Introduction
Lung cancer is a common malignancy with high
morbidity and mortality rates. According to a survey by Chen et
al (1), the incidence of lung
cancer in China ranks now first among all types of cancer. Lung
cancer also presents a high rate of metastases (2,3).
Metastasis is a complex multi-step process that comprises numerous
genes and several factors, including angiogenesis factors,
extracellular metal matrix proteases and adhesion molecules
(4,5). The development of lung cancer involves
many tumor suppressor genes that are downregulated, such as p53
(6), Rb (7) and Fhit (8), as well as the abnormal overexpression
of oncogenes, such as CDCA7 (9),
KIF20A (10) and CCNB2 (11). Non-small cell lung carcinoma (NSCLC),
which is a type of lung cancer, is divided into four histological
subtypes, including adenocarcinoma, squamous cell carcinoma, large
cell carcinoma, and NSCLC not otherwise specified (12). Adenocarcinoma and squamous cell
carcinoma represent ~80% of all NSCLC cases (13). The various types of lung cancer
possess distinct histological features and display different
biological behavior (14), which
influence the choice of treatment and the prognosis of patients
with lung cancer (15). Determining
the molecular profiles of all types of lung cancer in order to
develop novel therapies is therefore essential (16). The present study aimed to investigate
the effect of hypoxia on all histological types of lung cancer. It
has been demonstrated that a hypoxic microenvironment can inhibit
tumor apoptosis and promote DNA repair, increasing therefore cancer
invasion and metastasis and promoting radiochemotherapy resistance
(17,18).
HIF-1α is a crucial transcription factor that
regulates oxygen homeostasis, serving therefore a pivotal role in
tumor hypoxia (19). An increased
expression of HIF-1α has been observed in various types of human
cancer, including NSCLC, and can be associated with poor prognosis
in some cases (20,21). HIF-1α level is regulated by hypoxic
factors, such as limited oxygen concentration, and is associated
with tumor differentiation and invasion (22,23).
Radiofrequency ablation (RFA) is a minimally-invasive
interventional treatment for local tumors that promotes tumor cell
apoptosis and necrosis through high temperatures (24). RFA also stops blood supply to the
peripheral blood vessels of the tumor in order to reduce metastasis
(25). In addition, RFA has
demonstrated satisfactory clinical effects in the treatment of
patients with primary lung cancer and lung metastases (26). Subsequently, the 5-year survival rate
of patients with lung cancer is 25–61% (27). The 3-year survival rate of patients
with lung cancer reaches 57% when radiotherapy and chemotherapy are
combined (28). Although RFA is
effective for the treatment of lung cancer, it also induces several
complications that can severely affect the prognosis of patients
(29–32). The results from our previous study
demonstrated that local recurrences of lung cancer caused by the
overgrowth of residual tumor following RFA treatment are driven by
HIF-1α (33). However, whether
HIF-1α could be considered a prognostic factor for patients with
lung cancer following RFA treatment remains unknown. The present
study analyzed the clinical data and survival time of 80 patients
with lung cancer who underwent RFA in order to investigate the
effect of HIF-1α expression on the prognosis of these patients.
Materials and methods
Clinical data collection
A total of 80 patients diagnosed with lung cancer by
histopathological analysis and who had received RFA treatment
between January 2011 and October 2016 at the Department of Thoracic
Surgery of The Shenzhen People's Hospital were included in the
present study. The cohort consisted of 66 men and 14 women with an
average age of 62.14±9.41 years (age range, 41–80 years). Of the 80
patients, 61 patients were smokers or ever smokers (76.32%). The
inclusion criteria were as follows: i) Patients unable to undergo
or unsuitable for surgical treatment due to multiple tumors,
recurrences and severe cardiovascular disease as confirmed by
pathological or cytological examination (including percutaneous
biopsy, lymph node biopsy, fiberoptic bronchoscopy brushing and
biopsy, pleural effusion cytology and sputum cytology); ii)
patients with complete clinical data; and iii) patients with
definite staging. Patients with second primary malignancy and who
were lost during follow-up were excluded. During the surgery, tumor
tissues and tumor adjacent tissues (located 2 cm way from tumors)
were collected. The baseline characteristics of all patients are
summarized in Table I. Following RFA
treatment tissue samples were stained with hematoxylin and eosin
(H&E) and examined for pathological changes by light
microscopy. The ablation zone was treated by needle biopsy to
evaluate the expression of HIF-1α by immunohistochemistry (IHC).
Peripheral blood (10 ml) was collected at 1 week following RFA and
directly sent to the laboratory for the analysis of T lymphocyte
subsets (CD4+/CD8+). Their association with
the prognosis of patients with lung cancer was also analyzed.
 | Table I.Univariate analysis of the clinical
characteristics of patients with lung cancer. |
Table I.
Univariate analysis of the clinical
characteristics of patients with lung cancer.
|
Characteristics | Number | Median survival
time (days) | χ2
value | P-value |
|---|
| Sex |
|
| 1.035 | 0.596 |
|
Male | 66 | 578 |
|
|
|
Female | 14 | 432 |
|
|
| Age (years) |
|
| 11.769 | 0.001 |
|
<60 | 30 | 983 |
|
|
|
≥60 | 50 | 480 |
|
|
| Primary or
metastasis |
|
| 32.387 | <0.001 |
| Primary
cancer | 67 | 836 |
|
|
|
Metastatic cancer | 13 | 356 |
|
|
| UICC stage (67
cases) |
|
| 58.084 | <0.001 |
|
I/II | 34 | 1,124 |
|
|
|
III/IV | 33 | 467 |
|
|
| Postoperative blood
CD4+/CD8+ |
|
| 83.053 | <0.001 |
|
≤1.65 | 47 | 457 |
|
|
|
>1.65 | 33 | 1,145 |
|
|
| Chemotherapy |
|
| 3.967 | 0.042 |
| <3
cycles | 16 | 516 |
|
|
| ≥3
cycles | 23 | 813 |
|
|
| ECOG rating |
|
| 51.674 | <0.001 |
| 0 | 13 | 1,145 |
|
|
| 1 | 24 | 983 |
|
|
| 2 | 31 | 480 |
|
|
| 3 | 12 | 301 |
|
|
| Smoking |
|
| 0.326 | 0.568 |
|
Yes | 61 | 568 |
|
|
| No | 19 | 578 |
|
|
| HIF-1α
expression |
|
| 79.266 | <0.001 |
| Low
expression | 36 | 1,124 |
|
|
| High
expression | 44 | 438 |
|
|
Main instruments and reagents
HIF-1α (1:500; cat. no. RM242) rabbit anti-human
monoclonal antibody and SP immunoassay kit were purchased from
Boster Biological Technology. The goat anti-rabbit secondary
antibody (1:1,000; cat. no. BA1056) was provided by Wuhan Boster
Biological Engineering Technology Co., Ltd. Fluorescein
isothiocyanate- and antigen-presenting cells-CD4 and CD8 were
purchased from BD Biosciences. Inverted Microscope (Olympus
Corporation). The tissue embedder (Sakura), slicer (Leica
Microsystems GmbH), oscillator (Shanghai Jinghong Experimental
Equipment Co., Ltd.), plastic staining frame (Fuzhou Maixin Biotech
Co., Ltd.), computer image analysis system (HP), expandable anchor
RF electrode needle (Beijing Blade Photoelectric Technology
Development Co., Ltd.). A total of 12 microelectrode needles can be
released radially with a diameter of 4.0 cm, when fully opened to
cover the ablation area as much as possible, the local temperature
can reach 90–100°C during treatment and the thermal coagulation
necrosis diameter was 5–6 cm), RF-2000 radiofrequency treatment
instrument (Beijing Blade Photoelectric Technology Development Co.,
Ltd.; radiofrequency, 500 kHz; maximum output power, 200 W) and
Siemens 64 row SOMATOM Perspective dual source spiral CT (Siemens,
AG were used in experiments.
RFA treatment
All the patients undergoing lung RFA were admitted
as inpatients at the Department of Thoracic Surgery of The Shenzhen
People's Hospital and placed in observation for a minimum of one
night. All patients completed at least one post-procedure chest
radiograph. A total of 57 patients received lung RFA under general
anesthesia, whereas 23 patients were treated under local
anesthesia. Briefly, CT-guided radiofrequency needle was inserted
into the lung cancer tissue and the microelectrode was opened to
connect the radiofrequency current. The average duration of the RFA
treatment was 50 min with a mean and median impedance of 439 and
446 units, respectively. Technical success was defined as complete
ablation of all target lesions. When the RFA operation was
completed, a needle biopsy was performed immediately for the
detection of HIF-1α expression.
Patients' follow-up
The follow-up (6–66 months) was performed by
electronic medical record inquiry and telephone calls. Overall
survival was calculated from the first treatment with RFA (not from
the diagnosis of lung cancer). The association between HIF-1α
expression and patient prognosis was analyzed by unpaired
two-tailed Student's t-test. Kaplan-Meier (KM) analysis was used to
construct the survival curve. Cox proportional hazards model was
applied for multifactor survival analysis.
IHC
The dissected tissue was fixed in 4%
polyoxymethylene for two days, decalcified in 10% EDTA at 48°C for
2 weeks and sliced into 5-µm sections. Sections were subjected to
antigen retrieval in sodium citrate buffer (1 M, pH 6.0) at 99°C
for 20 min and incubated with primary antibody against HIF-1α
(1:1,000; cat. no. RM242; Boster Biological Technology) overnight
at 4°C. The slides were then incubated with a biotin-conjugated
goat anti-rabbit secondary antibody (1:1,000; cat. no. BA1003;
Wuhan Boster Biological Engineering Technology Co., Ltd.) at room
temperature for 45 min. The slides were examined with a Nikon
Eclipse Ti light microscope under a ×40 objective (Nikon
Instruments). A positive expression for HIF-1α was detected when
brownish-yellow particles appeared in the nucleus, membrane or
cytoplasm. Results of the IHC were analyzed using semi-quantitative
scoring method as described by Lin et al (34). Briefly, staining was scored as
negative (−), mild (+), moderate (++) or strong (+++) for <1,
1–10, 10–50 or >50% of cell nuclear stain, respectively. In the
present study, moderate (++) and strong (+++) scores defined high
HIF-1α expression, whereas negative (−) and mild (+) scores defined
low HIF-1α expression.
CD4+/CD8+
measurement by flow cytometry
FACS lysing solution (BD Biosciences) was used to
lyse red blood cells in the peripheral blood, which was followed by
two washes twice with PBS. CD4+/CD8+ was
measured using the BD Simultest™ CD4/CD8 kit (BD Biosciences),
according to manufacturer's instructions, through incubation with
FITC-labeled CD4 and PE-labeled CD8 antibody for 15–30 min at room
temperature. Flow cytometry was used to analyze the residual white
blood cells, and the proportions of the lymphocyte subsets were
calculated using FlowJo software version 10 (FlowJo LLC).
Statistical analysis
SPSS 19.0 software (IBM Corp.) was used for data
analysis. All data are expressed as the means ± standard error of
the mean. Statistical differences were analyzed by unpaired
two-tailed Student's t-test for the comparison between two groups.
The skewed distribution was described by median ± interquartile
range (M ± Q). Independent samples were compared by rank sum test.
All values described represent the means ± standard deviation. KM
method was used to construct the survival curve of patients with
lung cancer. Significance test was based on the log-rank method.
Cox ratio risk model was applied for the multivariate survival
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
A total of 80 patients with lung cancer were
included in the present study (Table
I). The median age of the patients was 61.6±9.8 (range, 41–80)
years. The cohort consisted of 66 men (82.5%) and 14 women (17.5%).
Furthermore, 61 patients were smokers or ever-smokers (76.3%). The
Eastern Cooperative Oncology Group (ECOG) performance status was
used to assess the patient's physical condition and the score of
all patients was <3. A total of 13 patients (16.3%) suffered
from metastatic pulmonary cancer. According to the results from the
survival rate, no patients with metastatic cancer and stage III/IV
primary cancer (according to The Eighth Edition of TNM Staging of
Lung Cancer) survived for 5 years (35), and only two patients with stage I/II
primary cancer survived for 5 years (Table II). The overall 5-year survival rate
was only 2.5% (Table II).
 | Table II.Comparison of the survival rate of
patients with lung cancer after radiofrequency ablation
treatment. |
Table II.
Comparison of the survival rate of
patients with lung cancer after radiofrequency ablation
treatment.
| Group | Number | 1 year (%) | 2 years (%) | 3 years (%) | 4 years (%) | 5 years (%) |
|---|
| Primary cancer | 67 |
|
|
|
|
|
| Stage I/II | 34 | 100.00 | 94.12 | 55.88 | 23.53 | 5.88 |
| Stage III/IV | 33 | 72.73 | 9.09 | 3.03 | 0.00 | 0.00 |
| Metastatic
cancer | 13 | 46.15 | 0.00 | 0.00 | 0.00 | 0.00 |
| Total | 80 | 80.00 | 43.75 | 25.00 | 10.00 | 2.50 |
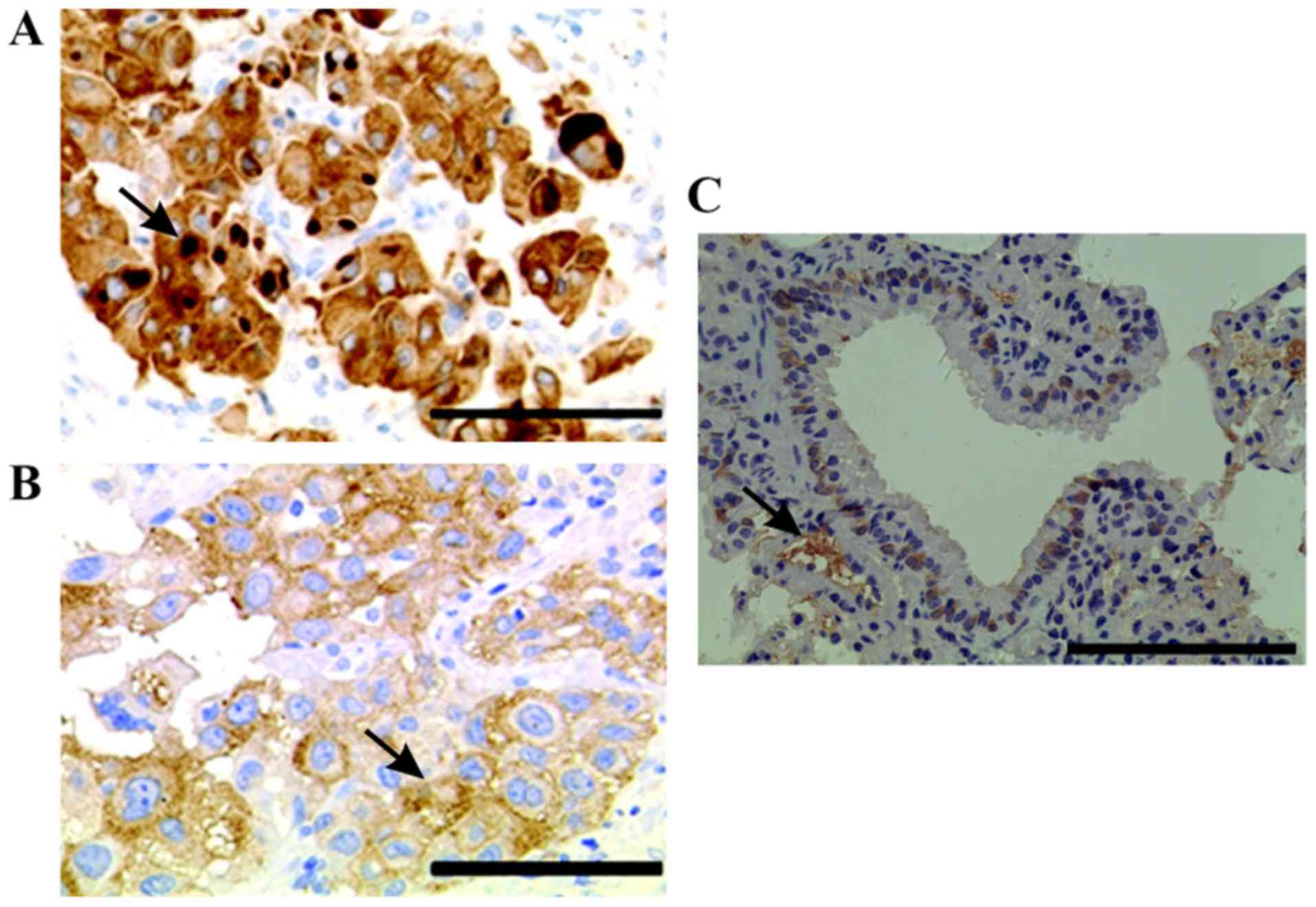
HIF-1α expression in lung cancer
tissues
The results demonstrated that HIF-1α was mainly
localized in the nucleus and cytoplasm of lung cancer cells
(Fig. 1A). Furthermore, only little
HIF-1α expression was detected in normal lung tissues (Fig. 1C). In addition, certain cancer cells
containing intracytoplasmic macroglobular spots were strongly
positive for HIF-1α (Fig. 1B), and
some tumor cells exhibited a positive perinuclear aureole.
Representative image of HIF-1α staining is presented in Fig. 1B. Among the 80 cases of lung cancer
tissues analyzed, the level of HIF-1α expression was as follows: 9
cases (11.3%) had negative staining (−), 27 cases (33.8%) had mild
staining (+); 13 cases (16.3%) had moderate staining (++), and 31
cases (38.8%) had strong staining (+++). Subsequently, among all
patients with lung cancer, 36 (45%) displayed low HIF-1α expression
and 44 (55%) presented high HIF-1α expression.
Prognostic value of HIF-1α expression
and other factors in patients with lung cancer treated by RFA
The follow-up time of the whole group was 6–66
months. Two patients survived until the end of the follow-up. The
median survival time was 573 days. The KM model and log-rank test
were used to analyze the effect of clinical factors on the overall
survival (OS). The results demonstrated that factors, including
age, Union for International Cancer Control (UICC) stage, primary
or metastatic cancer, chemotherapy, postoperative
CD4+/CD8+, ECOG rating and HIF-1α expression
significantly affected the OS (Table
I). The average value of CD4+/CD8+ was
1.65 (range, 0.15–4.58). Furthermore, decreased survival was
observed in patients with older age, high UICC stage, lower
CD4+/CD8+ level (expression of CD4 and CD8 by
flow cytometry are presented in Fig.
S1), high ECOG rating and high HIF-1α expression (Table I). In addition, a significant
difference between the OS of patients with primary cancer stage
I/II and of patients with primary cancer stage III/IV was observed
(χ2=58.084; P<0.001; Table II). Furthermore, a clear difference
was observed in the survival rate of patients with primary and
metastatic carcinoma (P<0.001; χ2=32.387; Table II). The associations between HIF-1α
expression, postoperative blood CD4+/CD8+ or
UICC staging and the OS of patients with lung cancer at different
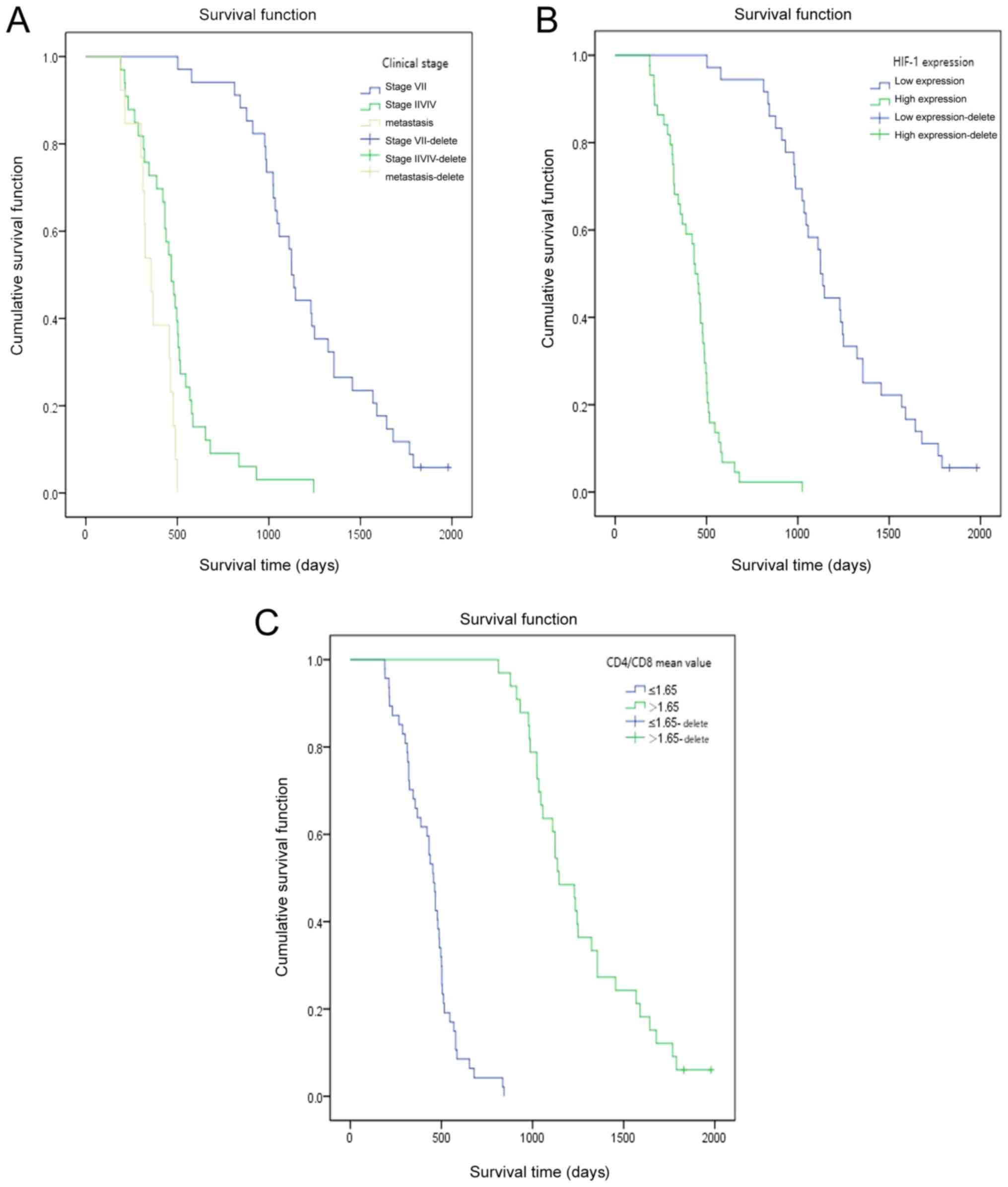
time points following RFA treatment are presented in Fig. 2. The results from KM survival curves
demonstrated that patients with high HIF-1α expression, high UICC
stage and low CD4+/CD8+ had a significantly
shorter OS.
Associations between HIF-1α expression
and clinicopathological characteristics in patients with lung
cancer treated by RFA
The association between HIF-1α expression and the
clinical characteristics of patients, including sex, age, tumor
metastasis, UICC stage, ECOG rating and smoking status was
evaluated. As presented in Table
III, HIF-1α expression was not associated with sex, age or
smoking status (P>0.05). However, HIF-1α expression was
significantly associated with tumor metastasis, UICC stage or ECOG
rating (P<0.05) in patients with lung cancer treated with
RFA.
 | Table III.Association between HIF-1α expression
and patients' clinical characteristics. |
Table III.
Association between HIF-1α expression
and patients' clinical characteristics.
|
Characteristics | Number | Low HIF-1α
expression (%; n=36) | High HIF-1α
expression (%; n=44) | χ2
value | P-value |
|---|
| Sex |
|
|
| 1.011 | 0.315 |
|
Male | 66 | 28 (42) | 38 (58) |
|
|
|
Female | 14 | 8 (57) | 6 (43) |
|
|
| Age (years) |
|
|
| 0.485 | 0.486 |
|
<60 | 30 | 15 (50) | 15 (50) |
|
|
|
≥60 | 50 | 21 (42) | 29 (58) |
|
|
| Primary or
metastasis |
|
|
| 8.729 | 0.003 |
| Primary
cancer | 67 | 35 (52) | 32 (48) |
|
|
|
Metastatic cancer | 13 | 1 (8) | 12 (92) |
|
|
| UICC stage (67
cases) |
|
|
| 18.6 | <0.001 |
|
I/II | 34 | 24 (71) | 10 (29) |
|
|
|
III/IV | 33 | 6 (18) | 27 (82) |
|
|
| ECOG rating |
|
|
| 45.801 | <0.001 |
| 0 | 13 | 12 (92) | 1 (8) |
|
|
| 1 | 24 | 20 (83) | 4 (17) |
|
|
| 2 | 31 | 6 (19) | 25 (81) |
|
|
| 3 | 12 | 2 (17) | 10 (83) |
|
|
| Smoking |
|
|
| 0.67 | 0.413 |
|
Yes | 61 | 29 | 32 |
|
|
| No | 19 | 7 | 12 |
|
|
Determination of independent factor of
prognosis in patients with lung cancer by Cox regression
analysis
The results from Cox regression analysis
demonstrated that the high expression of HIF-1α, advanced age,
clinical stage and chemotherapy could be considered as independent
risk factors for the prognosis of patients with lung cancer
following RFA treatment (Table IV).
Among all cases, the OR (odds ratio) value of high HIF-1α
expression, advanced age, clinical staging and chemotherapy was
5.910-, 2.307-, 0.029- and 0.026-fold, respectively (P=0.014 and
95% confidence interval (CI), 1.441–24.230 for high expression of
HIF-1α; P=0.003 and 95% CI, 1.340–3.397 for advanced age; P=0.001
and 95% CI, 0.003–0.254 for clinical staging; and P<0.001 and
95% CI, 0.110–0.463 for chemotherapy). Furthermore, the OR value of
high HIF-1α expression was higher than that of advanced age,
clinical staging and chemotherapy. HIF-1α expression may therefore
represent a more effective independent prognostic factor in
patients with lung cancer treated by RFA compared with age,
clinical stage and chemotherapy.
 | Table IV.Cox regression analysis of
independent factors in the prognosis of patients with lung
cancer. |
Table IV.
Cox regression analysis of
independent factors in the prognosis of patients with lung
cancer.
| Variables | β | SE | Wald
χ2 | P-value | OR value | 95% CI |
|---|
| HIF-1α
expression | 1.777 | 0.720 | 6.090 | 0.014 | 5.910 | 1.441 | 24.230 |
| Advanced age | 0.836 | 0.277 | 9.086 | 0.003 | 2.307 | 1.340 | 3.397 |
| Clinical stage | −3.548 | 1.111 | 10.202 | 0.001 | 0.029 | 0.003 | 0.254 |
| Chemotherapy or
not | −1.488 | 0.366 | 16.523 | <0.001 | 0.226 | 0.110 | 0.463 |
Discussion
RFA becomes a widely accepted treatment for primary
lung cancer in patients who are not candidates for subsegment
resection or lobectomy (36).
Compared with radiotherapy and chemotherapy, RFA focuses
exclusively on the tumor area without causing any damage to the
normal surrounding tissue (37). In
addition, compared with surgical treatment, RFA is a
minimally-invasive method (38). In
advanced lung cancers, RFA is extremely effective for palliative
care (39); however, it induces
several complications, including local recurrence, which severely
affects the prognosis of patients. Previous studies indicated that
RFA causes deep hypoxia in the tissues that are adjacent to the
resected area (40). The presence of
hypoxia has been reported in several types of solid tumor and is
associated with the aggressiveness, proliferation, and angiogenesis
potential of the tumor (41). HIF-1α
is induced in hypoxia and serves a crucial role in the pathological
process of inflammation, hypoxia, vascular permeability, apoptosis,
pulmonary fibrosis and lung cancer (42,43). In
addition, HIF-1α regulates the expression of PGK-1, VEGF and
other target genes to induce blood vessel and tissue proliferation
that in turn promotes tumor growth (44,45).
Hypoxia inhibits HIF-1α degradation and enhances HIF-1α DNA binding
activity, leading to high HIF-1α expression. VEGF expression is
therefore elevated, binds to vascular endothelial surface receptors
and activates the ischemic transduction pathway (46,47).
It has been reported that HIF-1α expression is
increased in several types of cancer, including lung, prostate,
breast and colon carcinomas, which are the main causes of
cancer-associated mortality (48).
In the present study, 36 and 44 patients with lung cancer exhibited
a low and high HIF-1α expression, respectively. Furthermore, the
results from KM curve demonstrated that patients with high HIF-1α
expression exhibited significantly shorter OS compared with
patients with low HIF-1α expression. The results from KM model and
log-rank test demonstrated a significant effect of HIF-1α
expression on the OS of patients with lung cancer. The results from
Cox regression analysis indicated that high HIF-1α expression could
be considered as an independent risk factor for patients with lung
cancer following RFA treatment. Furthermore, HIF-1α expression may
affect tumor angiogenesis, proliferation and metabolism, which may
influence tumor cell sensitivity to RFA treatment. It has been
reported that HIF-1α level is increased under hypoxic condition,
which results in increased tumor angiogenesis via VEGF and a
subsequent increase in tumor size, expansion of hypoxic areas,
resistance to RFA treatment and poor prognosis of patients with
lung cancer (49). Consistently, the
results from the present study demonstrated that patients with
lower HIF-1α expression presented elevated survival time compared
with patients with higher HIF-1α expression. In addition, HIF-1α
expression was significantly associated with tumor metastasis, UICC
stage and ECOG rating.
Lung cancer cells secrete numerous immunosuppressive
factors that can inhibit the anti-tumor immunity (50,51).
Furthermore, RFA treatment of lung cancer is effective and can be
monitored in real time (52). A
previous study demonstrated that RFA therapy for lung cancer can
improve the cellular immune function, due to the direct killing
effect of antigens and synergistic killing effect of cytokines
released by sensitized T cells. This leads to the correction of
immune imbalance of Th1/Th2 cells, increase CD4+ and
CD8+ cell levels, eliminate the sources of tumor-derived
inhibitors and restore the anti-tumor immunity (53,54). For
example, the decreased proportions of CD4+ cells (T helper cells),
CD4/CD8 ratios and B cells, and the increased number of
CD8+/CD28 T lymphocytes and regulatory T (Treg) cells
have been demonstrated in patients with lung cancer (55,56). In
the present study, the levels of CD4+ and CD8+ cells
were increased following RFA treatment, which suggested that RFA
treatment may restore the imbalanced immune function in patients
with lung cancer. In addition, results from the KM model and
log-rank test demonstrated that some factors, including age, UICC
stage, primary or metastatic cancer, chemotherapy, postoperative
blood levels of CD4+/CD8+, ECOG rating and
HIF-1α expression had a significant effect on the OS of patients
with lung cancer following RFA treatment. Furthermore, Cox
regression analysis revealed that high HIF-1α expression, advanced
age, clinical staging and chemotherapy may be considered as
independent risk factors for the prognosis of patients with lung
cancer after RFA treatment, which indicated that changes in T
lymphocyte subsets after treatment may be associated with patient
prognosis. UICC staging may also be considered as a prognostic
factor for patients with lung cancer after RFA treatment. In
addition, the long-term effect of treatment with RFA combined with
chemotherapy for patients with advanced lung cancer was better than
that of chemotherapy or RFA treatment alone. However, since the
present study only included a limited number of patients, further
investigation including a larger sample size is therefore required
in order to confirm the prognostic value of HIF-1α expression in
patients with lung cancer. Although the present study determined
the effect of HIF-1α expression on the prognosis of patients with
lung cancer after RFA treatment, the underlying mechanisms involved
and the correlation between HIF-1α expression and patients'
immunity required further investigation.
In conclusion, the present study analyzed HIF-1α
expression in patients with lung cancer. The results demonstrated
that patients treated with RFA and with high HIF-1α expression had
significantly shorter OS compared with patients with low HIF-1α
expression. Furthermore, high HIF-1α expression was significantly
associated with tumor metastasis, UICC stage and ECOG rating.
HIF-1α expression may therefore be considered as an effective
independent prognosis factor in patients with lung cancer treated
by RFA. The detection of HIF-1α expression may therefore be crucial
for evaluating the prognosis of patients with lung cancer who
underwent RFA treatment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Guangdong Province (grant no.
2018A0303130247) awarded to Jun Wan.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW and GD designed the experiments. JW, XL and ZR
performed the experiments. GD and BP analyzed the data. JW and XL
wrote the manuscript. All authors read and approved the final
version of the manuscript and agreed to be accountable for all
aspects of the research to ensure that the accuracy or integrity of
any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
This study was approved by the Clinical Medical
Research Ethics Committee of the First Affiliated Hospital of Anhui
Medical University (approval number AF/SC-08/02.0). All patients
provided informed consent prior to the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen WQ, Zuo TT, Zheng RS, Zeng HM, Zhang
SW and He J: Lung cancer incidence and mortality in China in 2013.
Zhonghua Zhong Liu Za Zhi. 39:795–800. 2017.(In Chinese).
PubMed/NCBI
|
|
2
|
Wang X and Adjei AA: Lung cancer and
metastasis: New opportunities and challenges. Cancer Metastasis
Rev. 34:169–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Riihimäki M, Hemminki A, Fallah M, Thomsen
H, Sundquist K, Sundquist J and Hemminki K: Metastatic sites and
survival in lung cancer. Lung Cancer. 86:78–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan X, Jiao SC, Zhang GQ, Guan Y and Wang
JL: Tumor-associated immune factors are associated with recurrence
and metastasis in non-small cell lung cancer. Cancer Gene Ther.
24:57–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wan J and Wu W: Hyperthermia induced
HIF-1α expression of lung cancer through AKT and ERK signaling
pathways. J Exp Clin Cancer Res. 35:1192016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou H, Chen A, Shen J, Zhang X, Hou M, Li
J, Chen J, Zou H, Zhang Y, Deng Q, et al: Long non-coding RNA
LOC285194 functions as a tumor suppressor by targeting p53 in
non-small celllung cancer. Oncol Rep. 41:15–26. 2019.PubMed/NCBI
|
|
7
|
Sasaki M, Sugio K, Kuwabara Y, Koga H,
Nakagawa M, Chen T, Kaneko K, Hayashi K, Shioyama Y, Sakai S and
Honda H: Alterations of tumor suppressor genes (Rb, p16, p27 and
p53) and an increased FDG uptake in lung cancer. Ann Nucl Med.
17:189–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee TG, Jeong EH, Kim SY, Kim HR, Kim H
and Kim CH: Fhit, a tumor suppressor protein, induces autophagy via
14-3-3τ in non-small cell lung cancer cells. Oncotarget.
8:31923–31937. 2017.PubMed/NCBI
|
|
9
|
Wang H, Ye L, Xing Z, Li H, Lv T, Liu H,
Zhang F and Song Y: CDCA7 promotes lung adenocarcinoma
proliferation via regulating the cell cycle. Pathol Res Pract.
215:1525592019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qian X, Song X, He Y, Yang Z, Sun T, Wang
J, Zhu G, Xing W and You C: CCNB2 overexpression is a poor
prognostic biomarker in Chinese NSCLC patients. Biomed
Pharmacother. 74:222–227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duan J, Huang W and Shi H: Positive
expression of KIF20A indicates poor prognosis of glioma patients.
Onco Targets Ther. 9:6741–6749. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
E L, Lu L, Li L, Yang H, Schwartz LH and
Zhao B: Radiomics for classification of lung cancer histological
subtypes based on nonenhanced computed tomography. Acad Radiol.
26:1245–1252. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yeo MK, Choi SY, Seong IO, Suh KS, Kim JM
and Kim KH: Association of PD-L1 expression and PD-L1 gene
polymorphism with poor prognosis in lung adenocarcinoma and
squamous cell carcinoma. Hum Pathol. 68:103–111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haga A, Takahashi W, Aoki S, Nawa K,
Yamashita H, Abe O and Nakagawa K: Classification of early stage
non-small cell lung cancers on computed tomographic images into
histological types using radiomic features: Interobserver
delineation variability analysis. Radiol Phys Technol. 11:27–35.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee G, Lee HY, Park H, Schiebler ML, van
Beek EJR, Ohno Y, Seo JB and Leung A: Radiomics and its emerging
role in lung cancer research, imaging biomarkers and clinical
management: State of the art. Eur J Radiol. 86:297–307. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen R, Olshen AB and Ladanyi M:
Integrative clustering of multiple genomic data types using a joint
latent variable model with application to breast and lung cancer
subtype analysis. Bioinformatics. 25:2906–2912. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui H, Seubert B, Stahl E, Dietz H,
Reuning U, Moreno-Leon L, Ilie M, Hofman P, Nagase H, Mari B and
Kruger A: Tissue inhibitor of metalloproteinases-1 induces a
pro-tumourigenic increase of miR-210 in lung adenocarcinoma cells
and their exosomes. Oncogene. 34:3640–3650. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Person RJ, Tokar EJ, Xu Y, Orihuela R,
Ngalame NN and Waalkes MP: Chronic cadmium exposure in vitro
induces cancer cell characteristics in human lung cells. Toxicol
Appl Pharmacol. 273:281–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goda N, Dozier SJ and Johnson RS: HIF-1 in
cell cycle regulation, apoptosis, and tumor progression. Antioxid
Redox Signal. 5:467–473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wan J, Chai H, Yu Z, Ge W, Kang N, Xia W
and Che Y: HIF-1α effects on angiogenic potential in human small
cell lung carcinoma. J Exp Clin Cancer Res. 30:772011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee CH, Lee MK, Kang CD, Kim YD, Park DY,
Kim JY, Sol MY and Suh KS: Differential expression of hypoxia
inducible factor-1 alpha and tumor cell proliferation between
squamous cell carcinomas and adenocarcinomas among operable
Non-small cell lung carcinomas. J Korean Med Sci. 18:196–203. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang H, Zhang Z, Xu Y, Xing L, Liu J, Li
J and Tan Q: The expression of hypoxia inducible factor 1-alpha in
lung cancer and its correlation with P53 and VEGF. J Huazhong Univ
Sci Technolog Med Sci. 24:124–127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Khan MN, Haggag YA, Lane ME, McCarron PA
and Tambuwala MM: Polymeric Nano-encapsulation of curcumin enhances
its anti-cancer activity in breast (MDA-MB231) and lung (A549)
cancer cells through reduction in expression of HIF-1α and nuclear
p65 (Rel A). Curr Drug Deliv. 15:286–295. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jun W and Jian W: Current progression of
radiofrequency ablation (RFA) in clinical application of lung
cancer therapy. J Biomater Tissue Eng. 9:417–426. 2019. View Article : Google Scholar
|
|
25
|
Luo W, Zhou P and Li W: Advances in
diagnosis and treatment of multiple primary lung cancer. Zhongguo
Fei Ai Za Zhi. 18:640–643. 2015.(In Chinese). PubMed/NCBI
|
|
26
|
de Baère T, Aupérin A, Deschamps F,
Chevallier P, Gaubert Y, Boige V, Fonck M, Escudier B and
Palussiére J: Radiofrequency ablation is a valid treatment option
for lung metastases: Experience in 566 patients with 1037
metastases. Ann Oncol. 26:987–991. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hiraki T, Gobara H, Iguchi T, Fujiwara H,
Matsui Y and Kanazawa S: Radiofrequency ablation for early-stage
nonsmall cell lung cancer. Biomed Res Int. 2014:1520872014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qi H and Fan W: Value of ablation therapy
in the treatment of lung metastases. Thorac Cancer. 9:199–207.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li G, Xue M, Chen W and Yi S: Efficacy and
safety of radiofrequency ablation for lung cancers: A systematic
review and meta-analysis. Eur J Radiol. 100:92–98. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu B, Ye X, Fan W, Li X, Feng W, Lu Q,
Mao Y, Lin Z, Li L, Zhuang Y, et al: Expert consensus for
image-guided radiofrequency ablation of pulmonary tumors (2018
version). Zhongguo Fei Ai Za Zhi. 21:76–88. 2018.(In Chinese).
PubMed/NCBI
|
|
31
|
Palussière J, Catena V and Buy X:
Percutaneous thermal ablation of lung tumors-Radiofrequency,
microwave and cryotherapy: Where are we going? Diagn Interv
Imaging. 98:619–625. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamamoto A, Hamamoto S, Matsuoka T,
Kageyama K, Jogo A, Sohgawa E, Okuma T, Hamuro M, Toyoshima M,
Kawabe J, et al: Spontaneous regression of untreated tumors with
immuno-radiofrequency ablation, RF ablation in combination with
local injection of OK-432, in a patient with lung metastases of
colon cancer. J Vasc Interv Radiol. 28:477–479. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wan J, Wu W and Zhang RQ: Local recurrence
of small cell lung cancer following radiofrequency ablation is
induced by HIF-1α expression in the transition zone. Oncol Rep.
35:1297–1308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin CS, Liu TC, Lee MT, Yang SF and Tsao
TC: Independent prognostic value of hypoxia-inducible factor
1-alpha expression in small cell lung cancer. Int J Med Sci.
14:785–790. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shin JW, Cho DG, Choi SY, Park JK, Lee KY
and Moon Y: Prognostic factors in stage IIB Non-small cell lung
cancer according to the 8th edition of TNM Staging System. Korean J
Thorac Cardiovasc Surg. 52:131–140. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Beland MD, Wasser EJ, Mayo-Smith WW and
Dupuy DE: Primary non-small cell lung cancer: Review of frequency,
location, and time of recurrence after radiofrequency ablation.
Radiology. 254:301–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Poch FGM, Rieder C, Ballhausen H, Knappe
V, Ritz JP, Gemeinhardt O, Kreis ME and Lehmann KS: Finding optimal
ablation parameters for multipolar radiofrequency ablation. Surg
Innov. 24:205–213. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Prud'homme C, Deschamps F, Moulin B,
Hakime A, Al-Ahmar M, Moalla S, Roux C, Teriitehau C, de Baere T
and Tselikas L: Image-guided lung metastasis ablation: A literature
review. Int J Hyperthermia. 36:37–45. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baisi A, Raveglia F, De Simone M and
Cioffi U: Palliative role ofpercutaneous radiofrequency ablation
for severe hemoptysisin an elderly patient with inoperable lung
cancer. J Thorac Cardiovasc Surg. 140:1196–1197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tong Y, Yang H, Xu X, Ruan J, Liang M, Wu
J and Luo B: Effect of a hypoxic microenvironment after
radiofrequency ablation on residual hepatocellular cell migration
and invasion. Cancer Sci. 108:753–762. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mees G, Sathekge M, Maes A and Van de
Wiele C: Radiolabelled probes targeting tumor hypoxia for
personalized medicine. Curr Pharm Des. 20:2308–2318. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wan J, Wu W, Chen Y, Kang N and Zhang R:
Insufficient radiofrequency ablation promotes the growth of
non-small cell lung cancer cells through PI3K/Akt/HIF-1α signals.
Acta Biochim Biophys Sin (Shanghai). 48:371–377. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wan J, Wu W, Huang Y, Ge W and Liu S:
Incomplete radiofrequency ablation accelerates proliferation and
angiogenesis of residual lung carcinomas via HSP70/HIF-1α. Oncol
Rep. 36:659–668. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pinato DJ, Black JR, Trousil S, Dina RE,
Trivedi P, Mauri FA and Sharma R: Programmed cell death ligands
expression in phaeochromocytomas and paragangliomas: Relationship
with the hypoxic response, immune evasion and malignant behavior.
Oncoimmunology. 6:e13583322017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cai FF, Xu C, Pan X, Cai L, Lin XY, Chen S
and Biskup E: Prognostic value of plasma levels of HIF-1α and
PGC-1a in breast cancer. Oncotarget. 7:77793–77806. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wan L, Huang J, Chen J, Wang R, Dong C, Lu
S and Wu X: Expression and significance of FOXP1, HIF-1α and VEGF
in renal clear cell carcinoma. J BUON. 20:188–195. 2015.PubMed/NCBI
|
|
47
|
Wachters JE, Schrijvers ML,
Slagter-Menkema L, Mastik M, de Bock GH, Langendijk JA, Kluin PM,
Schuuring E, van der Laan BF and van der Wal JE: Prognostic
significance of HIF-1α, CA-IX, and OPN in T1-T2 laryngeal carcinoma
treated with radiotherapy. Laryngoscope. 123:2154–2160. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression ofhypoxia-inducible factor 1alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
49
|
Kong J, Kong J, Pan B, Ke S, Dong S, Li X,
Zhou A, Zheng L and Sun WB: Insufficient radiofrequency ablation
promotes angiogenesis of residual hepatocellular carcinoma via
HIF-1α/VEGFA. PLoS One. 7:e372662012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Morine Y, Shimada M, Utsunomiya T, Imura
S, Ikemoto T, Mori H, Hanaoka J, Kanamoto M, Iwahashi S and Miyake
H: Hypoxia inducible factor expression in intrahepatic
cholangiocarcinoma. Hepatogastroenterology. 58:1439–1444. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kitajima Y and Miyazaki K: The Critical
impact of HIF-1α on gastric cancer biology. Cancers (Basel).
5:15–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhao X, Fu X, Blumenthal C, Wang YT,
Jenkins MW, Snyder C, Arruda M and Rollins AM: Integrated RFA/PSOCT
catheter for real-time guidance of cardiac radio-frequency
ablation. Biomed Opt Express. 9:6400–6411. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu W, Wan J, Xia R, Huang Z, Ni J and Yang
M: Functional role of regulatory T cells in B cell lymphoma and
related mechanisms. Int J Clin Exp Pathol. 8:9133–9139.
2015.PubMed/NCBI
|
|
54
|
Bagrodia A, Krabbe LM, Gayed BA, Kapur P,
Bernstein I, Xie XJ, Wood CG, Karam JA, Weizer AZ, Raman JD, et al:
Evaluation of the prognostic significance of altered mammalian
target of rapamycin pathway biomarkers in upper tract urothelial
carcinoma. Urology. 84:1134–1140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Karagoz B, Bilgi O, Gumus M, Erikci AA,
Sayan O, Turken O, Kandemir EG, Oztürk A and Yaylaci M:
CD8+CD28-cells and CD4+CD25+ regulatory T cells in the peripheral
blood of advanced stage lung cancer patients. Med Oncol. 27:29–33.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ke X, Zhang S, Xu J, Liu G, Zhang L, Xie
E, Gao L, Li D, Sun R, Wang F and Pan S: Non-small-cell lung
cancer-induced immunosuppression by increased human regulatory T
cells via Foxp3 promoter demethylation. Cancer Immunol Immunother.
65:587–599. 2016. View Article : Google Scholar : PubMed/NCBI
|