Introduction
Gastric cancer is a severe malignant disease that
develops in the lining of the stomach, and is the third leading
cause of cancer-associated mortality worldwide (1). In 2012, there were ~951,600 cases of
gastric cancer and 723,100 deaths (2). During the later stages of disease,
patients with gastric cancer usually display severe symptoms, such
as upper abdominal pain, weight loss and difficulty swallowing,
which results from metastasis to other organs, such as the lymph
nodes, liver and lungs (3). Previous
epidemiological studies demonstrated that gastric cancer
development has been associated with a number of causative factors,
including Helicobacter pylori infection, cigarette smoking,
dietary habits and genetic mutations, as well as pathogenic
conditions such as pernicious anemia, diabetes and chronic atrophic
gastritis (4,5). Among the causative factors, chronic
infections induced by the bacterium Helicobacter pylori have
been established as the most common cause of gastric cancer, and
are responsible for ~90% of noncardia gastric cancer worldwide
(6). Due to a lack of specific
symptoms during the early stages of disease, gastric cancer is
often diagnosed at an advanced stage, and this late diagnosis is
the primary reason for the poor prognosis observed in the majority
of patients (7). There is an urgent
requirement for the development of new diagnostic methods and novel
therapeutics to decrease gastric cancer-associated mortality and
improve the clinical outcomes of patients.
Currently, the primary methods used to treat gastric
cancer are surgery, chemotherapy and radiotherapy (8–10). The
only known curative therapies for gastric cancer are surgical
procedures such as endoscopic mucosal resection and endoscopic
submucosal dissection (11);
however, these methods are only suitable for patients with
early-stage gastric cancer. Chemotherapy, radiotherapy and newly
developed targeted therapies have primarily been used to treat
patients with later stage disease or those where the cancer has
metastasized to other organs (10,12,13). In
addition, chemotherapy has been used to shrink gastric tumors prior
to surgery, or to eradicate any remaining cancerous cells following
surgery (10). A number of different
chemotherapeutic agents have been used in the treatment of gastric
cancer, including fluorouracil, carmustine, doxorubicin, mitomycin
C, taxotere and cisplatin (DDP) (10,14). DDP
is one of the chemotherapy agents most widely used to treat number
of different types of cancer, but its use is limited by the
occurrence of multiple side effects and the frequent development of
resistance (15).
DDP resistance has been associated with changes in
its cellular uptake and efflux, increased DNA repair efficiency,
decreased rates of cell apoptosis and increased cellular
detoxification activity (15,16). A
number of reports have provided new insights into the molecular
processes that mediate DDP resistance in gastric cancer cells;
microRNA (miR)-21 was demonstrated to promote DDP resistance in
gastric cancer cells by suppressing the expression of the
phosphatase and tension homolog deleted on chromosome 10 gene and
activating the protein kinase B (AKT) signaling pathway (17). Furthermore, AKT signaling cascades,
together with hypoxia-inducible factor 1α, may enhance the
expression of the survivin gene, which contributes to the
development of DDP resistance in gastric cancer cells (18). Other molecular factors that may
contribute to DDP resistance in these cells include miR-1271
(19), X-ray repair cross
complementing group 1, thioredoxin-like protein 1 (20) and numerous other functional proteins
associated with cell proliferation and apoptosis. However, the
mechanisms of DDP resistance in gastric cancer cells are yet to be
fully elucidated.
Ras-related protein Rap-2A (RAP2A), is a member of
the small GTPase protein superfamily and a target of the p53
transcription factor, which is associated with multiple cellular
processes including cell proliferation, adhesion and migration
(21,22). Furthermore, RAP2A was demonstrated to
promote cancer cell migration, invasion and metastasis by
activating the AKT signaling pathway (21,22).
However, the role of RAP2A in the development of cellular
resistance to chemotherapy remains largely unknown. In the present
study, the potential roles of RAP2A in regulating the induced
resistance of gastric cancer cells to DDP were investigated, with
the aim of gaining new insights into the molecular mechanisms
underlying chemotherapy resistance.
Materials and methods
Cell culture and reagents
The human gastric cancer cell line MGC803
(BNCC100665) was purchased from the BeNa Culture Collection and
cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS; cat. no. 26140079;
Gibco; Thermo Fisher Scientific, Inc.), at 37°C in a humidified
atmosphere (5% CO2). MGC803/DDP cells with induced
resistance to cisplatin (DDP; cat. no. 15663-27-1; Sigma-Aldrich;
Merck KGaA) were obtained by exposing MGC803 cells to a
concentration gradient of DDP as previously described (23).
RAP2A knockdown
In order to suppress RAP2A expression, cultured
gastric cancer cells were transfected with RAP2A small interfering
(si)RNA. The siRNAs targeting the RAP2A gene were designed using
the Whitehead Institute Web Server (http://jura.wi.mit.edu/bioc/siRNAext) and synthesized
by Sangon Biotech Co., Ltd. An additional non-targeting scrambled
siRNA served as a negative control (NC) was also designed and
synthesized by Sangon Biotech Co., Ltd. The sequences of the RAP2A
and NC siRNAs were as follows: RAP2A siRNA #1,
5′-AUACUUCUCUCUCACUUUCCA-3′; RAP2A siRNA #2,
5′-ACUCUUAGCGGAAGUUUCCAU-3′; RAP2A siRNA #3,
5′-UCGUAUUUCUCGAUGAAGGUG-3′; NC siRNA, 5′-UUCGUCUGUACUCCACAUATT-3′.
Gastric cancer cells were transfected with the siRNAs using
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. At 2–7 days post-transfection, the levels of RAP2A
protein expression were determined using western blot analyses, and
further cellular assays were subsequently conducted. All
experiments were independently repeated ≥3 times.
Cell Counting Kit-8 (CCK-8) assay and
determination of the half maximal inhibitory concentration
(IC50) of DDP
Cell viability was analyzed using the CCK-8 assay
(Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. For the determination of the
IC50 value of DDP, MGC803 and MGC803/DDP cells (3,000
cells/well) were seeded into 96-well plates and treated with a DDP
concentration gradient of 0, 0.5, 1, 2, 4, 6, 8 and 10 µg/ml for 24
h. After cultivation in RPMI-1640 medium (Thermo Fisher Scientific,
Inc.) with 10% FBS (cat. no. 26140079; Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified incubator with 5%
CO2 for 24 h, CCK-8 solution was added to each well and
subsequently incubated at the same culture conditions for 3 h. The
absorbance of each well at 450 nm (OD450) was measured
with a microplate reader, and SPSS software (version 18.0; SPSS
Inc.) was used to calculate the IC50 value. In order to
assess the influence of DDP on cell viability, MGC803 and
MGC803/DDP cells were treated with 2 µg/ml DDP for 24, 48 and 72 h,
respectively, after which their viability was assessed using the
aforementioned CCK-8 method.
Cell migration and invasion
The migration and invasion capabilities of gastric
cancer cells treated with DDP and/or RAP2A siRNAs were determined
using the Transwell system (Corning Inc.) as previously described
(24), but with minor modifications.
For the migration assay, the treated MGC803 cells were starved
overnight in serum free medium then seeded (200 µl;
1×105 cells/ml) in the upper chamber of the Transwell
system, and 500 µl RPMI-1640 medium (Thermo Fisher Scientific,
Inc.) with 15% FBS (cat. no. 26140079; Gibco; Thermo Fisher
Scientific, Inc.) was plated in the lower chamber. After
cultivation at 37°C with 5% CO2 for 24 h, the cells were then
allowed to vertically migrate through the membrane and into the
lower chamber. The migrated cells in the lower chamber were fixed
with 4% paraformaldehyde (cat. no. P1110; Beijing Solarbio Science
and Technology Co., Ltd.) at room temperature for 10 min and
stained with 0.5% crystal violet (cat. no. C-6158; Sigma-Aldrich;
Merck KGaA) at room temperature for 5 min. The results were
carefully counted under an inverted light microscope (Olympus IX
73; Olympus Corporation) at ×100 magnification. For the analysis of
invasion capability, the treated MGC803 cells in serum-free medium
were seeded (1×105 cells/ml) into the upper chambers of
a Corning Transwell system coated with Matrigel. Invaded cells were
also stained with crystal violet (5%) at room temperature for 5
min. The remaining steps were the same as the migration assay. The
numbers of cells in ≤8 randomly selected visual fields were counted
and each experiment was performed in triplicate.
Cell apoptosis assay
The apoptotic rates of gastric cancer cells were
determined via flow cytometry using the Dead Cell Apoptosis kit
(Thermo Fisher Scientific, Inc.), containing Annexin V FITC and
propidium iodide (PI) according to the manufacturer's protocol.
Following treatment with DDP, gastric cancer cells were washed
three times in PBS solution and resuspended in Annexin-binding
buffer, and subsequently incubated with FITC Annexin V and PI
solution for 14 min at room temperature. The cells were incubated
in 300 µl Annexin-binding buffer, and the numbers of apoptotic
cells were determined by a FACS Calibur flow cytometry (BD
Biosciences). The apoptosis rate was quantitatively analyzed using
BD CellQuest software version 3.3 (BD Biosciences). The percentage
of apoptotic cells was determined for at ≤3 independent
experiments.
DNA damage assay
As previously described (25), the degree of DNA damage in the
gastric cancer cells was determined by staining with Hoechst 33342
and DAPI (both Thermo Fisher Scientific, Inc.). Briefly, the
treated cells were fixed with 4% paraformaldehyde (cat. no. P1110;
Beijing Solarbio Science and Technology Co., Ltd.) at room
temperature for 20 min, and then stained with Hoechst 33342 Working
Solution at room temperature in the dark for 10 min. After washing
with PBS, the fixed cells were stained with DAPI at room
temperature in the dark for 5 min. The level of DNA damage was
assessed using a laser confocal microscope (A1; Nikon Corporation)
at ×200 magnification.
Western blot analysis
Cultured gastric cancer cells were collected and the
total proteins were extracted using cell lysis buffer (Beyotime
Institute of Biotechnology) according to the manufacturer's
protocol. The protein concentration of each extract was determined
using a Modified Bradford Protein Assay kit (Sangon Biotech Co.,
Ltd.). Aliquots of total protein (25 µg per lane) were boiled at
100°C in loading buffer (cat. no. P0015; Beyotime Institute of
Biotechnology), and then separated via 10% SDS-PAGE. The separated
proteins were transferred onto a PVDF membrane (EMD Millipore) that
was then blocked with a 5% lipid-free milk solution for 2 h at room
temperature with gentle rotation. The membrane was incubated with
the appropriate primary antibodies diluted in TBST for 1–2 h at
room temperature, after which it was washed three times with TBST,
and then incubated with goat anti-rabbit horseradish peroxidase
(HRP)-conjugated secondary antibody (dilution, 1:3,000; cat. no.
ab6721; Abcam) and goat anti-mouse HRP-conjugated secondary
antibody (dilution, 1:3,000; cat. no. ab205719; Abcam) at room
temperature for 1 h. The membranes were developed using Pierce™ ECL
Plus Western Blotting Substrate (Pierce; Thermo Fisher Scientific,
Inc.) and a total of three independent experiments were performed
with GAPDH as the internal standard. The primary antibodies used
were as follows: Anti-RAP2A (dilution, 1:1,000; cat. no. ab49685;
Abcam), anti-multidrug resistance-associated protein (MRP;
dilution, 1:1,000; cat. no. PA5-18315; Thermo Fisher Scientific,
Inc.), anti- cleaved caspase-3 (dilution, 1:1,000; cat. no. ab2302;
Abcam), and anti-GAPDH (dilution, 1:4,000; cat. no. ab9483;
Abcam).
Statistical analysis
All statistical analyses were performed using SPSS
Statistics for Windows (version 18.0; SPSS Inc.). Quantitative data
are presented as the mean ± standard deviation. Differences between
groups were analyzed using the unpaired Student's t-test or
analysis of variance, as appropriate. P<0.05 was considered to
indicate a statistically significant result.
Results
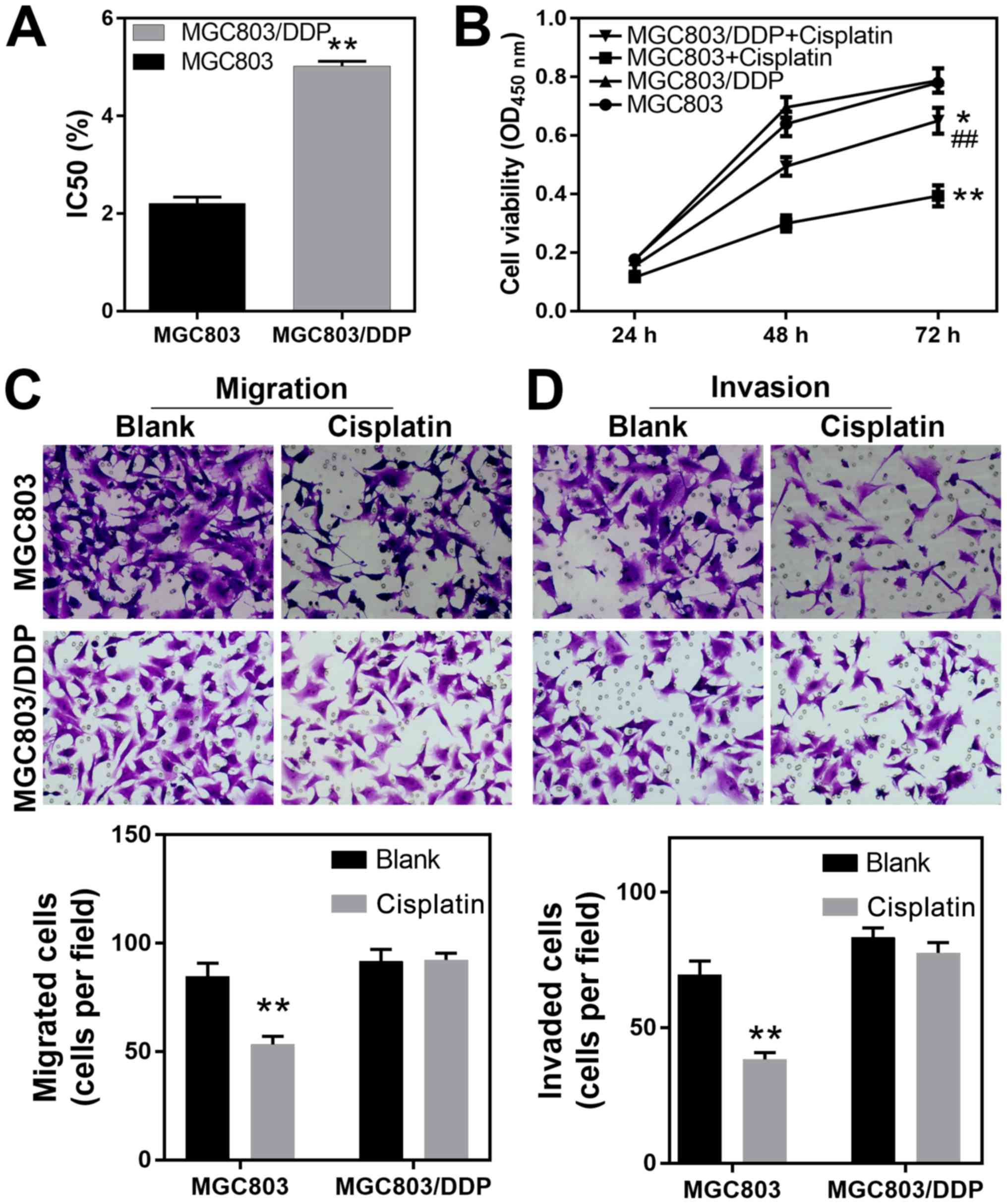
DDP-resistant MGC803/DDP cells exhibit
increased viability and migrational capacity
The present study used the MGC803 and corresponding
DDP-resistant MGC803/DDP cell lines to investigate the molecular
processes that mediate DDP resistance in gastric cancer cells. Both
cell lines were treated with a DDP concentration gradient for 24 h,
after which viability was assessed using the CCK-8 method, and the
DDP IC50 values were determined. The IC50
values for DDP when treating MGC803 and MGC803/DDP cells were
2.21±0.13 and 5.02±0.10 µg/ml, respectively, reflecting a higher
level of resistance to DDP in the MGC803/DDP cells (P<0.01;
Fig. 1A). Furthermore, following
treatment with 1.8 µg/ml (0.8-fold of IC50) DDP for 24,
48 and 72 h according to the cell growth rate, the MGC803/DDP cells
were significantly more viable than the MGC803 cells, further
reflecting their increased resistance to DDP (Fig. 1B). In order to further elucidate the
cellular processes associated with DDP tolerance, Transwell
matrigel assays were performed to investigate the migration and
invasion capabilities of the two cell lines. The results revealed
that the migration capability of the MGC803 cells was significantly
decreased by DDP (P<0.01) while the MGC803/DDP cells exhibited
no significant change in their ability to migrate following
treatment with DDP (Fig. 1C). The
matrigel assays demonstrated that the invasive capability of
MGC803/DDP cells was relatively insensitive to DDP when compared
with that of the MGC803 cells (P<0.01; Fig. 1D). These results demonstrated that
elevated levels of cell viability, and also elevated migration and
invasion capabilities, were associated with DDP resistance in
gastric cancer cells.
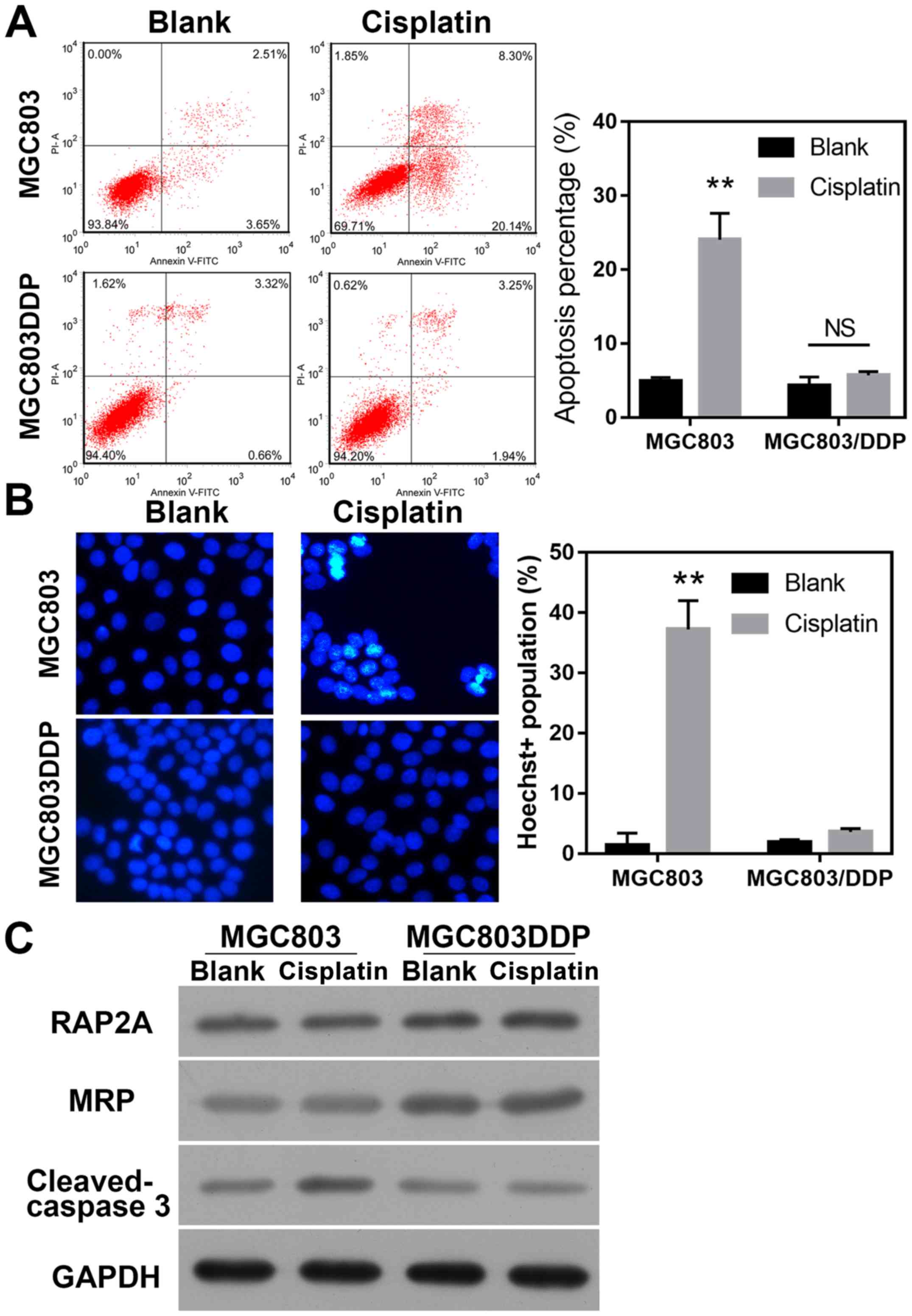
DDP-resistant MGC803/DDP cells exhibit
suppressed levels of apoptosis and DNA damage
Apoptotic retardation is a common feature of
chemo-resistant cancer cells. In order to test the involvement of
apoptotic regulation in the DDP-induced resistance of gastric
cancer cells, MGC803 and MGC803/DDP cells were treated with DDP,
and their respective apoptotic rates were determined. The results
revealed that treatment with DDP significantly increased the
apoptotic rate of MGC803 cells, but not MGC803/DDP cells
(P<0.01; Fig. 2A). Hoechst
staining was then used to evaluate DNA damage, and it was observed
that the degree of DNA damage caused by DDP treatment in MGC803/DDP
cells was less than that in MGC803 cells (P<0.01; Fig. 2B). Furthermore, the levels of MRP in
the MGC803/DDP cells were higher than those in the MGC803 cells,
and no DDP-induced upregulation of cleaved-caspase-3 expression was
observed in MGC803/DDP cells; this further confirmed the roles of
apoptosis regulation in the induction of DDP resistance.
Furthermore, it was also observed that the levels of RAP2A protein
in MGC803/DDP cells were higher than those in the MGC803 cells,
suggesting an association between RAP2A expression and DDP
resistance in gastric cancer cells (Fig.
2C).
RAP2A silencing attenuates DDP
resistance in MGC8033/DDP cells
In order to investigate the potential role of the
RAP2A gene in DDP resistance, siRNA transfection was used to knock
down the expression of RAP2A in DDP-resistant MGC8033/DDP cells.
siRNA #1, #2 and #3 were used to knock down RAP2A, and the results
revealed that siRNA #3 effectively targeted and blocked the
expression of RAP2A (Fig. 3A).
Therefore, siRNA #3 was used for subsequent experimentation. The
suppression of RAP2A protein expression in gastric cancer cells was
confirmed via western blotting (Fig. 3B
and C). The expression levels of MRP were notably decreased by
RAP2A siRNA in MGC8033/DDP cells, when compared with those in
MGC8033/DDP cells transfected with the NC siRNA (Fig. 3B). By contrast, the expression level
of cleaved-caspase-3 in MGC8033/DDP cells was significantly
increased by transfection with RAP2A siRNA compared with DPP+NC
(P<0.05; Fig. 3B and C). The
molecular phenotypes induced by RAP2A siRNA also suggested that
alterations in cellular function had occurred. It was observed that
the viability of DDP-treated MGC8033/DDP cells was decreased by
RAP2A knockdown, when compared with the viability of MGC8033/DDP
cells treated with the NC siRNA (Fig.
3D). Furthermore, the migrational capability of RAP2A knockdown
MGC8033/DDP cells was suppressed following DDP treatment, when
compared with that of cells transfected with the NC siRNA (Fig. 3E). Similarly, the invasion capability
of DDP-treated MGC8033/DDP cells was also decreased by RAP2A siRNA
when compared with that of cells transfected with the NC siRNA
(P<0.05; Fig. 3E). These results
directly indicate that RAP2A expression in gastric cancer cells
promotes the induction of DDP resistance, which may be mediated by
the enhanced migration and invasion capabilities of cancerous
cells.
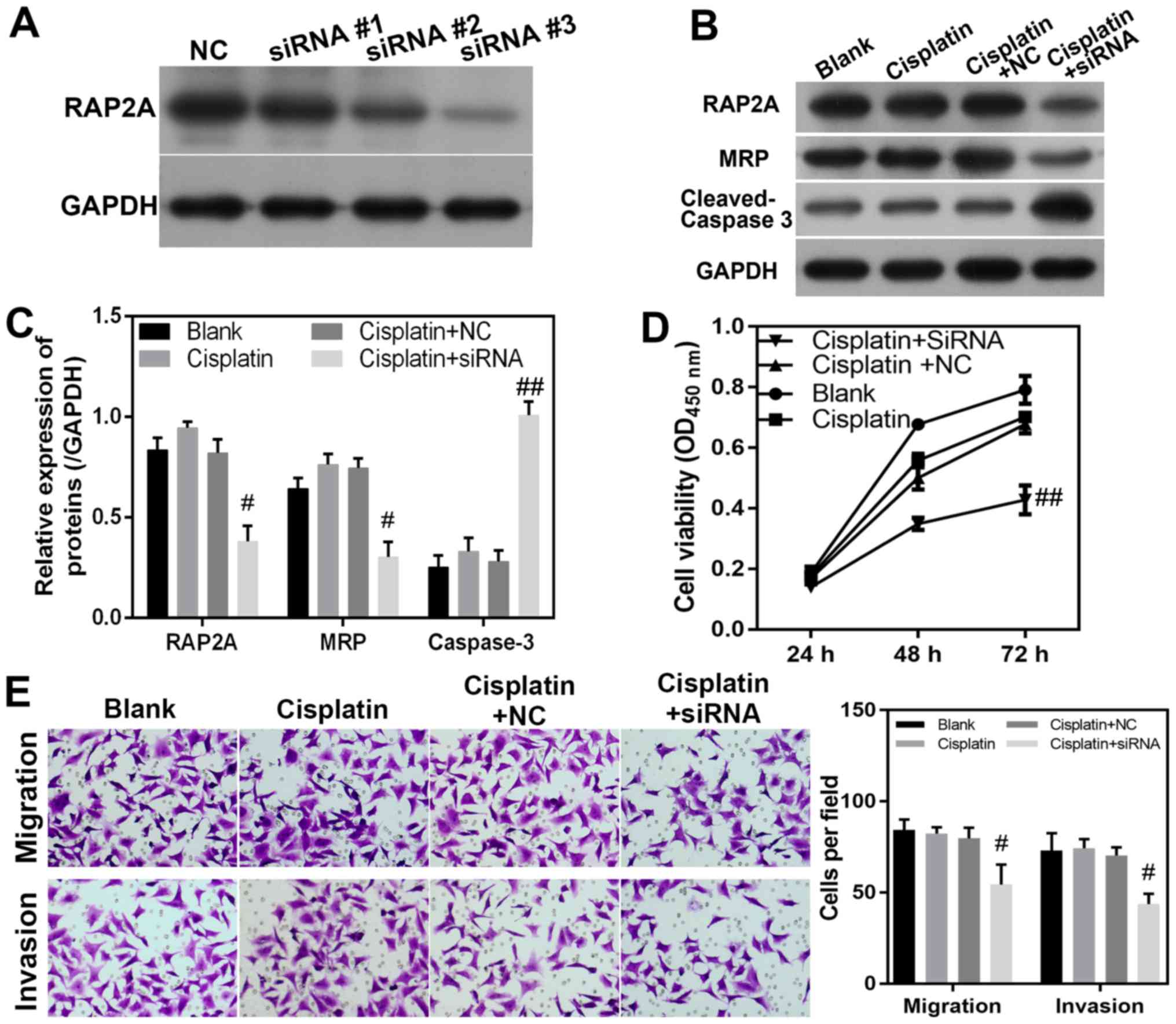 | Figure 3.RAP2A promotes migration, invasion
and DDP resistance in MGC803/DDP cells. (A) RAP2A expression was
knocked down by siRNA. (B and C) Levels of RAP2A, MRP and
cleaved-caspase-3 in RAP2A-knockdown MGC803/DDP cells during DDP
treatment. Relative levels of protein expression were determined
via western blotting, with GAPDH serving as an internal standard.
(D) Effect of RAP2A siRNA knockdown on MGC803/DDP cell viability
during DDP treatment. Cell viability was measured using the Cell
Counting Kit-8 assay. (E) Migration and invasion of MGC803/DDP
cells with RAP2A siRNA knockdown during DDP treatment for 24 h
(×100 magnification). Cell migration and invasion were analyzed
using the Transwell system. Statistical analysis of MGC803/DDP cell
migration and invasion. #P<0.05,
##P<0.01 vs. cisplatin+NC group. RAP2A, Ras-related
protein Rap-2a; DDP, cisplatin; siRNA, small interfering RNA; MRP,
multidrug resistance-associated protein; NC, negative control; OD,
optical density. |
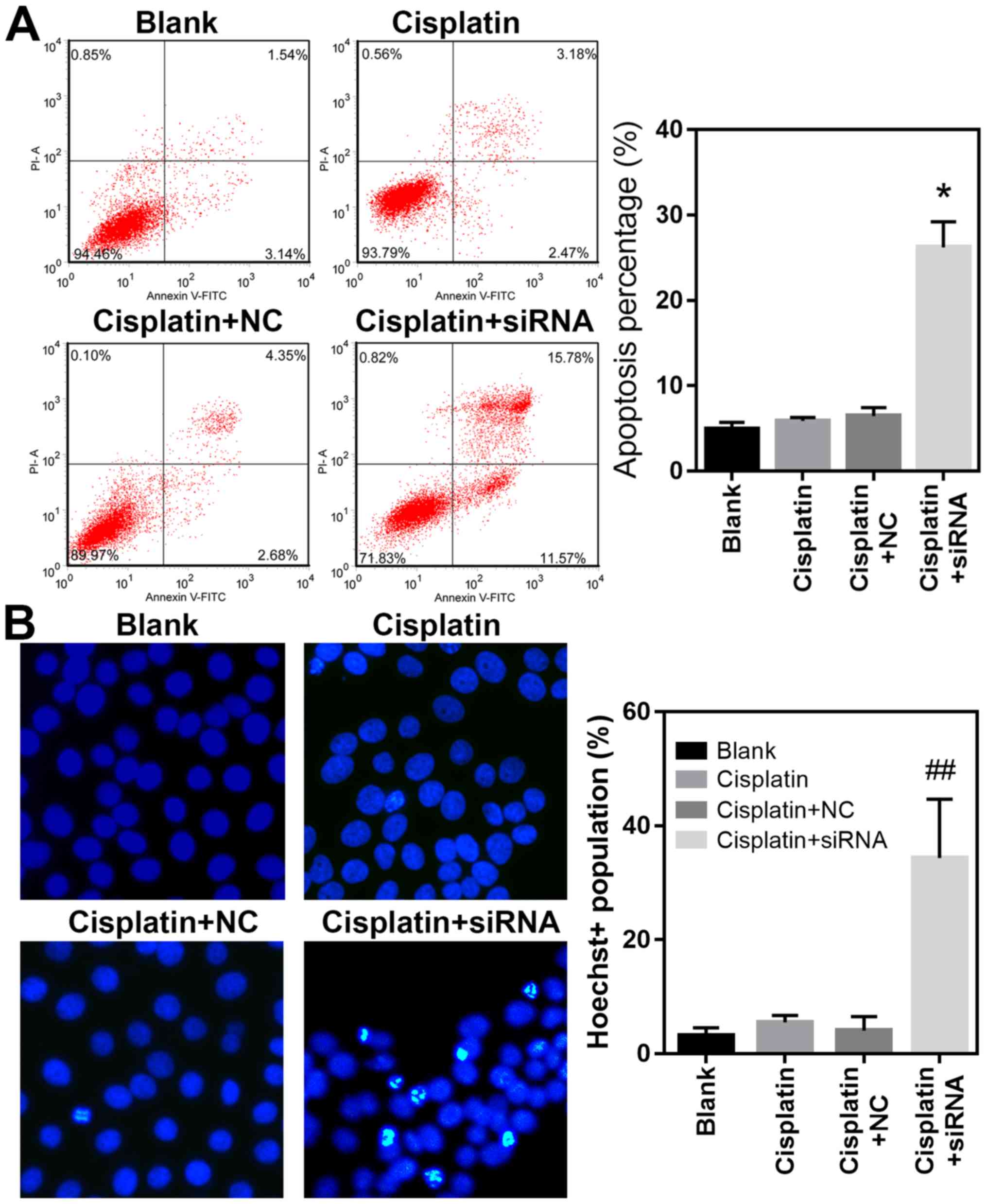
RAP2A knockdown promotes MGC803/DDP
cell apoptosis during DDP treatment
In order to gain additional information regarding
the cellular mechanisms underlying RAP2A-regulated DDP resistance
in gastric cancer cells, the apoptotic rates of RAP2A knockdown
MGC803/DDP cells were further analyzed using flow cytometry. The
results revealed that the percentage of apoptotic MGC803/DDP cells
during DDP treatment was significantly elevated following RAP2A
siRNA transfection, when compared with cells transfected with the
NC siRNA (P<0.05; Fig. 4A).
Furthermore, Hoechst 33342 staining revealed that the degree of DNA
damage in MGC803/DDP cells under DDP treatment was higher following
RAP2A knockdown, which was in contrast to the results of the NC
siRNA group (P<0.01; Fig. 4B).
These results suggested that RAP2A repressed DDP-induced gastric
cancer cell apoptosis and DNA damage, which were both closely
associated with the development of resistance to various
chemotherapeutic agents, including DDP. Collectively, the present
study suggested that RAP2A promoted DDP resistance in gastric
cancer cells, potentially by modulating cellular proliferation,
migration and invasion, as well as apoptosis and DNA damage during
DDP treatment.
Discussion
Chemotherapy is a major type of cancer treatment
that uses a single drug or the combination of multiple agents. It
has been widely applied in clinical oncology as a means of curative
therapy, or to decrease the symptoms and prolong the survival time
of cancer patients (26,27). Chemotherapy has also been
administered in combination with other cancer treatments, such as
surgery and radiation, or as neoadjuvant or adjuvant therapy given
prior to or following other types of cancer treatment (28). However, resistance to chemotherapy is
a primary cause of treatment failure, an issue that has become the
focus of research in recent decades (29). Previous studies have suggested that
specific pump proteins on the surface of cancerous cells (such as
p-glycoprotein, which effectively transports chemotherapeutic
agents back out of the cell) are critically involved in the
resistance to various chemotherapeutic drugs, including DDP
(30,31). Further cellular and molecular events,
including gene amplification, defective cell apoptosis and the
promotion of DNA damage repair, also mediate DDP resistance in
cancer cells (32–34). Despite recent progress, the
underlying molecular mechanisms responsible for the high
adaptability of cancer cells and the development of cancer cell
resistance to chemotherapeutic agents have only been investigated
to a limited extent.
In the present study, DDP resistance in MGC803/DDP
cells was confirmed by verifying their increased viability,
migration and invasion capabilities, and decreased levels of
apoptosis and DNA damage while under treatment with DDP. Subsequent
western blot analyses demonstrated increased levels of RAP2A
expression in DDP-resistant cells, suggesting that RAP2A may be a
GTPase protein able to regulate DDP resistance in gastric cancer
cells. Small GTPases, also known as G-proteins, constitute a large
group of hydrolase enzymes that hydrolyze GTP to form GDP, and are
associated with a number of biological processes, such as cell
growth, proliferation, differentiation, movement, lipid vesicle
transport and tumorigenesis (35).
Notably, numerous members of the G-protein family are reportedly
involved in cancer chemotherapy resistance. For example, Rho GTPase
enhances the rigidity of ovarian cancer cells and regulates the
actin remodeling mechanism to promote resistance to DDP (36). Furthermore, downregulation of RhoB
GTPase expression levels in laryngeal carcinoma cells was
demonstrated to promote the development of acquired DDP resistance
(36). Finally, Rho GDP dissociation
inhibitor 2, a regulator of the Rho family of GTPases, contributes
to DDP resistance in gastric cancer cells by enhancing the
expression of the Bcl-2 gene (37).
GTPases and their activating proteins, such as the 76 kDa
Ral-binding GTPase activating protein, are involved in the
development of resistance to multiple chemotherapeutic drugs
(including vinorelbine, doxorubicin and DDP) via their ability to
transport these drugs back out of cancerous cells (38–40).
Furthermore, autophagy and other cellular processes regulated by
GTPase members such as the Rac3 GTPase (41,42),
were demonstrated to mediate the development of cancer cell
resistance to antitumor drugs such as DDP (43,44). As
a GTPase family member, the significant increase of RAP2A
expression levels in MGC803/DDP cells suggested that RAP2
contributes to the resistance of gastric cancer cells to DDP
treatment.
To test this hypothesis, the present study knocked
down RAP2A in DDP-resistant MGC803/DDP cells via transfection with
specific siRNAs. In addition to the subsequent decrease in RAP2A
expression level, the viability and invasion capabilities of the
MGC803/DDP cells were also suppressed by RAP2A siRNA under DDP
treatment. The inhibition of DDP resistance in RAP2A-knockdown
MGC803/DDP cells was also confirmed by increases in cell apoptosis
and DNA damage. The cellular analysis of DDP-resistant cells
demonstrated that RAP2A may be a positive regulator of DDP
resistance in gastric cancer cells. A previous study demonstrated
that p53 was able promote RAP2A expression in cancer cells, and
that this further activated the matrix metalloproteinase (MMP)
enzymes MMP2 and MMP9 via phosphorylation of AKT (22). In addition, the RAP2A protein can be
specifically ubiquitinated by E3 ubiquitin ligase neuronal
precursor cell expressed and developmentally downregulated protein
4-1, and subsequently contributes to the increased migration and
invasion capabilities of glioma cells (45). These mechanisms that enable RAP2A to
regulate tumor development may also mediate gastric cancer
progression and the development of DDP resistance, and therefore
deserve further investigation. Additional investigation into the
roles of RAP2A expression in the resistance to other
chemotherapeutic drugs may also broaden the current understanding
of cancer chemotherapy resistance.
In summary, the present study demonstrated that
RAP2A gene expression was increased in DDP-resistant gastric cancer
cells. This enhanced level of RAP2A expression promoted gastric
cancer cell resistance to DDP by regulating cell viability,
migration, invasion, apoptosis and DNA damage. These findings
provide novel insights into the molecular mechanisms underlying DDP
resistance in gastric cancer, as well as the pathogenic roles
played by GTPase proteins during the development of chemotherapy
resistance. However, the present study was not performed in
vivo, and further studies are required in order to demonstrate
the effects of RAB2P expression on drug resistance in gastric
cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Program of Huzhou, Applied Research for Public Welfare
(grant no. 2017GYB40) and Zhejiang Medical and Health Science and
Technology Plan Project (grant no. 2019ZD050).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and JM designed the experiments. JZ performed the
experiments and drafted the manuscript. YWe and HS were involved in
flow cytometry experiments. JZ, YWa, LY and GC collected the data
and performed data analysis. JM revised the manuscript. All authors
approved the final version before submission.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stewart BW and Wild CP: World Cancer
Report 2014IARC; Lyon, France: pp. 839–851. 2014
|
|
2
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kamran SC, Hong TS and Wo JY: Advances in
the management of gastric and gastroesophageal cancers. Curr Oncol
Rep. 18:132016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee YY and Derakhshan MH: Environmental
and lifestyle risk factors of gastric cancer. Arch Iran Med.
16:358–365. 2013.PubMed/NCBI
|
|
5
|
Wang K, Yuen ST, Xu J, Lee SP, Yan HH, Shi
ST, Siu HC, Deng S, Chu KM, Law S, et al: Whole-genome sequencing
and comprehensive molecular profiling identify new driver mutations
in gastric cancer. Nat Genet. 46:573–582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Venerito M, Vasapolli R, Rokkas T,
Delchier JC and Malfertheiner P: Helicobacter pylori, gastric
cancer and other gastrointestinal malignancies. Helicobacter. 22
(Suppl 1):2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoon H and Kim N: Diagnosis and management
of high risk group for gastric cancer. Gut Liver. 9:5–17. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aurello P, Sagnotta A, Terrenato I,
Berardi G, Nigri G, D'Angelo F and Ramacciato G: Oncologic value of
laparoscopy-assisted distal gastrectomy for advanced gastric
cancer: A systematic review and meta-analysis. J Minim Access Surg.
12:199–208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen K, Xu XW, Zhang RC, Pan Y, Wu D and
Mou YP: Systematic review and meta-analysis of laparoscopy-assisted
and open total gastrectomy for gastric cancer. World J
Gastroenterol. 19:5365–5376. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Janunger KG, Hafström L and Glimelius B:
Chemotherapy in gastric cancer: A review and updated meta-analysis.
Eur J Surg. 168:597–608. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Othman MO and Wallace MB: Endoscopic
mucosal resection (EMR) and endoscopic submucosal dissection (ESD)
in 2011, a Western perspective. Clin Res Hepatol Gastroenterol.
35:288–294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liang L, Fang JY and Xu J: Gastric cancer
and gene copy number variation: Emerging cancer drivers for
targeted therapy. Oncogene. 35:1475–1482. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Valentini V and Cellini F: Radiotherapy in
gastric cancer: A systematic review of literature and new
perspectives. Expert Rev Anticancer Ther. 7:1379–1393. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wagner AD, Unverzagt S, Grothe W, Kleber
G, Grothey A, Haerting J and Fleig WE: Chemotherapy for advanced
gastric cancer. Cochrane Database Syst Rev. CD0040642010.PubMed/NCBI
|
|
15
|
Stordal B and Davey M: Understanding
cisplatin resistance using cellular models. IUBMB Life. 59:696–699.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jacobsen C and Honecker F: Cisplatin
resistance in germ cell tumours: Models and mechanisms. Andrology.
3:111–121. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang SM, Huang C, Li XF, Yu MZ, He Y and
Li J: miR-21 confers cisplatin resistance in gastric cancer cells
by regulating PTEN. Toxicology. 306:162–168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun XP, Dong X, Lin L, Jiang X, Wei Z,
Zhai B, Sun B, Zhang Q, Wang X, Jiang H, et al: Up-regulation of
survivin by AKT and hypoxia-inducible factor 1α contributes to
cisplatin resistance in gastric cancer. FEBS J. 281:115–128. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang M, Shan X, Zhou X, Qiu T, Zhu W, Ding
Y, Shu Y and Liu P: miR-1271 regulates cisplatin resistance of
human gastric cancer cell lines by targeting IGF1R, IRS1, mTOR, and
BCL2. Anticancer Agents Med Chem. 14:884–891. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu W, Wang S, Chen Q, Zhang Y, Ni P, Wu X,
Zhang J, Qiang F, Li A, Røe OD, et al: TXNL1-XRCC1 pathway
regulates cisplatin-induced cell death and contributes to
resistance in human gastric cancer. Cell Death Dis. 5:e10552014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gloerich M, ten Klooster JP, Vliem MJ,
Koorman T, Zwartkruis FJ, Clevers H and Bos JL: Rap2A links
intestinal cell polarity to brush border formation. Nat Cell Biol.
14:793–801. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu JX, Zhang DG, Zheng JN and Pei DS:
Rap2a is a novel target gene of p53 and regulates cancer cell
migration and invasion. Cell Signal. 27:1198–1207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang D, Duan H, Huang H, Tong X, Han Y,
Ru G, Qu L, Shou C and Zhao Z: Cisplatin resistance in gastric
cancer cells is associated with HER2 upregulation-induced
epithelial-mesenchymal transition. Sci Rep. 6:205022016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kramer N, Walzl A, Unger C, Rosner M,
Krupitza G, Hengstschläger M and Dolznig H: In vitro cell migration
and invasion assays. Mutat Res. 752:10–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Erba E, Ubezio P, Broggini M, Ponti M and
D'Incalci M: DNA damage, cytotoxic effect and cell-cycle
perturbation of Hoechst 33342 on L1210 cells in vitro. Cytometry.
9:1–6. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Redondo-Blanco S, Fernández J,
Gutiérrez-Del-Río I, Villar CJ and Lombó F: New insights toward
colorectal cancer chemotherapy using natural bioactive compounds.
Front Pharmacol. 8:1092017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zitvogel L, Apetoh L, Ghiringhelli F and
Kroemer G: Immunological aspects of cancer chemotherapy. Nat Rev
Immunol. 8:59–73. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meeks JJ, Bellmunt J, Bochner BH, Clarke
NW, Daneshmand S, Galsky MD, Hahn NM, Lerner SP, Mason M, Powles T,
et al: A systematic review of neoadjuvant and adjuvant chemotherapy
for muscle-invasive bladder cancer. Eur Urol. 62:523–533. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sui X, Chen R, Wang Z, Huang Z, Kong N,
Zhang M, Han W, Lou F, Yang J, Zhang Q, et al: Autophagy and
chemotherapy resistance: A promising therapeutic target for cancer
treatment. Cell Death Dis. 4:e8382013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Terek RM, Schwartz GK, Devaney K, Glantz
L, Mak S, Healey JH and Albino AP: Chemotherapy and P-glycoprotein
expression in chondrosarcoma. J Orthop Res. 16:585–590. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gibalová L, Sereš M, Rusnák A, Ditte P,
Labudová M, Uhrík B, Pastorek J, Sedlák J, Breier A and Sulová Z:
P-glycoprotein depresses cisplatin sensitivity in L1210 cells by
inhibiting cisplatin-induced caspase-3 activation. Toxicol In
Vitro. 26:435–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu LZ, Zhou XD, Qian G, Shi X, Fang J and
Jiang BH: AKT1 amplification regulates cisplatin resistance in
human lung cancer cells through the mammalian target of
rapamycin/p70S6K1 pathway. Cancer Res. 67:6325–6332. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bauer JA, Kumar B, Cordell KG, Prince ME,
Tran HH, Wolf GT, Chepeha DB, Teknos TN, Wang S, Eisbruch A, et al:
Targeting apoptosis to overcome cisplatin resistance: A
translational study in head and neck cancer. Int J Radiat Oncol
Biol Phys. 69 (2 Suppl):S106–S108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu WK, Wang Z, Fong CC, Liu D, Yip TC, Au
SK, Zhu G and Yang M: Chemoresistant lung cancer stem cells display
high DNA repair capability to remove cisplatin-induced DNA damage.
Br J Pharmacol. 174:302–313. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Okada T, Sinha S, Esposito I, Schiavon G,
López-Lago MA, Su W, Pratilas CA, Abele C, Hernandez JM, Ohara M,
et al: The Rho GTPase Rnd1 suppresses mammary tumorigenesis and EMT
by restraining Ras-MAPK signalling. Nat Cell Biol. 17:81–94. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sharma S, Santiskulvong C, Rao J,
Gimzewski JK and Dorigo O: The role of Rho GTPase in cell stiffness
and cisplatin resistance in ovarian cancer cells. Integr Biol
(Camb). 6:611–617. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cho HJ, Baek KE, Park SM, Kim IK, Nam IK,
Choi YL, Park SH, Im MJ, Choi J, Ryu J, et al: RhoGDI2 confers
gastric cancer cells resistance against cisplatin-induced apoptosis
by upregulation of Bcl-2 expression. Cancer Lett. 311:48–56. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Awasthi S, Sharma R, Yang Y, Singhal SS,
Pikula S, Bandorowicz-Pikula J, Singh SV, Zimniak P and Awasthi YC:
Transport functions and physiological significance of 76 kDa
Ral-binding GTPase activating protein (RLIP76). Acta Biochim Pol.
49:855–867. 2002.PubMed/NCBI
|
|
39
|
Stuckler D, Singhal J, Singhal SS, Yadav
S, Awasthi YC and Awasthi S: RLIP76 transports vinorelbine and
mediates drug resistance in non-small cell lung cancer. Cancer Res.
65:991–998. 2005.PubMed/NCBI
|
|
40
|
Vatsyayan R, Chaudhary P, Lelsani PC,
Singhal P, Awasthi YC, Awasthi S and Singhal SS: Role of RLIP76 in
doxorubicin resistance in lung cancer. Int J Oncol. 34:1505–1511.
2009.PubMed/NCBI
|
|
41
|
Wu R, Murali R, Kabe Y, French SW, Chiang
YM, Liu S, Sher L, Wang CC, Louie S and Tsukamoto H: Baicalein
targets GTPase-mediated autophagy to eliminate liver tumor
initiating stem cell-like cells resistant to mTORC1 inhibition.
Hepatology. 68:1726–1740. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu WL, Hossain MS, Guo DY, Liu S, Tong H,
Khakpoor A, Casey PJ and Wang M: A role for Rac3 GTPase in the
regulation of autophagy. J Biol Chem. 286:35291–35298. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sirichanchuen B, Pengsuparp T and
Chanvorachote P: Long-term cisplatin exposure impairs autophagy and
causes cisplatin resistance in human lung cancer cells. Mol Cell
Biochem. 364:11–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kumar P, Zhang DM, Degenhardt K and Chen
ZS: Autophagy and transporter-based multi-drug resistance. Cells.
1:558–575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang L, Zhu B, Wang S, Wu Y, Zhan W, Xie
S, Shi H and Yu R: Regulation of glioma migration and invasion via
modification of Rap2a activity by the ubiquitin ligase Nedd4-1.
Oncol Rep. 37:2565–2574. 2017. View Article : Google Scholar : PubMed/NCBI
|