Introduction
Granulomatous lobular mastitis (GLM) is a rare
idiopathic chronic inflammatory lesion of the breast that usually
masquerades as breast carcinoma, both clinically and
mammographically (1). It was first
described as a separate entity by Kessler and Wolloch (1) in 1972, although certain cases
describing GLM may also have been reported before 1972 (1–3). The
term ‘postpartum lobular granulomatous mastitis’ was proposed by
Davies and Burton (4) 10 years
later. Considering that certain cases developed GLM 15 years after
their last pregnancy, the term ‘GLM’ was formally recommended in
1987 by Going et al (5). This
term described the distinctive histological features of GLM more
accurately, avoiding the vagueness of ‘granulomatous mastitis’
(5). GLM is also known today as
idiopathic GLM (6). The etiology and
pathogenesis of GLM remains unclear. An association with pregnancy,
lactation, local autoimmune processes, infection,
hyperprolactinemia and chemical reaction induced by oral
contraceptive pills has been reported in the literature (4,7–10). The clinical and radiological features
of GLM are very similar to those of breast carcinoma. The most
common clinical manifestation is a unilateral, tender, painful,
extra-areolar breast lump (11,12).
Histopathologically, centrilobular granulomas and microabscess
formation may be observed (13,14).
Granulomatous lobulitis is not associated with trauma, specific
infection or exogenous material (1).
GLM treatment is associated with recurrent risks that may require
close medical attention for long periods of time (15). Management strategies include
observation, steroids, and partial or total mastectomy (16). Complete excision of inflammatory
tissue is the most effective treatment method, but systemic
corticosteroids, methotrexate and antibiotics can also have an
effect (6).
Despite the lack of definite epidemiological
evidence of ethnic predisposition, a prevalence of GLM in specific
racial populations has been observed (17,18). GLM
is not absent in Chinese populations, but it is usually hard to
differentiate from mammary duct ectasia (MDE). A retrospective
controlled study was performed in order to further illustrate the
clinicopathological features of GLM and MDE. The authors'
experiences on therapeutic strategies for GLM have also been
presented in this paper.
Materials and methods
Patients
The patients included in the present study were 118
women aged 15–55 years, who underwent treatment in the Qilu
Hospital of Shandong University between January 2010 and January
2012. Written informed consent was obtained from all patients prior
to the study start. The present study was approved by the Ethics
Committee on Scientific Research of Shandong University Qilu
Hospital (Jinan, China). In order to confirm the diagnosis, slides
of all cases were reviewed by a prudent pathologist of our
hospital; 29 cases were histopathologically diagnosed as GLM, 77 as
MDE and 12 as GLM accompanied with MDE. They were divided into the
GLM, MDE and overlapping groups, respectively, according to their
histological characteristics.
The individual medical history of all cases,
including age, smoking, pregnancy, parity, lactation, abortion,
time since the patient last gave birth, family history of breast
cancer, oral contraceptive and ethnic background, was reviewed. The
clinical manifestations, including mass, nipple retraction,
galactorrhea, abscess formation, skin ulcers, peau d'orange, pain
and enlargement of ipsilateral axillary lymph nodes, were all
considered. Sonography, mammography and fine needle aspiration were
performed selectively, depending on the symptomology. The previous
history and combined disease were elucidated simultaneously.
All patients underwent surgery with or without
preoperative antibiotics. Appropriate surgery, such as lumpectomy,
segmental mastectomy, subcutaneous mastectomy or subtotal
mastectomy, was performed based on the extent of the lesion of each
individual. A decline in the risk of recurrence was observed
following wide resection. Follow-ups were carried out and continued
2 years after the patients' hospitalization.
Histopathological evaluation
The hematoxylin and eosin-stained paraffin
histological sections were evaluated in detail. All the sections
were obtained and stained when the surgery was performed. The
paraffin-embedded specimens were deparaffinized, then submerged
into citrate antigen retrieval buffer, and heated for antigen
retrieval for 8 min. The sections were then treated with 3%
hydrogen peroxide at 37°C for 10 min to inactivate the endogenous
peroxidase. All the terms are listed as follows: Duct dilation,
periductal inflammation, periductal fibrosis, intraductal
secretion, ductal hyperplasia, periductal or intralobular
infiltration of inflammatory cells, intralobular microabscess
formation and perilobular granulomatous inflammation, granuloma or
microabscess formation in the surrounding tissue, multinuclear
giant and foam cell infiltration in the surrounding tissue,
necrosis. In the present study, GLM was defined as ‘perilobular
granulomatous inflammation, accompanied by predominant infiltration
of neutrophils with or without intralobular microabscess
formation’. MDE was defined as ‘periductal inflammation
characterized by extralobular irregular ductal dilation with
periductal fibrosis’. Special staining and identification of
microorganisms was not performed in the present study.
Statistical analysis
Experiments were performed in triplicate, and all
data were analyzed using SPSS statistical software (version 18.0;
SPSS Inc.). The data are presented as the mean ± standard
deviation. χ2 test was used for group comparisons
between categorical variables such as age, time of abortion. The
P-value was adjusted for multiple comparisons. ANOVA and a
Games-Howell post-hoc pairwise comparison were used for group
comparisons between continuous variables. P≤0.05 was considered to
indicate a statistically significant difference.
Results
During the study period, 1,173 patients were
histopathologically diagnosed with benign breast disease in
Department of Breast Surgery, Qilu Hospital. GLM accounted for
~3.5% (41/1,173, including 12 patients diagnosed with GLM
accompanied by MDE) of all the homochromous benign breast diseases
in the results of the present study. All 118 patients included in
the present study were Han Chinese, without a history of smoking.
No patient had a history of oral contraceptive use.
Patients were divided into three groups, according
to their histopathological characteristics: GLM, MDE and
overlapping groups. The GLM group contained patients with
perilobular granulomatous inflammation, accompanied by predominant
infiltration of neutrophils with or without intralobular
microabscess. The MDE group contained patients with periductal
inflammation characterized by extralobular irregular ductal
dilation with periductal fibrosis. The overlapping group contained
patients diagnosed with GLM, accompanied by MDE.
The age of the 29 patients in the GLM group ranged
from 15–53 years, with an average age of 31 years. A total of 28
cases were aged <40 years, and 27 had a history of both
pregnancy and parity. The highest number of pregnancies and number
of times giving birth was three. All 27 parous women had a history
of lactation. Of the 27 patients, nine had a history of abortion
with the largest frequency up to two times. The date of last
delivery for 32 patients had been within the last 5 years. The
majority of data from the MDE group were similar to those from the
GLM group, as presented in Table I.
However, the percentage of patients who had given birth within the
last 5 years in the MDE group was lower than that in the GLM group.
In the overlapping group, 3/12 patients were >40 years old, with
50% of the last births occurring within the last 5 years. In
addition, as a duct-centered process, the central portion of the
breast was more frequently affected in the duct ectasia group,
while in the GLM group, the breast peripheral parts were more
frequently affected. The location of the lesions within the breast
was analyzed, as it can also help distinguish among the three
diseases. The data revealed that the duct ectasia lesions were more
common in the central duct of the breast than the GLM lesions
(χ2=9.345; P=0.002). The medical history of the 118
cases was thoroughly reviewed. A total of four patients had
previously suffered from pituitary adenoma and three patients had
received pituitary adenoma resection. All four cases had been
histopathologically diagnosed with MDE. The serum prolactin level
dropped to normal in one patient following surgery, while that of
the other three patients remained twice as high as the upper limit
of the normal value. Another patient had a history of schizophrenia
for 14 years and underwent continuous treatment with risperidone
and neurolithium. This patient was diagnosed with GLM accompanied
by MDE.
 | Table I.Clinical characteristics of GLM, MDE
and overlapped groups. |
Table I.
Clinical characteristics of GLM, MDE
and overlapped groups.
|
|
|
|
| P-value |
|---|
|
|
|
|
|
|
|---|
| Characteristics | GLM (n=29) | MDE (n=77) | Overlapped
(n=12) | GLM vs. MDE | GLM vs.
overlapped | MDE vs.
overlapped |
|---|
| Age |
|
|
| 0.052 | 0.034a | 0.576 |
| Mean
(range), years | 31 (15–53) | 35 (18–55) | 34 (24–44) |
|
|
|
| ≤40
years, n (%) | 28 (96.6) | 63 (81.8) | 9 (75.0) |
|
|
|
| >40
years, n (%) | 1 (3.4) | 14 (18.2) | 3 (25.0) |
|
|
|
| Pregnancy history,
n (%) | 27 (93.1) | 71 (92.2) | 12 (100.0) | 0.876 | 0.351 | 0.317 |
| Frequency of
pregnancy, n (range) | 1.96 (1–3) | 2.23 (1–6) | 2.58 (1–8) | 0.389 | 0.155 | 0.338 |
|
Delivery, n (%) | 27 (93.1) | 71 (92.2) | 12 (100.0) | 0.876 | 0.351 | 0.317 |
| Number
of births | 1.48 (1–3) | 1.36 (1–3) | 1.42 (1–2) | 0.274 | 0.744 | 0.568 |
| Years
postpartum |
| ≤5, n
(%) | 26 (89.7) | 45 (58.4) | 6 (50.0) | 0.002a | 0.017a | 0.813 |
| >5,
n (%) | 3 (10.3) | 32 (41.6) | 6 (50.0) |
|
|
|
|
Lactation, n (%) | 27 (93.1) | 67 (87.0) | 11 (91.7) | 0.378 | 0.872 | 0.649 |
|
Abortion history, n (%) | 9 (31.0) | 36 (46.8) | 6 (50) | 0.144 | 0.251 | 0.834 |
|
Frequency of abortion, n
(range) | 1.25 (1–2) | 1.72 (1–4) | 2.33 (1–6) | 0.156 | 0.414 | 0.931 |
| Location |
| Around
areola | 21 (27.7) | 18 (62.1) |
| 0.002a |
|
|
|
Peripheral part | 56 (72.3) | 11 (37.9) |
|
|
|
|
Following comparative analysis, no statistically
significant difference was observed in pregnancy, delivery,
lactation and abortion between the GLM and MDE groups; however, a
last delivery within 5 years was observed more frequently in
patients of the GLM group (χ2=9.878; P=0.002). Compared
with the overlapping group, the percentage of patients aged <40
years old was higher in the GLM group (χ2=4.478;
P=0.034). Furthermore, more patients in the GLM group had had their
last delivery within the last 5 years (χ2=6.36;
P=0.012).
Local manifestations were evaluated. The majority of
patients were unilateral, excluding four bilateral cases diagnosed
with MDE. Nipple retraction was observed in 17 patients of the GLM,
43 of the MDE and 10 of the overlapping groups. Nipple discharge
was observed in two cases from the GLM and 13 from the MDE group.
None of the 12 patients in the overlapping group complained of
galactorrhea. A breast lump with or without pain was observed in
the majority of patients from all three groups. Abscess formation
occurred in 8 cases in the GLM, 38 in the MDE and 9 in the
overlapping group. Some cases subsequently developed skin ulcers
(Fig. 1). Ipsilateral axillary lymph
node enlargement was sometimes observed. The detailed local
manifestations are presented in Table
II.
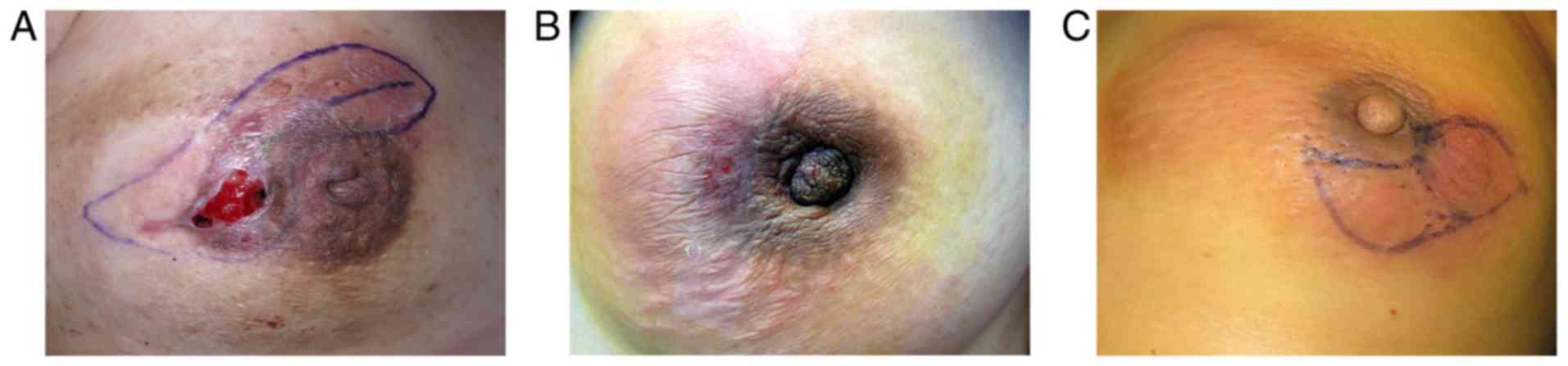 | Figure 1.(A) Granulomatous lobular mastitis
manifested as a painful tender breast lump, accompanied by nipple
retraction, redness of skin, abscess and ulcers on the surface of
the skin. (B) A patient with MDE suffering from hypophysoma
presenting with a painful, tender breast lump, accompanied by
nipple retraction, abscess formation and redness of surface skin.
(C) An overlapping case manifested as a widely distributed breast
lump, with nipple retraction, abscess formation, and redness of the
skin surface. MDE, mammary duct ectasia. |
 | Table II.Local manifestation of GLM, MDE and
overlapped groups. |
Table II.
Local manifestation of GLM, MDE and
overlapped groups.
|
|
|
|
| P-value |
|---|
|
|
|
|
|
|
|---|
|
Characteristics | GLM (n=29) | MDE (n=77) | Overlapped
(n=12) | GLM vs. MDE | GLM vs.
overlapped | MDE vs.
overlapped |
|---|
| Side, n (%) |
|
|
| 0.437 | 0.183 | 0.185 |
|
Right | 11 (37.9) | 30 (39.0) | 2 (16.7) |
|
|
|
|
Left | 18 (62.1) | 43 (55.8) | 10 (83.3) |
|
|
|
| Bilateral | 0 (0.0) | 4 (5.2) | 0 (0.0) |
|
|
|
| Nipple Retraction,
n (%) | 17 (58.6) | 43 (55.8) | 10 (83.3) | 0.797 | 0.129 | 0.071 |
| Galactorrhea, n
(%) | 2 (6.9) | 13 (16.9) | 0 (0.0) | 0.188 | 0.351 | 0.123 |
| Diameter of mass,
(cm) |
|
|
|
<0.001a | 0.002a |
<0.001a |
|
Mean | 6.23 | 4.00 | 6.27 |
|
|
|
|
Range | 3.0–10.0 | 0.6–12.0 | 2.0–14.0 |
|
|
|
| Abscess/ulceration,
n (%) | 8 (27.6) | 38 (49.4) | 9 (75.0) | 0.044a | 0.005a | 0.098 |
| Pain | 27 (93.1) | 52 (67.5) | 12 (100.0) | 0.007a | 0.351 | 0.020a |
| Lymph node
enlargement | 6 (20.7) | 12 (15.6) | 3 (25.0) | 0.533 | 0.762 | 0.418 |
The statistical analysis revealed no difference in
nipple retraction and galactorrhea between the GLM and MDE groups.
The mass size of GLM was usually larger than that of MDE
(P<0.001), while patients in the MDE group had a predisposition
for breast abscess and skin ulcers (χ2=4.062; P=0.044).
Patients in the GLM group more frequently complained of breast pain
(χ2=7.256; P=0.007). Axillary lymph node enlargement
occurred analogously in the three groups. Similar results in terms
of developing breast abscess were obtained between the overlapping
and MDE groups (χ2=7.862; P=0.005).
A total of 23 patients from the GLM group received
sonographic examination. The majority of patients exhibited an
irregular hypoechogenicity or inhomogeneous echos with obscured
margins. A sonolucent fluid-filled area containing lots of
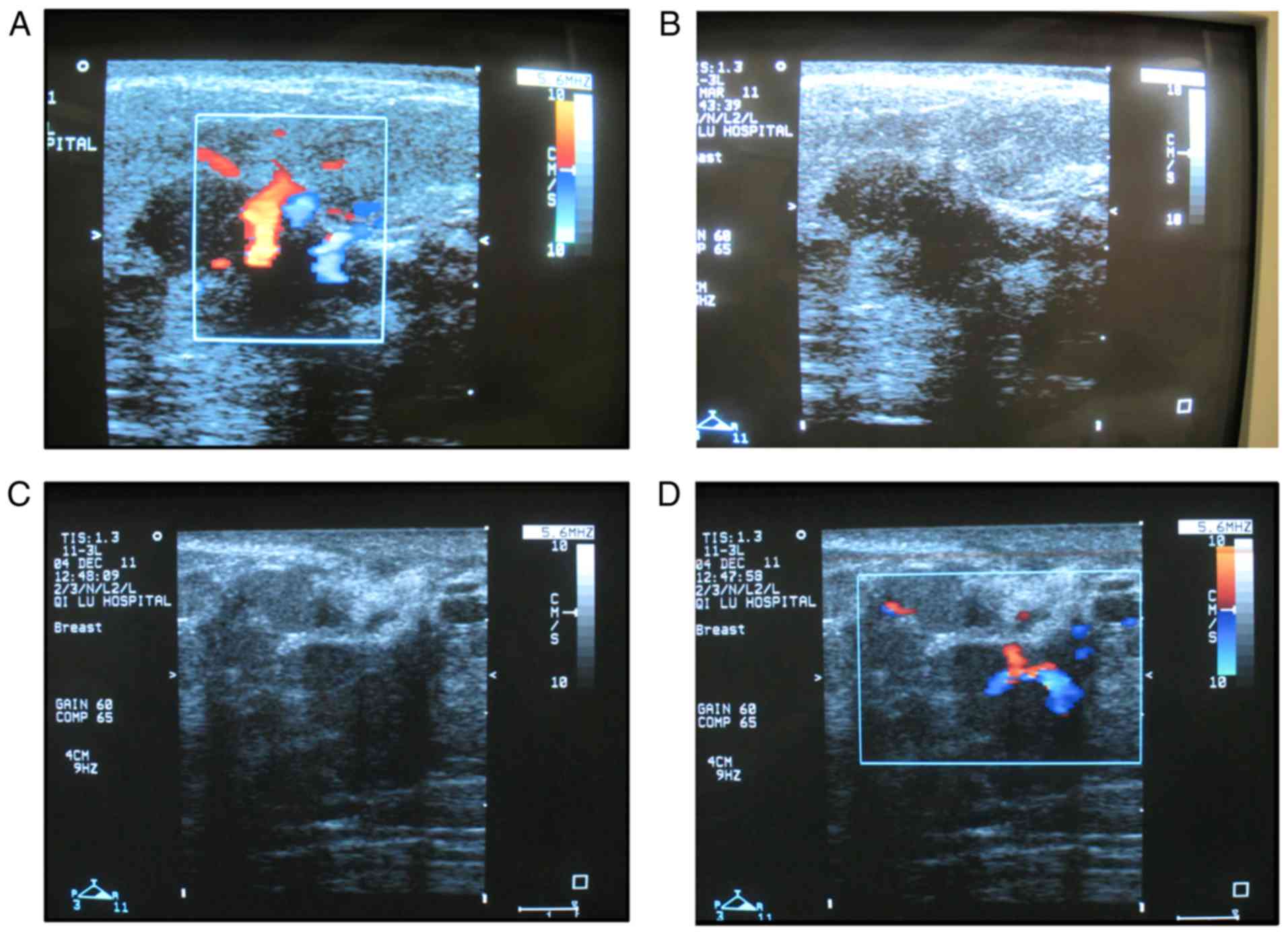
spot-like echos was observed (Fig. 2A
and B). A total of 72 cases from the MDE group received
sonographic examination. The sonographic features of MDE were
similar to those of GLM (Fig. 2C and
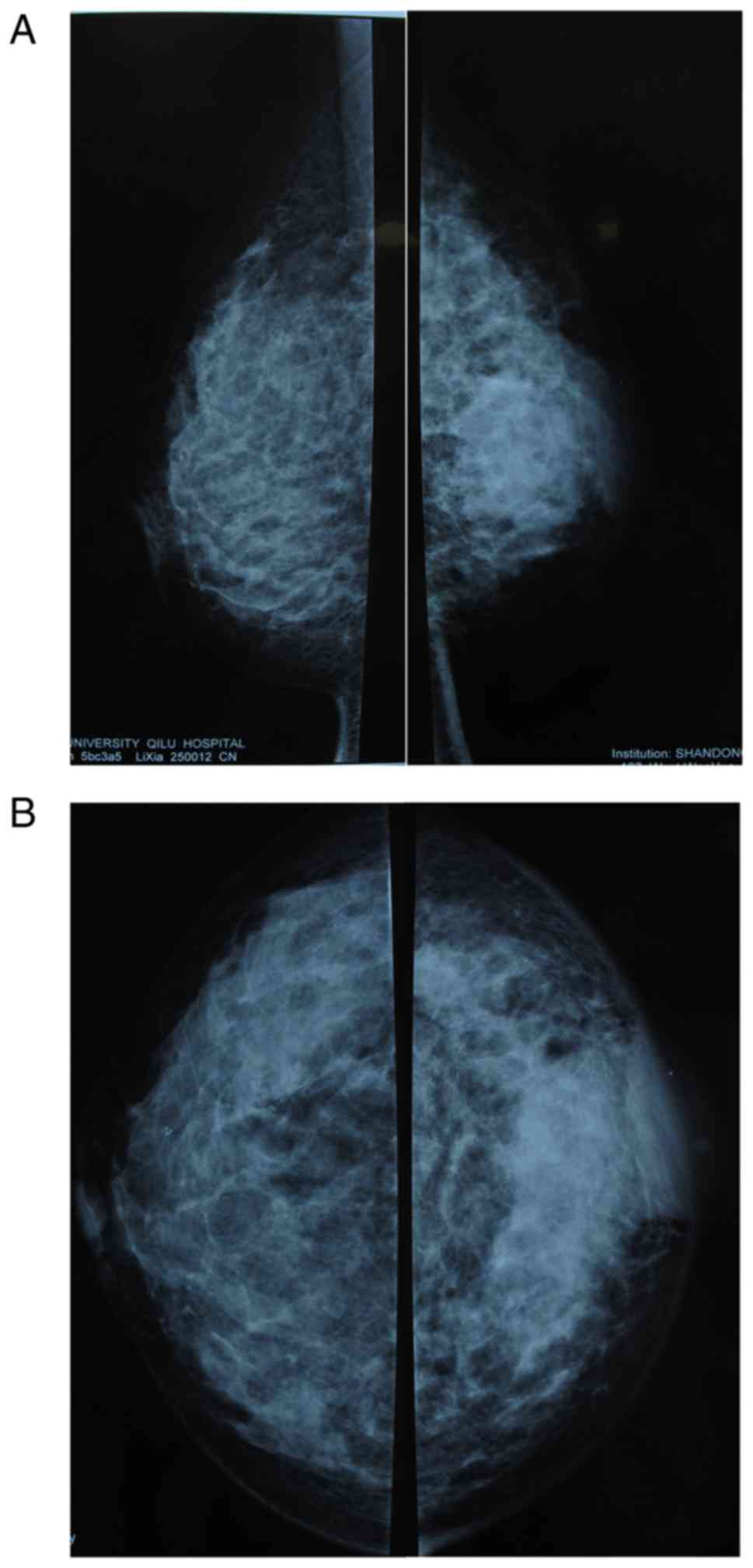
D). Local high density or architectural distortion can be seen
on the mammogram. Thickening and edema of the surface skin of the
inflammation or nipple retraction can also be observed (Fig. 3). It is believed that local
manifestation and sonography played an important role in the
diagnosis of these patients and the differential diagnosis from
breast carcinoma. However, the differentiation of GLM from MDE was
a clinical dilemma. The detailed histopathological features are
presented in Table III.
 | Table III.Histopathological characteristics of
GLM, MDE and overlapped groups. |
Table III.
Histopathological characteristics of
GLM, MDE and overlapped groups.
|
|
|
|
| P-value |
|---|
|
|
|
|
|
|
|---|
|
Characteristics | GLM (n=29) | MDE (n=77) | Overlapped
(n=12) | GLM vs. MDE | GLM vs.
overlapped | MDE vs.
overlapped |
|---|
| Duct dilation | 6 (20.7) | 75 (97.4) | 12 (100.0) |
<0.001a |
<0.001a | 0.572 |
| Periductal
inflammation | 1 (3.45) | 68 (88.3) | 11 (91.7) |
<0.001a |
<0.001a | 0.732 |
| Periductal
fibrosis | 1 (3.45) | 72 (93.5) | 12 (100.0) |
<0.001a |
<0.001a | 0.364 |
| Intraductal
secretion | 1 (3.45) | 69 (89.6) | 10 (83.3) |
<0.001a |
<0.001a | 0.522 |
| Duct
hyperplasia | 0 (0.0) | 56 (72.7) | 7 (58.3) |
<0.001a |
<0.001a | 0.308 |
| Periductal foam
cell | 0 (0.0) | 52 (67.5) | 6 (50.0) |
<0.001a |
<0.001a | 0.236 |
| Plasmocyte | 5 (17.2) | 26 (33.8) | 5 (41.7) | 0.095 | 0.098 | 0.593 |
| Neutrophile
granulocyte | 16 (55.2) | 9 (11.7) | 3 (25.0) |
<0.001a | 0.078 | 0.209 |
| Perilobular
granulomatous inflammation | 29 (100.0) | 9 (11.7) | 11 (91.6) |
<0.001a | 0.116 |
<0.001a |
| Intralobular
inflammatroy cell |
|
N,P,L | 13 (44.8) | 3 (3.9) | 4 (33.3) |
<0.001a | 0.479 |
<0.001a |
|
N,L/N | 14 (48.3) | 5 (6.5) | 6 (50.0) |
<0.001a | 0.920 |
<0.001a |
|
L,P/L | 1 (3.45) | 10 (13.0) | 1 (8.33) | 0.151 | 0.509 | 0.649 |
| Intralobular
Microabscess | 25 (86.2) | 7 (9.1) | 11 (91.7) |
<0.001a | 0.627 |
<0.001a |
| Features in
Surrounding tissue |
|
Granulomas | 26 (89.7) | 27 (35.1) | 10 (83.3) |
<0.001a | 0.574 | 0.002a |
|
Microabscess | 24 (82.8) | 23 (29.9) | 8 (66.7) |
<0.001a | 0.257 | 0.013a |
|
Multinuclear Giant Cell | 27 (93.1) | 32 (41.6) | 10 (83.3) |
<0.001a | 0.337 | 0.007a |
| Foam
Cell | 16 (55.2) | 43 (55.8) | 4 (33.3) | 0.951 | 0.203 | 0.146 |
| Cholesterol
crystal | 3 (10.3) | 4 (5.19) | 0 (0.0) | 0.341 | 0.247 | 0.419 |
| Calcification | 1 (3.45) | 1 (1.30) | 0 (0.0) | 0.468 | 0.515 | 0.691 |
| Necrosis | 1 (3.45) | 5 (6.49) | 0 (0.0) | 0.545 | 0.515 | 0.364 |
The most significant characteristic of GLM was
perilobular granulomatous inflammation (χ2=71.44;
P<0.001). Compared with the MDE group, intralobular microabscess
and granulomas were more common in patients with GLM (P<0.001).
Multinuclear giant cell infiltration in the local inflammatory
focus was observed more frequently in GLM (χ2=22.68;
P<0.001). Neutrophils were the most commonly observed
intralobular inflammatory cells (χ2=24.99; P<0.001),
with lymphocytes a close second. Plasmocytes were occasionally
observed. MDE was identified as mammary duct dilation with
inflammation of the duct wall and periductal tissue (P<0.001).
Foam cells could be found in or around the duct wall
(χ2=38.44; P<0.001). Intraductal secretion,
periductal fibrosis and duct hyperplasia were more common in MDE
than in GLM (P<0.001). Lymphocytes were the main type of
inflammatory cells infiltrating the local inflammatory focus in
MDE. A large number of plasmocytes was observed. Necrosis was
identified in both the GLM and MDE groups, but the difference was
not statistically significant. As expected, patients in the
overlapping group exhibited the histopathological characteristics
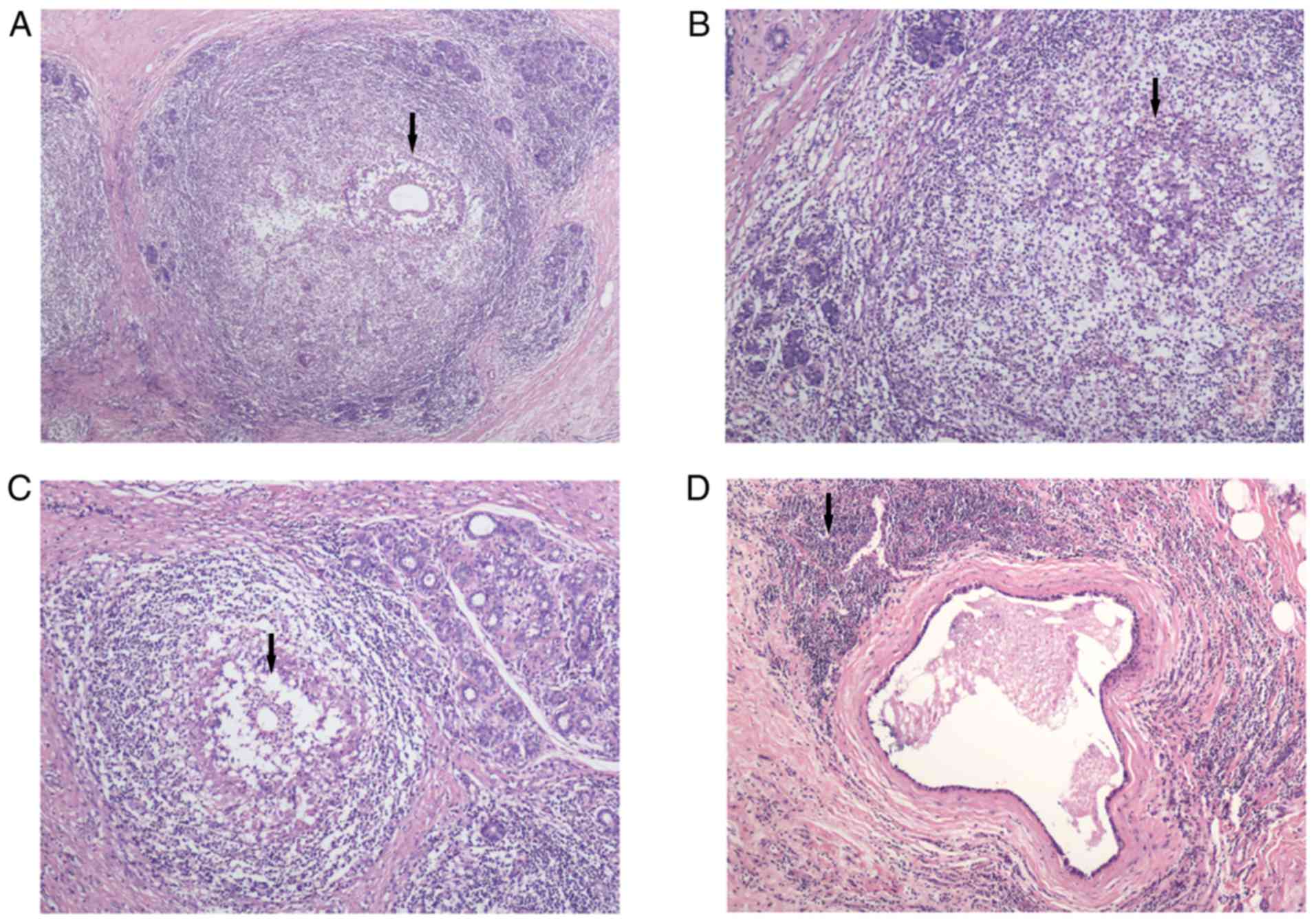
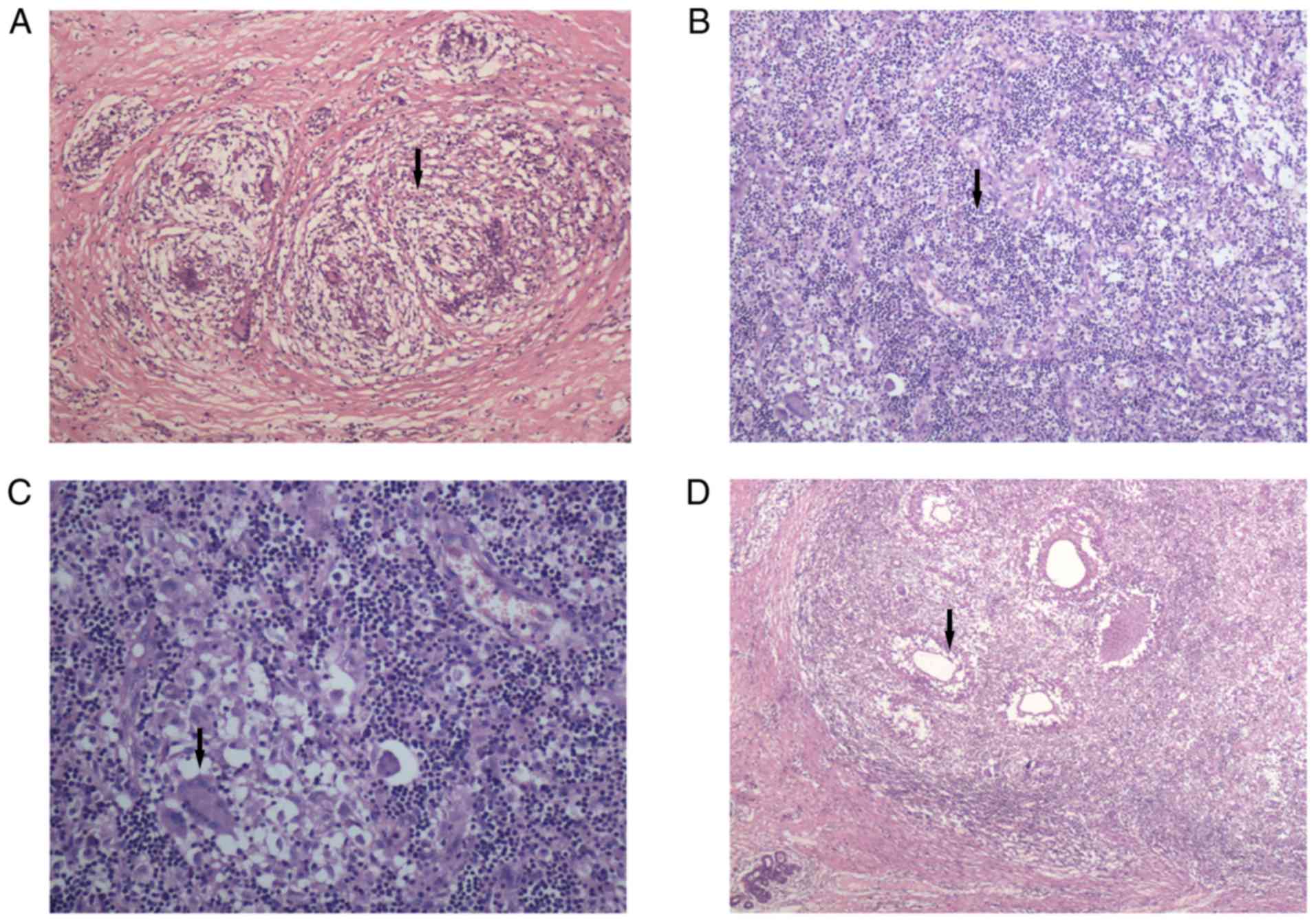
of both GLM and MDE simultaneously (Figs. 4 and 5).
The treatment strategy that followed for all three
groups was wide resection of the tissue involved in inflammation
and granulomas, with the administration of the necessary
preoperative antibiotics (such as cefazolin sodium) for 3–7 days.
Antibiotics sensitive to cocci bacteria were administered for 2–3
days following surgery. Prior to their final admission to hospital,
5 patients in the GLM, 21 in the MDE and 2 in the overlapping group
had a history of abscess incision and drainage. Certain patients
had previously experienced repeated recurrence, up to three times
prior to treatment. Corticosteroids were not used in the treatment
of all patients.
Discussion
GLM is considered as a rare chronic non-specific
inflammatory lesion of the breast (19). It is histopathologically
characterized by the presence of epithelioid and multinucleated
giant cell granulomas confined to the central lobules with
microabscesses in the absence of obvious etiology (20). MDE is commonly associated with
pathological nipple discharge, according to previously published
literature (21). MDE is a type of
periductal inflammatory disease of the breast. It is characterized
by extralobular irregular ductal dilation with periductal fibrosis.
Its clinical manifestations are usually similar to those of GLM,
making the differential diagnosis, via both physical examination
and imaging, challenging (22). Both
of these chronic inflammatory diseases mimic breast carcinoma. The
experience of the authors of the present study has demonstrated
that they may also benefit from the same therapeutic strategy. The
etiology and pathogenesis of GLM remains unclear. However, an
association with pregnancy, lactation, locally autoimmune process,
infection, hyperprolactinemia and chemical reaction induced by oral
contraceptive pills has been reported in previously published
articles (4,7–10).
After reviewing the literature, it was revealed that
the majority of patients are of Mediterranean (Turkey and Jordan)
and Asian (Arabia, China and Malaysia) origin (17). Although no obvious ethnic
predisposition has been previously reported, a prevalence of GLM in
specific ethnic populations has been mentioned in a number of
reports (18,23). According to the results of the
present study, patients with GLM account for 3.5% of all cases of
benign breast disease in Han Chinese women. The incidence rate in
the Han Chinese population was higher than that in other
populations, linking the occurrence of GLM to an ethnic
predisposition (17).
Although factors such as pregnancy, lactation,
locally autoimmune process, infection, hyperprolactinemia and
chemical reaction induced by oral contraceptive pills have been
considered as possible reasons for GLM, its etiology and
pathogenesis remains unclear (4,7–10). A previous study supported the
conclusion that patients with GLM are usually parous women with a
recent history of pregnancy and delivery (15). According to the results of the
present study, the percentage of parous women was similar between
the GLM and MDE groups, but the interval between the onset of the
disease and the patient's last delivery was statistically different
(P=0.002). Patients with GLM have usually had a delivery within the
last 5 years prior to developing the disease, suggesting that GLM
may be associated with pregnancy and lactation. This was consistent
with the conclusion of Al-Khaffaf et al (18). Marriott et al (22) mentioned that extravasated lactational
secretions may elicit a granulomatous inflammatory response by
themselves. Cserni et al (8)
reported that high levels of serum prolactin and subsequent
excessive stimulation and lactational change may be potential
causative factors for IGM.
Although it has been reported that the age of
patients with GLM may range from 11 to 80 years (15), the high-risk group are women of
childbearing age, between 30 and 40 years (6). In the results of the present study, no
significant differences were observed in the mean age between those
patients with GLM and those with MDE (P=0.052), but the majority of
patients with GLM were aged <40 years. Based on the authors'
clinical experience, the local presentation of GLM is very similar
to that of MDE, with breast lump being the primary complaint from
the patient. However, accompanying breast pain occurs more
frequently in GLM (P<0.01), and the mean diameter of a GLM mass
is 6.23 cm, which is larger than that of an MDE mass (P<0.001).
This result is comparable with that obtained by Gurleyik et
al (11). The present study also
revealed that the prevalence of abscess or skin ulcers in patients
with GLM was lower than that in patients with MDE (P=0.044). To the
best of our knowledge, this difference had not been well
illustrated in previous articles.
The lack of specificity in the imaging techniques,
such as ultrasound, mammography, MRI or CT, makes the diagnosis of
GLM and MDE challenging. The exact diagnosis of GLM is even
difficult when using fine needle aspiration cytology (24); however, it is feasible if the
clinical presentation and imageology characteristics are also
considered (25). Fortunately,
significant histopathological differences between GLM and MDE were
observed in the present study. The present study revealed that the
most outstanding histological feature of GLM was perilobular
granulomatous inflammation. Microabscess formation can be commonly
observed in the center of the lobule or in the normal breast tissue
surrounding the lesion. Multinuclear giant cells are commonly
observed in the lesion, while inflammatory cells infiltrating the
center of the lobule are usually neutrophil granulocytes.
Lymphocytes are also common, but plasmocyte infiltration is rare.
MDE is characterized by mammary duct dilatation, accompanied by
inflammatory responses inside the duct wall or tissues surrounding
the duct. The most common inflammatory cells infiltrating or
surrounding the mammary duct is foam cells. Secretion inside the
duct, fibrosis surrounding the duct, and ductal hyperplasia were
significantly more common in MDE than in GLM (P<0.001). Duct
dilatation may occur in GLM lesions, with the presence of
inflammation inside or surrounding the duct, but the inflammatory
response is not usually apparent. Both cholesterol crystals and
calcification may occur in GLM. MDE may also be accompanied by
cholesterol crystals and calcification, but this is not
statistically different when compared with GLM.
Controversy remains when regarding the most
effective therapeutic strategy of GLM. A number of conservative
therapies have been demonstrated to be effective, including
glucocorticoids, immunosuppressive drugs, antibiotics and so on
(26–29). However, all conservative treatments
are associated with a high risk of recurrence, and the majority of
patients eventually require surgery. For the majority of surgeons,
surgery is the most effective treatment for GLM and should be the
preferred first-line approach. Thorough excision of the
inflammatory tissue is the determinant of successful treatment
(26–29). In the literature, recurrence can
still not be completely avoided following surgery, with recurrence
rates at 5.5–50.0% (30–32). The experience of Akça et al
(33) demonstrated that wide
surgical excision was associated with a lower complication rate
than that of limited excision. Negative surgical margins of
inflammatory tissue are associated with a low recurrence rate
(31); however, it is believed that
it is difficult to accurately judge the margin during the
operation. Therefore, excessive excision is inevitable in order to
lower the risk of recurrence. In the present study, all patients
received thorough excision of the inflammatory tissue, and even
partial excision of the retracted nipple followed by nipple
reconstruction. No recurrence was observed after the follow-up.
However, the damage to the normal breast shape had a significant
psychological effect on certain patients. Breast implants or breast
reconstruction may be necessary in such cases.
In conclusion, GLM is a rare chronic non-specific
inflammatory lesion of the breast. It is more prevalent in Han
Chinese women than in other ethnicities. GLM and MDE have a very
similar clinical presentation and may benefit from the same
therapeutic strategy. There are, however, significant differences
in the histopathological characteristics of GLM and MDE. The lack
of specificity of imaging techniques makes it difficult to
differentiate GLM from MDE or breast carcinoma in certain patients,
unless histopathological diagnosis is used. Thorough excision of
the inflammatory tissue, and even partial excision of the retracted
nipple followed by breast implants or breast reconstruction,
remains the most effective therapeutic strategy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81672613 and
81602329), the Scientific Research Foundation of Shandong Province
for Outstanding Young Scientist Award (grant no. BS2014YY055), the
China Postdoctoral Science Foundation (grant no. 2015M572050), and
the Key Research and Development Program of Shandong Province
(grant nos. 2015GSF118035, 2016GGE2775 and 2015GSF118093).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LJ and XL designed the study. QY revised the
experimental design. LJ, XL and BS acquired, analyzed and
interpreted the data. XK and TM validated the experimental data.
LJ, XL and QY verified the results of the experiment. All authors
wrote the manuscript and revised it for important intellectual
content.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee on Scientific Research of Shandong University Qilu
Hospital. Written and informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kessler E and Wolloch Y: Granulomatous
mastitis: A lesion clinically simulating carcinoma. Am J Clin
Pathol. 58:642–646. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Milward TM and Gough MH: Granulomatous
lesions in the breast presenting as carcinoma. Surg Gynecol Obstet.
130:478–482. 1970.PubMed/NCBI
|
|
3
|
Miller F, Seidman I and Smith CA:
Granulomatous mastitis. N Y State J Med. 71:2194–2195.
1971.PubMed/NCBI
|
|
4
|
Davies JD and Burton PA: Postpartum
lobular granulomatous mastitis. J Clin Pathol. 36:3631983.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Going JJ, Anderson TJ, Wilkinson S and
Chetty U: Granulomatous lobular mastitis. J Clin Pathol.
40:535–540. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pereira FA, Mudgil AV, Macias ES and
Karsif K: Idiopathic granulomatous lobular mastitis. Int J
Dermatol. 51:142–151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tournemaine N, Nomballais F, Weber J,
Digabel-Chabay C, Bertrand AF and Cousin C: Granulomatous lesions
of the breast. Their role in inflammatory breast pathology and
their relations to lobular granulomatous mastitis. J Gynecol Obstet
Biol Reprod (Paris). 16:75–83. 1987.(In French). PubMed/NCBI
|
|
8
|
Cserni G and Szajki K: Granulomatous
lobular mastitis following drug-induced galactorrhea and blunt
trauma. Breast J. 5:398–403. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hmissa S, Sahraoui W, Missaoui N, Stita W,
Mokni M, Yacoubi MT, Khairi H and Korbi S: Lobular idiopathic
granulomatos mastitis. About 10 cases. Tunis Med. 84:353–357.
2006.(In French). PubMed/NCBI
|
|
10
|
Al Nazer MA: Idiopathic granulomatus
lobular mastitis. A forgotten clinical diagnosis. Saudi Med J.
24:1377–1380. 2003.PubMed/NCBI
|
|
11
|
Gurleyik G, Aktekin A, Aker F, Karagulle H
and Saglamc A: Medical and surgical treatment of idiopathic
granulomatous lobular mastitis: A benign inflammatory disease
mimicking invasive carcinoma. J Breast Cancer. 15:119–123. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Galea MH, Robertson JF, Ellis IO, Elston
CW and Blamey RW: Granulomatous lobular mastitis. Aust N Z J Surg.
59:547–550. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kfoury H and Al Bhlal L: Granulomatous
lobular mastitis: A clinicopathological study of 112 cases. Ann
Saudi Med. 17:43–46. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mote DG, Gungi RP, Satyanarayana V and
Premsunder T: Granulomatous mastitis-a diagnostic dilemma. Indian J
Surg. 70:241–243. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bani-Hani KE, Yaghan RJ, Matalka II and
Shatnawi NJ: Idiopathic granulomatous mastitis: Time to avoid
unnecessary mastectomies. Breast J. 10:318–322. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wilson JP, Massoll N, Marshall J, Foss RM,
Copeland EM and Grobmyer SR: Idiopathic granulomatous mastitis: In
search of a therapeutic paradigm. Am Surg. 73:798–802.
2007.PubMed/NCBI
|
|
17
|
Baslaim MM, Khayat HA and Al-Amoudi SA:
Idiopathic granulomatous mastitis: A heterogeneous disease with
variable clinical presentation. World J Surg. 31:1677–1681. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Al-Khaffaf B, Knox F and Bundred NJ:
Idiopathic granulomatous mastitis: A 25-year experience. J Am Coll
Surg. 206:269–273. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sheybani F, Sarvghad M, Naderi HR and
Gharib M: Treatment for and clinical characteristics of
granulomatous mastitis. Obstet Gynecol. 125:801–807. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tuli R, O'Hara BJ, Hines J and Rosenberg
AL: Idiopathic granulomatous mastitis masquerading as carcinoma of
the breast: A case report and review of the literature. Int Semin
Surg Oncol. 4:212007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rahal RM, de Freitas-Júnior R and
Paulinelli RR: Risk factors for duct ectasia. Breast J. 11:262–265.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marriott DA, Russell J, Grebosky J,
Wallace AM, Joste N and Royce ME: Idiopathic granulomatous lobular
mastitis masquerading as a breast abscess and breast carcinoma. Am
J Clin Oncol. 30:564–565. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Centers for Disease and Control and
Prevention (CDC): Idiopathic granulomatous mastitis in Hispanic
women-Indiana, 2006–2008. MMWR Morb Mortal Wkly Rep. 58:1317–1321.
2009.PubMed/NCBI
|
|
24
|
Tse GM, Poon CS, Law BK, Pang LM, Chu WC
and Ma TK: Fine needle aspiration cytology of granulomatous
mastitis. J Clin Pathol. 56:519–521. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gupta RK: Fine needle aspiration cytology
of granulomatous mastitis: A study of 18 cases. Acta Cytol.
54:138–141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
DeHertogh DA, Rossof AH, Harris AA and
Economou SG: Prednisone management of granulomatous mastitis. N
Engl J Med. 303:799–800. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmajuk G and Genovese MC: First report
of idiopathic granulomatous mastitis treated with methotrexate
monotherapy. J Rheumatol. 36:1559–1560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lai EC, Chan WC, Ma TK, Tang AP, Poon CS
and Leong HT: The role of conservative treatment in idiopathic
granulomatous mastitis. Breast J. 11:454–456. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stary CM, Lee YS and Balfour J: Idiopathic
granulomatous mastitis associated with corynebacterium sp.
Infection. Hawaii Med J. 70:99–101. 2011.PubMed/NCBI
|
|
30
|
Schelfout K, Tjalma WA, Cooremans ID,
Coeman DC, Colpaert CG and Buytaert PM: Observations of an
idiopathic granulomatous mastitis. Eur J Obstet Gynecol Reprod
Biol. 97:260–262. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adler NR, Wolfe R, McArthur GA, Kelly JW,
Haydon A, McLean CA and Mar VJ: Tumour mutation status and melanoma
recurrence following a negative sentinel lymph node biopsy. Br J
Cancer. 118:1289–1295. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ocal K, Dag A, Turkmenoglu O, Kara T,
Seyit H and Konca K: Granulomatous mastitis: Clinical, pathological
features, and management. Breast J. 16:176–182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Akça T, Çolak T, Çağlıkülekçi M, Öcal K
and Aydın S: Intestinal perforation in Wegener's granulomatosis: A
case report. Ulus Travma Acil Cerrahi Derg. 11:348–351.
2005.PubMed/NCBI
|