Introduction
Thyroid carcinoma is the most common endocrine
malignancy, and its incidence has increased globally in recent
decades (1). In Korea, thyroid
carcinoma diagnoses have also risen rapidly, accounting for 14.2%
of all cancer cases in 2014 (2).
Papillary thyroid carcinoma (PTC) accounts for the majority of the
newly diagnosed cases (3). PTC has a
very good prognosis; however, even after appropriate treatment,
10–20% of patients experience recurrence, with 2–5% developing
distant metastases between several years and several decades
following treatment (4). Since a
number of patients who experience recurrence eventually succumb to
cancer, the establishment of prognostic factors for recurrence is
an important part of PTC treatment.
The cancer stem cell (CSC) theory attracted interest
when it was first introduced in the 1990s as a novel target for
potential curative treatment for cancer (5). However, the discovery that CSCs have
plasticity and may exhibit a reversible phenotype with non-CSCs,
along with the observation that CSCs are not a rare population in
tumors, makes this theory complicated and difficult to adopt in
clinical practice. Despite these limitations, the CSC theory still
has clinical relevance, as these cells are believed to be able to
sustain or generate new tumors. In our previous study in
collaboration with the MD Anderson Cancer Center,
CD44+/CD24− were proposed as phenotypic
markers for CSCs in PTC (6).
However, since PTC is insufficiently aggressive to generate new
cancers in animal models, the tumorigenic potential of PTC CSCs
could not be proven. The present study aimed to define the
prognostic significance of CD44 and CD24 expression using
immunohistochemistry (IHC) on samples from patients with PTC, in
order to identify the clinical significance of these CSC
markers.
Materials and methods
Patients and tissue samples
Between July 2003 and December 2012, PTC samples
were collected from 500 patients with PTC (tumor size, >1 cm)
that underwent successful surgical resection at Seoul National
University Bundang Hospital; these samples were analyzed in this
retrospective study. Patients who received prior treatment or could
not commit to 6 months of follow-up care were excluded. This study
was approved by the Institutional Review Board at Seoul National
University Bundang Hospital (approval no. B-1507/306-310).
Previously prepared paraffin blocks of surgical specimens were
examined by two pathologists. Paraffin blocks of primary tumor
tissues were not available for 46 of the 500 patients; therefore,
samples from 454 patients were selected for the generation of
tissue microarrays (TMAs). The clinicopathological characteristics
of the patients are summarized in Table
I.
 | Table I.Clinicopathological characteristics of
the patients (n=454). |
Table I.
Clinicopathological characteristics of
the patients (n=454).
| Variable | Median (range) | N (%) |
|---|
| Sex |
|
|
| Male |
| 107 (23.6) |
|
Female |
| 347 (76.4) |
| Age at surgery,
years | 48.0 (10–87) |
|
|
<60 |
| 92 (20.3) |
| ≥60 |
| 362 (79.7) |
| cN1b |
| 94 (20.7) |
| pN1 |
|
|
| ≤5 |
| 351 (77.3) |
|
>5 |
| 103 (22.7) |
| T stage |
|
|
| T1 |
| 101 (22.2) |
| T2 |
| 14 (3.1) |
| T3 |
| 332 (73.1) |
| T4 |
| 7 (1.5) |
| Pathological tumor
size, cm | 1.4 (1.0–7.0) |
|
| ≤2 |
| 332 (73.1) |
|
>2 |
| 122 (26.9) |
| Multifocality |
| 204 (44.9) |
| Extrathyroidal
extension |
|
|
| No |
| 117 (25.8) |
|
Microscopic |
| 235 (51.8) |
|
Macroscopic |
| 102 (22.5) |
| Surgery |
|
|
|
Lobectomy/total |
| 21/433 |
|
thyroidectomy |
| (4.6/95.4) |
| CND/CND
+ LND |
| 281/96 |
|
|
| (61.9/21.1) |
| First relapse |
|
|
| Thyroid
remnant or bed |
| 3 (0.7) |
| Central
compartment LN |
| 5 (1.1) |
| Lateral
compartment LN |
| 27 (5.9) |
| Distant
site |
| 8 (1.8) |
| Time to first
relapse, months | 22 (2–101) |
|
| Follow-up time,
months | 70 (6–141) |
|
| Death |
|
|
|
Cancer |
| 0 (0) |
| Other
causes |
| 2 (0.4) |
TMA
Following the review of 454 tumor tissues,
representative core tissue sections (diameter, 2 mm) were extracted
from the paraffin blocks and arranged in new TMA blocks using a
trephine apparatus (Superbiochips Laboratories), according to the
manufacturer's protocol. The TMA blocks were sectioned into 4-µm
slices for IHC.
IHC staining
This study evaluated the expression of two proteins
in tumor tissues: CD44 and CD24. The following antibodies were
used: Rabbit monoclonal anti-CD44 (1:600; Boster Biological
Technology Co., Ltd.; cat. no. PA1021-2) and mouse monoclonal
anti-CD24 (1:50; Abcam; cat. no. MA5-11833). Using the Discovery XT
automated IHC instrument (Ventana Medical Systems, Inc.), the
sections were stained using the following procedures. Firstly,
detection was performed using a Ventana Chromo Map kit (Ventana
Medical Systems, Inc.). Sections were deparaffinized using an EZ
Prep solution included in the Chromo Map kit. CC1 standard (Tris,
borate and EDTA buffer; pH 8.4) was used for antigen retrieval (at
95°C for 44 min). Treatment with Inhibitor D (3%
H2O2) for 4 min at 37°C was used to block
endogenous peroxidase. Sections were then incubated with primary
antibodies for 32 min at 37°C and with an OmniMap anti-mouse
secondary antibody (Ventana Medical Systems, Inc.; cat. no.
760-4310) for 20 min at 37°C. Sections were incubated in
3,3′-diaminobenzidine + H2O2 substrate for 8
min at 37°C, followed by hematoxylin and eosin reagent counterstain
for 2 min at 37°C. Reaction buffer (Tris buffer; pH 7.6) was used
as a washing solution. Slides were evaluated on a Zeiss Axioskop
light microscope (Carl Zeiss) equipped with Zeiss Plan-Neofluar
objective lenses (×40, ×200).
IHC grades
Immunostaining was evaluated by two independent
pathologists, who were blind to the experimental design, and the
IHC scores were determined semi-quantitatively based on staining
intensity and proportion. IHC expression was graded according to
the following staining intensity criteria: 0, no staining; 1, weak
staining; 2, moderate staining; and 3, strong staining.
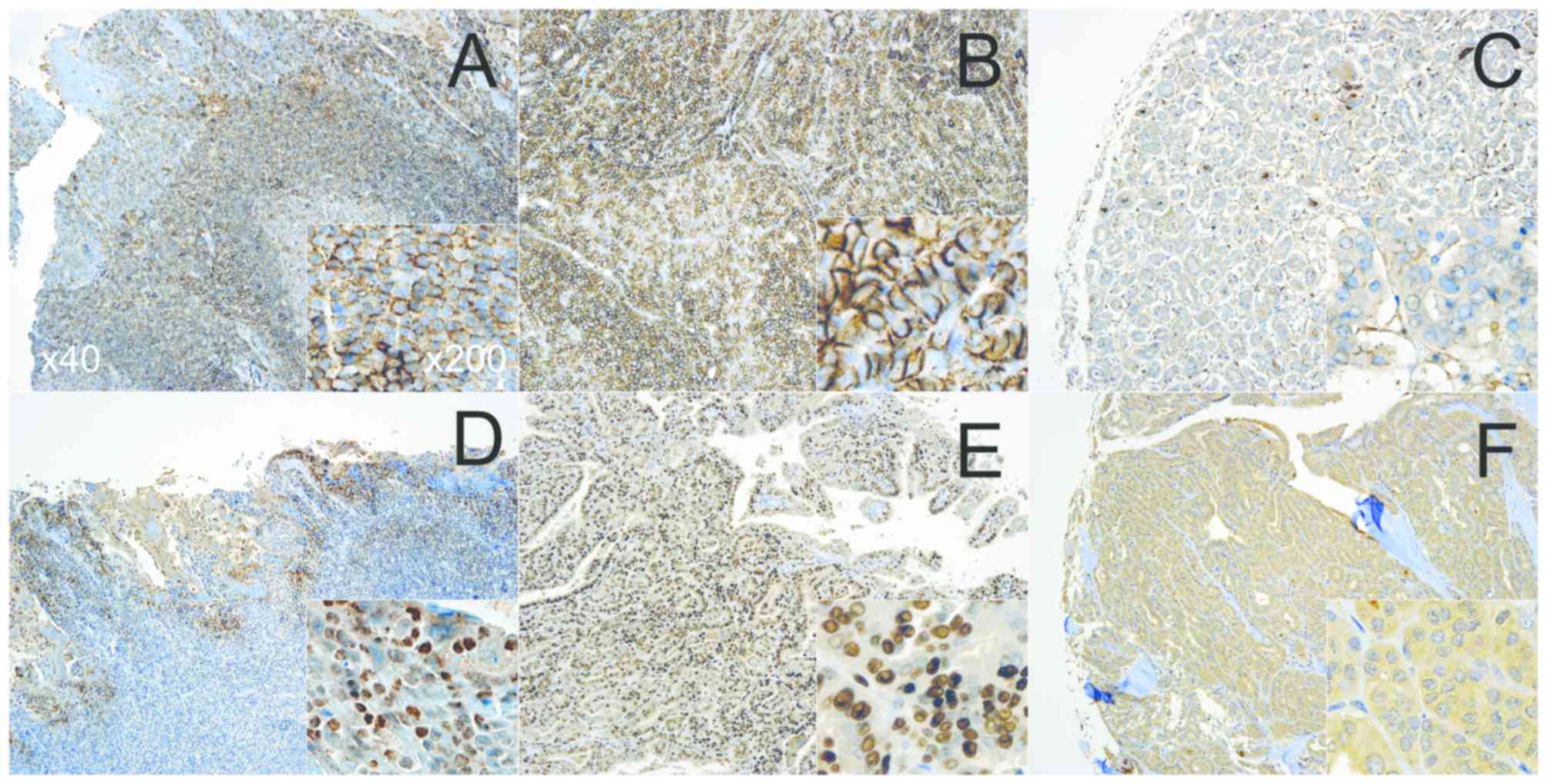
Tonsils from children undergoing tonsillectomy were
used as a positive control (Fig. 1)
(7). Tissues that scored ≤1 were
considered negative (−), whereas >1 was marked as positive (+)
for statistical analysis (Fig.
S1)
Statistical analysis
SPSS software (version 19.0; IBM Corp.) was used for
statistical analysis. Pearson's χ2 test was used to
analyze the relationship between protein expression and
clinicopathological data. Recurrence-free survival (RFS) was
determined based on the positive and negative expressions of
proteins using Kaplan-Meier survival analysis and univariate
log-rank test. In addition, a multivariate Cox regression test was
performed to identify factors affecting RFS. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient clinicopathological
characteristics
A total of 454 patients with PTC were included in
this study. Relevant demographic, clinical and pathological data,
as well as management and survival data of the patients were
retrieved and summarized in Table I.
A higher number of female compared with male patients with PTC
[female, 347 (76.4%) vs. male, 107 (23.6%)] were enrolled. Their
age ranged between 10 and 87 (median, 48.0) years. A total of 92
patients (20.3%) were <60 years old, whereas the remaining 362
patients (79.7%) were ≥60 years old. Of the 454 patients, 94
(20.7%) were suspected to have lateral lymph node metastasis at the
time of diagnosis (cN1b). Histopathologically, 351 patients (77.3%)
exhibited ≤5 lymph node metastases, whereas 103 patients (22.7%)
exhibited >5 lymph node metastases. According to the
Tumor-Node-Metastasis (TNM) staging system (8), 101 patients (22.2%) were classed as T
stage 1, 14 (3.1%) as stage 2, 332 (73.1%) as stage 3 and seven as
stage 4 (1.5%). There were 332 patients (73.1%) with primary tumor
diameters ≤2 cm in size and 122 patients (26.9%) with tumor
diameters >2 cm; 204 (44.9%) patients exhibited multifocality. A
total of 117 patients (25.8%) did not exhibit an extrathyroidal
extension, 235 (51.8%) exhibited a microscopic extrathyroidal
extension and 102 (22.5%) exhibited a gross extrathyroidal
extension. Total thyroidectomy was performed in the majority of
patients (95.4%). Central lymph node dissection was performed in
377 patients (83.0%), of which 96 patients (21.1%) also underwent
lateral lymph node dissection. The median follow-up period was 70
months and two patients were lost to the follow-up due to unrelated
causes. PTC recurred in 39 (8.6%) patients, with certain patients
exhibiting multiple instances of recurrence in different locations.
The median time to first recurrence was 22 months. Recurrence sites
were as follows: Three cases in the thyroid remnant or bed, five
cases in the central lymph node area, 27 cases in the lateral lymph
node area and eight cases of distant metastases.
Association of IHC results with
clinical data in patients with PTC
The majority of clinicopathological characteristics
did not demonstrate a statistically significant association with
single CSC markers; however, age (P=0.001), extrathyroidal
extension (P=0.039) and cancer recurrence (P<0.001) exhibited a
significant association with CD24 expression (Table II).
 | Table II.Association between CD44 and CD24 and
the clinical data of patients with papillary thyroid carcinoma. |
Table II.
Association between CD44 and CD24 and
the clinical data of patients with papillary thyroid carcinoma.
| Variable |
CD44− |
CD44+ | χ2 | P-value |
CD24− |
CD24+ | χ2 | Ρ-value | Others |
CD44+/CD24− | χ2 | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
|
|
|
|
|
|
<60 | 8 | 82 | 0.590 | 0.574 | 50 | 44 | 11.889 | 0.001a | 45 | 44 | 9.079 | 0.004a |
|
≥60 | 41 | 308 |
|
| 125 | 244 |
|
| 235 | 112 |
|
|
| Sex |
|
|
|
|
|
|
|
|
|
|
|
|
|
Male | 13 | 91 | 0.246 | 0.597 | 40 | 69 | 0.073 | 0.822 | 68 | 36 | 0.081 | 0.815 |
|
Female | 36 | 299 |
|
| 135 | 219 |
|
| 212 | 120 |
|
|
| Tumor size, cm |
|
|
|
|
|
|
|
|
|
|
|
|
| ≤2 | 34 | 287 | 0.391 | 0.608 | 132 | 206 | 0.840 | 0.389 | 203 | 116 | 0.176 | 0.675 |
|
>2 | 15 | 103 |
|
| 43 | 82 |
|
| 77 | 40 |
|
|
| cN1b |
|
|
|
|
|
|
|
|
|
|
|
|
| No | 41 | 306 | 0.714 | 0.461 | 136 | 233 | 0.684 | 0.407 | 225 | 120 | 0.715 | 0.393 |
|
Yes | 8 | 84 |
|
| 39 | 55 |
|
| 55 | 36 |
|
|
| pN1 |
|
|
|
|
|
|
|
|
|
|
|
|
| ≤5 | 42 | 297 | 2.182 | 0.140 | 128 | 221 | 2.141 | 0.143 | 224 | 113 | 3.266 | 0.071 |
|
>5 | 7 | 92 |
|
| 46 | 57 |
|
| 56 | 43 |
|
|
| Multifocality |
|
|
|
|
|
|
|
|
|
|
|
|
| No | 29 | 211 | 0.454 | 0.545 | 91 | 165 | 1.233 | 0.289 | 161 | 78 | 2.275 | 0.134 |
|
Yes | 20 | 179 |
|
| 84 | 123 |
|
| 119 | 78 |
|
|
| Extrathyroidal
extension |
|
|
|
|
|
|
|
|
|
|
|
|
| No +
Micro | 40 | 302 | 0.445 | 0.587 | 127 | 233 | 4.368 | 0.039a | 227 | 113 | 4.351 | 0.041a |
|
Macro | 9 | 88 |
|
| 48 | 55 |
|
| 53 | 44 |
|
|
| Recurrence |
|
|
|
|
|
|
|
|
|
|
|
|
| No | 48 | 352 | 3.191 | 0.105 | 148 | 274 | 15.061 |
<0.001a | 268 | 129 | 20.858 |
<0.001a |
|
Yes | 1 | 38 |
|
| 27 | 14 |
|
| 12 | 27 |
|
|
Associations of the combined status of
CD44+ and CD24− with clinicopathological data
in patients with PTC were also determined. A statistically
significant association was identified between the recurrence of
cancer for all combinations of CSC markers. Particularly, the
combination of CD44+/CD24− exhibited a
significant association with age and gross extrathyroidal
extensions (Table II).
RFS according to clinical data and
IHC
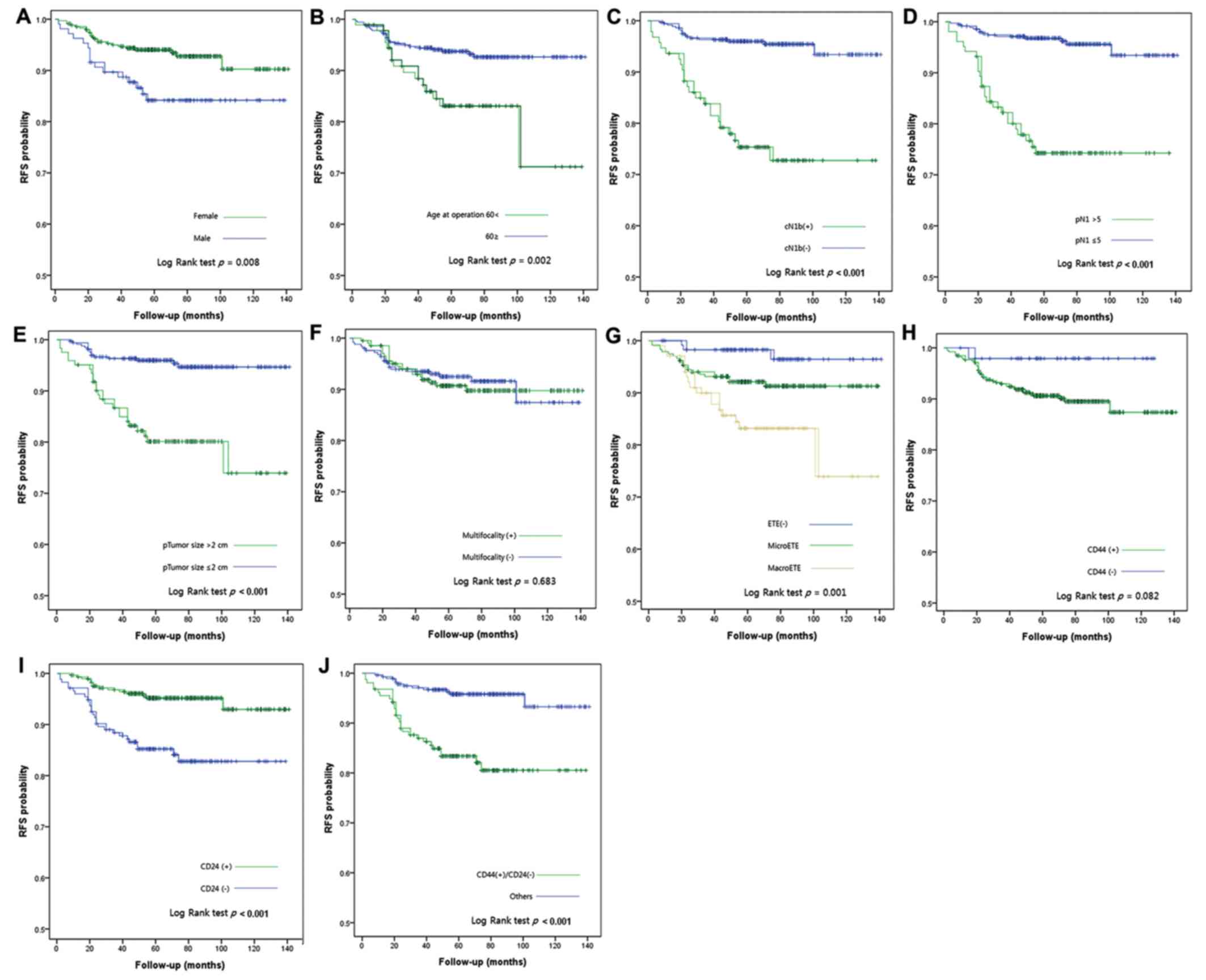
RFS curves according to clinical data and IHC
results are presented in Fig. 2. As
determined using Kaplan-Meier survival analysis and univariate
log-rank test, sex (P=0.008), age (P=0.002), cN1b (P<0.001), pN1
>5 (P<0.001), tumor size >2 cm (P<0.001),
extrathyroidal extension (P=0.001) and CD24−
(P<0.001) were prognostic factors for RFS. The CSC marker
combination CD44+/CD24− also exhibited
statistical significance in the log-rank test. In multivariate
analysis, CD44+/CD24− was identified as an
independent prognostic factor for PTC with a hazard ratio of 4.207
(Table III).
 | Table III.Multivariate analysis of
recurrence-free survival. |
Table III.
Multivariate analysis of
recurrence-free survival.
| Variable | Hazard ratio | 95% CI | P-value |
|---|
| Age (>60) | 1.911 | 0.937–3.895 | 0.075 |
| Sex (male) | 2.262 | 1.174–4.359 | 0.015a |
| Size (>2
cm) | 2.576 | 1.200–5.527 | 0.015a |
| cN1b | 2.606 | 0.909–7.474 | 0.075 |
| pN1 (>5) | 2.426 | 0.858–6.861 | 0.095 |
| Gross ETE | 1.259 | 0.573–2.769 | 0.566 |
|
CD44+/CD24− | 4.207 | 2.088–8.479 |
<0.001a |
Discussion
Cancer prognosis and treatment efficacy are
ultimately determined by survival of the patient, and the criteria
for staging are based on factors related to survival. However, in
the case of a differentiated thyroid carcinoma, cancer progression
is very slow (9); therefore, even in
the case of recurrence, a cure is possible following further
operations and treatment with radioactive iodine (10). If thyroid carcinoma is not cured, a
long and considerable period of palliative care may follow.
Therefore, in the case of differentiated thyroid carcinoma, the
prognosis may not be determined based on survival alone; instead,
analyzing RFS may be a more reasonable approach (11). In the present study, no patients
succumbed to PTC during the 70-month median follow-up period, and
the recurrence rate was 8.6%, similar to previous prognostic
studies (3,12).
Thyroid CSCs can be distinguished by the expression
of specific biomarkers, the ability to produce thyrospheres in
vitro and the ability to induce tumors in vivo (13). Zito et al (14) first attempted to isolate CSCs in 2008
by analyzing the expression of CD133 through flow cytometry in
thyroid cancer cell lines. Subsequently, Friedman et al
(15) demonstrated that the
transplantation of CD133+ cells into immunodeficient
NOD/SCID mice is sufficient to induce tumor growth in vivo.
Our previous study on CSCs focused on CD44 and CD24, which are CSC
markers for certain cancers, including breast and colon cancer
(16). Using specific cancer cell
lines (TPC-1 and its derivatives), higher numbers of
CD44+/CD24− cells have been identified in
more aggressive cell lines (positivity rates: 86% in highly
tumorigenic TPC-1 mouse cells; >73% in moderately tumorigenic
TPC-1SC2 cells; and >21% in parental, poorly tumorigenic TPC-1
cells) (4). Subsequently, 4–70% of
dispersed cells from thyroid cancers have been determined to be
CD44+/CD24−. These cells form spheres;
however, CD44+/CD24−, but not
CD44+/CD24+ cells from these spheres are
spherogenic. The cells derived from thyrospheres
(≥1×104) form tumors following orthotopic injection in
an immunodeficient mice model (6).
However, the impact of these markers on clinical outcome could not
be assessed in the previous study. Therefore, the present study
used PTC surgical specimens in TMAs to conduct standardized IHC
experiments.
To the best of our knowledge, the present study is
the first to analyze the association between CD44 and CD24
expression status and the clinical prognosis of PTC. The results of
the present study demonstrated that the expression of CD44 or CD24,
as determined by IHC, was not associated with commonly known
prognostic factors in patients with PTC, with the exception of the
presence of gross extrathyroidal extension. Recently, the American
Joint Committee on Cancer (AJCC) 8th edition for thyroid cancers
downstaged a large number of patients by raising the age at
diagnosis cut off from 45 to 55 years (8). This change was confirmed by the
identification of a good prognosis in patients aged between 45 and
55 years in an international multi-institutional validation study
of 9,484 patients (17). Similarly,
in the present study, a difference in IHC outcome and prognostic
analysis at index age 45 years was not observed (data not shown).
However, when the index age was raised to 60 years, differences in
CD44+/CD24− expression status and prognosis
were detected.
A significant association between RFS and CD24
expression was identified using Kaplan-Meier analysis. CD44
exhibited an association with RFS, which was not statistically
significant. In addition, CSC marker combination analysis,
including CD44+/CD24−, exhibited a
statistically significant association with RFS. These results were
consistent with the findings of Bi et al (18), which revealed that the IHC results
for CD44+/CD133+ in medullary thyroid
carcinoma are correlated with survival; in addition,
CD44+/CD24− is associated with prognosis in
patients with other types of cancer, such as breast (19).
At present, surgery, radiotherapy, chemotherapy and
hormonal therapy are used to treat thyroid cancer; however, these
treatments often exhibit limited efficacy. Conventional therapies
target highly proliferating cells that form the majority of the
tumor mass, but are ineffective against slowly proliferating or
quiescent CSCs, which are responsible for drug resistance,
metastasis and recurrence (20).
However, the clinical importance of the presence of CSC markers,
evaluated by IHC, remains uncertain. Due to their plasticity,
whether the cells positive for these markers are actually CSCs is
unknown. Even if IHC evaluation precisely reflects cancer stemness,
the overall interpretation of such data is still challenging
(19). However, it is beneficial for
such efforts to be continued, since the ability to identify,
isolate and study thyroid CSCs has a number of implications with
potential novel therapeutic consequences.
In conclusion, the expression status of
CD44+ and CD24− in tissue samples was
associated with RFS of patients with PTC. Particularly, the
combination of CD44+ and CD24− exhibited a
significant association with RFS and gross extrathyroidal
extension. Therefore, measuring CD44+/CD24−
expression in order to evaluate the prognosis associated with RFS
may be of use in PTC.
Supplementary Material
Supporting Data
Acknowledgements
All data in the present study were reconstructed
based on a master's thesis prepared by Dr Yoon-Jong Ryu under
supervision of Professor Soon-Hyun Ahn (Department of
Otorhinolaryngology Head and Neck Surgery, Seoul National
University College of Medicine).
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJR and SHA conceived and designed the study. YJR
acquired and analyzed the data. JYC and KL contributed to the
interpretation of the data. YJR and SHA wrote and revised the
paper. JYC and KL provided administrative, technical, or material
support. SHA supervised the study.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board at Seoul National University Bundang Hospital
(approval no. B-1507/306-310). Written informed consent was waived
due to the retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ito Y, Nikiforov YE, Schlumberger M and
Vigneri R: Increasing incidence of thyroid cancer: Controversies
explored. Nat Rev Endocrinol. 9:178–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National_Cancer_Information_Center, .
Korea cancer registry statistics 2014National Cancer Information
Center; Goyang: 2016
|
|
3
|
Cho BY, Choi HS, Park YJ, Lim JA, Ahn HY,
Lee EK, Kim KW, Yi KH, Chung JK, Youn YK, et al: Changes in the
clinicopathological characteristics and outcomes of thyroid cancer
in Korea over the past four decades. Thyroid. 23:797–804. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer the American thyroid association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moharil RB, Dive A, Khandekar S and
Bodhade A: Cancer stem cells: An insight. J Oral Maxillofac Pathol.
21:4632017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ahn SH, Henderson YC, Williams MD, Lai SY
and Clayman GL: Detection of thyroid cancer stem cells in papillary
thyroid carcinoma. J Clin Endocrinol Metab. 99:536–544. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fedchenko N and Reifenrath J: Different
approaches for interpretation and reporting of immunohistochemistry
analysis results in the bone tissue-a review. Diagn Pathol.
9:2212014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perrier ND, Brierley JD and Tuttle RM:
Differentiated and anaplastic thyroid carcinoma: Major changes in
the American Joint Committee on Cancer eighth edition cancer
staging manual. CA Cancer J Clin. 68:55–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tubiana M, Schlumberger M, Rougier P,
Laplanche A, Benhamou E, Gardet P, Caillou B, Travagli JP and
Parmentier C: Long-term results and prognostic factors in patients
with differentiated thyroid carcinoma. Cancer. 55:794–804. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Coburn M, Teates D and Wanebo HJ:
Recurrent thyroid cancer. Role of surgery versus radioactive iodine
(I131). Ann Surg. 219:587–595. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim WG, Kim EY, Yim JH, Han JM, Jeon MJ,
Kim TY, Ryu JS, Gong G, Hong SJ, Kim WB and Shong YK: Comparison of
different staging systems for predicting recurrence of papillary
thyroid carcinoma. Endocrinol Metab. 26:53–61. 2011. View Article : Google Scholar
|
|
12
|
Hwangbo Y, Kim JM, Park YJ, Lee EK, Lee
YJ, Park DJ, Choi YS, Lee KD, Sohn SY, Kim SW, et al: Long-term
recurrence of small papillary thyroid cancer and its risk factors
in a Korean multicenter study. J Clin Endocrinol Metab.
102:625–633. 2017.PubMed/NCBI
|
|
13
|
Nagayama Y, Shimamura M and Mitsutake N:
Cancer stem cells in the thyroid. Front Endocrinol (Lausanne).
7:202016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zito G, Richiusa P, Bommarito A, Carissimi
E, Russo L, Coppola A, Zerilli M, Rodolico V, Criscimanna A, Amato
M, et al: In vitro identification and characterization of
CD133(pos) cancer Stem-like cells in anaplastic thyroid carcinoma
cell lines. PLoS One. 3:e35442008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Friedman S, Lu M, Schultz A, Thomas D and
Lin RY: CD133+ anaplastic thyroid cancer cells initiate tumors in
immunodeficient mice and are regulated by thyrotropin. PLoS One.
4:e53952009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sahlberg SH, Spiegelberg D, Glimelius B,
Stenerlöw B and Nestor M: Evaluation of cancer stem cell markers
CD133, CD44, CD24: Association with AKT isoforms and radiation
resistance in colon cancer cells. PLoS One. 9:e946212014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tuttle RM, Haugen B and Perrier ND:
Updated American Joint Committee on Cancer/Tumor-Node-Metastasis
staging system for differentiated and anaplastic thyroid cancer
(Eighth edition): What changed and why? Thyroid. 27:751–756. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bi Y, Meng Y, Wu H, Cui Q, Luo Y and Xue
X: Expression of the potential cancer stem cell markers CD133 and
CD44 in medullary thyroid carcinoma: A ten-year follow-up and
prognostic analysis. J Surg Oncol. 113:144–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Horimoto Y, Arakawa A, Sasahara N, Tanabe
M, Sai S, Himuro T and Saito M: Combination of cancer stem cell
markers CD44 and CD24 is superior to ALDH1 as a prognostic
indicator in breast cancer patients with distant metastases. PLoS
One. 11:e01652532016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eramo A, Haas TL and De Maria R: Lung
cancer stem cells: Tools and targets to fight lung cancer.
Oncogene. 29:4625–4635. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|