Introduction
Epithelial ovarian cancer (EOC) causes around
125,000 deaths globally per year (1). Approximately 70% of women with ovarian
cancer (OC) are diagnosed with locally advanced or metastatic
disease (stages III/IV), of whom only ~30% will survive more than 5
years. By contrast, women diagnosed with earlier stage (stage I)
disease have a 5-year survival rate >90%. Unfortunately, signs
and symptoms of OC are usually absent or too subtle to be easily
detected in the early stages of the disease. Despite of high
initial response rates to chemotherapy, approximately 80% of women
with advanced OC relapse within 2 years after initial drug
treatment (2,3).
The standard treatment for EOC involves maximal
cytoreductive surgery followed by platinum and taxane-based
chemotherapy. At first, most patients with advanced stage (III/IV)
EOC respond well to surgery and chemotherapy; however, within two
years after initial treatment, cancer frequently relapses with a
drug-resistant phenotype and most patients die of the disease
(4). Age and disease staging at
diagnosis, tumor histology, and performance status (PS) are the
best known prognostic factors (5),
albeit limited by our restricted understanding of EOC's biology and
complicated by disease heterogeneity.
Currently, there is a growing interest in finding
specific molecular markers that could function both in the
prognosis of the disease and the patient's response to
chemotherapy. Good candidates include heat shock proteins (HSPs)
because of their role in facilitating malignant transformation,
tumor progression, and tumor survival (6,7). These
evolutionarily conserved proteins are classified according to their
molecular weight and, in mammalian cells, are grouped into six main
classes: HSP27, HSP40, HSP60, HSP70, HSP90, and HSP110 (7). The contribution of HSPs to
tumorigenesis can be attributed to their activities governing
folding/unfolding, turn-over, and transport of client proteins as
well as assembly of multiprotein complexes. As a result, various
crucial and clinically important cell responses are vitally
influenced and modulated by HSPs, e.g., cell growth, apoptosis,
metastasis, and treatment resistance (6,7).
Although the existing data for HSP's function in OC
progression and drug resistance is appealing, it is still limited
and conflicting at times. For instance, the cytosolic HSPB1(HSP27),
HSP70 (HSPA1A, HSPA1L), and HSP90(TRAP1) as well as the
mitochondrial HSP60 proteins and the tumor necrosis factor
receptor-associated protein 1 (TRAP-1) have all been shown to be
induced by drug treatment and frequently associated with
cross-resistance to anticancer compounds of different classes in
ovarian and other cancer types (6–10).
Moreover, the levels of circulating HSP27 protein were decreased
after chemotherapy treatment in metastatic OC patients (11). Thus, drug-mediated regulation of HSPs
in OC may follow differentially controlled stress signaling
pathways. Due to our limited understanding of HSP's role in OC
biology, studies elucidating their potential to help the prognostic
evaluation of patients and the therapeutic strategy upon relapse
after platinum-based chemotherapy are warranted.
In the present study, we correlated the expression
of TRAP1, HSPB1, HSPA1, HSPAl, and HSPD1 genes and the
clinical and pathological aspects of patients with OC. To this end,
we compared the expression of these genes in the primary and
metastatic ovarian tumor and investigated the relationship between
the observed expression profile with other known prognostic factors
and with the patients' response to chemotherapy and relapse-free
survival.
Materials and methods
Ethics
This study was approved by the Research Ethics
Committee of Vera Cruz Hospital (Belo Horizonte, Minas Gerais,
Brazil), under the protocol CAAE: 01242212.2.0000.5135. All
participants voluntarily signed an informed consent form.
Patients and tumor tissue samples
We collected ovarian tissue from 51 women divided
into four groups: Primary Epithelial Ovarian Cancer EOC (n=14),
metastatic EOC (n=11), ovarian serous cystadenoma (n=7) and normal
ovary (n=19). The patients were recruited to our study using
convenience sampling and they did not match any of the following
exclusion criteria: Previously treated with chemotherapy and/or
radiotherapy; HIV positive; presenting any infectious process
diagnosed or not during laparotomy; present or previous history of
other malignant neoplasms; using or with a previous history of use
of immunosuppressives, systemic corticosteroids or non-steroidal
anti-inflammatory drugs in the three months prior to the study. All
cases were reevaluated blindly by a senior consultant
subspecialized in gynecologic pathology and a representative
portion of each tumor containing >80% tumor cells were selected
for storage until analysis. Clinical and pathologic information
documented at the time of surgery included disease stage, tumor
grade and histotype, residual tumor size and debulking success.
In the EOC patients, samples were collected from
primary tumors and of metastatic tumors, when extra pelvic disease
above 1 cm was observed. Tumor staging was performed according to
the FIGO recommendations (12).
Normal ovarian epithelial tissue samples were taken from
postmenopausal women who required a bilateral oophorectomy. After
excision, the samples were immediately frozen in liquid nitrogen
and stored at −80°C until use.
RNA extraction, cDNA synthesis and
gene expression analysis
Total RNA was extracted from 50 to 100 mg of each
ovarian tumor sample using the TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. The RNA yield and A260/280 ratio were determined by a
NanovueTM Plus Spectrophotometer (GE Healthcare Biosciences). RNA
integrity and quality were characterized through 1% agarose gel
electrophoresis. Subsequently, the samples were treated with
RNAse-Free DNAse Set® (Qiagen) to remove possible traces
of genomic DNA.
cDNAs were synthesized using M-MLV Reverse
transcriptase (Promega Corporation) according to the manufacturer's
recommendations and were subjected to RT-qPCR using
TaqMan® Universal PCR master mix and inventoried
TaqMan® Assays (Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to manufacturer's recommendation.
Taqman assays were selected for each target gene: TRAP1
(Hs00212476_m1), HSPB1 (Hs00356629_g1), HSPA1A
(Hs00359163_s1), HSPA1L (Hs00271466_s1), HSPD1
(Hs01036753_g1) and for TBP (Hs00427620_m1) used as
endogenous control. A sample without a template was included as a
control in each assay. Each 40-cycle reaction was performed in
duplicate using a Step OnePlus detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Two technical
replicates were adopted for each sample. Relative gene expression
was determined using the 2−ΔΔCq method (13).
Gene functional and Network pathway
analysis
The differentially expressed genes determined using
the 2−ΔΔCq method and for the pathway analysis of
gene-associated proteins, the Kyoto Encyclopedia of Genes and
Genomes (KEGG) was investigated by using STRING database, version
10.5 (14). STRING was also used to
evaluate protein-protein interactions (PPI) among the associated
genes.
Statistics
Student's t-test and ANOVA were used to compare gene
expression and qualitative variables (15). To detect correlation between the
genes and to compare their expression with the quantitative
variables, we used Pearson's correlation and Spearman's correlation
(16), respectively. To compare
disease free time curves and survival curves with gene expression,
the log-rank test (17) was used. It
is worth mentioning that the gene expressions were recoded as
greater than 1, less than 1, or equal to 1. To analyze the factors
influencing survival and the disease-free interval, a univariate
analysis was performed using the Cox Regression Model and the Risk
Ratio was computed (17). Different
from logistic regression, the Cox model has the advantage of
including the effect of time up to the death and relapse besides
allowing the interpretation through the risk ratio and not the odds
ratio. The probability of survival and significance were calculated
using the Kaplan-Meier method (18).
All statistical analyzes were performed using the statistical
software package R (version 3.4.1) (http://www.r-project.org/), using stats package
(19) to quantitative variables and
survival package to analyze the survival rates (20). P<0.05 was considered to indicate a
statistically significant difference.
Results
The general characteristics of the patients are
shown in Table I. The parity was
2.07 births with a range between 0 and 8 deliveries. The
clinicopathologic characteristics of the tumor samples are shown in
Table II. The stage was I in 7
patients (29.17%) and III/IV in 17 patients in the EOC group
(70.83%). All samples were identified as high-grade serous
carcinoma by histopathological evaluation.
 | Table I.General characteristics of
patients. |
Table I.
General characteristics of
patients.
| Variables | Cystadenoma mean ±
standard error | Primary EOC mean ±
standard error | Metastatic EOC mean
± standard error | Normal ovary mean ±
standard error |
P-valuea |
|---|
| Age (years) | 50.00±16.54 | 57.93±10.54 | 59.55±10.76 | 47.68±8.33 | 0.017 |
| Menarche | 12.57±1.13 | 12.79±1.37 | 13.10±1.45 | – | – |
| Parity
(births) | 2.14±3.08 | 2.00±1.24 | 2.10±1.20 | – | – |
| Period after
Menopause | 6.86±11.19 | 8.62±9.03 | 10.13±9.03 | – | – |
 | Table II.Clinicopathologic characteristics in
ovarian sample. |
Table II.
Clinicopathologic characteristics in
ovarian sample.
| Variables | Cystadenoma N
(%) | Primary EOC N
(%) | Metastatic EOC N
(%) | Normal ovary N
(%) |
P-valuea |
|---|
| Stage |
|
|
|
|
|
| I | 0 (0.0) | 5 (35.7) | 2 (20.0) | – | – |
|
III | 0 (0.0) | 6 (42.9) | 6 (60.0) | – |
|
| IV | 0 (0.0) | 3 (21.4) | 2 (20.0) | – |
|
| Menopause |
|
|
|
|
|
| No | 4 (57.1) | 3 (21.4) | 2 (20.0) | 17 (89.5) | <0.001 |
|
Yes | 3 (42.9) | 11 (78.6) | 8 (80.0) | 2
(10.5) |
|
| Ascites |
|
|
|
|
|
| No | 7 (100.0) | 4 (30.8) | 3 (37.5) | – | – |
|
Yes | 0 (0.0) | 9 (69.2) | 5 (62.5) | – |
|
| Tumor
differentiation grade |
|
|
|
|
|
| G2 | 0 (0.0) | 5 (35.7) | 4 (40.0) | – | – |
| G3 | 0 (0.0) | 9 (64.3) | 6 (60.0) | – |
|
| CA-125 |
|
|
|
|
|
| <35
U/ml | 3 (42.9) | 4 (28.6) | 1 (9.10) | – | – |
| >35
U/ml | 4 (57.1) | 10 (71.4) | 10 (90.9) | – |
|
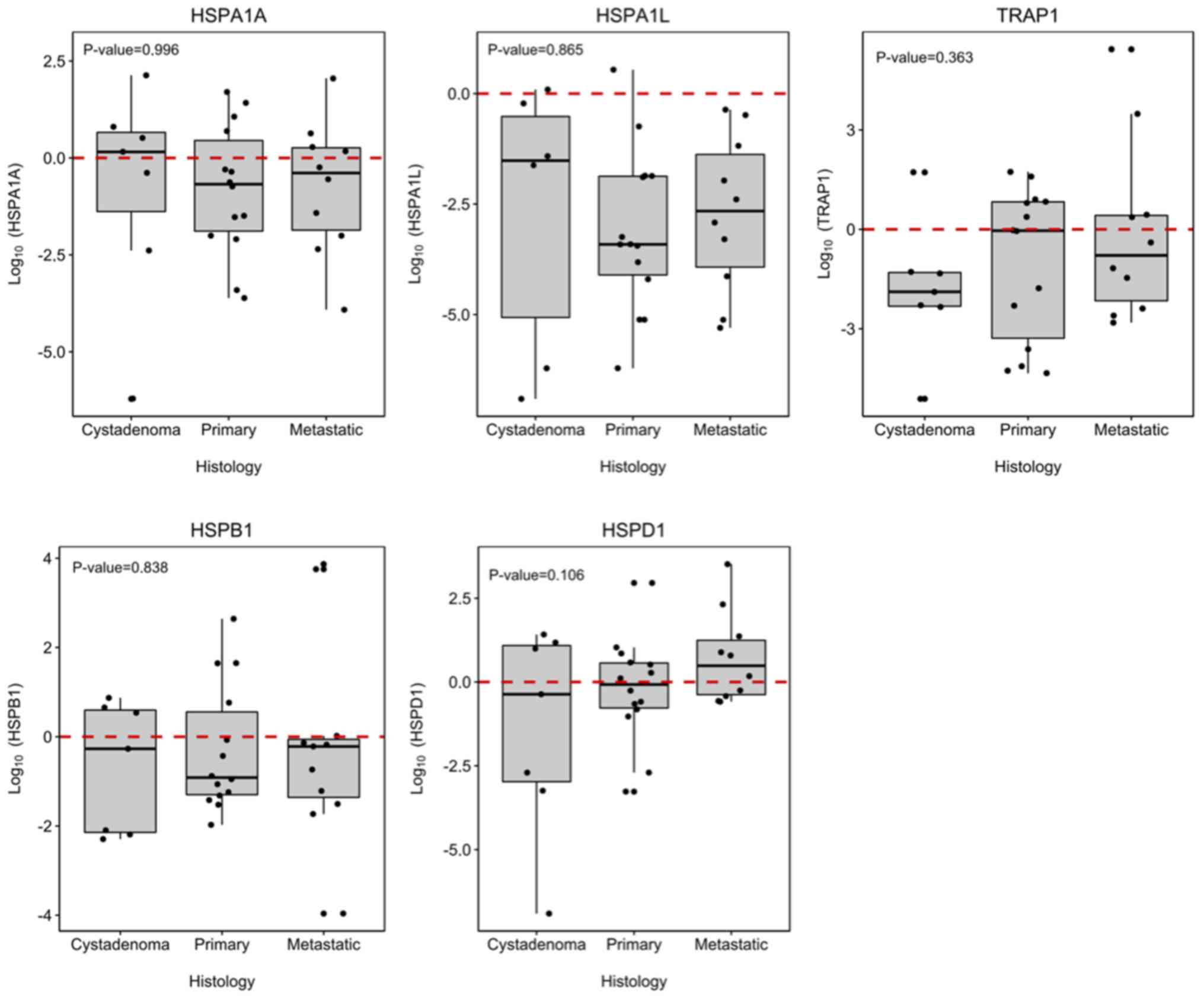
TRAP1, HSPA1A, HSPA1L, HSPD1 and HSPB1
genes showed differential expression between tumor samples of the
EOC group and samples from the cystadenoma, primary and metastatic
EOC samples. Although, no significantly differ among the groups
(P>0.050; Fig. 1). When the
groups were compared singly, HSPA1A, HSPA1L and TRAP1 were
significantly under-expressed in the primary and metastatic EOC
groups in comparison to the expression profile presented in normal
ovarian tissues, with HSPA1L showing the lowest expression
in both carcinoma groups (Fig.
2).
There was no correlation between the expression
levels of the analyzed genes and age, menarche, parity or period
after menopause initiation as well as between the seric levels of
the CA125 tumor marker and the expression of the HSP genes analyzed
(Table III).
 | Table III.Association between clinicopathologic
characteristics of EOC patients and TRAP1, HSPD1, HSPB1,
HSPA1L and HSPA1A gene expression profile. |
Table III.
Association between clinicopathologic
characteristics of EOC patients and TRAP1, HSPD1, HSPB1,
HSPA1L and HSPA1A gene expression profile.
|
| HSP gene expression
profile correlation |
|---|
|
|
|
|---|
| Clinicopathologic
characteristics | HSPA1A | HSPA1L |
HSPB1 |
HSPD1 |
TRAP1 |
|---|
| Histopathology | 0.996 | 0.865 | 0.838 | 0.106 | 0.363 |
| Age (years) | 0.797 | 0.309 | 0.723 | 0.287 | 0.451 |
| Menarche | 0.782 | 0.713 | 0.68 | 0.554 | 0.351 |
| Parity
(births) | 0.119 | 0.061 | 0.852 | 0.152 | 0.594 |
| Period after
Menopause | 0.804 | 0.643 | 0.486 | 0.632 | 0.409 |
| CA-125 | 0.222 | 0.806 | 0.539 | 0.842 | 0.315 |
| Stage | 0.962 | 0.327 | 0.075 | 0.193 | 0.040 |
| Menopause | 0.927 | 0.664 | 0.786 | 0.600 | 0.492 |
| Ascites | 0.562 | 0.573 | 0.585 | 0.174 | 0.798 |
| Tumor
differentiation grade | 0.397 | 0.305 | 0.035 | 0.080 | 0.163 |
| Cytoreduction | 0.797 | 0.772 | 0.239 | 0.824 | 0.422 |
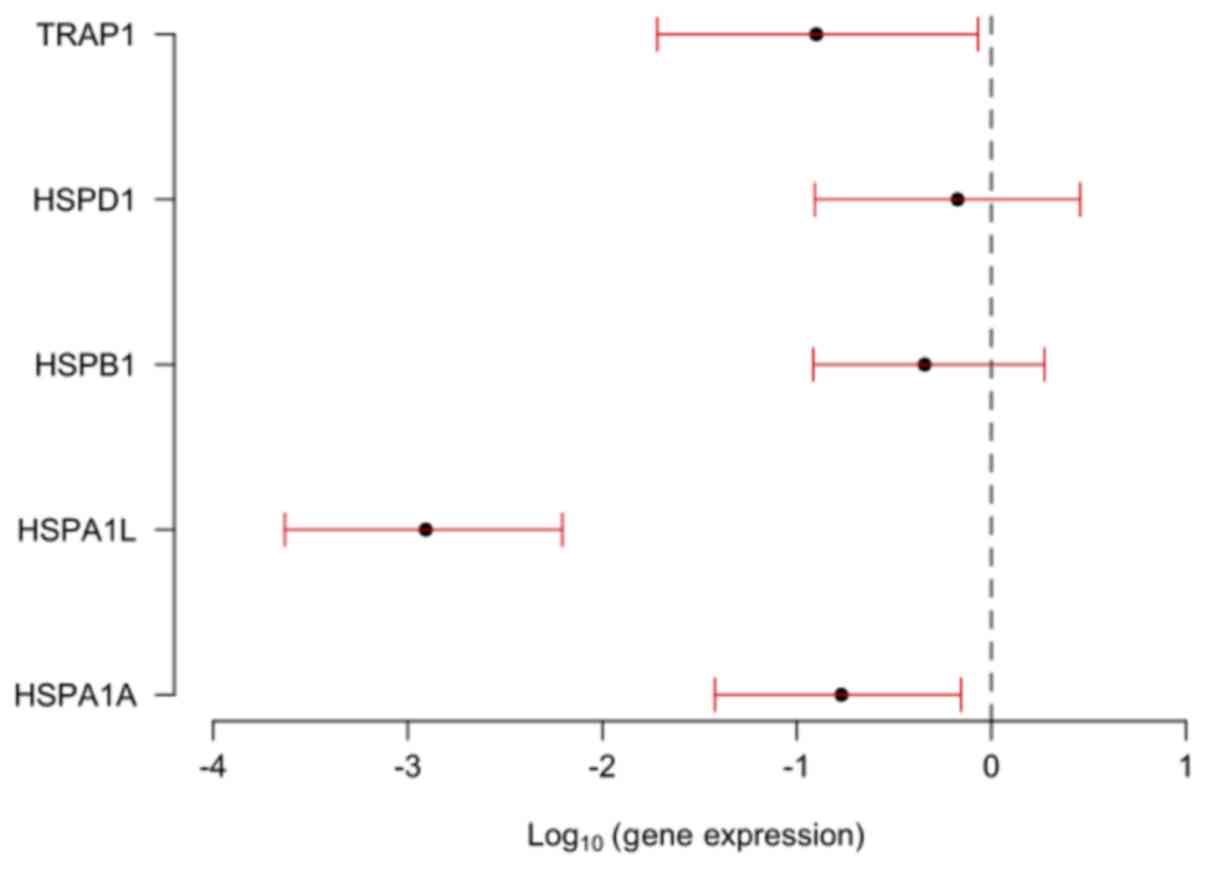
| Risk of dying | 0.73 | 1.09 | 0.92 | 1.02 | 0.94 |
| (P-value) | (0.048) | (0.491) | (0.527) | (0.892) | (0.511) |
A comparison between the expression profile of the
HSP genes and the OC staging showed that TRAP1 expression
was significantly greater in tumors at stage I than in tumors at
stages III and IV of EOC patients (P=0.040; Table III).
There was no correlation between cytoreduction and
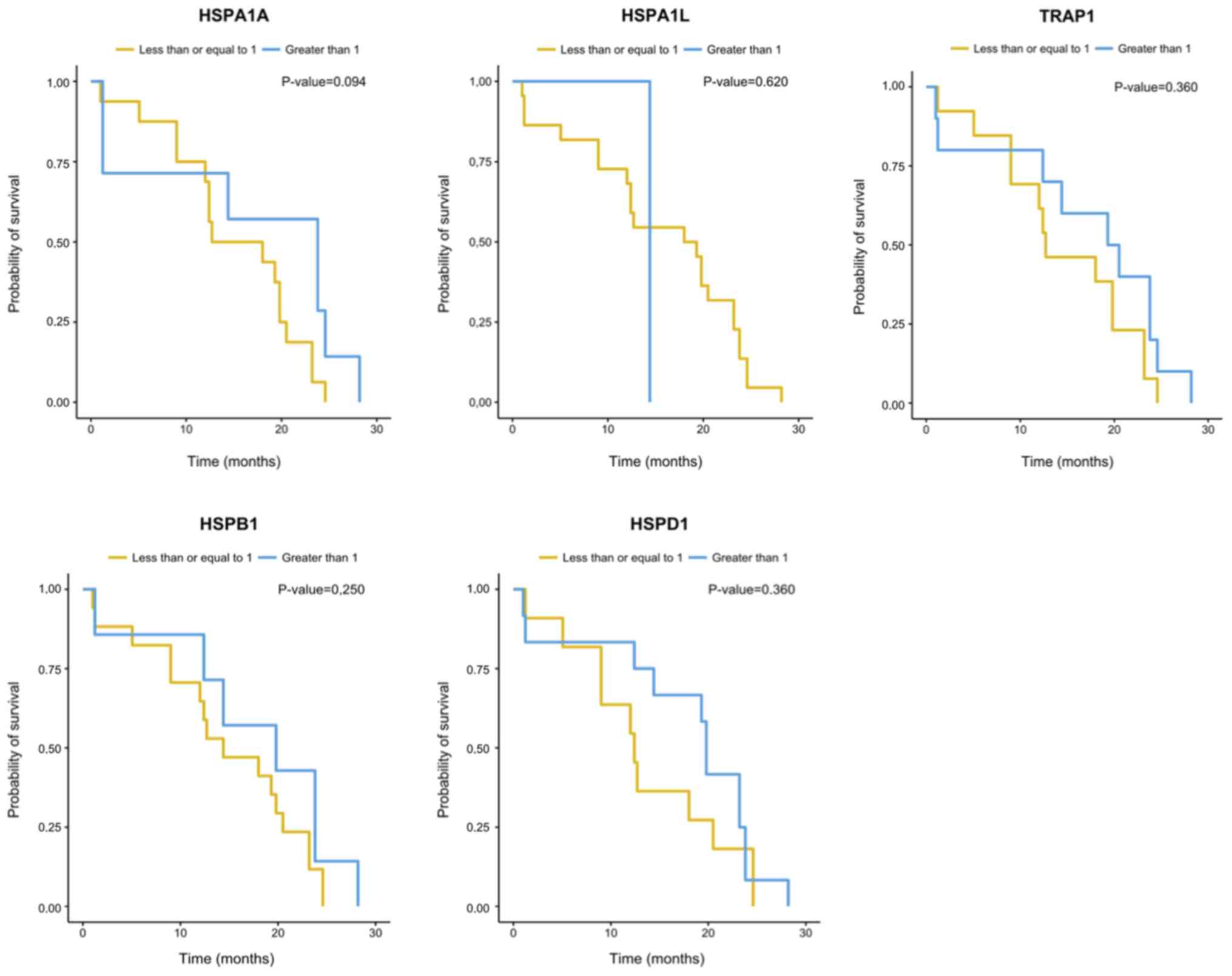
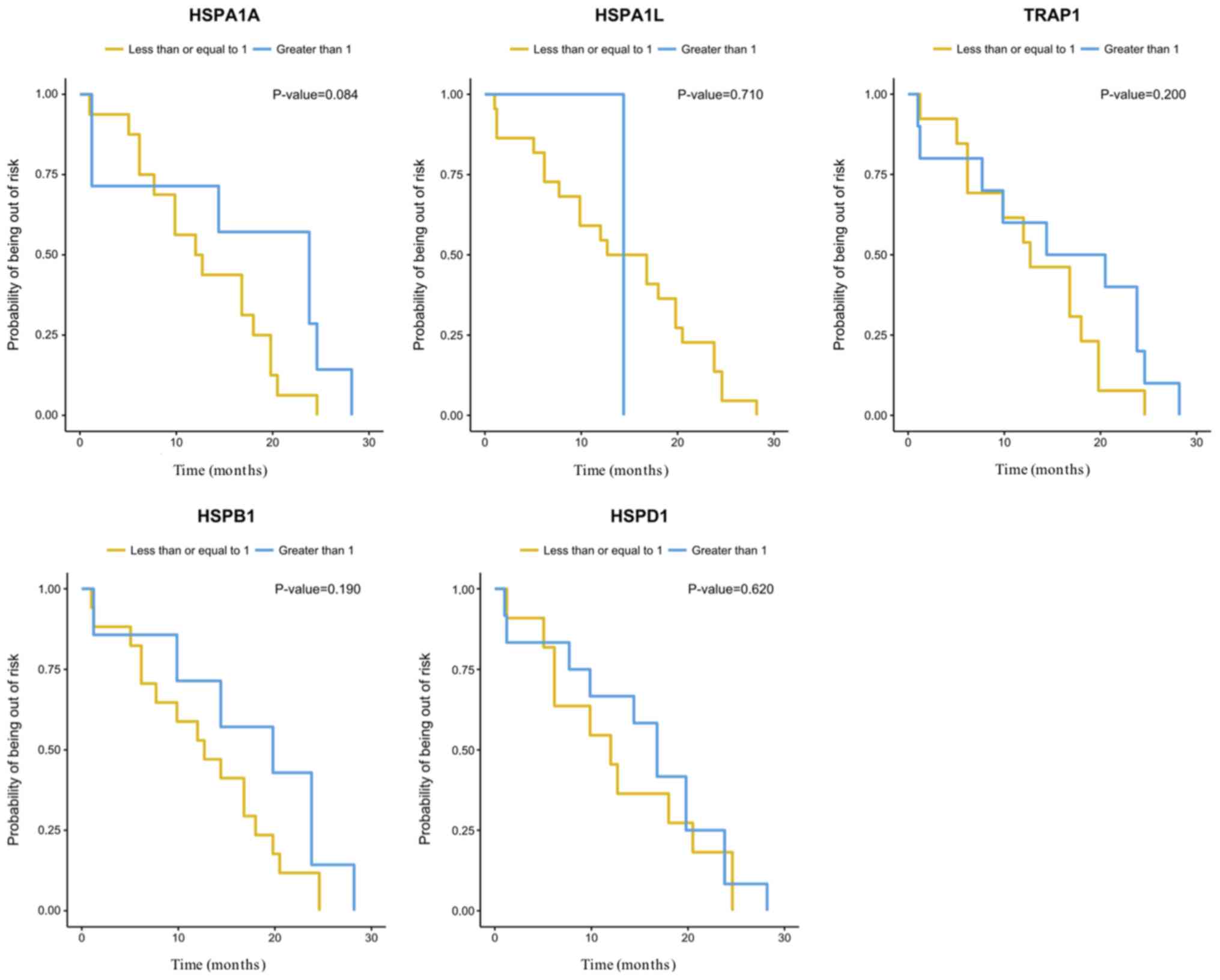
the expression of the HSP genes analyzed herein (Table III). There were no significant
differences (P=0.05) between the expression of the HSP genes
evaluated and overall survival (OS) or disease-free survival (DFS)
(Figs. 3 and 4). However, the gene expression analysis in
relation to OS suggested influence of HSPA1A expression
levels on the risk of dying of EOC (P=0.048). An increase of one
unit in the gene log decreased the risk of dying by 0.73 times
[0.53; 0.99] (Table III).
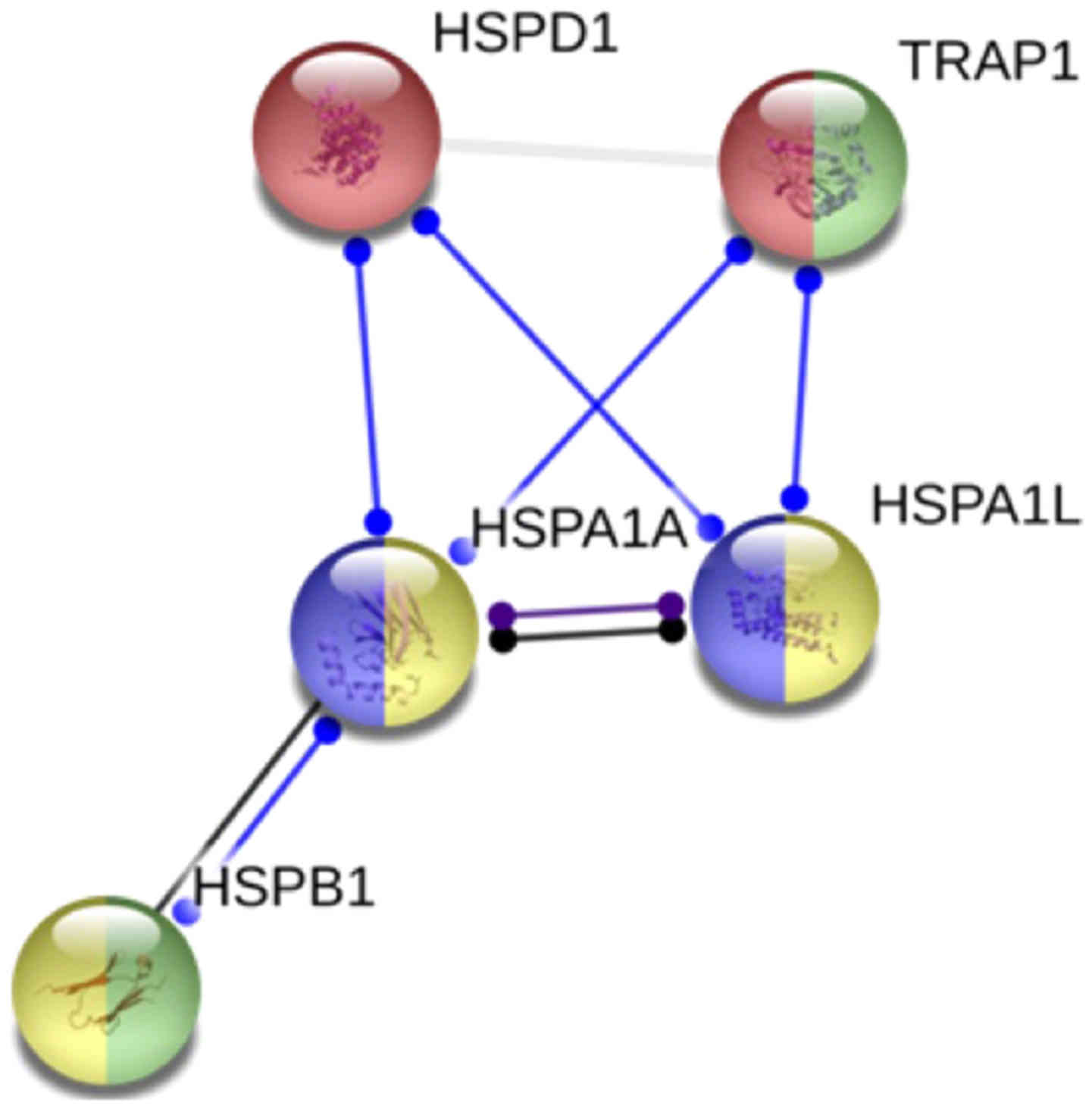
In silico network protein analysis made on
STRING database revealed the protein-protein interactions between
the proteins codified by the genes analyzed by us (Fig. 5).
Discussion
EOC is a very heterogeneous disease and the most
lethal gynecological neoplasia (21). Despite extensive effort, EOC
continues to be a poorly understood disease and patients survival
rates remain low. Therefore, new strategies for early diagnosis,
prognostic markers for clinical assessment and a better
understanding of the mechanisms related to ovarian carcinogenesis
are of extreme importance in order to obtain better outcomes for
the affected patients. In this study, we investigated whether a
gene signature among patients with and without EOC could be
identified. To this end, we evaluated the level of expression of
the genes TRAP1, HSPD1, HSPB1, HSPA1A, and HSPA1L in
tumor samples obtained from patients with cystadenoma, primary and
metastatic EOC in relation to baseline expression of these genes in
normal ovary (NO) tissues. Therefore, expression of each gene in NO
was assigned an arbitrary quantity of ‘1’ and their expression in
the tumor samples were expressed in terms of their fold difference
to NO (21). We found that these
five genes were differentially expressed between the groups, but
the prediction of EOC metastasis with gene expression profiling was
not better than chance alone. The comparison between the expression
levels of the studied genes in the tumor groups with the NO group
showed that HSPA1A, HSPA1L and TRAP1 were
significantly under-expressed in the EOC groups.
The under-expression of HSP70 isoforms was
previously observed in OC (22).
According to these authors, the genes HSPA1A and
HSPA1L reside on a particularly vulnerable CpG island, which
is subject to methylation and boosts the immune response. In
addition, the copy number variation (CNV) of the HSP genes
described for different tumors may also explain the
under-expression of the observed HSPA1A, HSPA1L and
TRAP1 genes in our study. TRAP1 expression is
correlated with the copy number, suggesting this could be one of
the driving mechanisms for the loss of TRAP1 expression in
OC (23).
Several gene expression studies identifying
molecular markers related to cancer progression have been
published. Overall, there is a considerable overlap between
previous studies and our study in terms of differentially expressed
genes between normal and tumor tissues. Furthermore, all studies
demonstrate the great diversity of tumor pathobiology, a feature
that makes cancer a difficult disease to treat effectively
(24–26).
TRAP1, HSPD1, HSPB1, HSPA1A, and
HSPA1L belong a stress or HSPs family of highly conserved
genes that are expressed in response to a wide variety of
physiological and environmental insults in order to maintain
cellular homeostasis or to contribute to cell survival to lethal
conditions. The stresses involving HSPs include such as hypoxia,
exposure to UV light and chemicals, viral agents, nutritional
deficiencies (e.g., glucose deprivation), surgical, emotional and
mechanical stress, among other stresses (27–30).
Beyond that, biological processes of proteins among HSP associated
genes analyzed by the Gene functional and Network pathway analysis
performed herein revealed their function in chaperone mediated
protein folding.
TRAP1 encodes a mitochondrial chaperone
protein that is a member of the heat shock family 90 (HSP90). The
protein has ATPase activity and interacts with tumor necrosis
factor type I (31). Interestingly,
alternate splicing results in multiple transcript variants
(32) and other study suggested that
TRAP1 has an oncogenic role in a variety of cancer types (33). In colorectal carcinoma, increased
expression of TRAP1 was correlated with increased lymph node
involvement, more advanced stages of the disease, and reduction in
overall survival. TRAP1 is currently a marker predicting worse
outcomes in colorectal cancer (34).
However, low levels of TRAP1 has been related to high tumor grade,
more advanced stage and resistance to platinum in OC (35). In OC cells lines and tissues, TRAP1
was shown to be associated with a metabolic shift, ultimately
causing the onset of resistance to cisplatin-based chemotherapy
(36). Furthermore, in OC clinical
samples, TRAP1 is often deleted in high-grade serous OC
patients and it is correlated directly with epithelial-mesenchymal
transition, which is an important determinant of the invasive
potential of tumor cells (23).
Therefore, TRAP1 downregulation is linked to tumor progression in
OC patients. Similarly, TRAP1 was under-expressed in the EOC
group compared with the NO group and higher expression was showed
in tumors at stages I/II than at III/IV (P=0.040), a finding that
could be correlate with a negative impact on the response to
chemotherapy and survival of patients with OC.
The HSPA1A, HSPA1L, HSPD1, and HSPB1
genes are expressed either constitutively or regulated inductively.
High molecular weight HSPs are ATP-dependent chaperones (HSPA1A,
HSPA1L, HSPD1), whereas small HSPs act in an ATP-independent
fashion (HSPB1). As molecular chaperones, the function of
HSPs is to regulate protein folding, transport, translocation and
assembly, particularly to refold misfolded proteins or assist their
elimination (27,30). The literature relates the
overexpression of these genes as a possible marker of worse
prognosis in other tumor types (37). It is known that in cancer there is a
need for ambiguous signal transduction, hence there are greater
demand for chaperones. The phenomenon is probably linked to the
drastic changes in protein homeostasis caused by the accumulation
of mutated proteins in cancer cells (27).
In our study, HSPA1A was the gene that
presented the lowest level of expression in relation to the NO
group. In addition, it showed a significant influence on the
overall survival of patients with EOC, who showed a decrease in
their risk of death by 0.73 times for every increase in one unit in
HSPA1A expression, suggesting a protective role for this
gene. This result highlights the potential of this gene as a
possible genetic marker to assist the clinical evaluation of the
prognosis of the disease.
The association between HSPB1 expression and
high-grade OC primary tumors and metastases was described
previously (38) and similar results
were already observed in cell lines (39). The immune response to HSPB1 is also
increased in women with OC and other gynecological tumors and some
studies suggested the use of anti-HSP27 antibody concentrations for
early diagnosis of relapse or disease progression (40). The association of HSPB1 with early
disease staging and longer survival of patients with OC, most
studies suggest an association between HSPB1 overexpression and
worse prognosis (41). There appears
to be a co-expression of HSPB1 or a positive correlation between
HSPB1 expression and resistance to chemotherapy and expression of
MDR1 (gene for resistance to multiple drugs) (42). Thus, there is evidence that the
overexpression and activity of HSP27 is associated with increased
carcinogenesis, metastatic potential, and resistance to
chemotherapy. However, in our series the expression of HSPB1
was not significantly different in the EOC group in comparison to
the control group.
This study has some limitations which have to be
considered. The small number of patients and controls do not allow
us to draw any meaningful conclusions regarding the relation
between gene expression and anatomic site or clinicopathologic
parameters. It is worth mentioning that, differently from tumors
studies in other sites, the low prevalence of ovarian tumors, along
with the ethical and biological determinants for control group
selection imposes a limiting factor of patient numbers in EOC
cohorts. So, our study was led with convenience samples, also known
as availability sampling, a specific type of nonprobability
sampling method that relies on data collection from a population
who are conveniently available to participate in study. Because of
that, it observed the unbalanced number of samples of each group.
Furthermore, the lack of immunohistochemistry to confirm the
expressions of HSP genes could be considered other limitation to
our study. However, it was designed to evaluate gene expression by
tracking messenger RNA using RT-qPCR, due access to standardized
protocols and automation ensures an accurate performance and fast
turn-around. Our findings highlight the importance of understanding
the role of HSP genes in the ovarian carcinogenesis process and
future investigations should be performed cloning HSP genes into OC
cell lines or using CRISPR gene editing to verify if
chemoresistance and others prognosis features can be altered in OC
by HSPs gene expression and confirm our results.
We can hypothesis that HSPA1A, HSPA1L and
TRAP1 downregulation seems to enhance the ability of the
cancer cells to die in a range of lethal conditions. Further
studies with a larger number of patients and longer follow-up are
necessary to assess the accuracy of the prognostic impact of these
results.
Acknowledgements
Not applicable.
Funding
The current study was financially supported from
grants from Fundação de Amparo à Pesquisa do Estado de Minas Gerais
(FAPEMIG; grant. no. CDS-APQ-02373-17), Coordenação de
Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho
Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Regarding the authorship of the manuscript, AWP,
SAL, BLC, GNG and SLM gave individual contribution in the concept
and design/analysis and interpretation of data, drafting the
manuscript or revised it critically for important intellectual
content and final approval. AWP and SAL were responsible for
patient recruitment. BLC, GNG and SLM were responsible for
performing the experimental assays.
Ethics approval and consent to
participate
The current study was approved by the Research
Ethics Committee of Vera Cruz Hospital, Belo Horizonte, Brazil,
under the protocol CAAE: 01242212.2.0000.5135. Informed consent
forms were obtained when the patients were accepted for the study
by the hospital.
Patient consent for publication
Consent for publication was obtained from all
participants of the current study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Corrado G, Salutari V, Palluzzi E,
Distefano MG, Scambia G and Ferrandina G: Optimizing treatment in
recurrent epithelial ovarian cancer. Expert Rev Anticancer Ther.
17:1147–1158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
du Bois A, Luck HJ, Meier W, Adams HP,
Möbus V, Costa S, Bauknecht T, Richter B, Warm M, Schröder W, et
al: A randomized clinical trial of cisplatin/paclitaxel versus
carboplatin/paclitaxel as first-line treatment of ovarian cancer. J
Natl Cancer Inst. 95:1320–1329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Agarwal R and Kaye SB: Prognostic factors
in ovarian cancer: How close are we to a complete picture? Ann
Oncol. 16:4–6. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu J, Liu T, Rios Z, Mei Q, Lin X and Cao
S: Heat shock proteins and cancer. Trends Pharmacol Sci.
38:226–256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ciocca DR, Arrigo AP and Calderwood SK:
Heat shock proteins and heat shock factor 1 in carcinogenesis and
tumor development: An update. Arch Toxicol. 87:19–48. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song TF, Zhang ZF, Liu L, Yang T, Jiang J
and Li P: Small interfering RNA-mediated silencing of heat shock
protein 27 (HSP27) Increases chemosensitivity to paclitaxel by
increasing production of reactive oxygen species in human ovarian
cancer cells (HO8910). J Int Med Res. 37:1375–1388. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Landriscina M, Amoroso MR, Piscazzi A and
Esposito F: Heat shock proteins, cell survival and drug resistance:
The mitochondrial chaperone TRAP1, a potential novel target for
ovarian cancer therapy. Gynecol Oncol. 117:177–182. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elstrand MB, Stavnes HT, Trope CG and
Davidson B: Heat shock protein 90 is a putative therapeutic target
in patients with recurrent advanced-stage ovarian carcinoma with
serous effusions. Hum Pathol. 43:529–535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao M, Ding JX, Zeng K, Zhao J, Shen F,
Yin YX and Chen Q: Heat shock protein 27: A potential biomarker of
peritoneal metastasis in epithelial ovarian cancer? Tumour Biol.
35:1051–1056. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim HS and Song YS: International
federation of gynecology and obstetrics (FIGO) staging system
revised: What should be considered critically for gynecologic
cancer? J Gynecol Oncol. 20:135–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res. 43
(Database Issue):D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Montgomery DC, Peck EA and Vining GG:
Introduction to linear regression analysis 5th editionJohn Wiley
& Sons; New York: 2012
|
|
16
|
Myles H and Wolfe DA: Nonparametric
Statistical MethodsJohn Wiley & Sons; New York: 1999
|
|
17
|
Colosimo EA and Giolo SR: ABE-Projeto
FisherEdgard Blücher; 2006
|
|
18
|
Kaplan EL and Meyer P: Nonparametric
estimation from incomplete observations. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
19
|
R Core Team, . A language and environment
for statistical computingR Foundation for Statistical Computing;
Vienna: 2018
|
|
20
|
Therneau TM and Grambsch PM: Modeling
survival data: Extending the Cox ModelSpringer; New York, NY:
2000
|
|
21
|
Kurman RJ and Shih Ie M: The origin and
pathogenesis of epithelial ovarian cancer: A proposed unifying
theory. Am J Surg Pathol. 34:433–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singh MK, Sharma B and Tiwari PK: The
small heat shock protein Hsp27: Present understanding and future
prospects. J Therm Biol. 69:149–154. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Amoroso MR, Matassa DS, Agliarulo I,
Avolio R, Lu H, Sisinni L, Lettini G, Gabra H, Landriscina M and
Esposito F: TRAP1 downregulation in human ovarian cancer enhances
invasion and epithelial-mesenchymal transition. Cell Death Dis.
7:e25222016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pfister K, Radons J, Busch R, Tidball JG,
Pfeifer M, Freitag L, Feldmann HJ, Milani V, Issels R and Multhoff
G: Patient survival by Hsp70 membrane phenotype: Association with
different routes of metastasis. Cancer. 110:926–935. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Braga Lda C, Silva LM, Ramos AP, Piedade
JB, Vidigal PV, Traiman P and da Silva Filho AL: Single CpG island
methylation is not sufficient to maintain the silenced expression
of CASPASE-8 apoptosis-related gene among women with epithelial
ovarian cancer. Biomed Pharmacother. 68:87–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de Lima AB, Silva LM, Goncales NG,
Carvalho MRS, da Silva Filho AL and da Conceicao Braga L:
Three-dimensional cellular arrangement in epithelial ovarian cancer
cell lines TOV-21G and SKOV-3 is associated with apoptosis-related
miRNA expression modulation. Cancer Microenviron. 11:85–92. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arrigo AP and Gibert B: HspB1, HspB5 and
HspB4 in human cancers: Potent oncogenic role of some of their
client proteins. Cancers (Basel). 6:333–365. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Beere HM: Stressed to death: Regulation of
apoptotic signaling pathways by the heat shock proteins. Sci STKE.
2001:re12001.PubMed/NCBI
|
|
29
|
Khalil AA, Kabapy NF, Deraz SF and Smith
C: Heat shock proteins in oncology: Diagnostic biomarkers or
therapeutic targets? Biochim Biophys Acta. 1816:89–104.
2011.PubMed/NCBI
|
|
30
|
Wang X, Chen M, Zhou J and Zhang X: HSP27,
70 and 90, anti-apoptotic proteins, in clinical cancer therapy
(Review). Int J Oncol. 45:18–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matassa DS, Amoroso MR, Maddalena F,
Landriscina M and Esposito F: New insights into TRAP1 pathway. Am J
Cancer Res. 2:235–248. 2012.PubMed/NCBI
|
|
32
|
Costantino E, Maddalena F, Calise S,
Piscazzi A, Tirino V, Fersini A, Ambrosi A, Neri V, Esposito F and
Landriscina M: TRAP1, a novel mitochondrial chaperone responsible
for multi-drug resistance and protection from apoptotis in human
colorectal carcinoma cells. Cancer Lett. 279:39–46. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aust S, Bachmayr-Heyda A, Pateisky P, Tong
D, Darb-Esfahani S, Denkert C, Chekerov R, Sehouli J, Mahner S, Van
Gorp T, et al: Role of TRAP1 and estrogen receptor alpha in
patients with ovarian cancer-a study of the OVCAD consortium. Mol
Cancer. 11:692012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Han JJ, Baek SK, Lee JJ, Kim GY, Kim SY
and Lee SH: Combination of TRAP1 and ERCC1 expression predicts
clinical outcomes in metastatic colorectal cancer treated with
Oxaliplatin/5-Fluorouracil. Cancer Res Treat. 46:55–64. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cappello F, Di Stefano A, David S, Rappa
F, Anzalone R, La Rocca G, D'Anna SE, Magno F, Donner CF, Balbi B
and Zummo G: Hsp60 and Hsp10 down-regulation predicts bronchial
epithelial carcinogenesis in smokers with chronic obstructive
pulmonary disease. Cancer. 107:2417–2424. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matassa DS, Amoroso MR, Lu H, Avolio R,
Arzeni D, Procaccini C, Faicchia D, Maddalena F, Simeon V,
Agliarulo I, et al: Oxidative metabolism drives
inflammation-induced platinum resistance in human ovarian cancer.
Cell Death Differ. 23:1542–1554. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vidyasagar A, Wilson NA and Djamali A:
Heat shock protein 27 (HSP27): Biomarker of disease and therapeutic
target. Fibrogenesis Tissue Repair. 5:72012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Elstrand MB, Kleinberg L, Kohn EC, Trope
CG and Davidson B: Expression and clinical role of antiapoptotic
proteins of the bag, heat shock, and Bcl-2 families in effusions,
primary tumors, and solid metastases in ovarian carcinoma. Int J
Gynecol Pathol. 28:211–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Langdon SP, Rabiasz GJ, Hirst GL, King RJ,
Hawkins RA, Smyth JF and Miller WR: Expression of the heat shock
protein HSP27 in human ovarian cancer. Clin Cancer Res.
1:1603–1609. 1995.PubMed/NCBI
|
|
40
|
Olejek A, Damasiewicz-Bodzek A, Bodzek P,
Wielkoszyński T, Zamłyński J, Stołtny P and Skutil M:
Concentrations of antibodies against heat shock protein 27 in the
sera of women with ovarian carcinoma. Int J Gynecol Cancer.
19:1516–1520. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Geisler JP, Tammela JE, Manahan KJ,
Geisler HE, Miller GA, Zhou Z and Wiemann MC: HSP27 in patients
with ovarian carcinoma: Still an independent prognostic indicator
at 60 months follow-up. Eur J Gynaecol Oncol. 25:165–168.
2004.PubMed/NCBI
|
|
42
|
Schneider J, Jimenez E, Marenbach K, Marx
D and Meden H: Co-expression of the MDR1 gene and HSP27 in human
ovarian cancer. Anticancer Res. 18:2967–2971. 1998.PubMed/NCBI
|