Introduction
Esophageal cancer is the eighth type of cancer most
frequently diagnosed and the sixth leading cause of death in the
world (1). Despite advances in
surgical techniques, postoperative managements, and treatment
strategies, the mortality rate of esophageal cancer remains high
and the 5-year survival rate was reported to range from 15 to 25%
(2,3). In Japan, neoadjuvant chemotherapy (NAC)
with a combination of cisplatin plus 5-fluorouracil (FP) followed
by surgery is a standard treatment strategy for resectable stage
II/III esophageal squamous cell carcinoma (ESCC) (4). However, in patients who respond
unfavorably, the inefficient preoperative therapy should be
discontinued and surgery should not be delayed. Conversely,
patients who respond favorably to preoperative therapy may benefit
from additional preoperative treatment. Thus, it is important to
establish a method that may be used to predict the pathological
response to NAC reliably to prevent wasting time and improve
surgical outcomes.
Recently, several systemic immuno-inflammatory
responses have been reported to be independent prognostic
biomarkers in many types of malignancies, such as stomach, colon,
hepatic, lung, and esophageal cancers (5–11).
However, the clinical impact of these systemic immuno-inflammatory
responses modulated by NAC on the response to NAC for esophageal
cancer remains unclear.
In this study, we investigated the clinical
significance of changes in systemic immuno-inflammatory measures
during NAC in terms of the response to NAC and long-term outcomes
in patients with esophageal cancer.
Materials and methods
Patients
A total of 85 consecutive patients underwent a
transthoracic esophagectomy for clinical stage II or III esophageal
cancer at the National Defense Medical College Hospital (Saitama,
Japan) between January 2009 and December 2014. All patients were
administered NAC before esophagectomy. Among the 85 patients, 9
(11%) were women and 76 (89%) were men. The mean age was 68.6±0.9
years (range, 43–86 years). The tumor node metastasis criteria from
the eighth edition of the Union for International Cancer Control
classification system were used for tumor staging (12). All patients were intended to be
administered 2 courses of NAC before esophagectomy. Patients who
failed to complete 2 courses of NAC were excluded from this study.
All patients including clinically stage IV were considered to be
resectable after NAC and underwent the resection with curative
intent. Patients who underwent the palliative surgery, such as
salvage surgery or bypass surgery, were excluded from this study.
Among the 85 patients, 7 patients could not achieve oral intake
because of the obstructing locally advanced esophageal cancer, and
underwent laparoscopic jejunostomy and adequate enteral nutrition
during NAC (13).
Neutrophil count, platelet count, lymphocyte count,
CRP level, albumin concentration, and psoas mass index (PMI) were
evaluated before and after NAC. The neutrophil-to-lymphocyte ratio
(NLR) was defined as the absolute neutrophil count divided by the
absolute lymphocyte count, the platelet-to-lymphocyte ratio (PLR)
was defined as the absolute platelet count divided by the absolute
lymphocyte count, and the C-reactive protein-to-albumin ratio (CAR)
was calculated by dividing the serum CRP level by the serum albumin
level. PMI (cm2/m2) was calculated by
dividing the area of the psoas muscle at the third lumbar vertebra
in a cross-sectional computed tomography (CT) image by height
squared as previously described (14). The blood test after NAC was performed
one month after the last chemotherapy and just before the
operation. No patients were administered granulocyte-colony
stimulating factor during NAC, which should affect the
immuno-inflammatory markers.
The association between patient demographics
(information obtained from our computer database) and overall
survival (OS) was evaluated in patients with esophageal cancer. OS
was measured from the date of esophagectomy to the date of death
from any cause. Patients who survived were defined to be censored
in our survival analyses. All patients were observed at our
hospital or outpatient clinic at 3- to 4-month intervals during the
first two years of the study, and every 6 or 12 months thereafter
for three years. A CT scan was performed, and tumor markers were
assessed every 6 months until five years after the resection. Five
years after surgery, annual follow-ups were conducted by telephone
with the patients, the patient's family members, or their
practitioners. The Institutional Review Board of the National
Defense Medical College Hospital approved this protocol. All
patients provided written informed consent prior to their inclusion
in the study.
NAC and surgical procedure
All patients were intended to be administered two
courses of NAC before esophagectomy. FP chemotherapy was repeated
twice every 3 weeks with a dose of 80 mg/m2 of cisplatin
was given by intravenous drip infusion on day one and
5-fluorouracil was administered at a dose of 800 mg/m2
by continuous infusion on days one through five. Esophageal
resection was performed approximately one month after the last
infusion.
In the open surgery patients, open transthoracic
esophagectomy was performed through the right fifth or sixth
thoracotomy with adequate lymphadenectomy. In the video-assisted
surgery patients, thoracoscopic esophagectomy was performed using
three ports with diameters of 12 mm as described previously
(15). Gastric tube reconstruction
was performed by laparoscopy-assisted surgery in all patients
(16).
Histopathological response
assessment
Histopathological response was graded into five
groups. Grade 0 response indicated no evidence of effect, Grade 1a
response indicated very slight effect (viable tumor cells occupy
more than 2/3 of the tumorous area), Grade 1b response indicated
slight effect (viable tumor cells remain in more than 1/3 but less
than 2/3 of the tumorous area), Grade 2 response indicated
considerable effect (viable tumor cells remain in less than 1/3 of
the tumorous area), and Grade 3 response indicated complete
response (12).
Statistical analysis
Statistical analyses were performed using the
Mann-Whitney U or chi-square tests. The Kaplan-Meier method
was used to make the OS curve, and the survival differences were
compared with the log-rank test. A multivariate analysis was
performed with the Cox proportional hazards model, and prognostic
variables were introduced in the model when the univariate analysis
revealed a significance level of P<0.05. All values with
P<0.05 were statistically significant. A time-dependent receiver
operating characteristics (ROC) curve for censored survival
outcomes was constructed to estimate the optimal cutoff value of
the inflammatory-nutritional measures before NAC in Tables I and II. JMP 12.0 (SAS Institute Inc., Cary, NC,
USA) was used to perform all analyses.
 | Table I.Association between pretreatment NLR,
PLR, CAR and PMI measures and histopathological response to
NAC. |
Table I.
Association between pretreatment NLR,
PLR, CAR and PMI measures and histopathological response to
NAC.
|
|
| NLR, n (%) |
| PLR, n (%) |
| CAR, n (%) |
| PMI (number, %) |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Histopathological
response | Cases, n (%) | Low (<3.84) | High (≥3.84) | P-value | Low (<107.3) | High (≥107.3) | P-value | Low (<0.075) | High (≥0.075) | P-value | Low (<4.53) | High (≥4.53) | P-value |
|---|
| Grade
0, 1a | 73 (85) | 56 (92) | 17 (71) | 0.03 | 9 (90) | 64 (85) | 1.00 | 19 (90) | 54 (84) | 0.72 | 29 (40) | 44 (60) | 0.34 |
| Grade
>1b | 12 (15) | 5 (8) | 7 (29) |
| 1 (10) | 11 (15) |
| 2 (10) | 10 (16) |
| 7 (58) | 5 (42) |
|
 | Table II.Association between pretreatment
individual components of systemic immuno-inflammatory measures and
histopathological response to NAC. |
Table II.
Association between pretreatment
individual components of systemic immuno-inflammatory measures and
histopathological response to NAC.
|
|
| Neutrophil, n
(%) |
| Lymphocyte, n
(%) |
| Platelet, n
(%) |
| CRP, n (%) |
| Albumin, n (%) |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Histopathological
response | Cases, n (%) | Low
(<4,701) | High (≥4,701) | P-value | Low
(<1,459) | High (≥1,459) | P-value | Low
(<344,000) | High
(≥344,000) | P-value | Low (<0.3) | High (≥0.3) | P-value | Low (<4.0) | High (≥4.0) | P-value |
|---|
| Grade 0, 1a | 73 (85) | 46 (94) | 27 (75) | 0.02 | 38 (83) | 35 (89) | 0.53 | 59 (85) | 14 (88) | >0.99 | 30 (91) | 43 (83) | 0.35 | 55 (83) | 18 (95) | 0.28 |
| Grade >1b | 12 (15) | 3 (6) | 9 (25) |
| 8
(17) | 4 (11) |
| 10 (15) | 2
(12) |
| 3 (9) | 9
(17) |
| 11 (17) | 1 (5) |
|
Results
The prediction of the pathological responses using
pretherapeutic inflammatory-nutritional measures is presented in
Table I. The pretherapeutic NLR was
a significant predictive marker of the pathological response to
NAC. However, pretherapeutic PLR, CAR, and PMI had no influence on
pathological responses to NAC. Among the individual components of
the systemic immuno-inflammatory parameters, high neutrophil counts
before NAC were associated with a favorable pathological response
to NAC (Table II). However, this
correlation was not observed with lymphocyte, platelet, CRP, and
albumin levels.
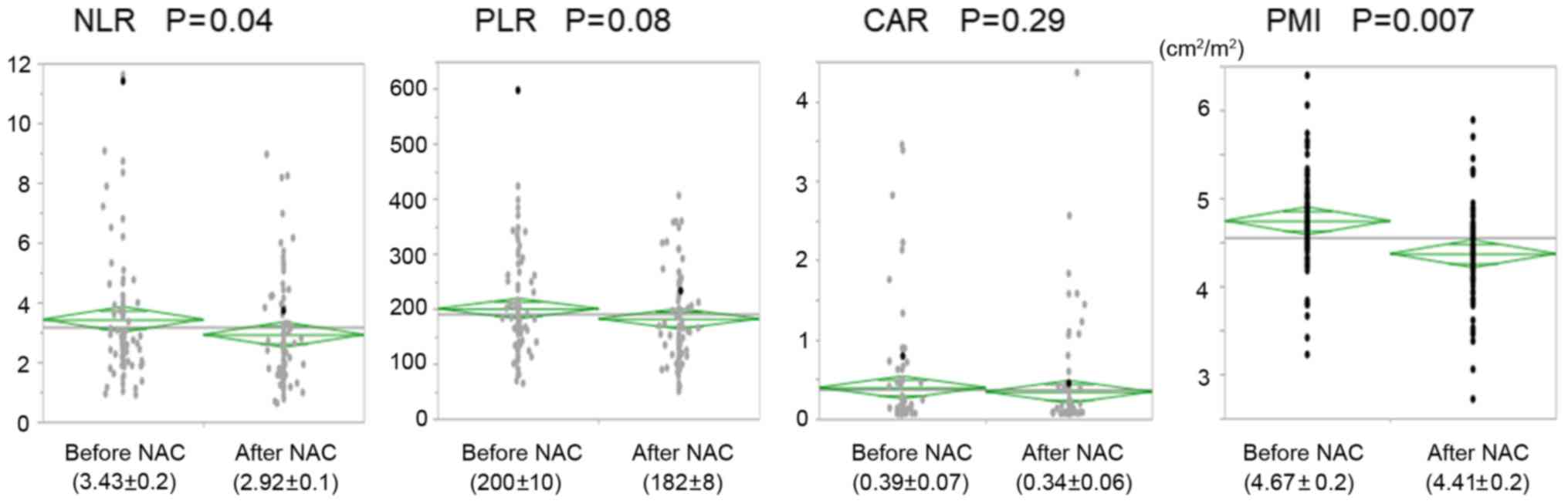
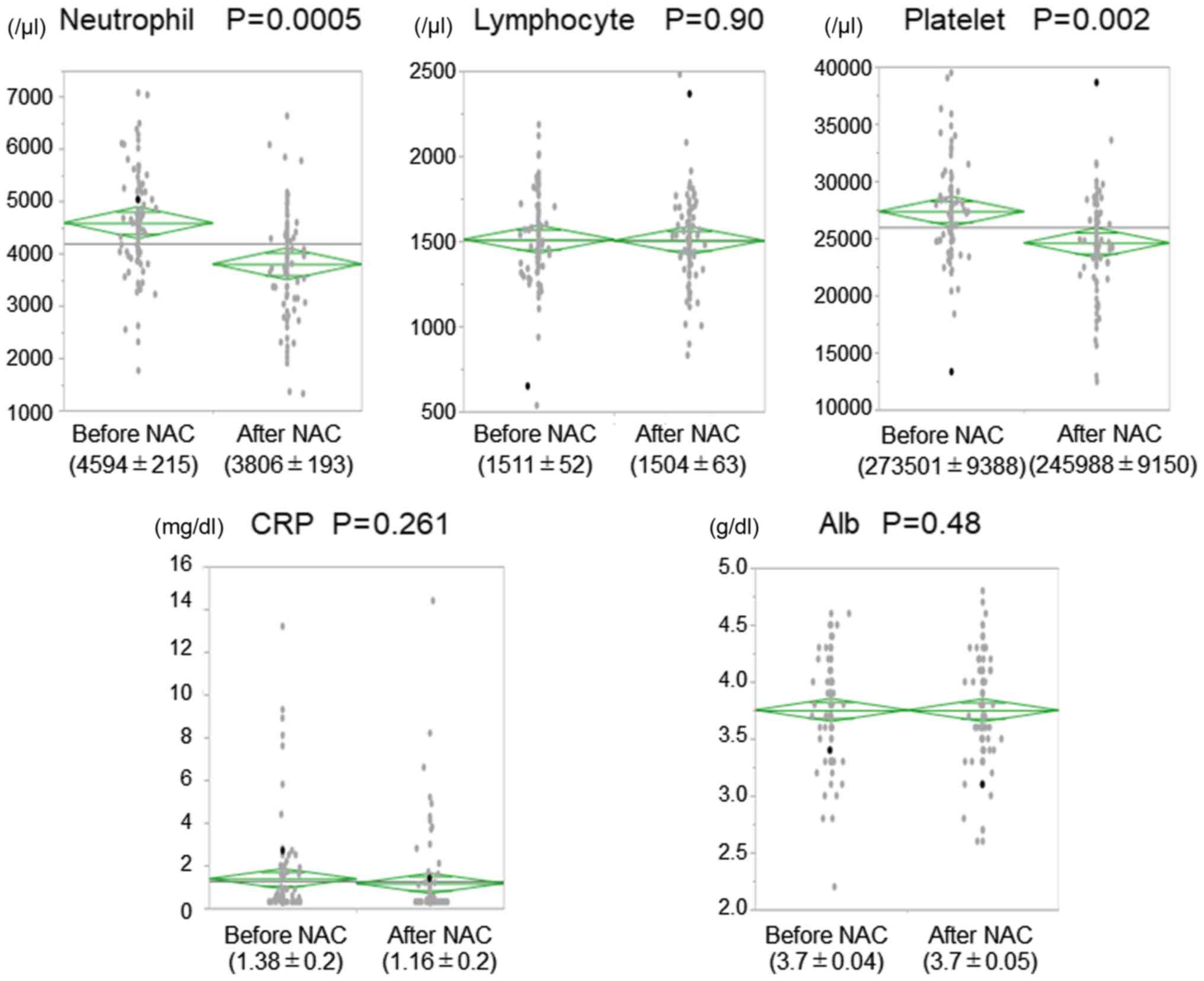
The NLR and PMI decreased significantly after NAC;
such decreases were not observed in the PLR and CAR (Fig. 1). Additionally, the neutrophil and
platelet counts were significantly decreased after NAC. However, no
changes in lymphocyte count, CRP levels, and albumin levels were
reported during NAC (Fig. 2).
Clinicopathological characteristics were compared
between patients grouped based on changes in the NLR, PLR, CAR, and
PMI after NAC (Table III).
Patients with a decreased NLR after NAC had pStage III/IV diseases
more frequently. Additionally, patients with a decreased CAR after
NAC had a better histopathological response to NAC compared to
those with an increased CAR. There were no correlations among
gender, age, surgical procedure, tumor depth, lymph node
metastasis, tumor location, histological type, tumor
differentiation, or response to NAC with the changes in the NLR,
PLR, CAR, and PMI after NAC. There were no correlations between the
postoperative complications and the changes in the NLR, PLR, CAR,
and PMI after NAC (Table SI).
 | Table III.Clinicopathological characteristics
according to the NLR, PLR, CAR and PMI measures. |
Table III.
Clinicopathological characteristics
according to the NLR, PLR, CAR and PMI measures.
|
|
| NLR, n (%) |
| PLR, n (%) |
| CAR, n (%) |
| PMI, n (%) |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Variable | Cases, n (%) | Decreased | Increased | P-value | Decreased | Increased | P-value | Decreased | Increased | P-value | Decreased | Increased | P-value |
|---|
| Sex |
|
|
| 0.48 |
|
| 0.73 |
|
| >0.99 |
|
| 0.46 |
|
Female | 9 (11) | 7 (13) | 2 (7) |
| 5 (10) | 4 (12) |
| 6 (11) | 3 (9) |
| 5 (9) | 4 (14) |
|
|
Male | 76 (89) | 48 (87) | 28 (93) |
| 47 (90) | 29 (88) |
| 47 (89) | 29 (91) |
| 52 (91) | 24 (86) |
|
| Age, years |
|
|
| 0.69 |
|
| 0.27 |
|
| 0.16 |
|
| 0.35 |
| mean ±
SD | 68.6±0.9 | 68.8±1.1 | 68.1±1.5 |
| 69.4±1.1 | 67.3±1.4 |
| 67.6±1.1 | 70.2±1.4 |
| 69.2±1.1 | 67.3±1.6 |
|
| Surgical
procedure |
|
|
| 0.65 |
|
| >0.99 |
|
| >0.99 |
|
| 0.18 |
|
Open | 34 (40) | 21 (38) | 13 (43) |
| 21 (41) | 13 (40) |
| 21 (40) | 13 (41) |
| 20 (35) | 14 (50) |
|
|
Video-assisted | 51 (60) | 34 (62) | 17 (56) |
| 31 (59) | 20 (60) |
| 32 (60) | 19 (59) |
| 37 (65) | 14 (50) |
|
| T stage |
|
|
| 0.58 |
|
| >0.99 |
|
| 0.57 |
|
| 0.40 |
|
T1/2 | 17 (20) | 10 (18) | 7 (23) |
| 10 (19) | 7 (21) |
| 12 (23) | 5 (16) |
| 13 (23) | 4 (14) |
|
|
T3/4 | 68 (80) | 45 (82) | 23 (77) |
| 42 (81) | 26 (79) |
| 41 (78) | 27 (84) |
| 44 (77) | 24 (86) |
|
| Lymph node
metastasis |
|
|
| 0.34 |
|
| >0.99 |
|
| 0.35 |
|
| 0.81 |
|
N0/1 | 55 (65) | 38 (69) | 17 (57) |
| 34 (65) | 21 (64) |
| 32 (60) | 23 (72) |
| 36 (63) | 19 (68) |
|
|
N2/3 | 30 (35) | 17 (31) | 13 (43) |
| 18 (35) | 12 (36) |
| 21 (40) | 9 (28) |
| 21 (37) | 9 (32) |
|
| pTNM stage |
|
|
| 0.02 |
|
| >0.99 |
|
| 0.45 |
|
| 0.44 |
|
I/II | 24 (28) | 20 (36) | 4 (13) |
| 15 (29) | 9 (27) |
| 17 (32) | 7 (22) |
| 18 (32) | 6 (21) |
|
|
III/IV | 61 (72) | 35 (64) | 26 (87) |
| 37 (71) | 24 (73) |
| 36 (68) | 25 (78) |
| 39 (68) | 22 (79) |
|
| Tumor location |
|
|
| 0.49 |
|
| 0.26 |
|
| >0.99 |
|
| 0.64 |
|
Upper/Middle | 49 (58) | 30 (55) | 19 (63) |
| 27 (52) | 22 (67) |
| 31 (58) | 18 (56) |
| 34 (60) | 15 (54) |
|
|
Lower | 36 (42) | 25 (45) | 11 (37) |
| 25 (48) | 11 (33) |
| 22 (42) | 14 (44) |
| 23 (40) | 13 (46) |
|
| Tumor type |
|
|
| >0.99 |
|
| 0.52 |
|
| 0.13 |
|
| >0.99 |
|
Adenocarcinoma/Others | 2 (2) | 1 (2) | 1 (3) |
| 2 (4) | 0 (0) |
| 0 (0) | 2 (6) |
| 1 (2) | 1 (4) |
|
|
Squamous | 83 (98) | 54 (98) | 29 (97) |
| 50 (96) | 33 (100) |
| 53 (100) | 30 (94) |
| 56 (98) | 27 (96) |
|
| Tumor
differentiation |
|
|
| 0.56 |
|
| 0.57 |
|
| >0.99 |
|
| >0.99 |
|
Well/Moderate | 69 (81) | 46 (84) | 23 (77) |
| 41 (79) | 28 (85) |
| 43 (81) | 26 (81) |
| 47 (81) | 22 (79) |
|
|
Poor | 16 (19) | 9 (16) | 7 (23) |
| 11 (21) | 5 (15) |
| 10 (19) | 6 (19) |
| 10 (19) | 6 (21) |
|
| Histological
response |
|
|
| 0.53 |
|
| 0.20 |
|
| 0.02 |
|
| 0.74 |
| Grade
0, 1a | 73 (86) | 46 (84) | 27 (90) |
| 47 (90) | 26 (79) |
| 42 (79) | 31 (97) |
| 48 (84) | 25 (89) |
|
| Grade
1b,2,3 | 12 (14) | 9 (16) | 3 (10) |
| 5 (10) | 7 (21) |
| 11 (21) | 1 (3) |
| 9 (16) | 3 (11) |
|
| RECIST |
|
|
| 0.23 |
|
| 0.70 |
|
| >0.99 |
|
| >0.99 |
|
PR,CR | 78 (92) | 52 (95) | 26 (87) |
| 47 (90) | 31 (94) |
| 49 (92) | 29 (91) |
| 52 (91) | 26 (93) |
|
|
SD,PD | 7 (8) | 3 (5) | 4 (13) |
| 5 (10) | 2 (6) |
| 4 (8) | 3 (9) |
| 5 (9) | 2 (7) |
|
Clinicopathological characteristics were compared
between patients who were grouped according to changes in the
individual components of the systemic immuno-inflammatory measures,
i.e., neutrophil, lymphocyte, and platelet counts; and CRP and
albumin levels after NAC (Table
IV). There were no relationships between clinicopathological
features and changes in the individual components of the systemic
immuno-inflammatory measures, except that patients with decreased
neutrophil counts after NAC had a better histological response.
 | Table IV.Clinicopathological characteristics
according to individual components of systemic immuno-inflammatory
measures. |
Table IV.
Clinicopathological characteristics
according to individual components of systemic immuno-inflammatory
measures.
|
|
| Neutrophil, n
(%) |
| Lymphocyte, n
(%) |
| Platelet, n
(%) |
| CRP, n (%) |
| Albumin, n
(%)s |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Variable | Cases, n (%) | Decreased | Increased | P-value | Decreased | Increased | P-value | Decreased | Increased | P-value | Decreased | Increased | P-value | Decreased | Increased | P-value |
|---|
| Sex |
|
|
| 0.83 |
|
| 0.73 |
|
| 0.28 |
|
| 0.49 |
|
| 0.49 |
|
Female | 9 (11) | 6 (11) | 3 (10) |
| 4 (9) | 5 (12) |
| 4 (8) | 5 (16) |
| 8 (12) | 1 (5) |
| 6 (13) | 3 (8) |
|
|
Male | 76 (89) | 48 (89) | 28 (90) |
| 40 (91) | 36 (88) |
| 49 (92) | 27 (84) |
| 57 (88) | 19 (95) |
| 40 (87) | 36 (92) |
|
| Age, years |
|
|
| 0.94 |
|
| 0.08 |
|
| 0.37 |
|
| 0.72 |
|
| 0.50 |
| mean ±
SD | 68.6±0.9 | 68.5±1.1 | 68.7±1.5 |
| 67.0±1.2 | 70.2±1.2 |
| 67.9±1.1 | 69.6±1.4 |
| 68.4±1 | 69.2±1.8 |
| 69.1±1.2 | 67.9±1.3 |
|
| Surgical
procedure |
|
|
| 0.49 |
|
| 0.65 |
|
| 0.82 |
|
| >0.99 |
|
| 0.51 |
|
Open | 34 (40) | 20 (37) | 14 (45) |
| 19 (43) | 15 (37) |
| 22 (42) | 12 (38) |
| 26 (40) | 8 (40) |
| 20 (43) | 14 (36) |
|
|
Video-assisted | 51 (60) | 34 (63) | 17 (55) |
| 25 (57) | 26 (63) |
| 31 (58) | 20 (62) |
| 39 (60) | 12 (60) |
| 26 (57) | 25 (64) |
|
| T stage |
|
|
| 0.39 |
|
| >0.99 |
|
| >0.99 |
|
| 0.06 |
|
| 0.10 |
|
T1/2 | 17 (20) | 9 (17) | 8 (26) |
| 9 (20) | 8 (20) |
| 11 (21) | 6 (19) |
| 16 (24) | 1 (5) |
| 6 (13) | 11 (28) |
|
|
T3/4 | 68 (80) | 45 (83) | 23 (74) |
| 35 (80) | 33 (80) |
| 42 (79) | 26 (81) |
| 49 (76) | 19 (95) |
| 40 (87) | 28 (72) |
|
| Lymph node
metastasis |
|
|
| >0.99 |
|
| >0.99 |
|
| >0.99 |
|
| 0.30 |
|
| 0.17 |
|
N0/1 | 55 (65) | 35 (65) | 20 (65) |
| 28 (64) | 27 (66) |
| 34 (64) | 21 (66) |
| 40 (62) | 15 (75) |
| 33 (72) | 22 (56) |
|
|
N2/3 | 30 (35) | 19 (35) | 11 (35) |
| 16 (36) | 14 (34) |
| 19 (36) | 11 (34) |
| 25 (38) | 5 (25) |
| 13 (28) | 17 (44) |
|
| TNM stage |
|
|
| 0.08 |
|
| 0.81 |
|
| 0.80 |
|
| 0.41 |
|
| 0.23 |
|
I/II | 24 (28) | 19 (35) | 5 (16) |
| 13 (30) | 11 (27) |
| 16 (30) | 8 (25) |
| 20 (31) | 4 (20) |
| 10 (22) | 14 (36) |
|
|
III/IV | 61 (72) | 35 (65) | 26 (84) |
| 31 (70) | 30 (73) |
| 37 (70) | 24 (75) |
| 45 (69) | 16 (80) |
| 36 (78) | 25 (64) |
|
| Tumor location |
|
|
| 0.65 |
|
| >0.99 |
|
| 0.26 |
|
| >0.99 |
|
| 0.06 |
|
Upper/Middle | 49 (58) | 30 (56) | 19 (61) |
| 25 (57) | 24 (59) |
| 28 (52) | 21 (66) |
| 37 (57) | 12 (60) |
| 22 (48) | 27 (69) |
|
|
Lower | 36 (42) | 24 (44) | 12 (39) |
| 19 (43) | 17 (41) |
| 25 (47) | 11 (34) |
| 28 (43) | 8 (40) |
| 24 (52) | 12 (31) |
|
| Tumor type |
|
|
| >0.99 |
|
| 0.49 |
|
| 0.52 |
|
| 0.06 |
|
| 0.49 |
|
Adenocarcinoma/Others | 2 (2) | 1 (2) | 1 (3) |
| 2 (5) | 0 (0) |
| 2 (4) | 0 (0) |
| 0 (0) | 2 (10) |
| 2 (4) | 0 (0) |
|
|
Squamous | 83 (98) | 53 (98) | 30 (97) |
| 42 (95) | 41 (100) |
| 51 (96) | 32 (100) |
| 65 (100) | 18 (90) |
| 44 (96) | 39 (100) |
|
| Tumor
differentiation |
|
|
| 0.25 |
|
| 0.58 |
|
| 0.58 |
|
| >0.99 |
|
| 0.41 |
|
Well/Moderate | 69 (81) | 46 (85) | 23 (74) |
| 37 (84) | 32 (78) |
| 44 (83) | 25 (78) |
| 53 (82) | 16 (80) |
| 39 (85) | 30 (77) |
|
|
Poor | 16 (19) | 8 (15) | 8 (26) |
| 7 (16) | 9 (22) |
| 9 (17) | 7 (22) |
| 12 (18) | 4 (20) |
| 7 (15) | 9 (23) |
|
| Histological
response |
|
|
| 0.04 |
|
| 0.12 |
|
| 0.52 |
|
| 0.06 |
|
| 0.13 |
| Grade
0, 1a | 73 (86) | 43 (80) | 30 (97) |
| 35 (80) | 38 (93) |
| 44 (83) | 29 (91) |
| 53 (82) | 20 (100) |
| 42 (91) | 31 (79) |
|
| Grade
1b, 2, 3 | 12 (14) | 11 (20) | 1 (3) |
| 9 (20) | 3 (7) |
| 9 (17) | 3 (9) |
| 12 (18) | 0 (0) |
| 4 (9) | 8 (21) |
|
| RECIST |
|
|
| 0.25 |
|
| 0.70 |
|
| >0.99 |
|
| 0.66 |
|
| 0.44 |
| PR | 78 (92) | 51 (94) | 27 (87) |
| 41 (93) | 37 (90) |
| 49 (92) | 29 (91) |
| 60 (92) | 18 (90) |
| 41 (89) | 37 (95) |
|
|
SD,PD | 7 (8) | 3 (6) | 4 (13) |
| 3 (7) | 4 (10) |
| 4 (8) | 3 (9) |
| 5 (8) | 2 (10) |
| 5 (11) | 2 (5) |
|
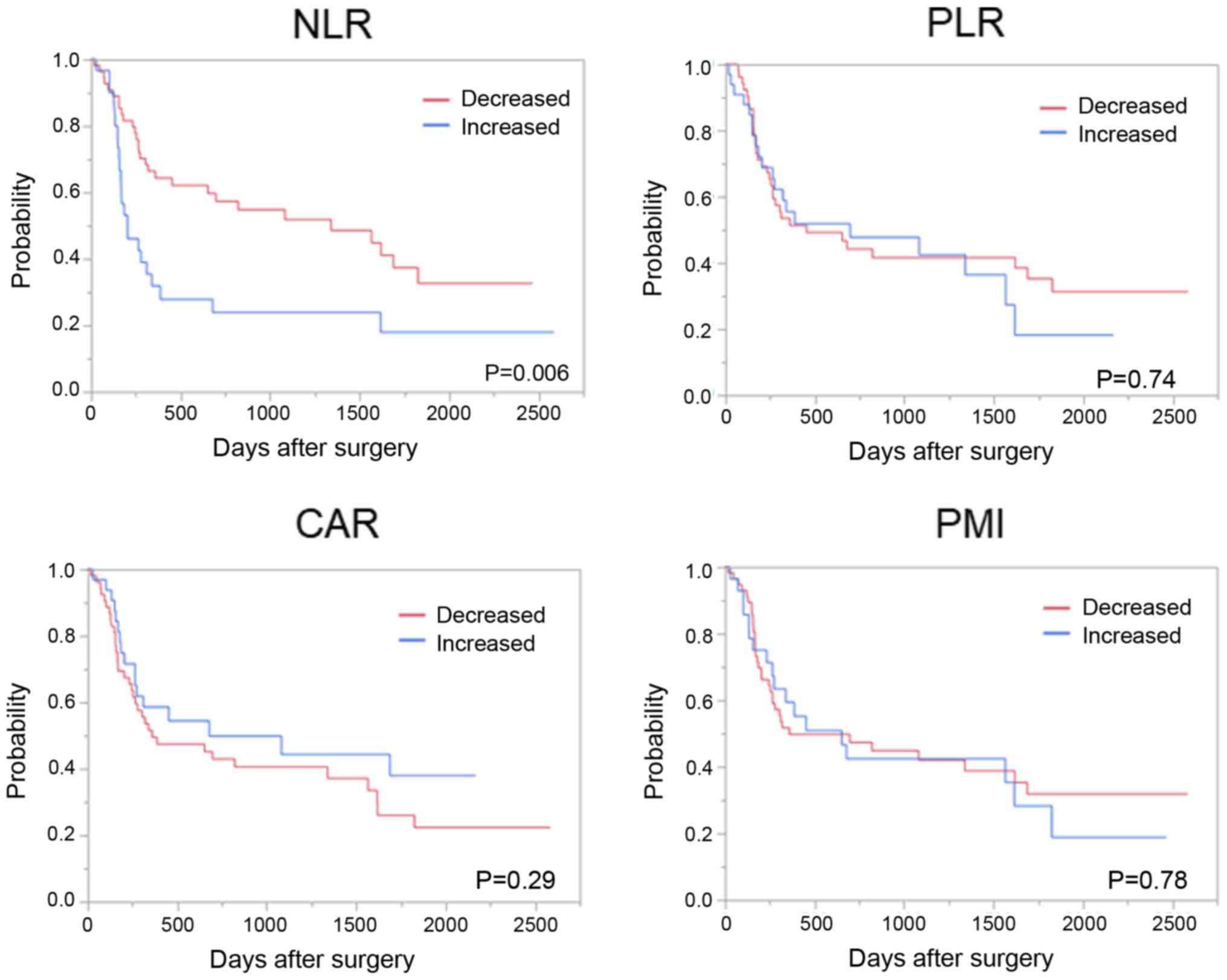
Patients with a decreased NLR after NAC had a
significantly favorable OS compared to those with an increased NLR;
however, such differences were not observed with changes in the
PLR, CAR, and PMI (Fig. 3).
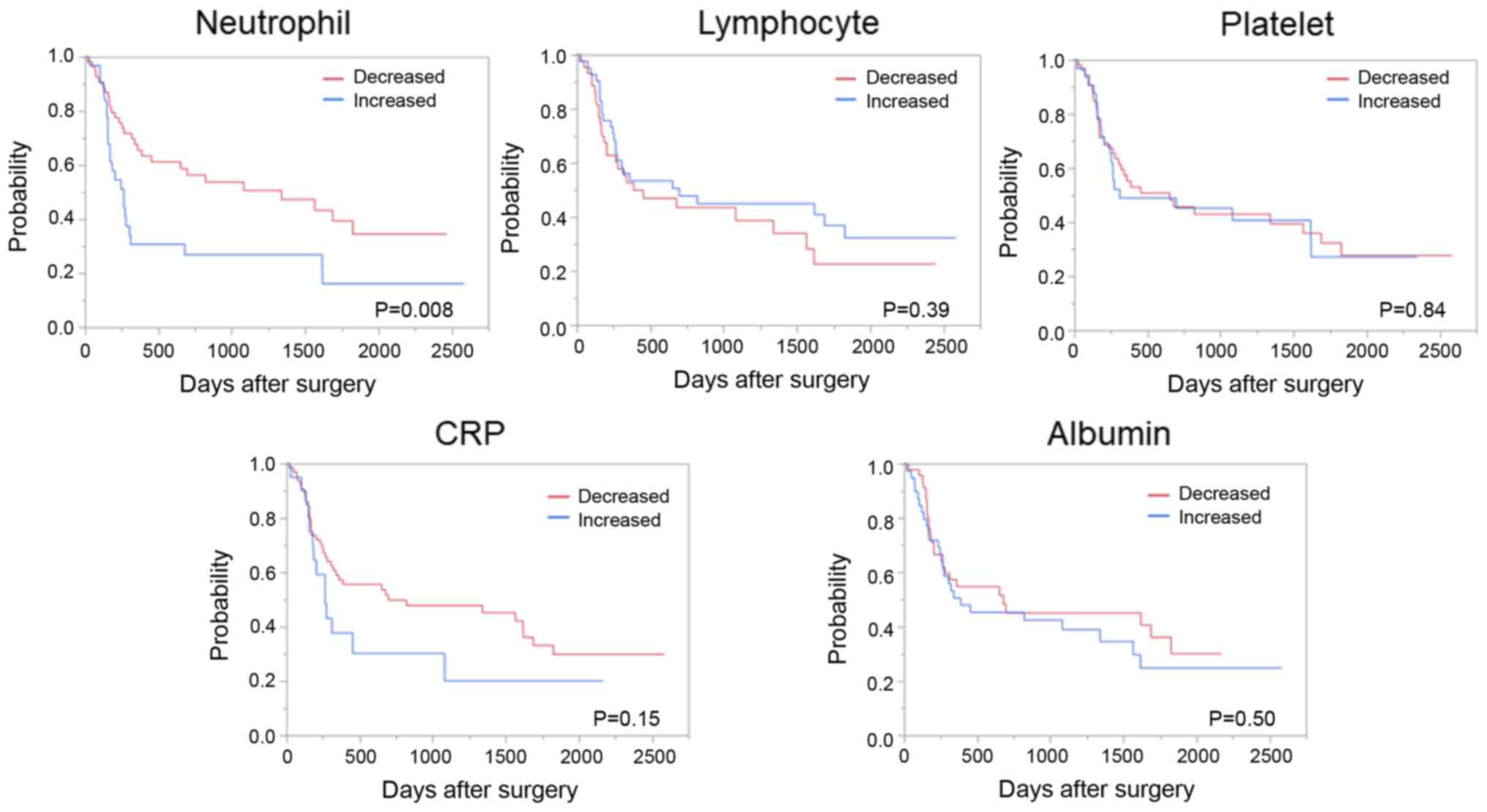
Similarly, patients with a decreased neutrophil count after NAC had
a significantly favorable OS compared to those with an increased
neutrophil count; however, such differences were not observed in
the changes in lymphocyte and platelet counts and CRP and albumin
levels (Fig. 4).
Univariate and multivariate analyses of factors that
might affect OS were performed separately for immune-inflammatory
measures (Table V) and their
individual components (Table VI)
due to their confounding. In the univariate analysis, the
pathological stage and decreased NLR after NAC were significantly
associated with favorable OS (Table
V). The multivariate analysis demonstrated that only a
decreased NLR after NAC was significantly associated with favorable
OS. Both the univariate and multivariate analyses, including the
individual components of the systemic immuno-inflammatory
parameters demonstrated that the pathological stage and decreased
neutrophil count after NAC were significantly associated with
favorable OS (Table VI).
 | Table V.Univariate and multivariate analyses
for association between overall survival of patients with
esophageal cancer and NLR, PLR CAR and PMI. |
Table V.
Univariate and multivariate analyses
for association between overall survival of patients with
esophageal cancer and NLR, PLR CAR and PMI.
| Variable | Univariate
analysis, HR (95% CI) | P-value | Multivariate
analysis, HR (95% CI) | P-value |
|---|
| Sex |
|
|
|
|
|
Female | Ref. | 0.420 |
|
|
|
Male | 1.483
(0.603–4.920) |
|
|
|
| Age, years+1 | 2.285
(0.473–12.45) | 0.320 |
|
|
| Surgical
procedure |
|
|
|
|
|
Open | Ref. | 0.830 |
|
|
|
Video-assisted | 0.944
(0.543–1.663) |
|
|
|
| p stage |
|
|
|
|
|
Stage1-2 | Ref. | 0.030 | Ref. | 0.070 |
|
Stage3-4 | 2.06
(1.093–4.227) |
| 1.811
(0.948–3.752) |
|
| Tumor location |
|
|
|
|
|
Lower/middle | Ref. | 0.210 |
|
|
|
Upper | 1.554
(0.735–2.978) |
|
|
|
| Tumor
differentiation |
|
|
|
|
|
Well/moderate | Ref. | 0.080 |
|
|
|
Poor | 1.794
(0.915–3.300) |
|
|
|
| Histopathological
response |
|
|
|
|
| Grade
1a | Ref. | 0.650 |
|
|
|
>Grade 1b | 1.181
(0.538–2.316) |
|
|
|
| NLR |
|
|
|
|
|
Increased | Ref. | 0.009 | Ref. | 0.020 |
|
Decreased | 0.468
(0.270–0.822) |
| 0.5200
(0.296–0.920) |
|
| PLR |
|
|
|
|
|
Increased | Ref. | 0.749 |
|
|
|
Decreased | 0.9099
(0.521–1.623) |
|
|
|
| CAR |
|
|
|
|
|
Increased | Ref. | 0.301 |
|
|
|
Decreased | 1.357
(0.771–2.482) |
|
|
|
| PMI |
|
|
|
|
|
Increased | Ref. | 0.790 |
|
|
|
Decreased | 0.92
(0.528–1.673) |
|
|
|
 | Table VI.Univariate and multivariate analyses
of overall survival of patients with esophageal cancer and
neutrophil, lymphocyte, platelet, CRP and Alb levels. |
Table VI.
Univariate and multivariate analyses
of overall survival of patients with esophageal cancer and
neutrophil, lymphocyte, platelet, CRP and Alb levels.
| Variable | Univariate
analysis, HR (95% CI) | P-value | Multivariate
analysis, HR (95% CI) | P-value |
|---|
| Sex |
|
|
|
|
|
Female | Ref. | 0.420 |
|
|
|
Male | 1.483
(0.603–4.920) |
|
|
|
| Age (years+1) | 2.285
(0.473–12.45) | 0.320 |
|
|
| Surgical
procedure |
|
|
|
|
|
Open | Ref. | 0.830 |
|
|
|
Video-assisted | 0.944
(0.543–1.663) |
|
|
|
| p stage |
|
|
|
|
|
Stage1-2 | Ref. | 0.030 | Ref. | 0.049 |
|
Stage3-4 | 2.06
(1.093–4.227) |
| 1.899
(1.002–3.913) |
|
| Tumor location |
|
|
|
|
|
Lower/middle | Ref. | 0.210 |
|
|
|
Upper | 1.554
(0.735–2.978) |
|
|
|
| Tumor
differentiation |
|
|
|
|
|
Well/moderate | Ref. | 0.080 |
|
|
|
Poor | 1.794
(0.915–3.300) |
|
|
|
| Histopathological
response |
|
|
|
|
| Grade
1a | Ref. | 0.650 |
|
|
|
>Grade 1b | 1.181
(0.538–2.316) |
|
|
|
| Neutrophil |
|
|
|
|
|
Increased | Ref. | 0.010 | Ref. | 0.022 |
|
Decreased | 0.484
(0.279–0.846) |
| 0.519
(0.298–0.910) |
|
| Lymphocyte |
|
|
|
|
|
Increased | Ref. | 0.395 |
|
|
|
Decreased | 1.268
(0.731–2.206) |
|
|
|
| Platelet |
|
|
|
|
|
Increased | Ref. | 0.841 |
|
|
|
Decreased | 0.944
(0.544–1.678) |
|
|
|
| CRP (mg/dl) |
|
|
|
|
|
Increased | Ref. | 0.175 |
|
|
|
Decreased | 0.641
(0.354–1.232) |
|
|
|
| Alb |
|
|
|
|
|
Increased | Ref. | 0.504 |
|
|
|
Decreased | 0.830
(0.479–1.436) |
|
|
|
Discussion
The present study is the first report to evaluate
the predictive value of the changes of various systemic
immuno-inflammatory and nutritional parameters on the therapeutic
effects of NAC and long-term outcomes in patients with esophageal
cancer. In this study, we demonstrated that patients with high
neutrophil counts before NAC had better histopathological responses
to NAC. Additionally, we reported that decreases in the NLR and
neutrophil counts after NAC were significant predictors of OS in
patients with resectable stage II/III esophageal cancer.
Although many studies have shown that systemic
immuno-inflammatory and nutritional parameters are useful
predictors of long-term outcomes, there are conflicting results
between these parameters and the response to NAC (5,17,18).
Sato et al (19) reported
that esophageal cancer patients with a lower NLR before NAC had a
better response to NAC than those with a higher NLR. There have
been similar reports for several malignancies (20,21).
Conversely, Lorente et al (22) and Graziano et al (23) did not find any significance of NLR as
a predictor of the response to NAC in breast cancer. In addition,
our present study demonstrated that a pretherapeutic higher values
of NLR and neutrophil count were related to a better response to
NAC in esophageal cancer patients. In addition, we previously
reported that the pretherapeutic higher values of NLR and CAR were
associated with unfavorable outcome (11). It is well known that
immuno-inflammatory parameters are easily affected by various
factors, such as obstruction of the tumor, anti-platelet drug use,
smoking history, and co-morbidities (11). Thus, we believe that it is important
to examine the changes in immuno-inflammatory parameters modulated
by NAC because there is a limitation to predicting the response of
NAC by these parameters just before NAC.
Konishi et al (24) reported that the severe neutrophil
decline after NAC is associated with a high histological response
in esophageal cancer, which is consistent with our present results.
There are also several reports describing how hematological
toxicity is correlated with a better response to NAC in breast and
colorectal cancers (25,26). The therapeutic effects of NAC for
cancer cells depend on whether a sufficient amount of therapeutic
agents can reach the cancer cells or whether the tumor is sensitive
to these agents. The same is true of healthy cells, particularly
hematopoietic cells. Thus, it is reasonable that hematological
toxicity is correlated with the response to chemotherapy (27).
In the present study, we presented that a
significant decline in the NLR after NAC was associated with
favorable OS and a better NAC response in patients with esophageal
cancer. Lee et al (28) also
demonstrated that a significant reduction in the NLR after
chemotherapy is associated with a better tumor response and a
favorable outcome in advanced lung cancer patients. However,
Konishi et al (24) did not
find a correlation between the grade of neutropenia and survival
outcome, although the severe neutrophil decline after NAC was
correlated with a high histological response. For this reason, they
speculated that severe neutropenia itself caused treatment-related
death and was associated with worse long-term outcomes.
PMI is a nutritional parameter and a marker of
sarcopenia that was recently reported for its prognostic value in
many malignancies (29,30). Liu et al (31) reported that a declined PMI after NAC
was associated with a poor prognosis in patients with esophageal
cancer. In our previous study, we found that decline of PMI until 6
months after esophagectomy was independently associated with the
incidence of pneumonia 6 months after resection for esophageal
cancer (32). In this study, NAC
decreased the PMI, but we could not find the relationship between
changes in the PMI during NAC and survival outcomes.
The present study was conducted at a single
institution using a retrospective design, a relatively small number
of patients, and was not included the dose intensity and
non-hematological adverse events of NAC, which are limitations of
this study. Therefore, prospective studies with more patients are
warranted to validate the results of this study.
In conclusion, among various systemic
immuno-inflammatory and nutritional parameters, a decreased NLR and
decreased neutrophil counts after NAC had clinical significance as
predictors of the response to NAC and of survival, respectively, in
esophageal cancer. Predicting responses to NAC at the early phase
of treatment, especially selecting non-responders, is significantly
important to reduce unnecessary chemotherapy or convert the
treatment to surgery before tumor progression. The systemic
immune-inflammatory and nutritional measures may help predicting
the efficacy of NAC to carefully investigate these changes during
NAC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YIs and HT conceived and designed the study. KK, ST,
YIt, NI, SN, YY, HH, ES, YK, SH and HU contributed to data
acquisition, data analysis and manuscript preparation. YIs, HT, HH,
ES, YK and HU contributed to writing of the manuscript and provided
supervision of the manuscript. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The Institutional Review Board of the National
Defense Medical College Hospital approved this protocol. All
patients provided written informed consent prior to their inclusion
in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pisani P, Parkin DM, Bray F and Ferlay J:
Estimates of the worldwide mortality from 25 cancers in 1990. Int J
Cancer. 83:18–29. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Ferlay J, Laversanne M, Brewster
DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E,
Swaminathan R, Antoni S, et al: Cancer Incidence in five
continents: Inclusion criteria, highlights from volume X and the
global status of cancer registration. Int J Cancer. 137:2060–2071.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ando N, Kato H, Igaki H, Shinoda M, Ozawa
S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, et al: A
randomized trial comparing postoperative adjuvant chemotherapy with
cisplatin and 5-fluorouracil versus preoperative chemotherapy for
localized advanced squamous cell carcinoma of the thoracic
esophagus (JCOG9907). Ann Surg Oncol. 19:68–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yodying H, Matsuda A, Miyashita M,
Matsumoto S, Sakurazawa N, Yamada M and Uchida E: Prognostic
significance of neutrophil-to-lymphocyte ratio and
platelet-to-lymphocyte ratio in oncologic outcomes of esophageal
cancer: A systematic review and meta-analysis. Ann Surg Oncol.
23:646–654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei XL, Wang FH, Zhang DS, Qiu MZ, Ren C,
Jin Y, Zhou YX, Wang DS, He MM, Bai L, et al: A novel
inflammation-based prognostic score in esophageal squamous cell
carcinoma: The C-reactive protein/albumin ratio. BMC Cancer.
15:3502015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shimada H, Takiguchi N, Kainuma O, Soda H,
Ikeda A, Cho A, Miyazaki A, Gunji H, Yamamoto H and Nagata M: High
preoperative neutrophil-lymphocyte ratio predicts poor survival in
patients with gastric cancer. Gastric Cancer. 13:170–176. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sarraf KM, Belcher E, Raevsky E, Nicholson
AG, Goldstraw P and Lim E: Neutrophil/lymphocyte ratio and its
association with survival after complete resection in non-small
cell lung cancer. J Thorac Cardiovasc Surg. 137:425–428. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagata T, Nakase Y, Nakamura K, Sougawa A,
Mochiduki S, Kitai S and Inaba S: Prognostic impact of a
nutritional index including muscle volume in stage 4 colorectal
cancer. In Vivo. 30:885–891. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kinoshita A, Onoda H, Imai N, Iwaku A,
Oishi M, Tanaka K, Fushiya N, Koike K, Nishino H and Matsushima M:
The C-reactive protein/albumin ratio, a novel inflammation-based
prognostic score, predicts outcomes in patients with hepatocellular
carcinoma. Ann Surg Oncol. 22:803–810. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ishibashi Y, Tsujimoto H, Hiraki S, Kumano
I, Yaguchi Y, Horiguchi H, Nomura S, Ito N, Shinto E, Aosasa S, et
al: Prognostic value of preoperative systemic immunoinflammatory
measures in patients with esophageal cancer. Ann Surg Oncol.
25:3288–3299. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM classification of malignant tumours. John Wiley
& Sons; 2016
|
|
13
|
Tsujimoto H, Hiraki S, Takahata R, Nomura
S, Ito N, Kanematsu K, Horiguchi H, Aosasa S, Yamamoto J and Hase
K: Laparoscopic jejunostomy for obstructing upper gastrointestinal
malignancies. Mol Clin Oncol. 3:1307–1310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hiraoka A, Aibiki T, Okudaira T, Toshimori
A, Kawamura T, Nakahara H, Suga Y, Azemoto N, Miyata H, Miyamoto Y,
et al: Muscle atrophy as pre-sarcopenia in Japanese patients with
chronic liver disease: Computed tomography is useful for
evaluation. J Gastroenterol. 50:1206–1213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsujimoto H, Takahata R, Nomura S, Yaguchi
Y, Kumano I, Matsumoto Y, Yoshida K, Horiguchi H, Hiraki S, Ono S,
et al: Video-assisted thoracoscopic surgery for esophageal cancer
attenuates postoperative systemic responses and pulmonary
complications. Surgery. 151:667–673. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsujimoto H, Ono S, Sugasawa H, Ichikura
T, Yamamoto J and Hase K: Gastric tube reconstruction by
laparoscopy-assisted surgery attenuates postoperative systemic
inflammatory response after esophagectomy for esophageal cancer.
World J Surg. 34:2830–2836. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu XL, Yu HQ, Hu W, Song Q and Mao WM: A
Novel inflammation-based prognostic score, the C-reactive
protein/albumin ratio predicts the prognosis of patients with
operable esophageal squamous cell carcinoma. PLoS One.
10:e01386572015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duan H, Zhang X, Wang FX, Cai MY, Ma GW,
Yang H, Fu JH, Tan ZH, Meng YQ, Fu XY, et al: Prognostic role of
neutrophil-lymphocyte ratio in operable esophageal squamous cell
carcinoma. World J Gastroenterol. 21:5591–5597. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sato H, Tsubosa Y and Kawano T:
Correlation between the pretherapeutic neutrophil to lymphocyte
ratio and the pathologic response to neoadjuvant chemotherapy in
patients with advanced esophageal cancer. World J Surg. 36:617–622.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng JF, Huang Y and Chen QX: Preoperative
platelet lymphocyte ratio (PLR) is superior to neutrophil
lymphocyte ratio (NLR) as a predictive factor in patients with
esophageal squamous cell carcinoma. World J Surg Oncol. 12:582014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qian Y, Tao J, Li X, Chen H, Lu Q, Yang J,
Pan H, Wang C, Zhou W and Liu X: Peripheral inflammation/immune
indicators of chemosensitivity and prognosis in breast cancer
patients treated with neoadjuvant chemotherapy. Onco Targets Ther.
11:1423–1432. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lorente D, Mateo J, Templeton AJ,
Zafeiriou Z, Bianchini D, Ferraldeschi R, Bahl A, Shen L, Su Z,
Sartor O and de Bono JS: Baseline neutrophil-lymphocyte ratio (NLR)
is associated with survival and response to treatment with
second-line chemotherapy for advanced prostate cancer independent
of baseline steroid use. Ann Oncol. 26:750–755. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Graziano V, Grassadonia A, Iezzi L, Vici
P, Pizzuti L, Barba M, Quinzii A, Camplese A, Di Marino P, Peri M,
et al: Combination of peripheral neutrophil-to-lymphocyte ratio and
platelet-to-lymphocyte ratio is predictive of pathological complete
response after neoadjuvant chemotherapy in breast cancer patients.
Breast. 44:33–38. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Konishi H, Fujiwara H, Shiozaki A,
Hiramoto H, Kosuga T, Komatsu S, Ichikawa D, Okamoto K and Otsuji
E: Effects of neutropenia and histological responses in esophageal
squamous cell carcinoma with neo-adjuvant chemotherapy. Int J Clin
Oncol. 21:95–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eryilmaz MK, Mutlu H, Salim DK, Musri FY,
Tural D and Coskun HS: The neutrophil to lymphocyte ratio has a
high negative predictive value for pathologic complete response in
locally advanced breast cancer patients receiving neoadjuvant
chemotherapy. Asian Pac J Cancer Prev. 15:7737–7740. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schuell B, Gruenberger T, Kornek GV,
Dworan N, Depisch D, Lang F, Schneeweiss B and Scheithauer W: Side
effects during chemotherapy predict tumour response in advanced
colorectal cancer. Br J Cancer. 93:744–748. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saarto T, Blomqvist C, Rissanen P, Auvinen
A and Elomaa I: Haematological toxicity: A marker of adjuvant
chemotherapy efficacy in stage II and III breast cancer. Br J
Cancer. 75:301–305. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee Y, Kim SH, Han JY, Kim HT, Yun T and
Lee JS: Early neutrophil-to-lymphocyte ratio reduction as a
surrogate marker of prognosis in never smokers with advanced lung
adenocarcinoma receiving gefitinib or standard chemotherapy as
first-line therapy. J Cancer Res Clin Oncol. 138:2009–2016. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Taguchi S, Akamatsu N, Nakagawa T, Gonoi
W, Kanatani A, Miyazaki H, Fujimura T, Fukuhara H, Kume H and Homma
Y: Sarcopenia evaluated using the skeletal muscle index is a
significant prognostic factor for metastatic urothelial carcinoma.
Clin Genitourin Cancer. 14:237–243. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meza-Junco J, Montano-Loza AJ, Baracos VE,
Prado CM, Bain VG, Beaumont C, Esfandiari N, Lieffers JR and Sawyer
MB: Sarcopenia as a prognostic index of nutritional status in
concurrent cirrhosis and hepatocellular carcinoma. J Clin
Gastroenterol. 47:861–870. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Motoyama S, Sato Y, Wakita A,
Kawakita Y, Saito H and Minamiya Y: Decreased skeletal muscle mass
after neoadjuvant therapy correlates with poor prognosis in
patients with esophageal cancer. Anticancer Res. 36:6677–6685.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nagata K, Tsujimoto H, Nagata H, Harada M,
Ito N, Kanematsu K, Nomura S, Horiguchi H, Hiraki S, Hase K, et al:
Impact of reduced skeletal muscle volume on clinical outcome after
esophagectomy for esophageal cancer: A retrospective study.
Medicine (Baltimore). 97:e114502018. View Article : Google Scholar : PubMed/NCBI
|