Introduction
Mesenchymal chondrosarcoma (MC) is a rare neoplasm
and represents only 2 to 9% of all chondrosarcomas. Almost 24% of
MCs originate from extraskeletal sites, such as head, neck, the
extremities, and trunk. The kidney is an extremely rare origin of
extraskeletal MC (EMC) (1). In a
recent report from Japan (2),
primary origins of MC were bone (58%) and soft tissues (42%). MC
develops at various sites in the body, including the head and neck
(12%), trunk (62%), and extremities (26%). The authors reported 5-
and 10-year overall survival rates of 66 and 56%, respectively
(2).
In the absence of specific tumor markers and
radiographic findings, the diagnosis of MC largely depends on
pathologic examination (3,4). The HEY1-NCOA2 gene fusion is
reported to be a specific chromosomal aberration for MC and may
play a critical role in diagnosis due to its high sensitivity
(3,5–7).
Physiologically, HEY1 is considered to function mainly as a
transcriptional repressor and NCOA2 encodes a
transcriptional coactivator protein for intranuclear receptors
(7). Recently, an
IRF2BP2-CDX1 fusion gene was implicated as another candidate
MC oncogene exclusive to the HEY1-NCOA2 fusion gene
(8).
We describe the first case of primary renal EMC
mimicking renal cell carcinoma at the locally advanced stage
(cT3bN0) accompanied by vena cava thrombus and multiple pulmonary
tumor emboli. The microscopic pathology was typical of MC but the
HEY1-NCOA2 and IRF2BP2-CDX1 gene fusions were not
detected.
Case report
Clinical summary
A 64-year-old woman visited our clinic complaining
of sudden onset asymptomatic gross hematuria and was hospitalized
for further examination. Flank pain and fever were absent. Routine
laboratory tests indicated no remarkable abnormality in hematology
and urine cytology was negative. No past history was noted, except
for liver cystectomy and right mastectomy.
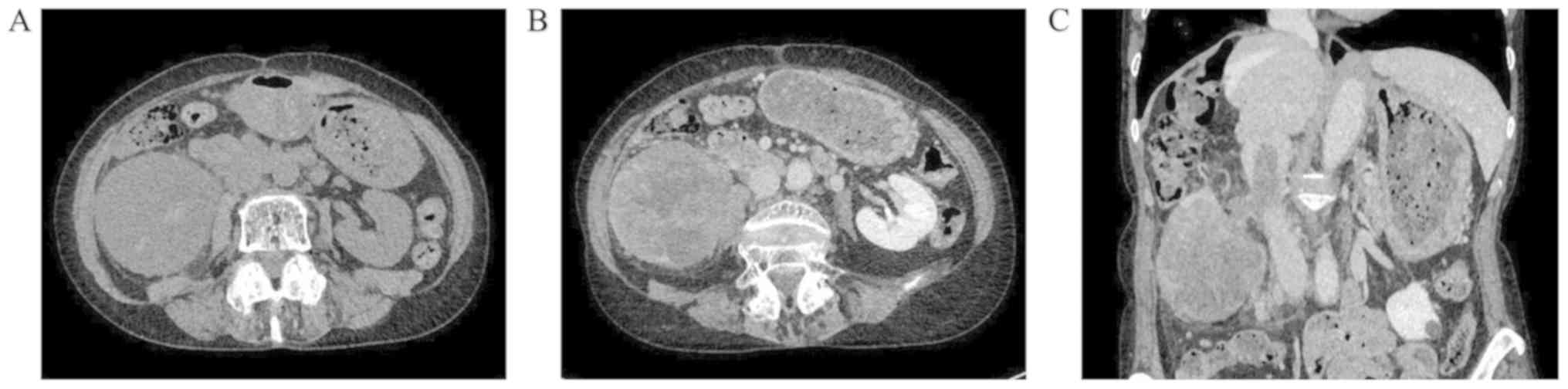
Computed tomography (CT) of the abdomen revealed a
giant mass (90×70×67 mm) in the mid to lower pole of the right
kidney. The tumor was hypovascular on contrast-enhanced CT images
compared with adjacent normal renal parenchyma, although tumor
vessels surrounding the mass were well-developed. In addition, the
tumor was characterized as internally heterogeneous, free of
calcification, and protruding into the inferior vena cava (IVC)
through the right renal vein to form a tumor thrombus up to the
level of the caudate lobe of the liver (Fig. 1). Tumor emboli were disseminated to
pulmonary arteries, but no distant metastases were identified. The
tumor presented with a high standardized uptake value of 12.00 at
the maximum in positron emission tomography (PET)-CT. Magnetic
resonance imaging (MRI) with delayed contrast-enhancement revealed
the heterogeneous nature of the tumor. On plain MRI, the tumor had
high, low, slightly low, and slightly low signal intensities on
T2-weighted, T1-weighted, diffusion-weighted, and apparent
diffusion coefficient mapping images, respectively. Histological
examination using percutaneous needle-biopsied specimens of the
tumor revealed that it contained atypical chondrocytes and spindle
cells with high nuclear-to-cytoplasmic ratio, suggesting renal
chondrosarcoma. Radical nephrectomy with intracaval thrombectomy
was performed for the initial treatment for the tumor. We
successfully en bloc resected the whole kidney and the attached IVC
thrombus using an artificial heart-lung machine and clamping IVC
just beneath the beginning of the hepatic veins in cooperation with
vascular surgeons.
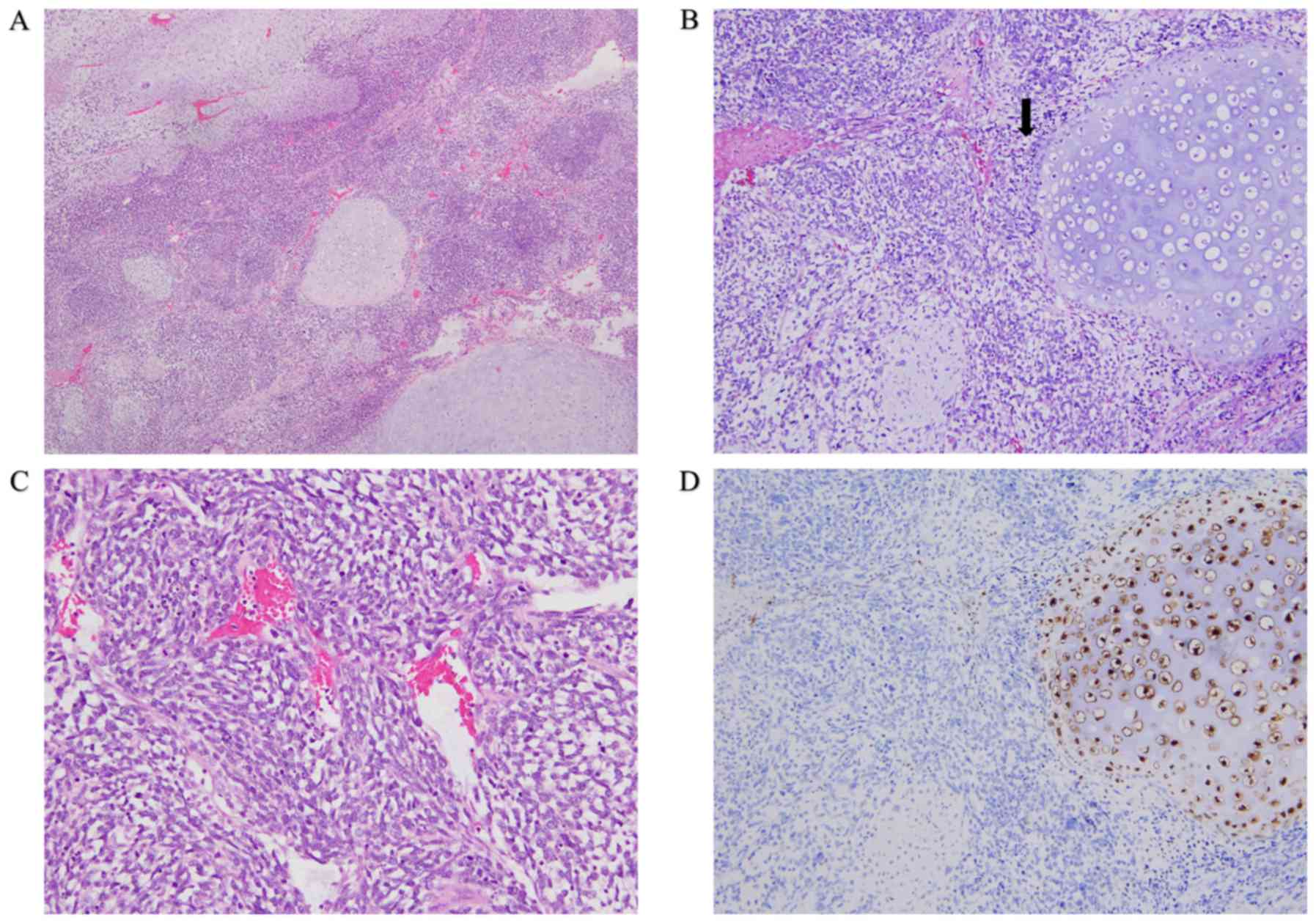
The results of the pathological investigation of the
resected specimens are presented in Fig.
2. They had biphasic morphological components with transition
zones between round and spindle-shaped hyperchromic tumor cells and
contained cartilaginous islands with good differentiation (Fig. 2A and B). The spindle-shaped cells
showed indistinct cytoplasmic borders, inconspicuous nucleoli, and
a hemangiopericytoma-like pattern (Fig.
2C). Tumor cells invaded into small lympho-vascular structures,
renal veins, and renal hilar fat tissues. No metastasis was
microscopically confirmed in any of the 11 local lymph nodes
surgically resected in total, including para-aortic/vena cava and
renal hilar lymph nodes. Immunohistochemically, the tumor was
positively stained with antibodies to vimentin, CD99, and Ki-67
(labelling index: 60–70%), without immunoreaction to epithelial
membrane antigen, CD34, chromogranin A, and synaptophysin.
Expression of S-100 protein was negative in mesenchymal spindle
cells, but was slightly positive only in some chondroid cells
(Fig. 2D). Based on these
pathological features, the tumor was diagnosed as primary renal
EMC.
Two months later, she experienced local recurrence
and multiple lung metastases. Systemic chemotherapy was delivered
and began with three courses of doxorubicin (30 mg/m2 on
day 1–2, every 3 weeks). This was followed by pazopanib (800
mg/day), but the therapy was discontinued due to drug eruption at
day 11. Eribulin mesilate was delivered in two courses (1.4
mg/m2 on day 1 and 8, every 3 weeks). The dose was
reduced to 1.1 mg/m2 in the second cycle because of
neutropenia. Palliative irradiation of 20 Gy was given to de novo
metastasis to the right 8th rib for pain control. The patient's
condition gradually worsened and she finally died of the malignancy
8 months after surgery.
HEY1-NCOA2 and IRF2BP2-CDX1 gene
fusion
Standard reverse transcription-polymerase chain
reaction (RT-PCR) were used to detect the HEY1-NCOA2 and
IRF2BP2-CDX1 chromosomal fusions, as described elsewhere
(9). In brief, RNA samples were
extracted from surgically resected and fleshly frozen MC tissues
with mirVana™ miRNA Isolation kit (Life Technologies). Total RNA (2
µg) was reverse-transcribed in a 20 µl reaction volume using a cDNA
Reverse Transcription Kit according to the manufacturer's
instructions (Applied Biosystems). RT-PCR was run using 1 µl cDNA
in a 50 µl PCR reaction using AmpliTaq Gold DNA polymerase (Applied
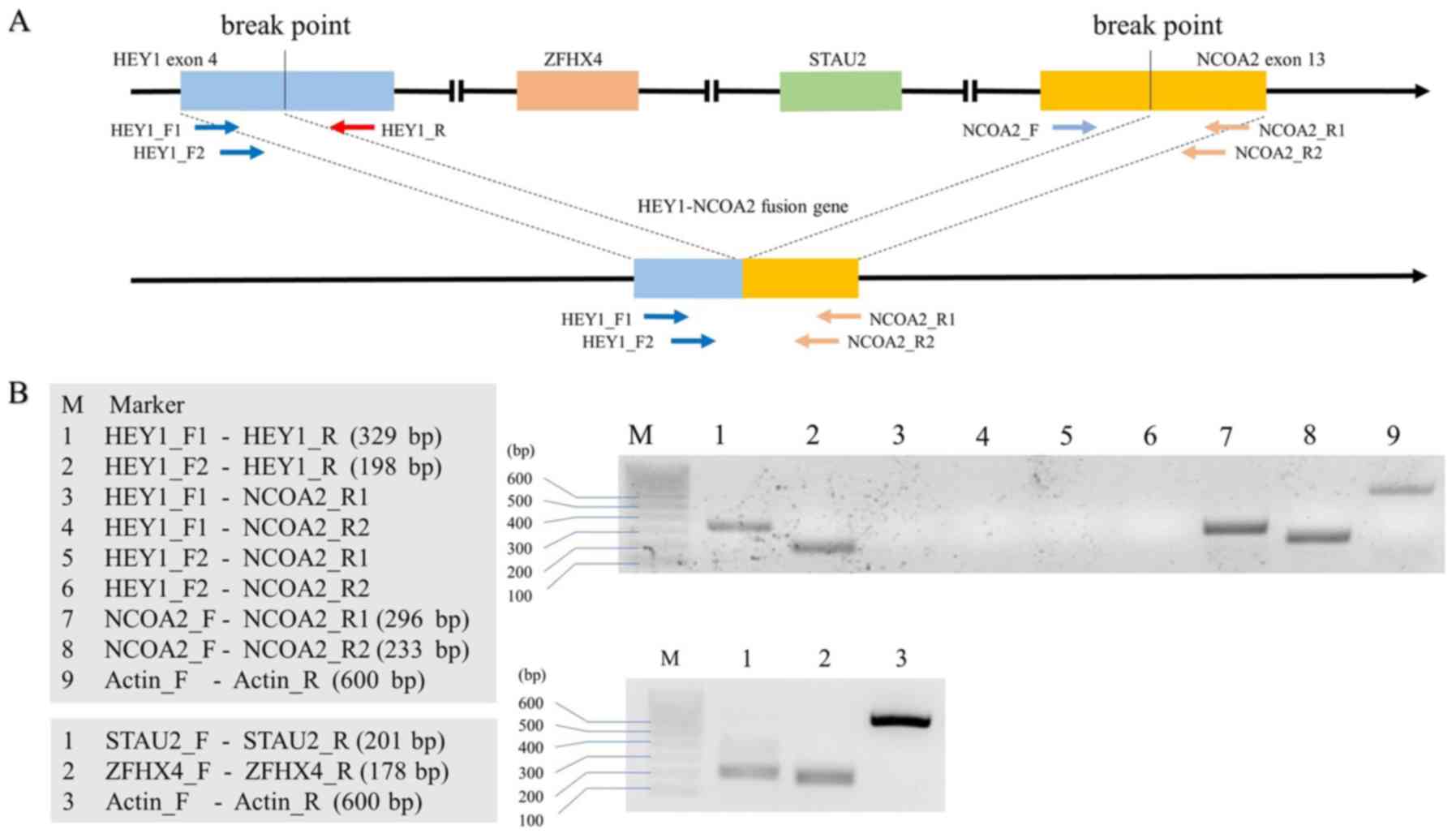
Biosystems). Primers for HEY1-NCOA2 fusion, STAU2,
and ZFHX4 are shown in Table
I and Fig. 3A (all were
purchased from Integrated DNA Technologies, IA, USA). The primers
for IRF2BP2-CDX1 were the same as reported previously
(Integrated DNA Technologies) (8).
The PCR conditions were 95°C for 30 sec, 55–60°C (according to the
Tm of each primer provided from the manufacturer) for 30 sec, and
72°C for 45 sec after initial denaturation at 95°C for 2 min.
Thirty-five cycles were run. RT-PCR of the housekeeping gene
encoding β-actin was run on all samples to check the RNA quality.
PCR products were checked using 2% agarose gel electrophoresis.
 | Table I.Primer sequences for PCR. |
Table I.
Primer sequences for PCR.
| Primer | 5′→3′ |
|---|
| HEY1_F1 |
CGAGGTGGAGAAGGAGAGTG |
| HEY1_F2 |
ACCGGATCAATAACAGTTTG |
| HEY1_R |
CCCGAAATCCCAAACTCCGA |
| NCOA2_F |
AGCTTTTCCCAGACACGAGG |
| NCOA2_R1 |
TCCTGGCTGAGGTATCAC |
| NCOA2_R2 |
AGTTGGGCTTTGCAATGTGA |
| STAU2_F |
ACTCCCCCTTGTTCTCCAGT |
| STAU2_R |
TGCCTGGTTATTGTCCGCTT |
| ZFHX4_F |
CCGCTGATGACTGGACAACT |
| ZFHX4_R |
GGTGTTGGTCTTCACCGCTA |
| βActin_F |
CCTCGCCTTTGCCGATCC |
| βActin_R |
GGATCTTCATGAGGTAGTCAGTC |
| IRF2BP2_F1 |
CAAGAGCCGCGGGTCTGGAGA |
| IRF2BP2_F2 |
GTCAACAGGCCCAAGACCGTGC |
| IRF2BP2_R |
GTGTGGTCCGGTTGGAATGAGGTG |
| CDX1_F |
CCGCAGTACCCCGACTTCTCCAG |
| CDX1_R1 |
GTTCAGTGAGCCCCAGATTGGCAG |
| CDX1_R2 |
TGATGTCGTGGGCCATCGGC |
The overall results of RT-PCR are presented in
Fig. 3B. The HEY1-NCOA2
fusion gene was not observed in this patient. Instead of the fusion
gene, intact HEY1 and NCOA2 genes were successfully
detected. The presence of intact STAU2 and ZFHX4
genes, each of which should be deleted from the chromosome at the
fusion of HEY1 and NCOA2 genes (Fig. 3A), was confirmed by RT-PCR in the
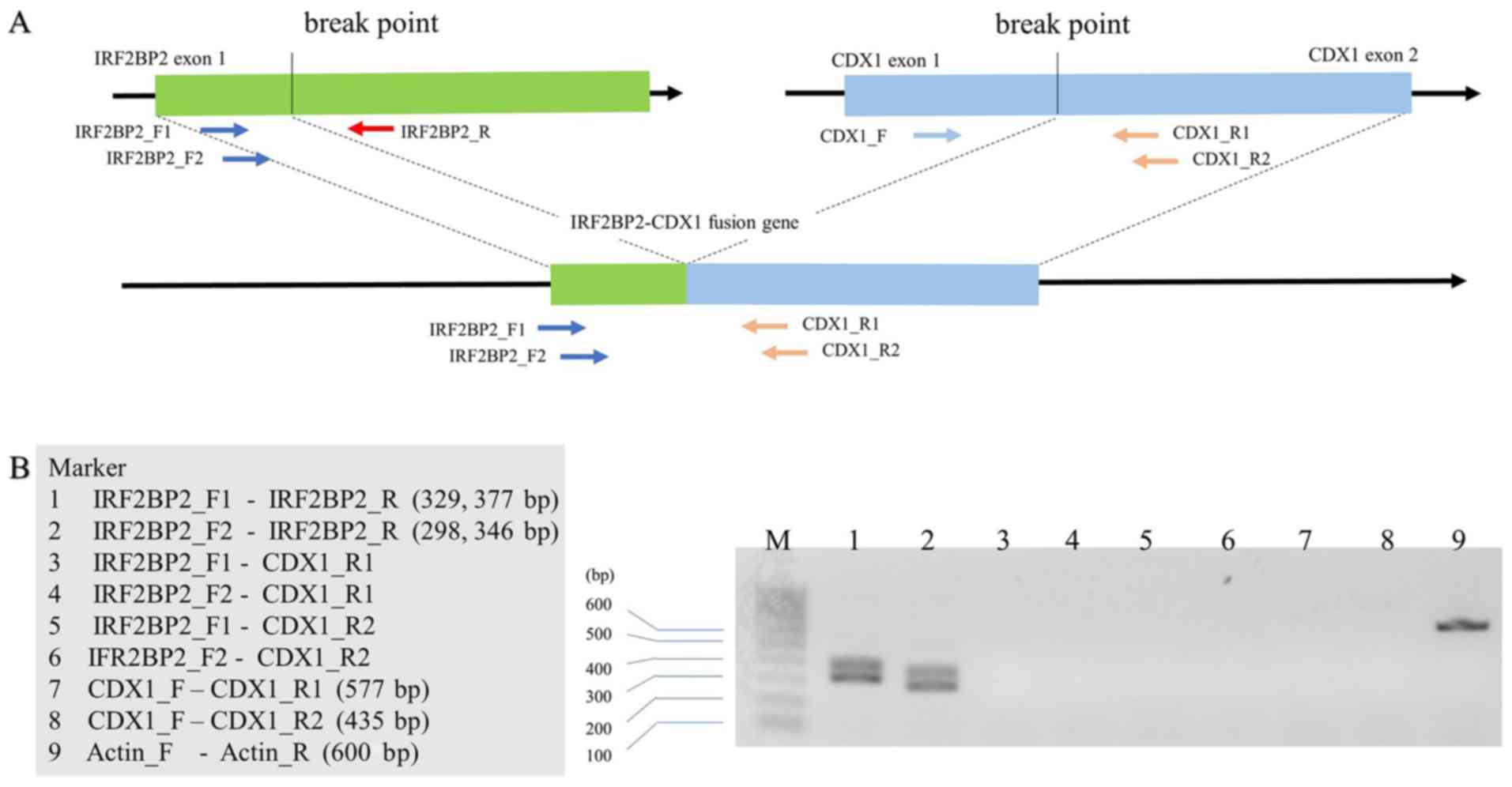
patient's samples. Similarly, the IRF2BP2-CDX1 fusion was
not detected in the patient (Fig.
4). Taken together, these findings demonstrate that the
HEY1-NCOA2 and IRF2BP2-CDX1 gene fusions were absent
or were not located at the chromosomal breakpoints that were
previously reported.
Discussion
Primary renal EMC is extremely rare (1). To our best knowledge, only 16 patients
with renal EMC, including the present case, have been reported in
the medical literature (Table II)
(1,10–23).
According to a review article on renal EMC (1), flank pain and hematuria are two most
common clinical symptoms. Renal EMC occurs from children to elderly
people with the peak incidence in the third decade, equally in men
and women (1). No clinical
differences in oncological behaviors are indicated between renal
and non-renal origins of EMC (1).
Despite a paucity of credible evidence for renal EMC, a favorable
prognosis might be expected if the tumor is small, locally
confined, and completely resected (1). Mimicking advanced renal cell carcinoma
(cT3bN0), our case had tumor dissemination in pulmonary arteries at
the initial presentation and early metastatic recurrence occurred
in the bilateral lungs soon after radical nephrectomy with IVC
thrombectomy.
 | Table II.Case series about MC of the
kidney. |
Table II.
Case series about MC of the
kidney.
| No. | Year | Author | Age | Sex | Calcification | Size (cm) |
Metastasisa | Treatment | Follow | Outcome |
|---|
| 1 | 1981 | Pitfield J | 61 | M | + | 12 | − | − | 2 m | Dead |
| 2 | 1984 | Malhotra CM | 27 | M | + | 9 | − | RTx, CTx, Mx | 69 m | Alive |
| 3 | 1991 | Karanauskas S | 15 | M | + | ND | + | ND | ND | ND |
| 4 | 2001 | Gomez-B | 52 | F | + | 8 | − | − | 1 y | Alive |
| 5 | 2006 | Kaneko T | 61 | F | + | 2.5 | − | − | 6 y | Alive |
| 6 | 2008 | Dantonello TM | 24 | F | ND | 10 | − | NAC | 1.3 y | Alive |
| 7 | 2009 | Buse S | 23 | F | + | 7 | + | AC, RTx | 36 m | Alive |
| 8 | 2012 | Xu H | 64 | M | − | 11 | + | − | 2 m | Dead |
| 9 | 2014 | Gherman V | 67 | M | − | 30 | − | − | 9 m | Dead |
| 10 | 2014 | Tyagi R | 22 | F | − | 6.5 | + | CTx | ND | Alive |
| 11 | 2015 | Rothberg MB | 16 | F | + | 15.2 | + | ND | ND | ND |
| 12 | 2015 | Chen D | 17 | M | − | 15 | +b | CTx | 10 m | Alive |
| 13 | 2017 | Salehipour M | 22 | M | + | 9 | − | ND | ND | ND |
| 14 | 2017 | Pani K | 24 | M | + | 8.5 | − | AC | 6 m | Alive |
| 15 | 2018 | Valente P | 35 | M | + | 20 | − | − | 18 m | Alive |
| 16 | − | Present case | 64 | F | − | 9 | − | CTx, RTx | 8 m | Dead |
MC is often difficult to diagnose because of the
lack of specific findings. On clinical imaging, MC typically
exhibits low CT attenuation with calcification and heterogeneous
appearance, T1-low and T2-high/low intensities of MR signals, and
strongly metabolic activity on PET-CT (4,24). These
radiological findings are not specific to MC and its diagnosis
entirely depends on histology. For this reason, MC is usually
diagnosed with MC postoperatively. Renal MC is frequently
misdiagnosed as renal cell carcinoma, renal pelvic cancer, or other
rare malignant tumors, such as Wilms' tumor, in younger cases. In
our case, the kidney tumor was initially suspected to be advanced
RCC due to IVC thrombus and pulmonary tumor emboli. Low
contrast-enhancement in CT and MRI and lack of remarkable findings
of blood tests, including leukocyte and neutrophil counts, and
serum levels of lactate dehydrogenase and C-reactive protein seem
to be atypical for such advanced RCC. Percutaneous needle biopsy
helped us to make an accurate pathological diagnosis and formulate
sufficient treatment plans for the kidney tumor. However, not the
all cases of MC could be successfully diagnosed with
needle-biopsied specimens. Typically, MC histology shows a biphasic
pattern, which consists of sheets of undifferentiated, round to
spindle cell component, and islands of hyaline cartilage. The
diagnosis in a small portion of biopsied tissues remains uncertain
when the chondroid structures scattered within MC are not
incidentally identified (3). This
sampling error would be an intrinsic limitation in the histologic
diagnosis of biopsied tissue.
Chromosomal aberration of HEY1-NCOA2 fusion
is reportedly specific to MC and can be a highly sensitive
diagnostic tool (3,5,6).
Table III presents 12 articles on
the gene fusion in MC that were published in medical literature
(3,5,6,8,25–32). The
HEY1-NCOA2 gene fusion is supposed to be absent in another
histologic type of sarcoma (3,5,6), but its diagnostic sensitivity is not
perfect (67–100%) despite the characteristic histology of MC
(Table III). Interestingly, the
IRF2BP2-CDX1 gene fusion in MC may be exclusive to the
HEY1-NCOA2 fusion, suggesting heterogeneous oncogenesis of
MC (8,28). In the present study, we failed to
detect the HEY1-NCOA2 and IRF2BP2-CDX1 gene fusions
in renal EMC. Herein, CDX1 expression appeared to be absent in our
case (Fig. 4). Specifically
expressed in the intestines, CDX1 is a transcription factor to
induce epithelial cell differentiation (33,34). In
gastric, colorectal and hepatocellular carcinomas, CDX1 acts as a
tumor suppressor and low expression of CDX1 are related clinically
to poor prognosis of the malignancies (34). Thus, no expression of CDX1 might
potentiate malignant nature in the present renal MC. Our results
support the possible existence of another oncogenic mechanism in
such fusion-negative cases, reflecting on the genetic heterogeneity
in MC tumorigenesis to a typical microscopic phenotype.
 | Table III.Case series about gene fusion of
MC. |
Table III.
Case series about gene fusion of
MC.
|
|
|
HEY1-NCOA2 |
IRF2BP2-CDX1 |
|
|---|
|
|
|
|
|
|
|---|
| Year | Author | n | Positive | Positive ratio
(%) | n | Positive | Positive ratio
(%) | Assay |
|---|
| 2012 | Wang L | 15 | 10 | 67 | − | − | − | FISH, RT-PCR |
| 2012 | Nyquist KB | 4 | 3 | 75 | 4 | 1 | 25 | FISH, RT-PCR |
| 2012 | Nakayama R | 10 | 8 | 80 | − | − | − | FISH |
| 2013 | Fritchie KJ | 6 | 6 | 100 | − | − | − | RT-PCR |
| 2014 | Panagopoulos I | 1 | 1 | 100 | − | − | − | RT-PCR |
| 2014 | Andersson C | 1 | 1 | 100 | − | − | − | RT-PCR |
| 2014 | Moriya K | 1 | 1 | 100 | − | − | − | FISH |
| 2015 | Sajjad EA | 1 | 1 | 100 | 1 | 0 | 0 | RT-PCR |
| 2015 | Bishop MW | 6 | 6 | 100 | − | − | − | FISH |
| 2016 | Cohen JN | 2 | 2 | 100 | − | − | − | RT-PCR |
| 2018 | Folpe AL | 3 | 3 | 100 | − | − | − | RT-PCR |
| 2018 | Toki S | 1 | 1 | 100 | − | − | − | RT-PCR |
| Total |
| 51 | 43 | 84 | 5 | 1 | 20 |
|
It might be better to analyze HEY1, NCOA2,
STAU2 and ZFHX4 gene expression using the adjacent or
normal kidney tissues together with the renal MC for comparison
(Fig. 3B). However, we were unable
to do additional experiments due to the paucity of the
freshly-frozen tissue samples left behind in store. This is a
limitation in interpretation of the present results.
In conclusion, we report the first case of primary
renal EMC, which progressed to form tumor thrombus in IVC and
pulmonary arteries at diagnosis. On pathological inspection of
surgical specimens, the tumor exhibited typical microscopic
appearance of MC. However, the presence of the HEY1-NCOA2
nor IRF2BP2-CDX1 gene fusions in the tumor was detected by
RT-PCR, suggesting the possibility of genetically heterogeneous
pathways to MC oncogenesis. Further studies will be needed to
confirm the present findings and reinforce the molecular diagnosis
of MC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AY contributed to conception and design of the
study, acquisition of data, interpretation of data and drafted the
initial and revised versions of the manuscript and is the
corresponding author. OI did conception and design of the study,
interpretation of data and assisted with the writing of several
drafts of the manuscript. HI participated in analysis and
interpretation of data especially for the technical aspects. TK and
MY performed the histological examination of the tumor and revised
the pathological section of study. SN contributed to the
conception, design and intellectual content such as genomic
abnormality and tumorigenesis. NT contributed to conception, design
and supervision of the study and made final approval of the version
to be published. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the principles embodied in the Declaration of Helsinki and approved
by the Ethical Committee of Yamagata University Faculty of Medicine
(approval no. 28, 2019). Written informed consent for publication
was provided by the patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MC
|
mesenchymal chondrosarcoma
|
|
EMC
|
extraskeletal mesenchymal
chondrosarcoma
|
|
HEY1
|
hairy/enhancer-of-split related with
YRPW motif 1
|
|
NCOA2
|
nuclear receptor coactivator 2
|
|
STAU2
|
staufen double-stranded RNA binding
protein 2
|
|
ZFHX4
|
zinc finger homeobox 4
|
|
IRF2BP2
|
interferon regulatory factor 2 binding
protein 2
|
|
CDX1
|
caudal type homeobox 1
|
References
|
1
|
Chen D, Ye ZI, Wu X, Shi B, Zhou L, Sun S,
Wei B, Yang S, Mao X and Lai Y: Primary mesenchymal chondrosarcoma
with bilateral kidney invasion and calcification in renal pelvis: A
case report and review of the literature. Oncol Lett. 10:1075–1078.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsuda Y, Ogura K, Hakozaki M, Kikuta K, Ae
K, Tsuchiya H, Iwata S, Ueda T, Kawano H and Kawai A: Mesenchymal
chondrosarcoma: A Japanese Musculoskeletal Oncology Group (JMOG)
study on 57 patients. J Surg Oncol. 115:760–767. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakayama R, Miura Y, Ogino J, Susa M,
Watanabe I, Horiuchi K, Anazawa U, Toyama Y, Morioka H, Mukai M and
Hasegawa T: Detection of HEY1-NCOA2 fusion by fluorescence in-situ
hybridization in formalin-fixed paraffin-embedded tissues as a
possible diagnostic tool for mesenchymal chondrosarcoma. Pathol
Int. 62:823–826. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shakked RJ, Geller DS, Gorlick R and
Dorfman HD: Mesenchymal chondrosarcoma: Clinicopathologic study of
20 cases Arch Pathol Lab Med. 136:61–75. 2012.PubMed/NCBI
|
|
5
|
Wang L, Motoi T, Khanin R, Olshen A,
Mertens F, Bridge J, Cin PD, Antonescu CR, Singer S, Hameed M, et
al: Identification of a novel, recurrent HEY1-NCOA2 fusion in
mesenchymal chondrosarcoma based on a genome-wide screen of
exon-level expression data. Genes Chromosomes Cancer. 51:127–139.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fritchie KJ, Jin L, Ruano A, Oliveira AM
and Rubin BP: Are meningeal hemangiopericytoma and mesenchymal
chondrosarcoma the same? A study of HEY1-NCOA2 fusion. Am J Clin
Pathol. 140:670–674. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
El Beaino M, Roszik J, Livingston JA, Wang
WL, Lazar AJ, Amini B, Subbiah V, Lewis V and Conley AP:
Mesenchymal chondrosarcoma: A review with emphasis on its
fusion-driven biology. Curr Oncol Rep. 20:372018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nyquist KB, Panagopoulos I, Thorsen J,
Haugom L, Gorunova L, Bjerkehagen B, Fosså A, Guriby M, Nome T,
Lothe RA, et al: Whole-transcriptome sequencing identifies novel
IRF2BP2-CDX1 fusion gene brought about by translocation
t(1;5)(q42;q32) in mesenchymal chondrosarcoma. PLoS One.
7:e497052012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ichiyanagi O, Ito H, Takai S, Naito S,
Kato T, Nagaoka A and Yamakawa M: A GRIA2 and PAX8-positive renal
solitary fibrous tumor with NAB2-STAT6 gene fusion. Diagn Pathol.
10:1552015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pitfield J, Preston BJ and Smith PG: A
calcified renal mass: Chondrosarcoma of kidney. Br J Radiol.
54:2621981. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Malhotra CM, Doolittle CH, Rodil JV and
Vezeridis MP: Mesenchymal chondrosarcoma of the kidney. Cancer.
54:2495–2499. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karanauskas S, Wells RG and Sty JR:
Extraosseus uptake with bone scintigraphy. Renal mesenchymal
chondrosarcoma with metastasis. Clin Nucl Med. 16:375–377. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gomez-Brouchet A, Soulie M, Delisle MB and
Escourrou G: Mesenchymal chondrosarcoma of the kidney. J Urol.
166:23052001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaneko T, Suzuki Y, Takata R, Takata K,
Sakuma T and Fujioka T: Extraskeletal mesenchymal chondrosarcoma of
the kidney. Int J Urol. 13:285–286. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dantonello TM, Int-Veen C, Leuschner I,
Schuck A, Furtwaengler R, Claviez A, Schneider DT, Klingebiel T,
Bielack SS, Koscielniak E, et al: Mesenchymal chondrosarcoma of
soft tissues and bone in children, adolescents, and young adults.
Cancer. 112:2424–2431. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Buse S, Behnisch W, Kulozik A, Autschbach
F and Hohenfellner M: Primary chondrosarcoma of the kidney: Case
report and review of the literature. Urol Int. 83:116–118. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu H, Shao M, Sun H and Li S: Primary
mesenchymal chondrosarcoma of the kidney with synchronous implant
and infiltrating urothelial carcinoma of the ureter. Diagn Pathol.
7:1252012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gherman V, Tomuleasa C, Bungardean C,
Crisan N, Ona V-D, Feciche B, Irimie A and Coman I: Management of
renal extraskeletal mesenchymal chondrosarcoma. BMC Surg.
14:1072014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tyagi R, Kakkar N, Vasishta RK and
Aggarwal MM: Mesenchymal chondrosarcoma of kidney. Indian J Urol.
30:225–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rothberg MB, Bhalodi AA, Reda EF, Zelkovic
P and Franco I: Primary renal mesenchymal chondrosarcoma: A case
report. Urology. 85:676–678. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Salehipour M, Hosseinzadeh M, Sisakhti AM,
Parvin VA, Sadraei A and Adib A: Renal extra skeletal mesenchymal
chondrosarcoma: A case report. Urol Case Rep. 12:23–25. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pani K, Yadav M, Priyaa P and Kumari N:
Extraskeletal mesenchymal chondrosarcoma at unusual location
involving spleen and kidney with review of literature. Indian J
Pathol Microbiol. 60:2622017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Valente P, Macedo-Dias J, Lobato C, Reis M
and Pina F: Primary mesenchymal chondrosarcoma of the kidney: A
case report and review of literature. J Cancer Res Ther.
14:6942018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Y, Wang X and Guo L, Li Y, Deng S,
Liu Y and Guo L: Radiological features and pathology of
extraskeletal mesenchymal chondrosarcoma. Clin Imaging. 36:365–370.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Panagopoulos I, Gorunova L, Bjerkehagen B,
Boye K and Heim S: Chromosome aberrations and HEY1-NCOA2 fusion
gene in a mesenchymal chondrosarcoma. Oncol Rep. 32:40–44. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Andersson C, Osterlundh G, Enlund F,
Kindblom LG and Hansson M: Primary spinal intradural mesenchymal
chondrosarcoma with detection of fusion gene HEY1-NCOA2: A
paediatric case report and review of the literature. Oncol Lett.
8:1608–1612. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moriya K, Katayama S, Onuma M, Rikiishi T,
Hosaka M, Watanabe M, Hasegawa T, Sasahara Y and Kure S:
Mesenchymal chondrosarcoma diagnosed on FISH for HEY1-NCOA2 fusion
gene. Pediatr Int. 56:e55–e57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sajjad EA, Sikora K, Paciejewski T,
Garbicz F, Paskal W, Szacht M, Grajkowska W and Wlodarski PK:
Intraparenchymal mesenchymal chondrosarcoma of the frontal lobe-a
case report and molecular detection of specific gene fusions from
archival FFPE sample. Clin Neuropathol. 34:288–293. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bishop MW, Somerville JM, Bahrami A, Kaste
SC, Interiano RB, Wu J, Mao S, Boop FA, Williams RF, Pappo AS and
Samant S: Mesenchymal chondrosarcoma in children and young adults:
A single institution retrospective review. Sarcoma.
2015:6082792015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cohen JN, Solomon DA, Horvai AE and Kakar
S: Pancreatic involvement by mesenchymal chondrosarcoma harboring
the HEY1-NCOA2 gene fusion. Hum Pathol. 58:35–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Folpe AL, Graham RP, Martinez A,
Schembri-Wismayer D, Boland J and Fritchie KJ: Mesenchymal
chondrosarcomas showing immunohistochemical evidence of
rhabdomyoblastic differentiation: A potential diagnostic pitfall.
Hum Pathol. 77:28–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Toki S, Motoi T, Miyake M, Kobayashi E,
Kawai A and Yoshida A: Minute mesenchymal chondrosarcoma within
osteochondroma: An unexpected diagnosis confirmed by HEY1-NCOA2
fusion. Hum Pathol. 81:255–260. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zheng H, Yang Y, Wang MC, Yuan SX, Tian T,
Han J, Ni JS, Wang J, Xing H and Zhou WP: Low CDX1 expression
predicts a poor prognosis for hepatocellular carcinoma patients
after hepatectomy. Surg Oncol. 25:171–177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fagerberg L, Hallström BM, Oksvold P,
Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S,
Danielsson A, Edlund K, et al: Analysis of the human
tissue-specific expression by genome-wide integration of
transcriptomics and antibody-based proteomics. Mol Cell Proteomics.
13:397–406. 2014. View Article : Google Scholar : PubMed/NCBI
|