Introduction
Adenoid cystic carcinoma (ACC) is a relatively
uncommon malignant tumor in various gland-bearing organs, most
commonly in the salivary glands. It accounts for ~1% of all
malignant head and neck tumors and 10% of all salivary gland tumors
(1). ACC of the salivary gland
(SACC) occurs more commonly in the major salivary glands and is
reported to be the most common cancer of the minor salivary glands
(2). Histologically, SACC is
presumed to originate from the intercalated duct. It is composed of
epithelial and myoepithelial neoplastic cells that form various
patterns, including tubular, cribriform, and solid form. SACC is
characterized by a high propensity to invade nerves and an
apparently indolent clinical course, with frequent local
recurrences, a late onset of metastases and often a poor long-term
survival. The genetics of primary SACC have been extensively
investigated, which has resulted in the identification of recurrent
gene fusions involving the MYB and MYBL1 genes
(3). However, as most SACCs are
resistant to radiation therapy, the identification of additional
prognostic biomarkers is needed to predict the response of SACC to
targeted therapy and/or identify targets for individual
therapy.
Lysosome-associated protein transmembrane 4β
(LAPTM4B), a newly identified oncogene (NM_018407, Gene ID:
55353), was first cloned in human hepatocellular carcinoma (HCC)
(4). It is located on chromosome
8q22.1, spanning at least 50 kb, including seven exons and six
introns. The full-length cDNA of LAPTM4B contains two
translational initiation codons (ATG) with an interval of 273 base
pairs, and encodes at least two protein isoforms, LAPTM4B-35 and
LAPTM4B-24, a type III transmembrane protein with four
transmembrane regions. LAPTM4B-35 differs from LAPTM4B-24; it
contains an extra 91 amino acid residues at the N terminus, which
harbors a proline-rich domain, PPRP. This serves as the binding
site of the SH3 domain of some signaling molecules and plays
critical roles in the proliferation and metastatic potential of
tumor cells (5). The LAPTM4B
gene has been found to be expressed at a fairly low level in normal
human tissues except the testis and muscles, but the LAPTM4B-35
protein is upregulated in various types of carcinomas. The
overexpression of LAPTM4B-35, rather than LAPTM4B-24, has been
suggested to be closely associated with high-grade HCC (6), and is inversely correlated with overall
survival and disease-free survival of patients with HCC (7,8),
gallbladder carcinoma (9),
colorectal carcinoma (10), ovarian
carcinoma (11,12), non-small cell lung cancer (13,14),
prostate cancer (15), endometrial
carcinoma of uterus (16) and
gastric cancer (17,18). So far there is no clear evidence
suggesting that there are any clinicopathological features
associated with upregulation of LAPTM4B-35 in SACC tissues. In the
present study, we explored LAPMT4B-35 expression in indolent SACC
to identify its potential relationship with clinicopathological
features. Our results suggest that LAPTM4B-35 overexpression is
associated with high histological grade and advanced clinical
stage.
Materials and methods
General
Archived formalin-fixed, paraffin-embedded samples
were obtained from patients with SACC who were surgically treated
in The Second Affiliated Hospital of Soochow University and outside
institutes between January 2010 and December 2017. The slides were
reviewed by two pathologists. The SACC tumors were
histopathologically classified as grade I, II or III according to
WHO classification; grade I tumors mainly showed only a tubular and
cribriform pattern without a solid component; grade II tumors were
defined as cribriform with solid components of <30%; grade III
tumors were those showing solid components of ≥30%. When there was
an area of histological transformation, it was designated as
transformed. Any differences in the scores were resolved by
discussion between the two pathologists. The Ethics Committee of
the Second Affiliated Hospital of Soochow University approved the
study. All the patients consented in writing to participate in the
study.
Immunohistochemical staining
LAPTM4B-35 expression was detected using
immunohistochemistry for paraffin-embedded specimens obtained from
106 patients with SACC. A total of five normal salivary glands and
106 SACC tissues was sectioned at 4 µm and stained with H&E for
confirmation. Sections adjacent to the H&E-stained sections
were used for LAPTM4B-35 immunohistochemical (IHC) staining.
Anti-human LAPTM4B-35 rabbit polyclonal antibody
(LAPTM4B-N1-99-pAb), purified by immuno-affinity and specifically
recognizing LAPTM4B-35 (but not LAPTM4B-24), was provided by
Professor Rou-Li Zhou from the Department of Cell Biology at Peking
University Health Science Centre. The IHC analysis was performed as
described previously (8). Briefly,
the sections were deparaffinized in xylene, rehydrated in ethanol
and incubated with 0.3% hydrogen peroxide
(H2O2) for 10 min to block endogenous
peroxidase activity, then non-specific immunoglobulin binding was
blocked by incubation with 10% non-immunized normal rabbit serum
for 10 min. After washing in Tris buffer, the slides were incubated
for 1 h at room temperature with the primary rabbit polyclonal
anti-LAPTM4B-35 antibody (1 mg/ml, dilution 1:100). The slides were
then washed and incubated for 30 min with biotin-labeled secondary
antibody (animal origin: goat, catalog no.: SP-9001). Color
development was performed by incubation with horseradish
peroxidase-conjugated streptavidin for 45 min, followed by
3,3′-diaminobenzidine tetrahydrochloride (Dako) in 0.01%
H2O2 for 10 min. Finally, the slides were
counterstained with Meyer's hematoxylin for 30 sec. IHC was
performed using an IHC kit purchased from Jingmei Inc. according to
the manufacturer's instructions. Negative control slides were
stained with normal rabbit IgG at the same dilution. The positive
controls were HCC specimens with a positive expression of
LAPTM4B-35.
Semi-quantitative analysis of
LAPTM4B-35 staining
IHC analysis of LAPTM4B-35 was performed as
previously described (10). All of
the stained tissues were reviewed separately by two pathologists
experienced in evaluating IHC (W.L. and Q.Y.) who were blinded to
the clinical status. LAPTM4B-35 expression was determined
semi-quantitatively by the sum of cytoplasm staining intensity and
the percentage of positively staining tumor cells. The percentage
of immune-reactive tumor cells was scored as follows: 0 represents
<10%; 1 represents 10–50%; 2 represents >50%. The staining
intensity was scored as 0, no staining or weak staining; 1,
moderate staining; and 2, strong staining. The overall score for
LAPTM4B-35 expression was the sum of points determined for the
percentage of positive cells and staining intensity, and an overall
score ranging from 0 to 4 was assigned. For the statistical
analysis, the patients were divided into two groups. Scores of 0–2
and 3–4 were, respectively, considered to be low and high/over
expression. Any difference in the scoring was resolved by
co-observation and discussion between the two pathologists.
Statistical analysis
Statistical analyses were performed using SPSS 16.0
software (SPSS, Inc.). The Chi-square test or the Fisher's exact
were used to examine the association between LAPTM4B-35 expression
and the clinicopathological factors of the patients. A two-sided
P<0.05 was considered statistically significant.
Results
Demographic characteristics of
patients with SACC
A total of 106 cases with SACC were assessed for
LAPTM4B-35 expression. There were 45 males and 61 females aged
20–87 years, with a median age of 50 years. The patients were
divided into two groups based on their age (≤50 and >50 years).
Of the 106 patients, 43 (40.57%) had tumors categorized as
histological grade I, 33 (31.13%) had grade II tumors, 26 (24.53%)
had grade III tumors, and 4 (3.77%) cases showed transformation. A
standardized neck dissection involving levels I, I to III, or I to
IV/V was performed in 22 patients (20.75%). There were no surgical
resection specimens in 11 cases diagnosed by biopsy only. Thus,
some clinical related information, such as tumor size and TNM
stage, was not available for these 11 cases. The main
characteristics of the patients are presented in Table I.
 | Table I.Relationship between LAPTM4B-35
overexpression and clinicopathological features of SACC. |
Table I.
Relationship between LAPTM4B-35
overexpression and clinicopathological features of SACC.
| Variables | Patients total
(n) | n | LAPTM4B-35
overexpression (%) | P-value |
|---|
| All cases | 106 |
|
|
|
| Sex |
| Male | 45 | 10 | 22.22 | 0.51 |
|
Female | 61 | 17 | 27.87 |
|
| Age (years) |
| ≤50 | 55 | 17 | 30.91 | 0.18 |
|
>50 | 51 | 10 | 19.61 |
|
| Tumor location |
| Major
salivary gland | 47 | 13 | 27.66 | 0.64 |
| Minor
salivary gland | 59 | 14 | 23.73 |
|
| Tumor size |
| ≤2
cm | 32 | 9 | 28.13 | 0.53a |
| >2
cm | 63 | 14 | 22.22 |
|
| Histopathological
grade |
| G1 | 43 | 6 | 13.95 | 0.001 |
| G2 | 33 | 6 | 18.18 |
|
| G3 and
transformed | 30 | 15 | 50.00 |
|
| TNM stage |
| I,
II | 77 | 15 | 19.48 | 0.026a |
| III,
IV | 18 | 8 | 44.44 |
|
| Lymph node
metastasis |
|
Absent | 89 | 20 | 22.47 | 0.62a |
|
Present | 6 | 2 | 33.33 |
|
Expression of LAPTM4B-35 in normal
salivary glands
Salivary glands are divided into major and minor
glands. According to the type of epithelial cell and their
secretory product, the acini are classified in three forms: serous,
mucoserous, and mucous. First, we detected the expression of
LAPTM4B-35 in five normal salivary glands by means of IHC analysis.
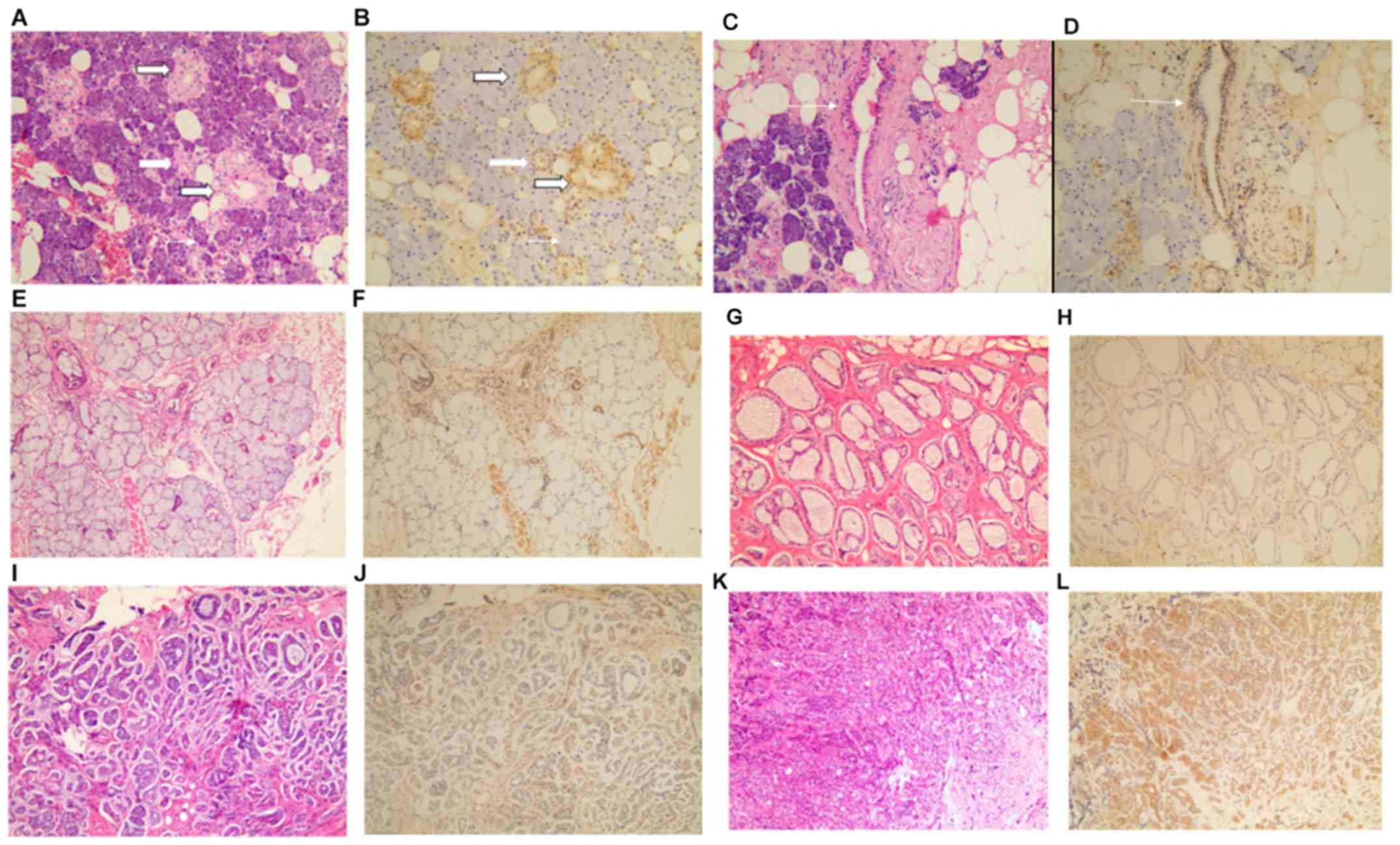
Representative examples of LAPTM4B-35 staining are shown in
Fig. 1. In all salivary glands
tested, LAPTM4B-35 expression was variable among different parts of
the glands and cell types. It was expressed at a very low level in
acini, both in serous and mucous acini, at a low level in
intercalated duct and excretory duct cells and moderately in
secretory/striated ducts (Fig.
1A-F). Consistent with previous studies, the positive
LAPTM4B-35 signal was mainly localized in the cell cytoplasm.
LAPTM4B-35 protein expression in SACC
and its relationship with clinicopathological characteristics
LAPTM4B-35 expression was low in 79 of 106 patients
(74.53%) and high in 27 of 106 patients (25.47%), according to the
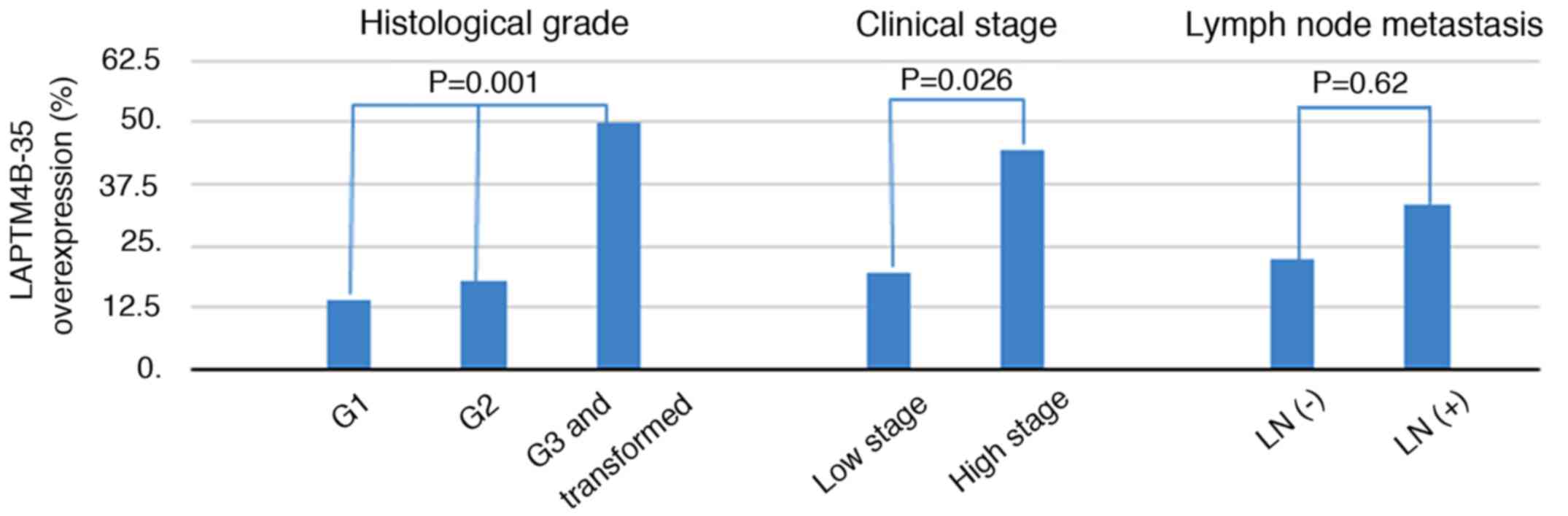
criteria mentioned above. Importantly, high LAPTM4B-35 expression
was identified in 6/43 (13.95%) of histological grade I samples,
6/33 (18.18%) of grade II, and 15/30 (50.00%) of grade III and
transformed tumor samples. As shown in Fig. 2, the LAPTM4B-35 immunoreactivity was
significantly associated with histological grade (P=0.001, Fig. 2). We then grouped grade I and II as
low grade, and grade III and transformed as high grade. High
LAPTM4B-35 expression was higher in high grade SACC (15/30, 50.0%),
compared to 12/76 (15.8%) in low grade SACC (P=0.0003). In
addition, as shown in Table I and
Fig. 2, a significant difference was
also found between high LAPTM4B-35 expression and advanced clinical
stage (8/18 vs. 15/77, P=0.026). As shown in Table I, although no statistically
significant differences were identified between LAPTM4B-35
expression and age or lymph node metastasis, there was a trend that
a high expression of LAPTM4B-35 was more common in younger patients
(17/55, 30.91% vs. 10/51, 19.61%, P=0.18), and lymph node
metastasis (2/6, 33.33% vs. 20/89, 22.47%, P=0.62) (Fig. 2). However, no significant association
was observed between LAPTM4B-35 immunoreactivity and sex, tumor
size or location (P>0.05).
Discussion
A number of previous studies have shown that
LAPTM4B-35 overexpression occurs with high frequency (62–88%) in a
large number of carcinomas that grow rapidly, and plays critical
roles in tumorigenesis, progression, metastasis, recurrence, and
drug resistance (5,9–11,13–18).
To the best of our knowledge, this is the first report of
LAPTM4B-35 expression in a clinically indolent malignancy, SACC. We
found that the total high expression frequency of LAPTM4B-35 in
SACC tested was only 25.47% (27/106). Most SACC express LAPTM4B-35
at relatively low level, but at a high level in 50% of SACC with
high pathological grade and advanced clinical stage. Although
growing slowly, SACC is a life-threatening malignancy for its
propensity of recurrence and tumor-related death continuously
occurring within 30 years after primary treatment. Thus, the
identification of novel prognostic biomarkers for SACC is warranted
to predict and improve the prognosis of SACC. In this study, we
demonstrated an association between high LAPTM4B-35 expression with
clinicopathological parameters of SACC. Previous studies have shown
that LAPTM4B-35 expression was dramatically elevated in high grade
(above grade III) carcinomas (5,8–11,13–18). In
line with previous findings, comparing SACC with the paired
compartment (Fig. 1), a higher
LAPTM4B-35 protein expression was correlated with more aggressive
features, including high histopathological grade and advanced
clinical stage. In addition, there was a propensity that a high
LAPTM4B-35 expression was associated with younger age of onset or
lymph node metastasis. Lymph node metastasis may be underestimated
based on the uncompleted specimen from lymph nodes and the limited
number of cases, which may affect the power of the statistical
analyses. A larger sample size remains to be studied to further
verify these results (19). There
was no association between LAPTM4B-35 expression and sex, age,
tumor location or size.
LAPTM4B has been reported widely expressed in
human tissues with a relatively high expression level in heart,
skeletal muscle, and testis, a moderate expression level in ovary,
kidney, and pancreas, a low expression level in liver, spleen, and
thymus, and the lowest expression in lung and peripheral leukocytes
(4). In this study, LAPTM4B-35
showed variable expression level in normal salivary glands. It was
negative in acini cells, weak in intercalated duct and excretory
duct cells, and moderate in striated ducts cells. The function of
LAPTM4B-35 in normal salivary glands remains to be clarified. On
the other hand, it is interesting to note that previous studies
focused on tumors derived from one cell type. As SACC is composed
to two types of cells, the epithelial and myoepithelial neoplastic
cells, LAPTM4B-35 might also play an important role in cancers that
originate from two cell types. The role of LAPTM4B-35 in epithelial
and myoepithelial neoplastic cells merits further
investigation.
Conventional prognostic factors for salivary gland
carcinomas include advanced stage, close or involved surgical
margins, vascular invasion, and high histological grade. However,
the infiltrative nature of SACC makes it a complex task to achieve
both tumor-free margins and preserve vital structures, function,
and acceptable cosmetic outcome. Recently, some significant
prognostic factors of SACC, including clinicopathological
characteristics and molecular markers, were identified.
Interestingly, intraneural but not perineural invasion has been
identified to be an independent predictor of poor prognosis
(20). In contrast to more common
malignancies, only very few molecular-based prognostic studies have
been carried out in SACC. Overall, SACC exhibit a low mutational
burden. Deletion of the chromosomal region 1p36 has been identified
predominantly in solid type ACC and was associated with an
aggressive clinical course (21).
NOTCH1 mutations were identified in a subgroup of patients
with solid subtype, advanced-stage disease and poor prognosis.
Preliminary data showed that a Notch1 inhibitor had antitumor
activity and may serve as a potential therapeutic target (22). On the other hand, it has been
reported that LAPTM4B-35 is a cancer driver gene, and its high
expression plays fundamental oncogenic and onco-progressive roles.
It has been shown to be an independent prognostic factor that may
be a potential biomarker for prognostic prediction and may be a
novel therapeutic target (23). The
present study suggests that this may also be true in the context of
SACC (24).
There are also some limitations to the present
study. First, due to unavailable commercial SACC cell lines, we
were not able to perform functional study to support our current
findings. Second, formalin fixed paraffin embedded (FFPE) tissue
was the only research material in the study. The results could not
be validated by western blot analysis or qPCR analysis due to the
poor quality of protein and mRNA extracted from FFPE tissue,
although previous studies showed increased expression of LPTM4B-35
by both IHC and western blot analysis in ovarian carcinoma
(12). Lastly, as SACC is an
indolent malignancy and progresses slowly, there is limited
follow-up data that could be used for survival analysis. A future
study is warranted to evaluate the association between LAPTM4B-35
expression and prognosis using long-term follow-up data.
Taken together, the present study shows that high
LPTM4B-35 expression is associated with high histological grade and
advanced clinical stage. LAPTM4B-35 may be a subtype-specific
marker for SACC and is a potential prognostic biomarker for the
management of SACC.
Acknowledgements
The authors would like to thank Professor Rou Li
Zhou for her help in the preparation of the manuscript.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (no. 31100944) and Postdoctoral
Science Foundation of Jiangsu Province (no. 1402080B).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JF wrote the manuscript. JF, JY and WQ led the
conception and design of this study. JF, WL and CX were responsible
for the data collection and analysis. JY and CX were in charge of
interpreting the immunohistochemistry results. JF and WQ helped
with statistical analysis. All the authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Second Affiliated Hospital of Soochow University, China. Signed
informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Coca-Pelaz A, Rodrigo JP, Bradley PJ,
Vander Poorten V, Triantafyllou A, Hunt JL, Strojan P, Rinaldo A,
Haigentz M Jr, Takes RP, et al: Adenoid cystic carcinoma of the
head and neck - An update. Oral Oncol. 51:652–661. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
International Head; Neck Scientific Group,
: Cervical lymph node metastasis in adenoid cystic carcinoma of the
sinonasal tract, nasopharynx, lacrimal glands and external auditory
canal: A collective international review. J Laryngol Otol.
130:1093–1097. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Togashi Y, Dobashi A, Sakata S, Sato Y,
Baba S, Seto A, Mitani H, Kawabata K and Takeuchi K: MYB and MYBL1
in adenoid cystic carcinoma: Diversity in the mode of genomic
rearrangement and transcripts. Mod Pathol. 31:934–946. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shao GZ, Zhou RL, Zhang QY, Zhang Y, Liu
JJ, Rui JA, Wei X and Ye DX: Molecular cloning and characterization
of LAPTM4B, a novel gene upregulated in hepatocellular carcinoma.
Oncogene. 22:5060–5069. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu X, Xiong F, Wei X, Yang H and Zhou R:
LAPTM4B-35, a novel tetratransmembrane protein and its PPRP motif
play critical roles in proliferation and metastatic potential of
hepatocellular carcinoma cells. Cancer Sci. 100:2335–2340. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu XR, Zhou RL, Zhang QY, Zhang Y, Jin
YY, Lin M, Rui JA and Ye DX: Structure analysis and expressions of
a novel tetratransmembrane protein, lysosoma-associated protein
transmembrane 4 beta associated with hepatocellular carcinoma.
World J Gastroenterol. 10:1555–1559. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang H, Xiong F, Qi R, Liu Z, Lin M, Rui
J, Su J and Zhou R: LAPTM4B-35 is a novel prognostic factor of
hepatocellular carcinoma. J Surg Oncol. 101:363–369. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang H, Xiong FX, Lin M, Yang Y, Nie X and
Zhou RL: LAPTM4B-35 overexpression is a risk factor for tumor
recurrence and poor prognosis in hepatocellular carcinoma. J Cancer
Res Clin Oncol. 136:275–281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou L, He XD, Chen J, Cui QC, Qu Q, Rui
JA and Zhao YP: Overexpression of LAPTM4B-35 closely correlated
with clinicopathological features and post-resectional survival of
gallbladder carcinoma. Eur J Cancer. 43:809–815. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang Y, Yin M, Jiang W, Zhang H, Xia B,
Xue Y and Huang Y: Overexpression of LAPTM4B-35 is associated with
poor prognosis in colorectal carcinoma. Am J Surg. 204:677–683.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yin M, Li C, Li X, Lou G, Miao B, Liu X,
Meng F, Zhang H, Chen X, Sun M, et al: Over-expression of LAPTM4B
is associated with poor prognosis and chemotherapy resistance in
stages III and IV epithelial ovarian cancer. J Surg Oncol.
104:29–36. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Y, Yang H, McNutt MA, Xiong F, Nie X,
Li L and Zhou R: LAPTM4B overexpression is an independent
prognostic marker in ovarian carcinoma. Oncol Rep. 20:1077–1083.
2008.PubMed/NCBI
|
|
13
|
Tang H, Tian H, Yue W, Li L, Li S, Gao C,
Si L, Qi L and Lu M: Overexpression of LAPTM4B is correlated with
tumor angiogenesis and poor prognosis in non-small cell lung
cancer. Med Oncol. 31:9742014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kong F, Gao F, Chen J, Sun Y, Zhang Y, Liu
H, Li X, Yang P, Zheng R, Liu G, et al: Overexpressed LAPTM4B-35 is
a risk factor for cancer recurrence and poor prognosis in
non-small-cell lung cancer. Oncotarget. 7:56193–56199. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang H, Wei Q, Liu R, Qi S, Liang P, Qi
C, Wang A, Sheng B, Li L and Xu Y: Overexpression of LAPTM4B-35: A
novel marker of poor prognosis of prostate cancer. PLoS One.
9:e910692014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meng FL, Yin MZ, Song HT, Yang H, Lou G
and Zhou RL: LAPTM4B-35 overexpression is an independent prognostic
marker in endometrial carcinoma. Int J Gynecol Cancer. 20:745–750.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng X, Zheng Z, Bu Z, Wu X, Zhang L,
Xing X, Wang X, Hu Y, Du H, Li L, et al: LAPTM4B-35, a
cancer-related gene, is associated with poor prognosis in TNM
stages I–III gastric cancer patients. PLoS One. 10:e01215592015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu L, Xu X, Jing L, Zhou G, Cao Z, Han Y
and Zhou R: Lysosomal-associated protein transmembrane 4 Beta-35
overexpression is a novel independent prognostic marker for gastric
carcinoma. PLoS One. 10:e01180262015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amit M, Binenbaum Y, Sharma K, Ramer N,
Ramer I, Agbetoba A, Glick J, Yang X, Lei D, Bjørndal K, et al:
Incidence of cervical lymph node metastasis and its association
with outcomes in patients with adenoid cystic carcinoma. An
international collaborative study. Head Neck. 37:1032–1037. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amit M, Binenbaum Y, Trejo-Leider L,
Sharma K, Ramer N, Ramer I, Agbetoba A, Miles B, Yang X, Lei D, et
al: International collaborative validation of intraneural invasion
as a prognostic marker in adenoid cystic carcinoma of the head and
neck. Head Neck. 37:1038–1045. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Šteiner P, Andreasen S, Grossmann P, Hauer
L, Vaněček T, Miesbauerová M, Santana T, Kiss K, Slouka D and
Skálová A: Prognostic significance of 1p36 locus deletion in
adenoid cystic carcinoma of the salivary glands. Virchows Arch.
473:471–480. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ferrarotto R, Mitani Y, Diao L, Guijarro
I, Wang J, Zweidler- McKay P, Bell D, William WN Jr, Glisson BS,
Wick MJ, et al: Activating NOTCH1 mutations define a distinct
subgroup of patients with adenoid cystic carcinoma who have poor
prognosis, propensity to bone and liver metastasis, and potential
responsiveness to Notch1 inhibitors. J Clin Oncol. 35:352–360.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li ZR: LAPTM4B: A novel diagnostic
biomarker and therapeutic target for hepatocellular carcinoma.
Hepatocellular Carcinoma. Lau WY: InTech Press; Melbourne, FL: pp.
1–34. 2012
|
|
24
|
Yang H, Xiong F, Wei X, Yang Y, McNutt MA
and Zhou R: Overexpression of LAPTM4B-35 promotes growth and
metastasis of hepatocellular carcinoma in vitro and in vivo. Cancer
Lett. 294:236–244. 2010. View Article : Google Scholar : PubMed/NCBI
|