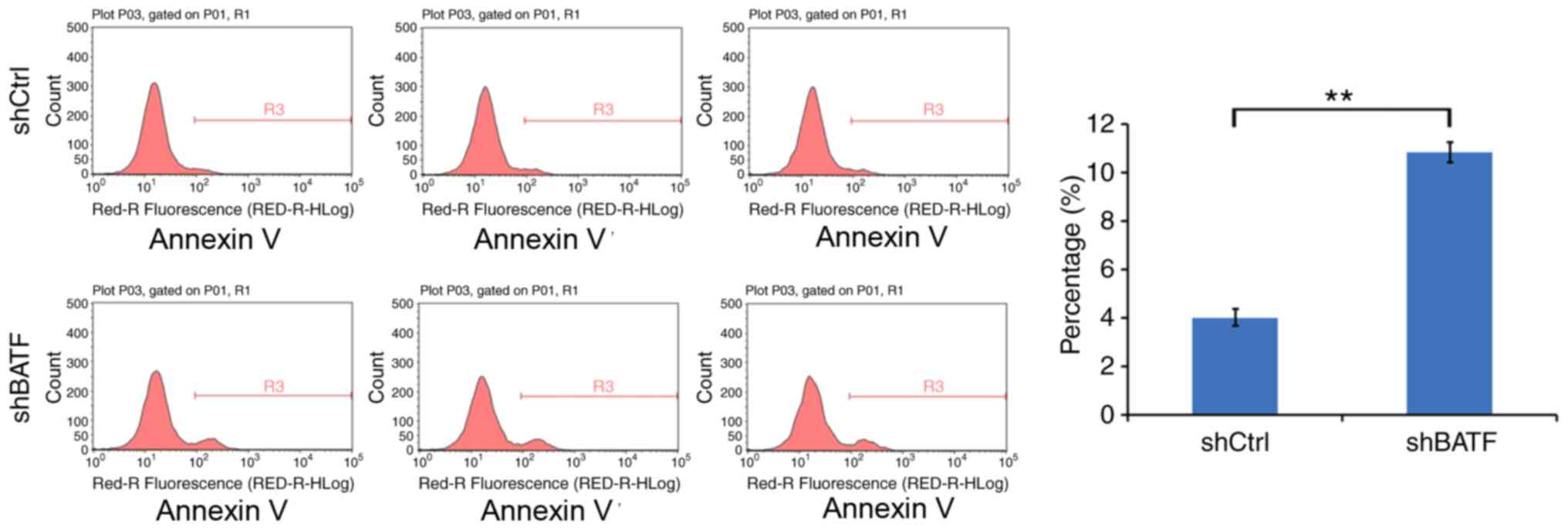
Knockdown of BATF induces apoptosis in
A549 cells
The effect of BATF knockdown on apoptosis was
detected by annexin V staining. Compared with the control group,
the apoptotic rate of the shBATF group was significantly increased
in the A549 cell line (P<0.01; Fig.
5), suggesting that knockdown of BATF promoted apoptosis in
A549 cells.
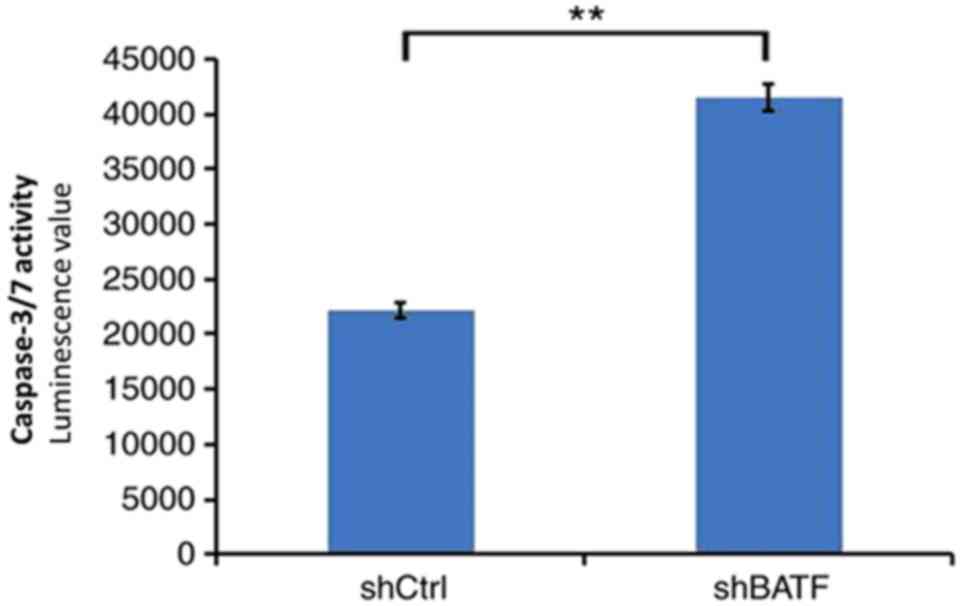
Knockdown of BATF increases caspase
3/7 activity in A549 cells
As shown in Fig. 6,
in the A549 cell line, caspase 3/7 activity was significantly
increased following shBATF infection compared with shCtrl infection
(P<0.01).
Discussion
The main aims of this study were to investigate the
biological roles of BATF in human NSCLC cells and to elucidate the
role of BATF in the development of lung cancer. Lung cancer is
often a multifactorial process involving processes such as ECM
degradation, transmission, basement membrane, cell adhesion,
vascular production and movement (21–25).
Therefore, identification of novel biomarkers is crucial for the
improvement of clinical outcomes for NSCLC patients.
Dorsey et al (19) first described BATF as a modulator of
the AP-1 transcription complex in specific human tissues following
identification in a cDNA library from Epstein-Barr virus-infected
human B cells. Analysis of polyadenylated mRNA from different human
tissues and established cell lines revealed a strong hybridization
in Raji Burkitt's lymphoma cells and in the healthy lung tissue
(26). BATF knockout mice have a
defect in TH17 development based on its direct regulation of both
RORγt and of RORγt target genes such as IL-17A. (27). BATF serves a crucial role in Th17
differentiation and exhibits distinct DNA binding specificity and
protein-protein interactions with Th17-specific factors (28). Th17 cells serve a key role in the
development of NSCLC (29).
In the present study, screening of TCGA database
revealed that BATF was expressed at significantly higher levels in
NSCLC tissues compared with adjacent non-tumor tissues. In
addition, BATF was moderately expressed in the A549 NSCLC cell
line. Therefore, lentivirus-mediated BATF shRNA was constructed and
transfected into A549 cells; the results revealed that cell
proliferation was inhibited in BATF-silenced A549 cells. Compared
with that of the control group, the apoptotic rate of the
BATF-shRNA group was significantly higher. These results suggested
that BATF may regulate NSCLC cell proliferation and apoptosis in
vitro. A number of studies provide support that BATF may play
an important role in the development of different types of cancer,
including colon cancer, lymphoma and multiple myeloma (16–18).
There were certain limitations to the present study.
Only one lung cancer cell line was used; the results will be
verified in other lung cancer cell lines and normal cell lines in a
future study. In addition, a larger sample size is required to
identify the association between BATF expression and clinical
features of NSCLC in the future.
In conclusion, the results of the present study
demonstrated that BATF was upregulated in NSCLC tissues in TCGA
database. In the human NSCLC A549 cell line, BATF knockdown
inhibited cell proliferation and promoted apoptosis. Although
detailed mechanisms remain to be elucidated, these results
suggested that BATF may serve an oncogenic role in the development
of NSCLC. Therefore, BATF may be a potential target for the
treatment of NSCLC.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81800279) and the Natural
Science Foundation of Jiangsu Province (grant no. BK20180197).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in The Cancer Genome Atlas repository
(http://cancergenome.nih.gov).
Authors' contributions
HM, YF and LP designed the experiments. HM wrote the
manuscript. LP and BZ acquired the data, and wrote and revised the
manuscript. HH was responsible for the analysis and discussion of
the data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Approval was obtained to review the data from
Institutional Ethical Committee of The First Affiliated Hospital of
Soochow University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Paz-Ares L: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sangodkar J, Katz S, Melville H and Narla
G: Lung adenocarcinoma: Lessons in translation from bench to
bedside. Mt Sinai J Med. 77:597–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vansteenkiste J, Crinò L, Dooms C,
Douillard JY, Faivre-Finn C, Lim E, Rocco G, Senan S, Van Schil P,
Veronesi G, et al: 2nd ESMO consensus conference on lung cancer:
Early-stage non-small-cell lung cancer consensus on diagnosis,
treatment and follow-up. Ann Oncol. 25:1462–1474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chabowski M, Polański J,
Jankowska-Polanska B, Lomper K, Janczak D and Rosinczuk J: The
acceptance of illness, the intensity of pain and the quality of
life in patients with lung cancer. J Thorac Dis. 9:2952–2958. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huo Y, Li A and Wang Z: LncRNA AWPPH
participates in the metastasis of non-small cell lung cancer by
upregulating TGF-β1 expression. Oncol Lett. 18:4246–4252.
2019.PubMed/NCBI
|
|
7
|
NSCLC Meta-analysis Collaborative Group, :
Preoperative chemotherapy for non-small-cell lung cancer: A
systematic review and meta-analysis of individual participant data.
Lancet. 383:1561–1571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frank L, Christodoulou E and Kazerooni EA:
Radiation risk of lung cancer screening. Semin Respir Crit Care
Med. 34:738–747. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chalela R, Curull V, Enríquez C, Pijuan L,
Bellosillo B and Gea J: Lung adenocarcinoma: From molecular basis
to genome-guided therapy and immunotherapy. J Thorac Dis.
9:2142–2158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jabeen R, Goswami R, Awe O, Kulkarni A,
Nguyen ET, Attenasio A, Walsh D, Olson MR, Kim MH, Tepper RS, et
al: Th9 cell development requires a BATF-regulated transcriptional
network. J Clin Invest. 123:4641–4653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ota T, Suzuki Y, Nishikawa T, Otsuki T,
Sugiyama T, Irie R, Wakamatsu A, Hayashi K, Sato H, Nagai K, et al:
Complete sequencing and characterization of 21,243 full-length
human cDNAs. Nat Genet. 36:40–45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Murphy TL, Tussiwand R and Murphy KM:
Specificity through cooperation: BATF-IRF interactions control
immune-regulatory networks. Nat Rev Immunol. 13:499–509. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hernandez JM, Floyd DH, Weilbaecher KN,
Green PL and Boris-Lawrie K: Multiple facets of junD gene
expression are atypical among AP-1 family members. Oncogene.
27:4757–4767. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wagner EF and Eferl R: Fos/AP-1 proteins
in bone and the immune system. Immunol Rev. 208:126–140. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schleussner N, Merkel O, Costanza M, Liang
HC, Hummel F, Romagnani C, Durek P, Anagnostopoulos I, Hummel M,
Jöhrens K, et al: The AP-1-BATF and -BATF3 module is essential for
growth, survival and TH17/ILC3 skewing of anaplastic large cell
lymphoma. Leukemia. 32:1994–2007. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dai L, Cui X, Zhang X, Cheng L, Liu Y,
Yang Y, Fan P, Wang Q, Lin Y, Zhang J, et al: SARI inhibits
angiogenesis and tumour growth of human colon cancer through
directly targeting ceruloplasmin. Nat Commun. 7:119962016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gil M, Pak HK, Park SJ, Lee AN, Park YS,
Lee H, Lee H, Kim KE, Lee KJ, Yoon DH, et al: Engagement of CD99
reduces AP-1 activity by inducing BATF in the human multiple
myeloma cell line RPMI8226. Immune Netw. 15:260–267. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duruisseaux M and Esteller M: Lung cancer
epigenetics: From knowledge to applications. Semin Cancer Biol.
51:116–128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zarogoulidis P, Tsakiridis K, Karapantzou
C, Lampaki S, Kioumis I, Pitsiou G, Papaiwannou A,
Hohenforst-Schmidt W, Huang H, Kesisis G, et al: Use of proteins as
biomarkers and their role in carcinogenesis. J Cancer. 6:9–18.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marchetti A, Felicioni L, Malatesta S,
Grazia Sciarrotta M, Guetti L, Chella A, Viola P, Pullara C,
Mucilli F and Buttitta F: Clinical features and outcome of patients
with non-small-cell lung cancer harboring BRAF mutations. J Clin
Oncol. 29:3574–3579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He L, Zhang Y, Sun H, Jiang F, Yang H, Wu
H, Zhou T, Hu S, Kathera CS, Wang X, et al: Targeting DNA flap
endonuclease 1 to impede breast cancer progression. EBioMedicine.
14:32–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thomas C, Ji Y, Lodhi N, Kotova E, Pinnola
AD, Golovine K, Makhov P, Pechenkina K, Kolenko V and Tulin AV:
Non-NAD-like poly(ADP-ribose) polymerase-1 inhibitors effectively
eliminate cancer in vivo. EBioMedicine. 13:90–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dorsey MJ, Tae HJ, Sollenberger KG,
Mascarenhas NT, Johansen LM and Taparowsky EJ: B-ATF: A novel human
bZIP protein that associates with members of the AP-1 transcription
factor family. Oncogene. 11:2255–2265. 1995.PubMed/NCBI
|
|
27
|
Ise W, Kohyama M, Schraml BU, Zhang T,
Schwer B, Basu U, Alt FW, Tang J, Oltz EM, Murphy TL and Murphy KM:
The transcription factor BATF controls the global regulators of
class-switch recombination in both B cells and T cells. Nat
Immunol. 12:536–543. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schraml BU, Hildner K, Ise W, Lee WL,
Smith WA, Solomon B, Sahota G, Sim J, Mukasa R, Cemerski S, et al:
The AP-1 transcription factor Batf controls T(H)17 differentiation.
Nature. 460:405–409. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma QY, Huang DY, Zhang HJ, Wang S and Chen
XF: Upregulation of bacterial-specific Th1 and Th17 responses that
are enriched in CXCR5+CD4+ T cells in
non-small cell lung cancer. Int Immunopharmacol. 52:305–309. 2017.
View Article : Google Scholar : PubMed/NCBI
|