Introduction
Gastric cancer (GC) is one of the most common cancer
types worldwide. In 2018, 26,240 new cases were identified in the
United States of America (1).
Although current treatment modalities include surgical resection
and chemotherapy, many patients are not eligible for radical
resection or have a poor response to chemotherapy (2). Overall, the 5-year survival rate for GC
patients is at an unacceptably low rate of 31% (1).
Natural products are one of the key foundations of
medical history. Due to the complex features of GC, there is a need
for complementary therapy such as herbal medication. Molecular
properties such as high chemical heterogeneity and biocompatible
features make herbal therapy an optimal scaffold for drug
discovery. More than 50% of commonly used drugs in clinical
practice are derived from natural products (3). In oncology, this rate increases to
>60% (4). Oridonin has been shown
to induce apoptosis and autophagy in several cancer cell lines
(5–7), including GC cell lines (8). Nevertheless, the molecular mechanisms
of how oridonin contributes to GC treatment remain poorly
understood.
Oridonin has been found to activate
mitogen-activated kinases (MAPKs) (9), which play important roles in cell
proliferation and apoptosis (10).
However, the exact MAPK pathway through which oridonin induces
apoptosis in GC remains elusive. Herein, the underlying mechanisms
through which oridonin contributes to apoptosis was investigated.
The HGC-27 cell line was used for this study, as the efficacy of
oridonin has already been reported (8). The effect of oridonin on proliferation
and apoptosis was assessed and the possible MAPK pathway
responsible for activating the observed caspase-dependent apoptosis
was investigated.
Materials and methods
Cell culture
The HGC-27 cell line, obtained from The Cell Bank of
Type Culture Collection of the Chinese Academy of Sciences was used
for this investigation. The cells were cultured with RPMI-1640
supplemented with 10% fetal bovine serum, 100 U/ml penicillin and
10 µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.) in a
humidified atmosphere at 37°C and 5% CO2 atmosphere.
SP600125 (Sigma-Aldrich; Merck KGaA) was used as a general
inhibitor of JNK. Cells (3×103 per well) were treated
with 5 µM SP600125 at 37°C for 24 h.
Cell Counting Kit-8 assay
The CCK-8 assay was utilized to investigate the
cytotoxic effect of oridonin on HGC-27 cells. Cells
(3×103 per well) were seeded onto 96-well plates.
Different concentrations of oridonin (2.5, 5, 7.5, 10 and 15 µM)
were added to the cells. Following an incubation period of 12, 24,
48 and 72 h, the cells were treated with 20 µl of CCK-8 solution.
These four time points were selected to assess the change in the
overall survival of HGC-27 cells under different time periods. The
optical absorbance was assessed at 450 nm utilizing a 96-well plate
reader (Thermo Fisher Scientific, Inc.). All experiments were
performed three times.
Colony formation
Various concentrations of oridonin were allocated to
the cells, after which cells were incubated for 7 days. For assay
termination, the cells were irrigated and stabilized in ice-cold
100% methanol for 5 min, followed by staining with crystal violet
dye (Beyotime Institute of Biotechnology) for 30 min. Images of
colonies were captured using an Epson scanner (Suwa).
Real-time cell impedance
The rate of cell proliferation in a real-time cell
analysis (RTCA) was performed at 24, 48, 72 and 96 h, as per the
conventional instructions given by Roche Applied Science. In brief,
100 µl of the mixture was added to every plate, which was already
filled with 2×103 cells. Cell impedance was assessed
with the xCELLigence RTCA DP instrument (ACEA Biosciences).
Cell cycle analysis
The GC cells were first treated with oridonin.
Thereafter, the cells were settled with 66% ethanol and kept at 4°C
overnight. The following day, the ethanol was removed, irrigated
and the cells were dyed with PBS and propidium iodide (PI)
respectively. Cell cycle distribution was subsequently assessed
utilizing FASCanto II flow cytometry (BD Biosciences), according to
the manufacturer's protocols.
Cell apoptosis analysis
The Annexin V-FITC/propidium iodide (PI) detection
kit (Nanjing KeyGen Biotech Co., Ltd.) was used to monitor cell
apoptosis. Cells were first treated in the presence or absence of
oridonin for the allocated period. Subsequently, the cells were
detached and resuspended in 500 µl binding buffer containing 5 µl
of PI and 5 µl of Annexin V-FITC. Thereafter, the cells were
incubated in the dark at 25°C for 15 min prior to analysis. Cell
apoptosis was analyzed at 24 h.
Western blotting
Proteins were extracted from the cells using RIPA
buffer (cat. no. 89901; Thermo Fisher Scientific, Inc.) at 4°C.
Protein concentration was measured using the BCA protein assay kit
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. Proteins (30 µg/lane) were separated by 10% SDS-PAGE and
transferred onto PVDF membranes. The membranes were subsequently
incubated overnight at 4°C with 5% nonfat dry milk and the primary
antibodies against PARP (cat. no. 9532; from Cell Signaling
Technology, Inc.; 1:1,000), pro-caspase-3 (cat. no. 9662, 1:1,000),
cleaved-caspase-3 (cat. no. 9502, 1:1,000), pro-caspase-8 (cat. no.
9746, 1:1,000), Mcl-1 (cat. no. 94296, 1:1,000), Bcl-xl (cat. no.
2764, 1:1,000), Bcl-2 (cat. no. 15071, 1:1,000), Xiap (cat. no.
15071, 1:1,000), survivin (cat. no. 2808, 1:1,000), cyclin B1 (cat.
no. 12231, 1:1,000), p53 (cat. no. 48818, 1:1,000), p21 (cat. no.
2947, 1:1,000; all from Cell Signaling Technology, Inc.),
cleaved-caspase-9 (cat. no. ab2324; Abcam; 1:1,000), pro-caspase-9
(cat. no. ab2013; Abcam; 1:1,000) and β-actin (cat. no. 3700,
1:10,000; Cell Signaling Technology, Inc.). The following day, the
membranes were washed with PBST and further incubated for 2 h at
4°C with anti-mouse IgG (cat. no. 16402-1-AP; ProteinTech Group,
Inc.; 1:1,500) and anti-rabbit IgG (cat. no. 13688-1-AP,
ProteinTech Group, Inc.; 1:1,500) horseradish peroxidase conjugated
secondary antibodies. Next, the membranes were bands were detected
using enhanced chemiluminescence substrate (Generay Biotech Co.,
Ltd.).
Statistical analysis
Results in this study represent three independent
experiments. All data are expressed as means ± standard deviation.
ANOVA followed by Tukey's multiple comparison tests were performed
utilizing GraphPad Prism (GraphPad Software, Inc.). P<0.05 were
considered to indicate a statistically significant difference.
Results
Cytotoxic effects of oridonin on
HGC-27 cells
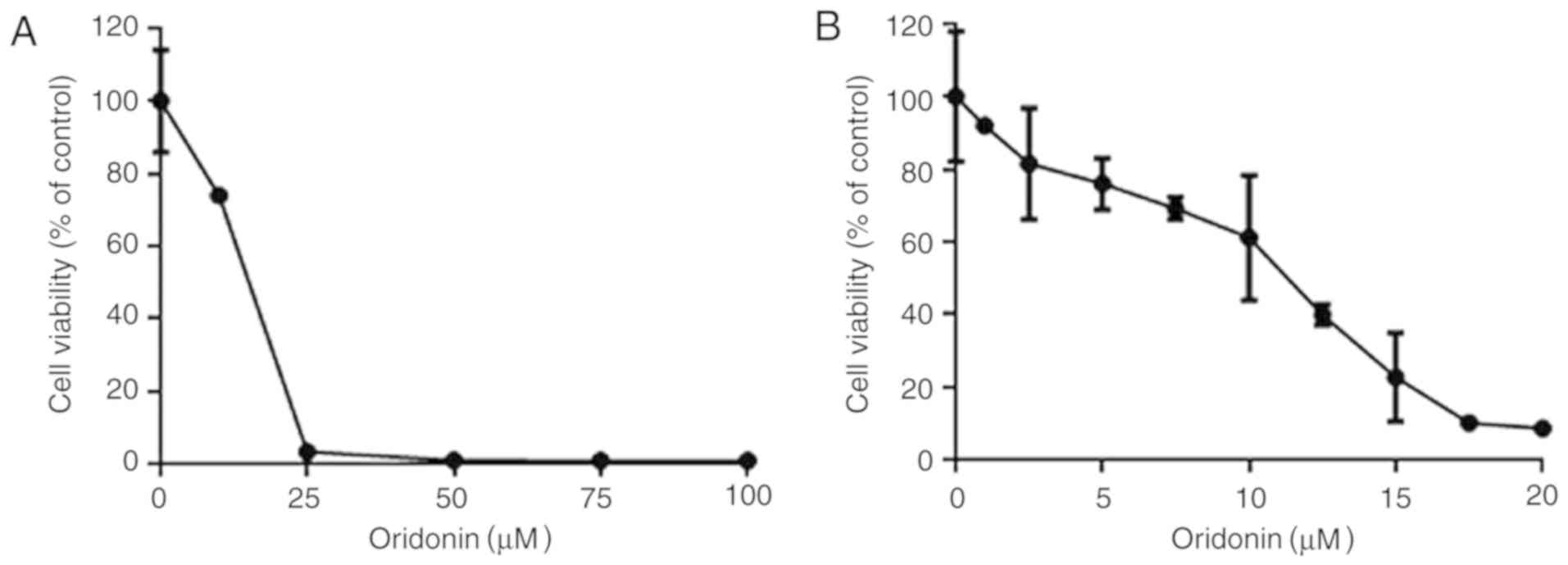
The CCK-8 assay was first used to determine whether
oridonin could effectively decrease the viability of HGC-27 cells.
Effectively, oridonin increased the cytotoxicity of GC cells in an
evident dose- and time-dependent manner in the HGC-27 line.
Following the treatment of cells with oridonin for 72 h, the IC50
values increased from 3.61 to 21.11 µM.
In order to assess the dose-response effect of
oridonin, the HGC-27 cells were cultured in 2.5, 5, 10, 15, 25, 50,
75 and 100 µM oridonin for 48 h to assess the cytotoxic effects of
the drug (Fig. 1A). Oridonin
decreased HGC-27 cell viability in a dose-dependent manner when
used at concentrations of between 2.5 and 25 µM (Fig. 1B).
Oridonin induces proliferation in
HGC-27 cells
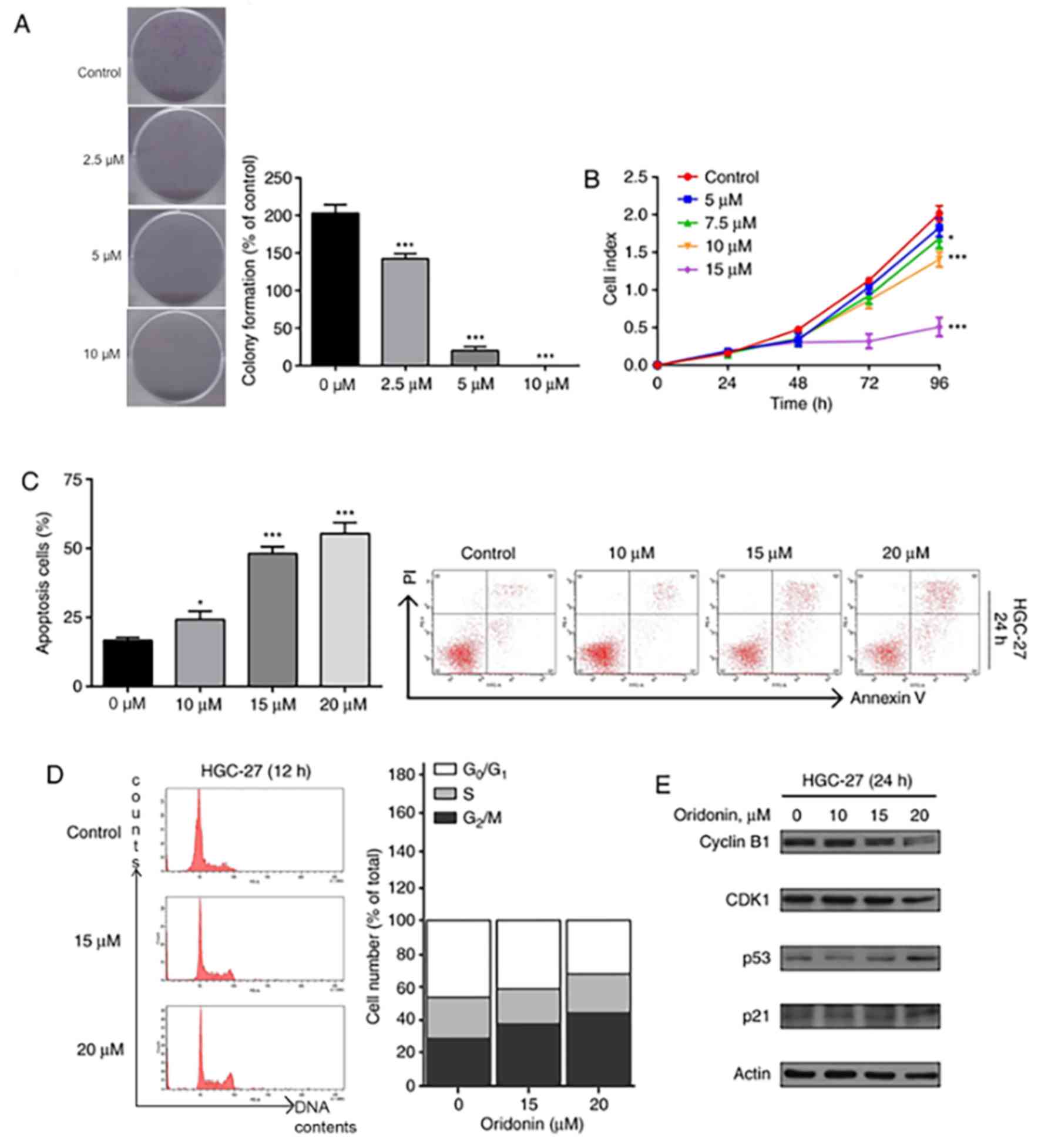
In order to investigate the effect of oridonin on
proliferation, HGC-27 cells were exposed to different doses of
oridonin (2.5–10 µM) for 7 days. The cells exposed to oridonin
formed fewer colonies (Fig. 2A) in
comparison with the control groups. These results further
demonstrate the dose-dependent inhibitory effect of oridonin on
HGC-27 cell proliferation (Fig.
2B).
Oridonin induces apoptotic cell death
in HGC-27 cells
In order to assess the properties of oridonin on
cell death in HGC-27 cells, flow cytometric analysis was conducted.
The apoptotic cell ratio was 15.7 in the control group. Upon adding
10, 15 and 20 µM oridonin to the cells, an increase in apoptotic
cells was observed at 24 h (26.3, 50.1 and 52.4, respectively;
P<0.05; Fig. 2C). Since apoptosis
was observed at 24 h, further time points were not investigated.
Thus, oridonin induces cell death in the HGC-27 cells via apoptosis
in a dose-dependent manner.
Oridonin arrests cell cycle
progression of GC cells
It was hypothesized that cell cycle arrest was the
underlying mechanism of oridonin-induced inhibition of cell
proliferation, which was investigated using flow cytometry.
Following exposure to 15 and 20 µM oridonin for 12 h, an increased
population of cells were observed at the G2/M phase
(Fig. 2D). The findings of the
present study suggest that oridonin induces cell death in HGC-27
cells by inducing apoptosis via cell cycle arrest. Furthermore, it
was speculated that the proteins responsible for apoptosis such as
cyclin B1, CDK1, p53 and p21 were involved in this process.
Effectively, western blot analysis demonstrated downregulation of
cyclin B1 and CDK1, and upregulation of p53 and p21 (Fig. 2E).
Oridonin induces caspase-dependent
apoptosis in HGC-27 cells through the JNK pathway
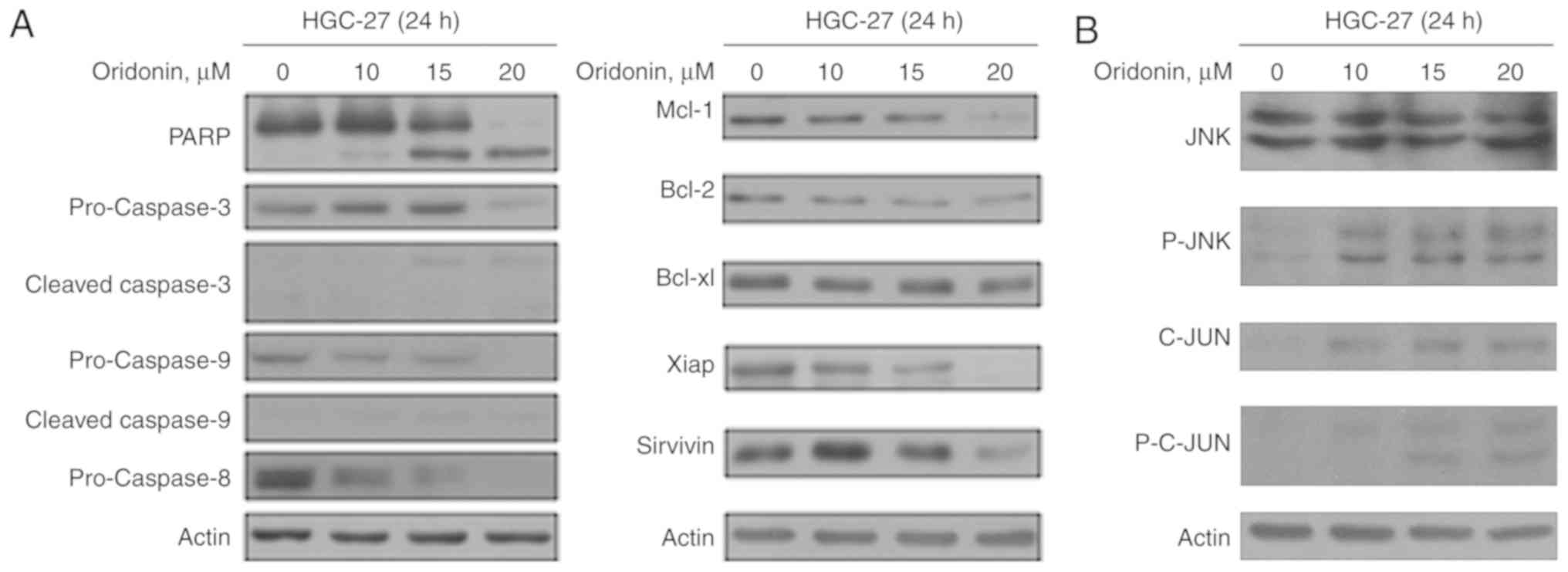
Several studies have reported that oridonin can
induce apoptosis (5,6). Following the treatment of HGC-27 cells
with oridonin, dose-dependent downregulation of the precursor forms
of caspase-3, −8, and −9, and a dose-dependent upregulation of the
cleaved forms of caspase-3, and −9 were observed (Fig. 3A). Furthermore, increased levels of
PARP was also observed. Furthermore, downregulation of the
anti-apoptotic proteins Mcl-1, Bcl-2, Bcl-xl, Xiap and survivin was
also demonstrated (Fig. 3A).
Effects of inhibiting phosphorylated
(p)-JNK, C-JUN, p-C-JUN in oridonin-treated cells
The effects of oridonin on the p-JNK pathway was
subsequently evaluated. JNK and C-JUN were activated following
oridonin treatment, and C-JUN activity was enhanced in a
dose-dependent manner (Fig. 3B).
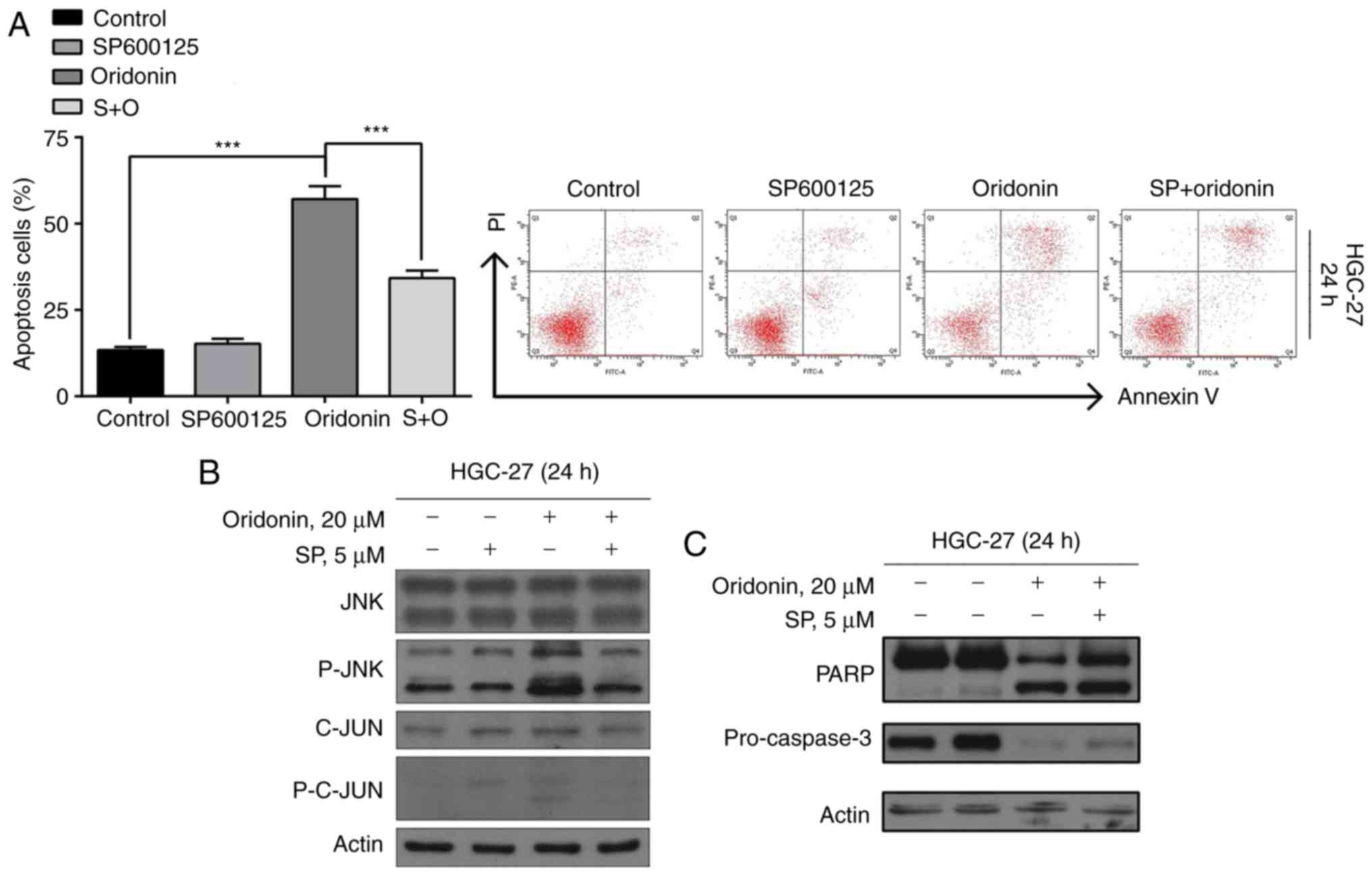
Flow cytometric analysis demonstrated that the JNK
pathway is involved in oridonin-induced apoptosis of GC cells.
SP600125 was used as a general inhibitor of JNK. Effectively,
SP600125 blocked the activation of JNK and lowered the rate of
apoptosis induced by oridonin (P<0.05; Fig. 4A).
Furthermore, the results of the western blot
analysis demonstrated that the oridonin-induced cleaved forms of
p-JNK, C-JUN, p-C-JUN (Fig. 4B),
PARP and the levels of precursor forms of caspase-3 (Fig. 4C) were restored upon the addition of
SP600125. In summary, these observations suggest that oridonin
induces apoptosis in HGC-27 cells via the p-JNK pathway.
Discussion
Oridonin is a commonly used drug in traditional
Chinese medicine. Isolated from Rabdosiarubenscens, the medicinal
drug was first recognized as having antitumor properties in 1967
(11). Since then, several studies
have found the herb to exhibit good anticancer effects on a wide
range of malignant cancer types such as colorectal cancer (12), esophageal cancer (13) and osteosarcoma (14). Previous findings have shown that
oridonin triggers apoptosis in GC through the mitochondrial pathway
(15). Herein, the findings of the
present study demonstrated that oridonin can effectively arrest
cells at the G2/M phase and induce apoptosis. Intrinsic
apoptosis was demonstrated by decreased expression of the precursor
levels of caspases-3, −8, and −9 along with increased expression of
cleaved forms of caspases-3, −8 and −9. Extrinsic apoptosis
activated the p-JNK/C-JUN pathway.
In the present study, decreased viability and
proliferation of HGC-27 cells was demonstrated, following treatment
with oridonin in a dose dependent manner. In eukaryotes, the cell
cycle is controlled by cyclins and cyclin-dependent kinases (CDKs).
Previous studies have found that cyclin B1 and CDK1 proteins
actively partake in regulating the G2/M phase (16). The overexpression of cyclin B1 and
CDK1 often results in uncontrolled cell growth. The treatment of
HGC-27 cells with different concentrations of oridonin demonstrated
the downregulation of cyclin B1 and CDK1, which led to a decreased
proportion of cells at the G2/M phase. The findings of
the present study are in accordance with previously published
studies, where cell cycle arrest was also observed at the
G2/M phase (8,16).
Subsequently, the ability of oridonin to induce
apoptosis in cells was evaluated. Apoptosis is an integral and
highly preserved mode of cell death that is essential for normal
development, host defense and inhibition of oncogenesis (6). Evading apoptosis is a distinct
characteristic of all cancer cells; thus agents that can activate
apoptosis in cancer cells are valuable in anticancer therapeutics.
In the present study, apoptosis was evaluated by flow cytometry, by
measuring the expression levels of anti-apoptotic and proapoptotic
proteins. Flow cytometry initially inferred that oridonin caused
apoptosis in HGC-27 cells. Programmed cell death was further
confirmed upon decreased expression of the precursor levels of
caspases-3, −8, and −9, increased expression of cleaved forms of
caspases-3, −8 and −9, as well as decreased expression of Mcl-1,
Bcl-2 and Bcl-xl.
A significant increase in the pro-apoptotic Bcl-2
proteins results in the formation of pores in the outer
mitochondrial membrane, which liberate apoptotic mitochondrial
proteins to activate caspases and induce apoptosis. Caspases are
activated during apoptosis in a self-amplifying cascade and play a
potent role in programmed cell death. The increase in p53 triggered
by increased expression of cleaved forms of caspases-3 and −9
suggests the activation of the intrinsic apoptosis pathway by
oridonin. Moreover, the downregulation of pro-caspase-8 suggests
that apoptosis was also triggered by the extrinsic pathway.
The findings of the present study show that oridonin
triggers the p-JNK/C-JUN pathway, which is an upstream signal for
caspase-associated apoptosis in HGC-27 cells. The association
between the signaling pathways involved in oridonin-induced
apoptosis should be further investigated. Further studies should
focus on in vivo experiments to further investigate the
anticancer mechanism of oridonin.
In conclusion, the present study indicates that
oridonin can efficiently inhibit cell proliferation and induce
caspase-dependent cell apoptosis by activating the p-JNK/C-JUN
pathway in HGC-27 cells. Moreover, these findings highlight the
importance of targeting the JNK/C-JUN signaling pathway as an
anticancer strategy. Thus, the potential of oridonin as an agent
for chemotherapeutic therapy to treat GC was demonstrated.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Program of Guangdong (grant no. 2017A010105004) and by
Outstanding Young Reserve Talents Program of the Eighth Affiliated
Hospital of Sun Yat-sen University (grant no. 2019006).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DR and RAG designed the experiments and wrote the
manuscript. XW, QZ, and HC conducted the experiments, generated
figures and contributed to the statistical analysis. XW first
conceived the concept of this study, and ensured the accuracy and
integrity of the manuscript. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Society, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs from 1981 to 2014. J Nat Prod. 79:629–661.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan W, Lu J, Huang M, Li Y, Chen M, Wu G,
Gong J, Zhong Z, Xu Z, Dang Y, et al: Anti-cancer natural products
isolated from chinese medicinal herbs. Chin Med. 6:272011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Wang Y, Wang S, Gao Y, Zhang X and
Lu C: Oridonin phosphate-induced autophagy effectively enhances
cell apoptosis of human breast cancer cells. Med Oncol. 32:3652015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bao R, Shu Y, Wu X, Weng H, Ding Q, Cao Y,
Li M, Mu J, Wu W, Ding Q, et al: Oridonin induces apoptosis and
cell cycle arrest of gallbladder cancer cells via the mitochondrial
pathway. BMC Cancer. 14:2172014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui Q, Tashiro S, Onodera S, Minami M and
Ikejima T: Oridonin induced autophagy in human cervical carcinoma
HeLa cells through Ras, JNK, and P38 regulation. J Pharmacol Sci.
105:317–325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun KW, Ma YY, Guan TP, Xia YJ, Shao CM,
Chen LG, Ren YJ, Yao HB, Yang Q and He XJ: Oridonin induces
apoptosis in gastric cancer through Apaf-1, cytochrome c and
caspase-3 signaling pathway. World J Gastroenterol. 18:7166–7174.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang H, Ye Y, Chui JH, Zhu GY, Li YW, Fong
DW and Yu ZL: Oridonin induces G2/M cell cycle arrest and apoptosis
through MAPK and p53 signaling pathways in HepG2 cells. Oncol Rep.
24:647–651. 2010.PubMed/NCBI
|
|
10
|
Burotto M, Chiou VL, Lee JM and Kohn EC:
The MAPK pathway across different malignancies: A new perspective.
Cancer. 120:3446–3456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujita E, Nagao Y, Node M, Kaneko K,
Nakazawa S and Kuroda H: Antitumor activity of the Isodon
diterpenoids: Structural requirements for the activity.
Experientia. 32:203–206. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao FH, Liu F, Wei W, Liu LB, Xu MH, Guo
ZY, Li W, Jiang B and Wu YL: Oridonin induces apoptosis and
senescence by increasing hydrogen peroxide and glutathione
depletion in colorectal cancer cells. Int J Mol Med. 29:649–655.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang JH, Pi J, Jin H and Cai JY:
Oridonin-induced mitochondria-dependent apoptosis in esophageal
cancer cells by inhibiting PI3K/AKT/mTOR and Ras/Raf pathways. J
Cell Biochem. 120:3736–3746. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang XH, Zhang SF, Bao JT and Liu FY:
Oridonin synergizes with Nutlin-3 in osteosarcoma cells by
modulating the levels of multiple Bcl-2 family proteins. Tumour
Biol. 39:10104283177016382017.PubMed/NCBI
|
|
15
|
Gao S, Tan H, Zhu N, Gao H, Lv C, Gang J
and Ji Y: Oridonin induces apoptosis through the mitochondrial
pathway in human gastric cancer SGC-7901 cells. Int J Oncol.
48:2453–2460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Zhu L, Feng X, Zhang H, Luo Q and
Chen F: Oridonin induces G2/M cell cycle arrest and apoptosis in
human oral squamous cell carcinoma. Eur J Pharmacol. 815:282–289.
2017. View Article : Google Scholar : PubMed/NCBI
|