Introduction
Gastric cancer remains one of the leading causes of
cancer-related deaths worldwide, and the clinical prognosis of
advanced gastric cancer remains poor despite multiple treatment
options (1–4). Against this background, various studies
have been performed to identify useful biomarkers to predict
gastric cancer prognosis (5–7). However, unlike HER2, few markers are
generally used in clinical practice, and further investigations of
novel markers are warranted.
The chromodomain helicase DNA binding 5
(CHD5) gene was first identified because of its distinctive
location on the short arm of chromosome 1p36 in a region of
frequent deletion in neuroblastomas (8,9).
CHD5 is the fifth of nine members of the CHD family,
which is considered to epigenetically regulate chromatin
organization and DNA transcription, translation, and replication
(10,11). CHD5 has been recognized as a
tumor suppressor gene in various malignancies. It has been
suggested that CHD5 is frequently down-regulated in tumor
cells through promoter hypermethylation, and its decreased
expression is associated with unfavorable clinical features and
poor outcomes (12–14).
Regarding gastric cancer, several studies
investigated the methylation status of CHD5 in gastric
cancer cell lines and tissue samples and revealed that CHD5
was down-regulated by promoter hypermethylation (15). However, only a few studies have
investigated the clinical implications of CHD5 protein expression
in gastric cancer (16), and the
mechanism of CHD5 function in gastric cancer has not yet been
clarified. In the present study, we used immunohistochemistry (IHC)
to investigate the association between CHD5 expression in tissue
samples and clinicopathological characteristics in patients with
gastric cancer, and clarified the mechanisms underlying the role of
CHD5 in tumor progression in gastric cancer cells in
vitro.
Materials and methods
Patients and tissue samples
We collected the data of 154 consecutive patients
with cT2-4 gastric cancer between January 2011 and December 2013.
Patients who received neoadjuvant chemotherapy or underwent
non-curative resection (R2) were excluded. All tumors were
histologically diagnosed as adenocarcinoma of the stomach. A total
of 154 gastric cancer tissue samples were analyzed after written
informed consent was obtained from each patient. We used the 14th
edition of the Japanese classification of gastric carcinoma to
determine the pathological stage (17). The present study was approved by the
Institutional Review Board of Osaka University Hospital (approval
number: 18227).
Cell lines and cell culture
conditions
We used six gastric cancer cell lines, as follows:
AGS, KATO III, MKN45, NCI-N87, NUGC-3, and OCUM-1. A human cervical
cancer cell line, HeLa, was used as the positive control. AGS,
MKN45, OCUM-1, NCI-N87, and NUGC-3 were cultured in Roswell Park
Memorial Institute (RPMI) 1640 medium (Nacalai Tesque), HeLa in
Dulbecco's modified eagle's medium (DMEM) (Wako Pure Chemical
Industries), and KATO III in mixed RPMI 1640 and DMEM (1:1), each
containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific,
Inc.). To induce cell growth, all cell lines were incubated in a
humidified atmosphere under 5% CO2 at 37°C.
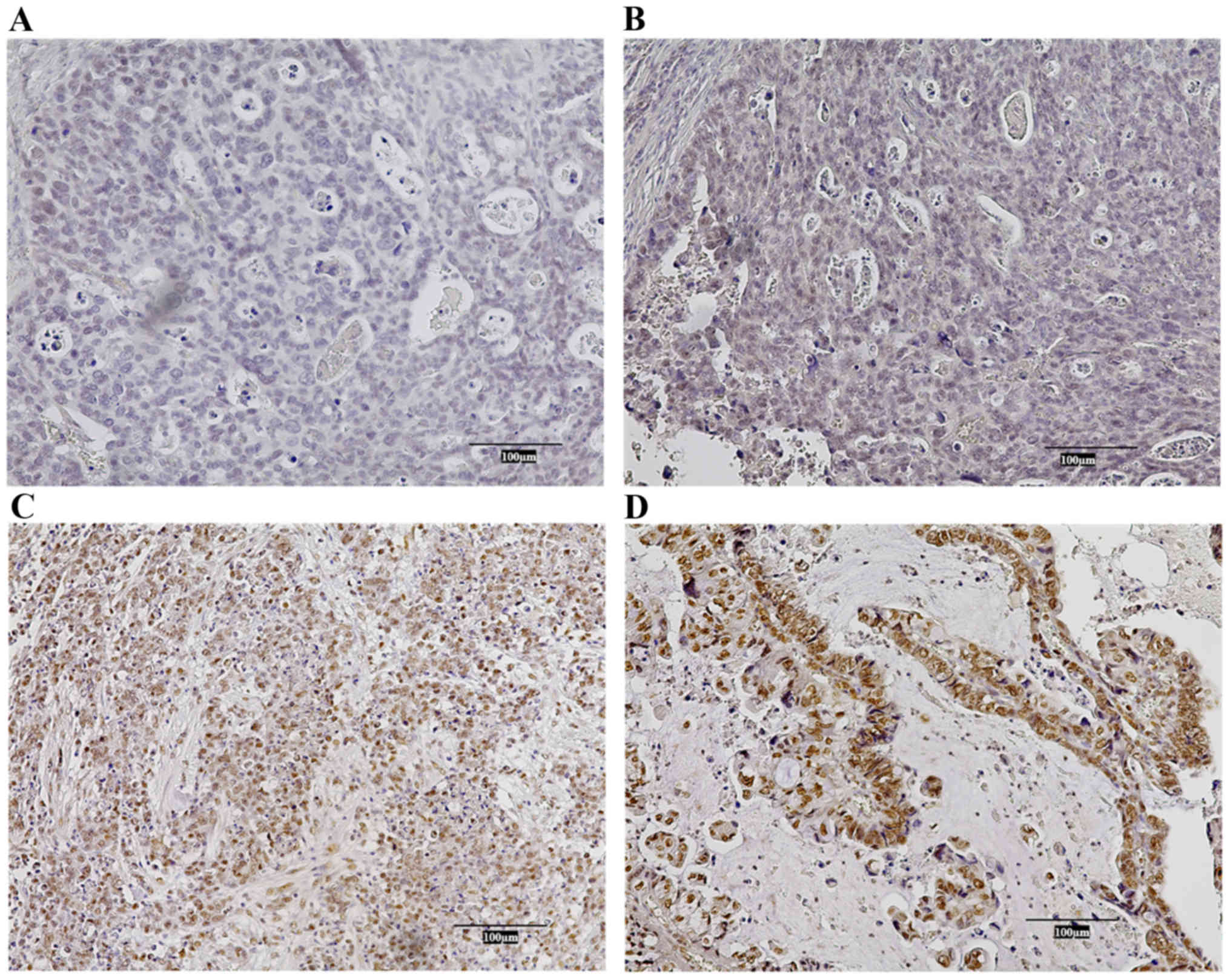
Immunohistochemical staining of
CHD5
We prepared 3.5-µm-thick sections of the resected
specimens from formalin-fixed paraffin-embedded blocks. These were
deparaffinized with xylene and then rehydrated with multistep
descending concentrations of ethanol. The sections were autoclaved
in citrate buffer at 115°C for 20 min, immersed in 0.3% hydrogen
peroxide to block endogenous peroxidase, and incubated in horse
serum for 20 min to avoid nonspecific staining. The slides were
incubated with monoclonal antibody against CHD5 (sc-271248;
dilution, 1:500; Santa Cruz Biotechnology, Inc.) overnight at 4°C,
with ABC peroxidase (Vector Laboratories) for 20 min, and with
diaminobenzidine tetrahydrochloride for 1.5 min to visualize the
reactions with CHD5. Normal human Purkinje cells in cerebellum
tissue were used as a positive control (Fig. S1). CHD5 expression was evaluated in
terms of the percentage and intensity of tumor cells that stained
positively for CHD5. As reported previously, the percentage and
intensity scores were defined as follows: percentage; 0%, score 0;
1–10%, score 1; 11–50%, score 2; 51–100%, score 3; and intensity;
negative, score 0; weak, score 1; moderate, score 2; strong, score
3 (18,19). The final score was estimated by
multiplying both scores and was classified into two groups: the
high CHD5 expression group (>3) and the low CHD5 expression
group (≤3). Representative IHC staining in each group can be found
in Fig. 1.
RNA extraction and quantitative
real-time reverse transcription PCR (RT-PCR)
Total RNA was extracted from cells and converted to
complementary DNA (cDNA). Primers for RT-PCR were as follows: CHD5
forward, CCAGTGGGCACCGAGGAG, and CHD5 reverse,
CTTCTTCCGCTTCCCTTTAC. GAPDH was used as an internal control. RT-PCR
was carried out with THUNDERBIRD SYBR qPCR Mix (Toyobo Life
Science) and the LightCycler 2.0 Instrument (Roche Life
Science).
CHD5 up-regulation with the
CRISPR/Cas9 system
The clustered regularly interspaced short
palindromic repeats (CRISPR)/CRISPR-associated protein (Cas9)
system was used to up-regulate CHD5. CHD5 CRISPR Activation Plasmid
(CHD5-plasmid) and Control CRISPR Activation Plasmid
(Control-plasmid) for negative control (Santa Cruz Biotechnology,
Inc.) were used based on the manufacturer's protocol.
Proliferation assay
A proliferation assay was performed using AGS,
MKN45, and NUGC-3 gastric cancer cells. Cells were seeded and
incubated for 24 h in 96-well plates, and then transfected with
CHD5-plasmid and Control-plasmid. After transfection, the
3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay (Sigma-Aldrich) was used to estimate the cell number every 24
h until cells were confluent.
Migration assay
A wound-healing assay was performed using AGS,
MKN45, and NUGC-3 gastric cancer cells. Cells transfected with
CHD5-plasmid or Control-plasmid were seeded and incubated until
confluent. Cell monolayers were scratched and then incubated in
medium with 0.5% FBS. Every 12 h after scratching, we evaluated the
percent reduction of the scratched area using the scientific
image-analysis program, ImageJ (National Institutes of Health,
Bethesda).
Invasion assay
We used a Corning BioCoat Matrigel Invasion Chamber
(Discovery Labware) to evaluate the invasion abilities of AGS and
NUGC-3 gastric cancer cells (20).
Cells were cultured in serum-free medium and then transferred to
wells filled with medium containing 10% FBS. After incubation for
24 h cells that invaded from the culture site to the opposite side
of the membrane were fixed and stained with Diff-Quick, and then
counted with a microscope.
Western blotting analysis
We extracted proteins from AGS, MKN45, and NUGC-3
gastric cancer cells. Proteins were resolved with SDS-PAGE gels
(Bio-Rad Laboratories), transferred onto PVDF membranes
(Millipore), and incubated with primary antibodies at 4°C
overnight. After incubation with secondary antibodies, signals were
detected with the ECL Prime Western Blotting Detection reagent (GE
Healthcare). The following antibodies (all at 1:1,000 dilution)
were used in the present study: ACTB (Sigma-Aldrich), CHD5 (Santa
Cruz Biotechnology, Inc.), p53 (DAKO), Murine Double Minute 2
(MDM2; Santa Cruz Biotechnology, Inc.), Enhancer of zeste homologue
2 (EZH2; Invitrogen), and tri-methylation at lysine 27 of histone
H3 (H3K27me3; Abcam).
Statistical analysis
We compared clinicopathological factors using the
Chi-squared test for categorical variables and the Mann-Whitney U
test for continuous variables. Overall survival (OS) was defined as
the time from the date of surgery to the date of death from any
cause. Recurrence-free survival (RFS) was defined as the time from
the date of surgery to either the date of recurrence or death from
any cause. OS and RFS were estimated by the Kaplan-Meier method and
tested with the log-rank test. Cox proportional hazards models were
used for both univariate and multivariate analyses. A value of
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using the SPSS
statistics software program, version 22 (IBM Corp).
Results
Patient clinicopathological
characteristics according to CHD5 expression in gastric cancer
tissues
Staining with IHC demonstrated CHD5 in cell nuclei,
but also occasionally in the cytoplasm or membrane. Most non-tumor
gastric epithelial cells were strongly or moderately stained with
the antibody (Fig. S2). Of the
tumor tissues, 45 (29.2%) were negatively or weakly stained,
whereas 109 (70.8%) were moderately or strongly stained. After
multiplication of the percentage scores, 97 (63.0%) and 57 (37.0%)
of 154 patients showed high and low CHD5 expression, respectively.
Clinicopathological characteristics according to CHD5 expression
are shown in Table I. Compared to
the high CHD5 expression group, a significantly higher proportion
of patients in the low CHD5 expression group demonstrated positive
lymphatic invasion (P=0.032) as well as more advanced pT status
(P=0.011) and pStage (P=0.014). There were no significant
differences between the two groups in terms of other factors.
 | Table I.Clinicopathologic characteristics of
gastric cancer patients according to CHD5 expression. |
Table I.
Clinicopathologic characteristics of
gastric cancer patients according to CHD5 expression.
|
| CHD5 expression |
|
|---|
|
|
|
|
|---|
| Characteristics | High (n=97) | Low (n=57) | P-value |
|---|
| Age (years) |
|
| 0.15 |
| Median
(range) | 71 (40–90) | 68 (30–88) |
|
| Sex |
|
| 0.21 |
|
Male | 70 (72.1%) | 35 (61.4%) |
|
|
Female | 27 (27.9%) | 22 (38.6%) |
|
| Histological
type |
|
| 0.74 |
|
Differentiated | 46 (47.4%) | 29 (50.9%) |
|
|
Undifferentiated | 51 (52.6%) | 28 (49.1%) |
|
| Lymphatic
invasion |
|
| 0.032 |
|
Yes | 68 (70.1%) | 49 (86.0%) |
|
| No | 29 (29.9%) | 8 (14.0%) |
|
| Venous
invasion |
|
| 0.067 |
|
Yes | 30 (30.9%) | 26 (45.6%) |
|
| No | 67 (69.1%) | 31 (54.4%) |
|
| pT status |
|
| 0.011 |
| T1 | 27 (27.8%) | 6 (10.5%) |
|
| T2 | 14 (14.4%) | 13 (22.8%) |
|
| T3 | 42 (43.2%) | 21 (36.8%) |
|
| T4 | 14 (14.4%) | 17 (29.8%) |
|
| pN status |
|
| 0.12 |
| N0 | 53 (54.6%) | 26 (45.6%) |
|
| N1 | 15 (15.5%) | 7 (12.3%) |
|
| N2 | 18 (18.6%) | 9 (15.8%) |
|
| N3 | 11 (11.3%) | 15 (26.3%) |
|
| pStage |
|
| 0.014 |
| I | 26 (26.8%) | 11 (19.3%) |
|
| II | 48 (49.5%) | 20 (35.1%) |
|
|
III | 20 (20.6%) | 18 (31.6%) |
|
| IV | 3 (3.1%) | 8 (14.0%) |
|
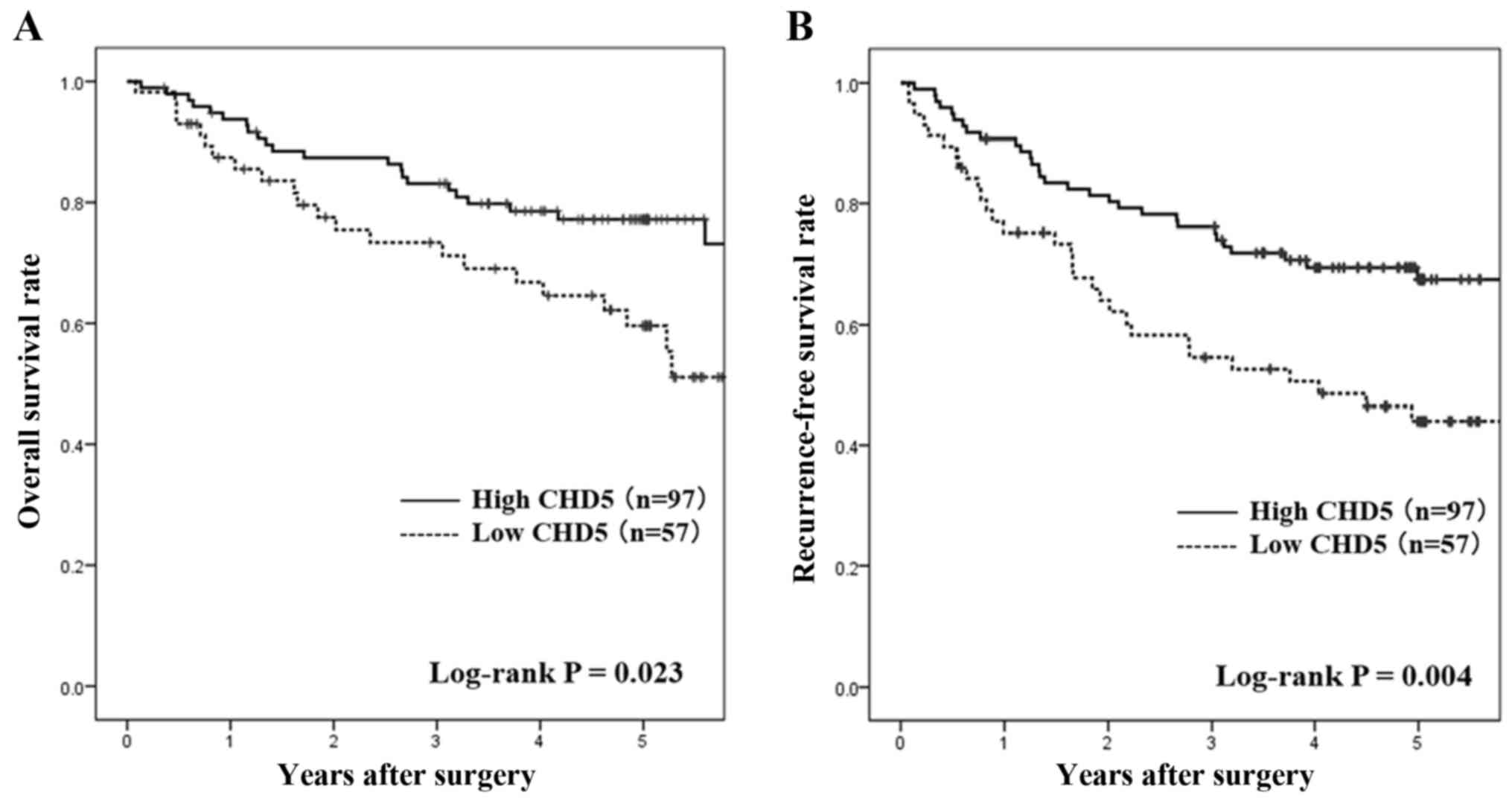
Survival analysis according to CHD5
expression
The OS and RFS at the median follow-up durations for
all censored patients was 60.9 and 61.1 months, respectively. OS in
the low CHD5 expression group was significantly shorter than that
in the high CHD5 expression group (hazard ratio [HR], 1.96; 95%
confidence interval [CI], 1.09–3.45; log-rank P=0.023; Fig. 2A). Similarly, RFS in the low CHD5
expression group was significantly shorter than that in the high
CHD5 expression group (HR, 2.04; 95% confidence interval [CI],
1.23–3.46; log-rank P=0.004; Fig.
2B).
A Cox multivariate analysis for OS using all
considerable confounding factors showed that CHD5 expression was an
independent prognostic factor, along with age and pathological N
status (Table II).
 | Table II.Cox multivariate analysis of overall
survival. |
Table II.
Cox multivariate analysis of overall
survival.
|
Characteristics | Hazard ratio (95%
CI) | P-value |
|---|
| Age (years) |
| 0.001 |
|
<70 | 1 |
|
|
≥70 | 2.91
(1.54–5.50) |
|
| Sex |
| 0.71 |
|
Female | 1 |
|
|
Male | 1.13
(0.59–2.14) |
|
| Histological
type |
|
|
|
Differentiated | 1 | 0.21 |
|
Undifferentiated | 1.48
(0.80–2.75) |
|
| Lymphatic
invasion |
| 0.16 |
| No | 1 |
|
|
Yes | 1.77
(0.79–3.92) |
|
| Venous
invasion |
| 0.44 |
| No | 1 |
|
|
Yes | 1.28
(0.69–2.38) |
|
| Pathological T
status |
| 0.13 |
|
<T3 | 1 |
|
|
≥T3 | 1.65
(0.86–3.15) |
|
| Pathological N
status |
| 0.001 |
| N0 | 1 |
|
|
N1-3 | 3.65
(1.72–7.72) |
|
| CHD5
expression |
| 0.009 |
|
High | 1 |
|
|
Low | 2.25
(1.23–4.10) |
|
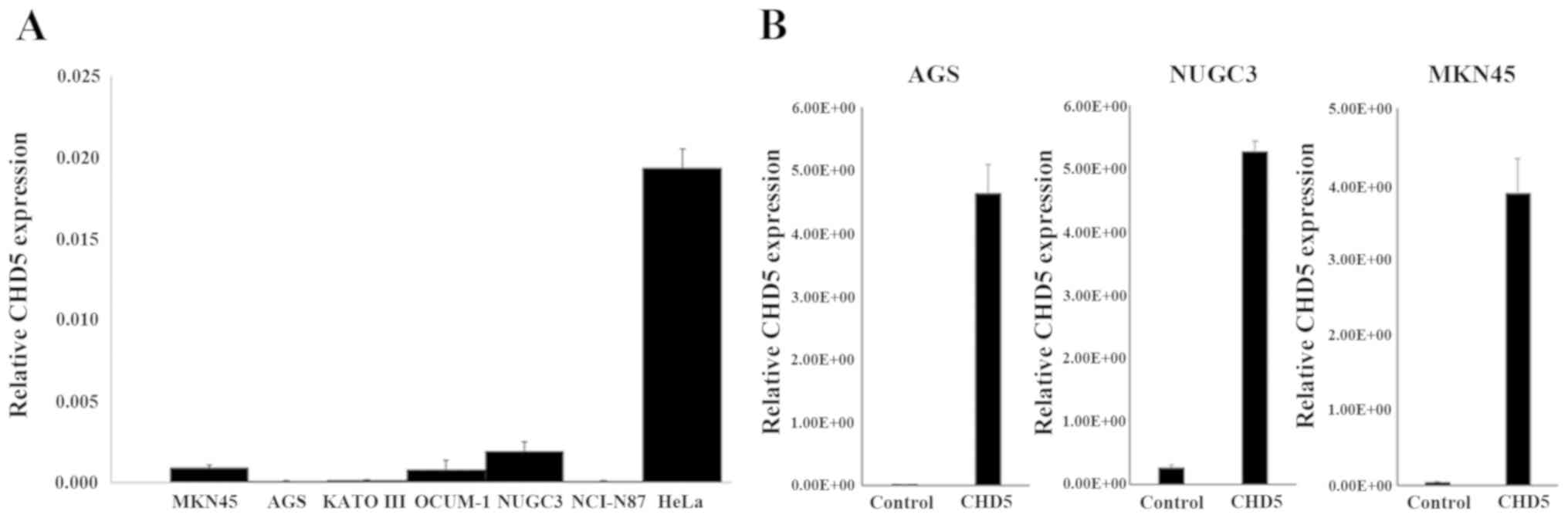
CHD5 baseline expression and
up-regulation using the CRISPR/Cas9 system in gastric cancer
cells
The expression of CHD5 in gastric cancer cell lines
was determined by quantitative RT-PCR. Although CHD5 was expressed
in HeLa cells, its expressions in six gastric cancer cell lines
(AGS, KATO III, MKN45, NCI-N87, NUGC-3, and OCUM-1) were
down-regulated (Fig. 3A). After
transfecting AGS, MKN45, and NUGC-3 cells with CHD5-plasmid, CHD5
expression was up-regulated in comparison with cells transfected
with Control-plasmid (Fig. 3B).
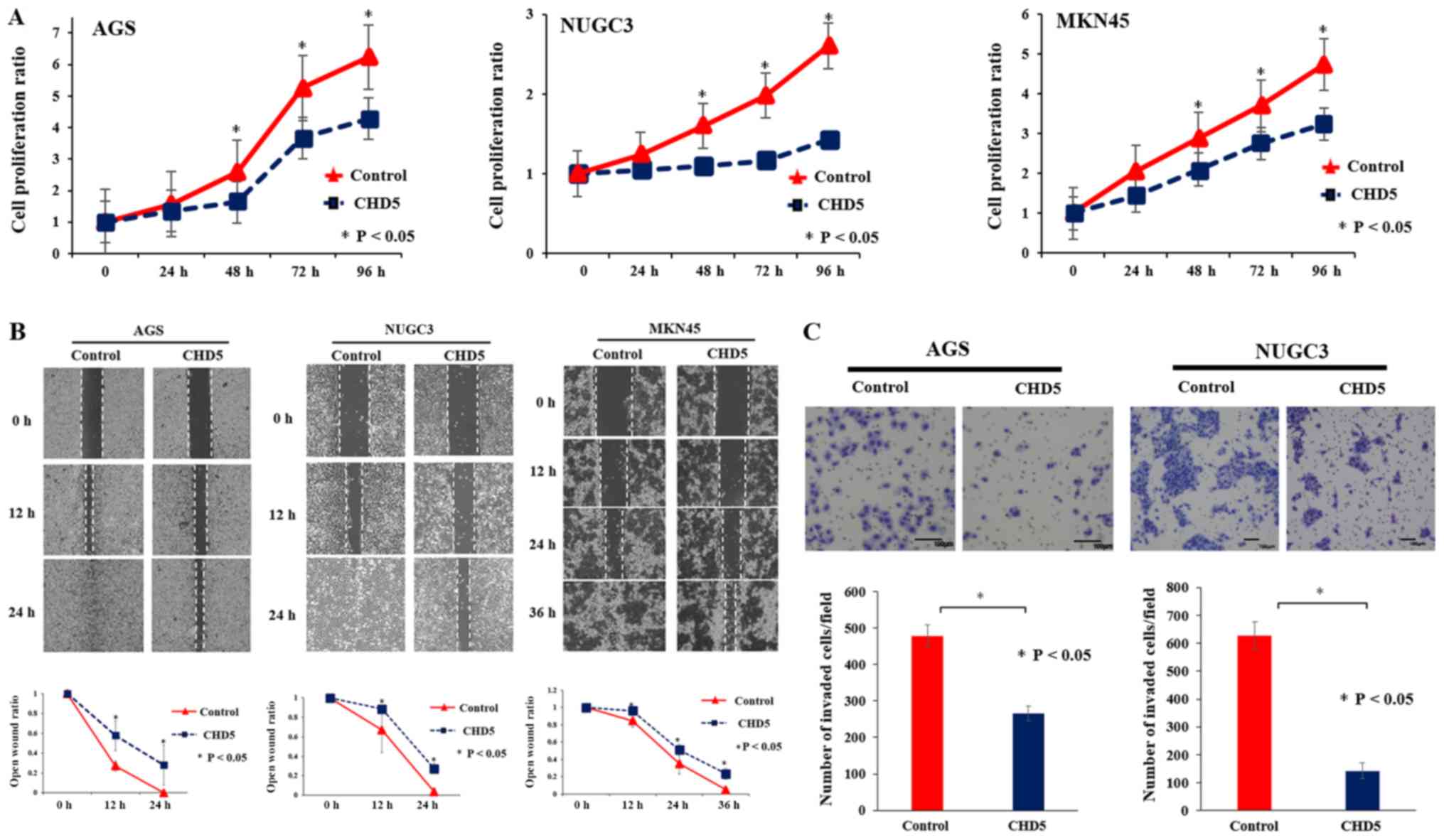
Proliferation, migration, and invasion
assays in gastric cancer cells
We assessed the effects of CHD5 up-regulation by the
CRISPR/Cas9 system on the proliferation, migration, and invasion of
gastric cancer cell lines. The proliferation assay showed that cell
growth abilities in AGS, MKN45, and NUGC-3 cells with up-regulated
CHD5 following CHD5-plasmid transfection were significantly
inhibited compared to cells transfected with Control-plasmid
(Fig. 4A). Next, the scratch
wound-healing assay showed that cell migration abilities of AGS,
MKN45, and NUGC-3 cells were significantly suppressed following
CHD5 up-regulation (Fig. 4B).
Finally, the Matrigel invasion assay showed that invasion abilities
of AGS and NUGC-3 cells were significantly decreased following CHD5
up-regulation (Fig. 4C).
Western blotting analysis of multiple
cancer genes regulated by CHD5
We examined the protein expression levels of
multiple cancer genes, including oncogenes (MDM2, p53) and
epigenetic master genes (EZH2, H3K27me3), in AGS, NUGC-3, and MKN45
cells transfected with CHD5-plasmid or Control-plasmid. Western
blotting showed that the expression levels of MDM2, EZH2, and
H3K27me3 were decreased in CHD5-transfected gastric cancer cells,
while the expression level of p53 was increased in CHD5-transfected
gastric cancer cells (Fig. S3).
Discussion
In the present study, we performed IHC in gastric
cancer specimens and revealed that the low CHD5 expression group
was significantly more likely to exhibit lymphatic invasion, more
advanced tumor stage, and worse prognosis than the high CHD5
expression group, and CHD5 expression was an independent prognostic
factor for gastric cancer. Furthermore, we found that in vitro
up-regulation of CHD5 with the CRISPR/Cas9 system suppressed the
proliferation, migration, and invasion abilities of gastric cancer
cells. We also showed in western blotting analysis that the
up-regulation of CHD5 suppressed the transcription of multiple
targets, including MDM2, EZH2, and H3K27me3, but up-regulated p53.
Thus, CHD5 is considered to be a tumor suppressor in gastric cancer
and its expression in tissue samples may be a novel biomarker to
predict prognosis in gastric cancer patients.
Previous studies reported that low CHD5 protein
expression was an independent predictive marker for poor prognosis
in patients with a variety of malignancies, including glioma,
neuroblastoma, pancreatic cancer, and lung cancer (18,19,21,22).
Meanwhile, in gastric cancer, few studies have investigated the
protein expression of CHD5 in tumor samples, although several
studies reported that CHD5 was down regulated through promoter
hypermethylation and might function as a tumor suppressor in
gastric cancer cell lines (16,23). Our
results support those of previous reports and indicate that IHC of
CHD5 may be clinically useful to more accurately characterize
patients with gastric cancer.
As for the mechanisms of CHD5 down-regulation, many
studies revealed that CHD5 was transcriptionally silenced by
DNA methylation of its promoter, silencing the remaining allele in
some cancers with 1p deletion (24–26).
Indeed, it was reported that in various cancers, the promoter of
CHD5 was more heavily methylated than those of the remaining
genes in the CHD family (27). Also, regarding gastric cancer, CHD5
was reported to be down regulated through promoter hypermethylation
in cell lines and tissue samples as described above. In the present
study, all gastric cancer cell lines, including those whose
methylation status has already been examined, showed suppressed
CHD5 expression, with promoter methylation speculated to be the
cause.
Additionally, the functional role of CHD5 has
been explored in several malignancies and it has been characterized
as a tumor suppressor gene, although the detailed mechanism remains
unclear. First, Bagchi et al suggested that CHD5 regulated
proliferation, apoptosis, and senescence through the p19(Arf)-p53
tumor suppressor pathway in a mouse model (12). In pancreatic cancer, Hall et
al showed that low CHD5 expression prolonged cancer survival by
activating the DNA damage response, and was a poor prognostic
factor in patients with pancreatic cancer treated with adjuvant
chemotherapy (19). Furthermore, in
renal cell carcinoma, Du et al. proposed that CHD5 might
epigenetically down-regulate the expression of various
cancer-related targets, such as oncogenes, epigenetic genes,
epithelial-mesenchymal transition factors, and stem cell markers,
by binding to their promoter areas (28). In addition, several recent studies
reported that some kinds of microRNA represented a potential
epigenetic mechanism to regulate CHD5 expression (16,29).
Against this background, we used western blotting analysis to
examine the p53 pathway, which has been most commonly suggested to
be associated with CHD5, and EZH2, which is a primary epigenetic
regulator of various tumor suppressor genes (30). Additionally, several studies reported
the mutual regulation of CHD5 and EZH2 (31). We found that CHD5 up-regulation was
associated with up-regulation of p53 and down-regulation of EZH2.
Based on these results, we hypothesize that down-regulation of CHD5
might promote gastric cancer tumorigenesis by down-regulating p53,
one of the most important tumor suppressor genes, and up-regulating
EZH2, thereby suppressing various tumor suppressor genes.
The present study has several limitations. First, it
was a retrospective study conducted at a single institution.
However, we collected the data of consecutive patients, and we
therefore believe that the issue of selection bias was minimized.
Second, we only assessed CHD5 in terms of proliferation, inhibitory
migration, and invasion. Further research is needed to yield more
detailed findings about the roles of CHD5. Third, we used western
blotting to examine the expression levels of only a limited number
of cancer-related genes and tumor suppressor genes. In the future,
it may be necessary to investigate other pathways and determine the
precise mechanisms of CHD5 in gastric cancer.
To the best of our knowledge, this is the first
study to investigate the clinical implications of CHD5 expression
and its functions as a tumor suppressor in gastric cancer. In
conclusion, our study suggested that CHD5 expression affects cancer
malignancy and prognosis by regulating oncogenes and epigenetic
modifiers in gastric cancer. CHD5 might function as a tumor
suppressor, and assessing its expression using IHC may be a useful
prognostic biomarker in gastric cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TH, YK, NW, and YD conceived and designed the study.
TH and YK analyzed the data and wrote the manuscript. TT, YM, KT,
TM, MY, KN and MM interpreted the data, were involved in drafting
the manuscript and revising it critically for important
intellectual content. All authors read and approved the final
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Osaka University Hospital (approved no. 18227), and
it conforms to the provisions of the Declaration of Helsinki.
Informed consent was obtained from all individuals in the current
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kurokawa Y, Doki Y, Mizusawa J, Terashima
M, Katai H, Yoshikawa T, Kimura Y, Takiguchi S, Nishida Y,
Fukushima N, et al: Bursectomy versus omentectomy alone for
resectable gastric cancer (JCOG1001): A phase 3, open-label,
randomised controlled trial. Lancet Gastroenterol Hepatol.
3:460–468. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colvin H, Mizushima T, Eguchi H, Takiguchi
S, Doki Y and Mori M: Gastroenterological surgery in Japan: The
past, the present and the future. Ann Gastroenterol Surg. 1:5–10.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hashimoto T, Kurokawa Y, Mori M, et al:
Update on the Treatment of gastric cancer. JMA J. 1:40–9. 2018.
View Article : Google Scholar
|
|
5
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3, openlabel,
randomized controlled trial. Lancet. 376:687–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartley AN and Hamilton SR: Select
biomarkers for tumors of the gastrointestinal tract: Present and
future. Arch Pathol Lab Med. 139:457–468. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hashimoto T, Kurokawa Y, Takahashi T,
Miyazaki Y, Tanaka K, Makino T, Yamasaki M, Nakajima K, Ikeda JI,
Mori M and Doki Y: Predictive value of MLH1 and PD-L1 expression
for prognosis and response to preoperative chemotherapy in gastric
cancer. Gastric Cancer. 22:785–792. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thompson PM, Gotoh T, Kok M, White PS and
Brodeur GM: CHD5, a new member of the chromodomain gene family, is
preferentially expressed in the nervous system. Oncogene.
22:1002–1011. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okawa ER, Gotoh T, Manne J, Igarashi J,
Fujita T, Silverman KA, Xhao H, osse YP, White PS and Brodeur GM:
Expression and sequence analysis of candidates for the 1p36.31
tumor suppressor gene deleted in neuroblastomas. Oncogene.
27:803–810. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kolla V, Zhuang T, Higashi M, Naraparaju K
and Brodeur GM: Role of CHD5 in human cancers: 10 years later.
Cancer Res. 74:652–658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oliver SS, Musselman CA, Srinivasan R,
Svaren JP, Kutateladze TG and Denu JM: Multivalent recognition of
histone tails by the PHD fingers of CHD5. Biochemistry.
51:6534–6544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bagchi A, Papazoglu C, Wu Y, Capurso D,
Brodt M, Francis D, Bredel M, Vogel H and Mills AA: CHD5 is a tumor
suppressor at human 1p36. Cell. 128:459–475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mulero-Navarro S and Esteller M: Chromatin
remodeling factor CHD5 is silenced by promoter CpG island
hypermethylation in human cancer. Epigenetics. 3:210–215. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baykara O, Tansarikaya M, Bulut P,
Demirkaya A and Buyru N: CHD5 is a potential tumor suppressor in
non small cell lung cancer (NSCLC). Gene. 618:65–68. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Lau KK, So LK and Lam YW: CHD5 is
down-regulated through promoter hypermethylation in gastric cancer.
J Biomed Sci. 16:952009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu G, Zhu H, Zhang M and Xu J: Histone
deacetylase 3 is associated with gastric cancer cell growth via the
miR-454-mediated targeting of CHD5. Int J Mol Med. 41:155–163.
2018.PubMed/NCBI
|
|
17
|
Japanese Gastric Cancer Association, .
Japanese classification of gastric carcinoma: 3rd English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, He S, Tu Y, Ji P, Zong J, Zhang J,
Feng F, Zhao J, Gao G and Zhang Y: Downregulation of chromatin
remodeling factor CHD5 is associated with a poor prognosis in human
glioma. J Clin Neurosci. 20:958–963. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hall WA, Petrova AV, Colbert LE, Hardy CW,
Fisher SB, Saka B, Shelton JW, Warren MD, Pantazides BG, Gandhi K,
et al: Low CHD5 expression activates the DNA damage response and
predicts poor outcome in patients undergoing adjuvant therapy for
resected pancreatic cancer. Oncogene. 33:5450–5456. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamamoto M, Takahashi T, Serada S, Sugase
T, Tanaka K, Miyazaki Y, Makino T, Kurokawa Y, Yamasaki M, Nakajima
K, et al: Overexpression of leucine-rich α2-glycoprotein-1 is a
prognostic marker and enhances tumor migration in gastric cancer.
Cancer Sci. 108:2052–2060. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garcia I, Mayol G, Rodriguez E, Suñol M,
Gershon TR, Ríos J, Cheung NK, Kieran MW, George RE, Perez-Atayde
AR, et al: Expression of the neuron-specific protein CHD5 is an
independent marker of outcome in neuroblastoma. Mol Cancer.
9:2772010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao R, Yan Q, Lv J, Huang H, Zheng W,
Zhang B and Ma W: CHD5, a tumor suppressor that is epigenetically
silenced in lung cancer. Lung Cance. 76:324–331. 2012. View Article : Google Scholar
|
|
23
|
Qu Y, Dang S and Hou P: Gene methylation
in gastric cancer. Clin Chim Acta. 424:53–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu X, Zhu Z, Li W, Fu X, Su D, Fu L, Zhang
Z, Luo A, Sun X, Fu L and Dong JT: Chromodomain helicase DNA
binding protein 5 plays a tumor suppressor role in human breast
cancer. Breast Cancer Res. 14:R732012. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fatemi M, Paul TA, Brodeur GM, Shokrani B,
Brim H and Ashktorab H: Epigenetic silencing of CHD5, a novel
tumor-suppressor gene, occurs in early colorectal cancer stages.
Cancer. 120:172–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wong RR, Chan LK, Tsang TP, Lee CW, Cheung
TH, Yim SF, Siu NS, Lee SN, Yu MY, Chim SS, et al: CHD5
downregulation associated with poor prognosis in epithelial ovarian
cancer. Gynecol Obstet Invest. 72:203–207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fujita T, Igarashi J, Okawa ER, Gotoh T,
Manne J, Kolla V, Kim J, Zhao H, Pawel BR, London WB, et al: CHD5,
a tumor suppressor gene deleted from 1p36.31 in neuroblastomas. J
Natl Cancer Inst. 100:940–949. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Du Z, Li L, Huang X, Jin J, Huang S, Zhang
Q and Tao Q: The epigenetic modifier CHD5 functions as a novel
tumor suppressor for renal cell carcinoma and is predominantly
inactivated by promoter CpG methylation. Oncotarget.
7:21618–216130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Naraparaju K, Kolla V, Zhuang T, Higashi
M, Iyer R, Kolla S, Okawa ER, Blobel GA and Brodeur GM: Role of
microRNAs in epigenetic silencing of the CHD5 tumor suppressor gene
in neuroblastomas. Oncotarget. 7:15977–15985. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim KH and Roberts CW: Targeting EZH2 in
cancer. Nat Med. 22:128–134. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie CR, Li Z, Sun HG, Wang FQ, Sun Y, Zhao
WX, Zhang S, Zhao WX, Wang XM and Yin ZY: Mutual regulation between
CHD5 and EZH2 in hepatocellular carcinoma. Oncotarget.
6:40940–40952. 2015. View Article : Google Scholar : PubMed/NCBI
|