Introduction
Pancreatic cancer is characterized by high mortality
and metastasis rates (1); it is one
of the four most common causes of cancer-associated mortality with
a reported 6.6% in Europe in 2018 (2). Based on the GLOBOCAN 2018 data, the
number of pancreatic cancer-associated deaths was 432,242 per year
in the USA (3). Pancreatic ductal
adenocarcinoma (PDAC) is the primary pathological type of
pancreatic cancer, and patients with the disease present with a
poor prognosis (4). A genetic study
revealed that the Kirsten rat sarcoma viral oncogene (KRAS)
mutation is an early event in pancreatic tumourigenesis and plays a
significant role in pancreatic cancer (5). Currently, surgery is the only means of
treating pancreatic cancer; however, it is not sufficient to
improve the survival rate of patients with pancreatic cancer
(6,7). Unfortunately, traditional chemotherapy
drugs are not effective due to the resistance of pancreatic cancer
cells. Despite the fact that the prognosis has been enhanced by
clinical standard therapies (8), the
5 year survival rates of patients with pancreatic cancer remain at
<5% (9). Thus, finding new
therapeutic methods for pancreatic cancer is imperative for future
preclinical research.
Ferroptosis, a recently discovered form of regulated
cell death (RCD), is dependent on the presence of intracellular
iron and the accumulation of reactive oxygen species (ROS)
(10). Ferroptosis has been
identified in numerous pathological diseases, such as
ischaemia-reperfusion injury, neurodegenerative diseases and a
series of different cancer types, including breast cancer,
hepatocellular carcinoma and pancreatic cancer (10–12).
Recently, a number of clinical studies have indicated that
traditional Chinese herbs exhibit potent anticancer effects in
pancreatic cancer by affecting ferroptosis, which suggests that
ferroptosis may also play an important role in the disease
(13–15). Furthermore, it has been demonstrated
that ferritinophagy, the lipid peroxidation pathway, the
glutathione (GSH) peroxidase 4 (GPX4) pathway and the system Xc-
pathway are closely associated with ferroptosis.
In the present review article, the potential
molecular mechanisms underlying ferroptosis in pancreatic cancer
are discussed, alongside the potential future directions for
ferroptosis research.
Molecular mechanism underlying ferroptosis
and relative regulators in cancer
In 2012, a new form of RCD called ferroptosis was
discovered and reported by Dixon et al (10). Ferroptosis is morphologically and
mechanistically different from apoptosis, necroptosis, autophagy
and other forms of cell death. Morphologically, it has been
demonstrated that in ferroptosis, the mitochondrial volume is
decreased compared with that of normal mitochondria, the
mitochondrial membrane density is increased, the mitochondrial
ridge disappears and the outer membrane ruptures (16). Biochemically, ferroptosis is
dependent on iron and ROS, which are characteristic of lipid
peroxidation (17). Currently,
studies indicate that the two main pathways involved in ferroptosis
are the iron metabolism pathway and the ROS metabolism pathway
(16,18). Iron metabolism in the cell consists
of the import, storage and export of iron. Ferric iron
(Fe3+) bound to transferrin is transported to the
endosome via transferrin receptor 1. Inside the endosome,
Fe3+ is reduced to ferrous iron (Fe2+), which
is finally gathered in a labile iron pool in the cytoplasm.
Cytoplasmic iron is retained as ferritin, which comprises ferritin
heavy chain (FtH) and ferritin light chain (FtL). Finally,
excessive iron is exported by ferroportin (19,20).
Ferroptosis is mediated by the Fenton reaction, in which
Fe2+ reacts with hydrogen peroxide to generate ROS
(21). ROS are a very important
secondary signal in cells, and are formed by the partial reduction
of molecular oxygen, including superoxide
(O2•–), peroxides (H2O2
and ROOH) and free radicals (HO• and RO•)
(17). ROS damage the stability of
DNA and promote cell death. ROS-induced ferroptosis may involve
multiple sources. In addition to the iron-dependent accumulation of
ROS, NADPH-dependent lipid peroxidation and GSH depletion are known
for the induction of ferroptosis (10,22).
Mechanistically, several molecules, called ferroptosis regulators,
have recently been identified to regulate ferroptosis by targeting
iron metabolism and lipid peroxidation. Among them, system Xc- and
GPX4 are negative regulators of ferroptosis (22,23). The
system Xc- is an anionic amino acid transport system composed of
the twelve-pass transmembrane transport protein cystine/glutamate
transporter (SLC7A11) and the single-pass transmembrane regulator
protein 4F2 cell-surface antigen heavy chain (SLC3A2). System Xc-
imports extracellular cysteine to exchange intracellular glutamate.
Therefore, the selective inhibition of system Xc- causes a decrease
in intracellular cysteine. Decreasing GSH synthesis results in
excessive toxic lipid ROS accumulation, which triggers ferroptosis
at the molecular level (23). GPX4
can directly decrease phospholipid hydroperoxide and prevent lipid
peroxidation-dependent cell death, which is an essential negative
regulator of ferroptosis. GPX4 is necessary to remove fatty oxygen
radical enzymes that can decrease the toxic lipid hydroperoxides
(L-OOH) to lipid alcohols (L-OH). Once GPG4 is inactivated, L-OOH
will gradually accumulate. At the same time, cellular L-OOH is
catalysed by iron into toxic lipid radicals, such as the alkoxy
radical L-O, resulting in cytotoxicity and cell death (22). By contrast, voltage-dependent anion
channel (VDAC)2/3 and NADPH oxidase (NOX), as positive regulators,
promote ferroptosis. Mitochondrial voltage-dependent anion channels
(VDACs) are novel targets for anticancer drugs. Cells with more
VDAC protein are more sensitive to erastin (24). Erastin, the classical inducer of
ferroptosis, interacts with VDAC proteins, leading to mitochondrial
dysfunction, the release of oxidative species and non-apoptotic
oxidative cell death (25). The NOX
protein family reduces oxygen to superoxide by transferring
electrons across biological membranes. The canonical NOX inhibitor
diphenyleneiodonium and the NOX1/4-specific inhibitor GKT137831
were both shown to suppress erastin-induced ferroptosis in Calu-1
cells in a preivious study (10).
The protein cellular tumour antigen p53 (p53) participates in
controlling cell survival and death, and plays double roles in
regulating ferroptosis through a transcriptional or
post-translational mechanism. Spermidine/spermine
N1-acetyltransferase 1 (SAT1) is an important regulator of
polyamine metabolism through acetylating spermidine and spermine
using acetyl-coenzyme A (26). The
expression of glutaminase 2 (GLS2) is responsible for p53-mediated
oxygen consumption, mitochondrial respiration and ATP generation in
cancer cells (27). p53 promotes
ferroptosis by inhibiting SLC7A11 expression and increasing SAT1
and GLS2 expression, while p53 also inhibits ferroptosis by
downregulating the expression of DPP4 and upregulating the
expression of CDKN1A/p21 (28). In
other words, ferroptosis is a non-apoptotic form of cell death and
is characterized by iron-dependent and ROS-dependent processes.
Ferroptosis is associated with a variety of
physiological and pathological processes, including
neurodegenerative disease, acute kidney failure, drug-induced
hepatotoxicity, hepatic and heart ischaemia/reperfusion injury, and
T-cell immunity, particularly in cancer cell death (16,22,29–33).
Ferroptosis has been identified in a number of different types of
tumour cell, including breast cancer (34), head and neck cancer (35), hepatocellular carcinoma (36), pancreatic cancer (15) and ovarian cancer (37). Therefore, preventing ferroptosis has
become an important strategy to prevent associated diseases and
cancer types.
Potential roles of ferroptosis in pancreatic
cancer
In recent years, it has proven difficult to identify
new ways to treat pancreatic cancer. Gemcitabine, as the first-line
drug, is used alone or in combination for the treatment of patients
with advanced PDAC. Heat shock 70-kDa protein 5 (HSPA5) improves
the anticancer activity of gemcitabine by inducing ferroptosis
(38). In addition to gemcitabine,
it has been demonstrated that certain traditional Chinese herbs
induce ferroptosis in pancreatic cancer. Furthermore, a number of
molecules have been demonstrated to induce ferroptosis in
pancreatic cancer cells, suggesting that they may offer new options
for pancreatic cancer treatment.
Iron metabolism and ferroptosis in
pancreatic cancer Ferritinophagy can promote ferroptosis in
pancreatic cancer
A number of studies have provided evidence to
support the association between autophagy and ferroptosis (39,40).
However, the underlying molecular mechanism between autophagy and
ferroptosis in pancreatic cancer remains unclear. Recently,
researchers have revealed that nuclear receptor coactivator 4
(NCOA4)-mediated ferritinophagy is an autophagic process that
contributes to ferroptosis via the degradation of ferritin in
pancreatic cancer (41,42). The iron storage protein ferritin
consists of two subunits of ferritin, FtH and FtL, which are
associated with intracellular iron storage and release (43). NCOA4 is a cargo receptor specifically
responsible for the selective autophagic turnover of ferritin in
ferritinophagy. Degradation of ferritin leads to increased
intracellular free iron, which triggers ROS generation and
consequent ferroptosis in pancreatic cancer. Notably, knockdown of
NCOA4 by specific shRNAs in pancreatic cancer inhibited
ferritinophagy and suppressed erastin-induced ferroptosis. By
contrast, overexpression of NCOA4 increased ferritin degradation
and promoted ferroptosis. Knockout or knockdown of
autophagy-related 5 (Atg5) and Atg7 in human pancreatic cancer cell
lines decreased both intracellular Fe2+ and the product
of L-OOH, and induced ferroptosis, which indicates that the Atg
genes play an essential role in the mediation of autophagy and
ferroptosis (42). Consequently,
autophagy plays a key role in promoting ferroptosis, and further
research is required in order to elucidate the association of
ferritinophagy and ferroptosis, which can provide innovative
treatments for pancreatic cancer.
Artesunate can induce ferroptosis in
PDAC
The natural compound Artesunate (ART) is a
noteworthy anti-malaria drug. It was previously demonstrated that
ART also exhibited an anti-tumour effect and was a specific inducer
of ferroptosis in a number of different types of cancer, including
pancreatic cancer (14,44,45). ART
exhibited higher cytotoxicity in PDAC cells with Ras mutation
compared with that in PDAC cell lines expressing wild-type KRas
(14). Despite the fact that the
underlying molecular mechanism is currently unknown, it was
discovered that the functional lysosome and iron metabolism are
involved in the ferroptosis induced by ART in PDAC and other types
of tumour cell (14,45). Ferritin binds with NCOA4 in the
autophagosome and is delivered into the lysozyme, and ART
accumulates in the lysosomes and increases ferritin protein
degradation (44). Degradation of
ferritin in lysosomes increases the volume of intracellular iron
and plays an essential role in ART-activated ferroptosis, which is
similar to ferritinophagy (44,46).
Yang et al (44) discovered
that ART activates lysosomal activity by increasing V-ATPase
assembly. Notably, the co-treatment of ART and transferrin
increased lysosomal free iron and promoted ferroptosis in PDAC
(14). Iron-dependent ROS generation
and accumulation are indispensable steps for ferroptosis in
response to ART (44,46). In summary, ferroptosis induced by ART
is dependent on the presence of intracellular iron.
ROS metabolism and ferroptosis in
pancreatic cancer
LOX is sufficient for ferroptosis in
pancreatic cancer
Recently, a very important association between lipid
peroxide and ferroptosis has been identified. Decreased levels of
GSH results in a deficiency of GPX4-reducing substrates, preventing
the conversion of L-OOH into L-OH. The gradual accumulation of
L-OOH leads to ferroptosis (23).
Two principal mechanisms for the formation of L-OOH are well known:
Free radical chain oxidation of organic compounds, called
autoxidation, and iron-dependent lipoxygenase (LOX)-mediated
activity (47,48). A recent study by Xie et al
(13) demonstrated that the
12/15-LOX inhibitor baicalein is effective in preventing
erastin-induced ferroptosis by protecting pancreatic cancer cells
from RSL3 toxicity, which indicates that LOX may regulate
ferroptosis. Another study also reported that LOX activity may
contribute to ferroptosis (49).
LOXs are non-haem iron-containing dioxygenases that catalyse
polyunsaturated fatty acids to produce fatty acid hydroperoxides
that damage cells (37). Shintoku
et al (50) demonstrated that
upon exposure to the ferroptosis inducers erastin and RSL3, ω-6
PUFA-mediated production of 4-hydroxy-2-nonenal was increased,
contributing to ferroptosis, whereas inhibition or silencing of
arachidonate 15-lipoxygenase (ALOX15) decreased both
erastin-induced and RSL3-induced ferroptotic cell death in
pancreatic cancer. During the process of inducing ferroptosis, the
ALOX15 protein consistently localized to cellular membranes,
suggesting that ALOX15 results in ferroptosis by inducing the
production of L-OOH in cell membranes (50). LOX is expressed in different kinds of
tissue and is associated with several different types of cancer.
Therefore, the induction of ferroptosis may be a new approach in
treating cancer by regulating the expression of LOX (51). In conclusion, these reports
implicated LOX as a key regulator of ferroptosis in PDAC.
Preventing mitochondrial lipid
oxidation suppresses ferroptosis in pancreatic cancer
It is well known that the occurrence of ferroptosis
is accompanied by morphological changes to the mitochondria
(10). Previous studies have
supported a hypothesis that ferroptosis is intrinsically associated
with a lipid oxidation pathway by intersecting with the
mitochondrial membrane (52,53). Recently, Krainz et al
(54) revealed that, as lipid
peroxidation mitigators, XJB-5-131, JP4-039 and selected analogues
influence the relative subcellular localization of nitroxide and
prevent ferroptosis in PANC-1 cells. Both XJB-5-131 and JP4-039,
mitochondrially targeted nitroxides, could prevent ROS accumulation
and protect against mitochondrial function. The protective effect
of JP4-039 was >20- to 30-fold weaker than that of XJB-5-131,
which coincides with the much lower concentration of JP4-039 in
mitochondrial enrichment than that of XJB-5-131. These results
suggest that mitochondria-targeted nitroxides inhibit ferroptosis,
and that preventing mitochondrial lipid oxidation may offer a
potential therapeutic opportunity in pancreatic cancer (54). To conclude, preventing mitochondrial
lipid oxidation protects against ferroptosis in pancreatic
cancer.
Non-oxidative dopamine inhibits
ferroptosis by modulating lipid peroxidation in pancreatic
cancer
It is already known that lipid peroxidation is an
important element in inducing ferroptosis (17). Erastin is a classic ferroptosis
inducer that inhibits system Xc-activity and VDAC (16). Dopamine is a powerful antioxidant and
has numerous functions in the nervous and immune systems (55,56).
However, the effect of dopamine on ferroptosis is currently
unknown. A recent study revealed that non-oxidative dopamine is an
inhibitor of ferroptosis that protects against erastin-induced
ferroptosis in pancreatic cancer (53). The levels of Fe2+ and
malondialdehyde production (MDA), one of the end products of lipid
peroxidation, were decreased following the dopamine treatment of
erastin-induced cell death in pancreatic cancer. In terms of the
mechanism, dopamine inhibits ferroptosis by modulating lipid
peroxidation in two separate ways in PANC-1 cells. On the one hand,
dopamine decreases the accumulation of Fe2+; on the
other hand, dopamine markedly inhibits GSH depletion and GPX4
degradation (57). GPX4 is a
GSH-dependent enzyme that cannot use GSH as a co-substrate to
reduce lipid peroxidation due to GSH depletion. Both the
iron-induced Fenton reaction and GSH depletion induce ferroptosis
(22). In conclusion, these findings
provide a new strategy for pancreatic cancer therapy by inhibiting
lipid peroxidation.
Cotylenin A and phenethyl
isothiocyanate induce ferroptosis by ROS accumulation in pancreatic
cancer
Previous studies have demonstrated that certain
drugs can induce ferroptosis in pancreatic cancer, providing a
feasible therapeutic strategy against pancreatic cancer (15,58).
Phenethyl isothiocyanate (PEITC) is a potent generator of ROS,
while cotylenin A (CN-A) exhibits potent anti-tumour activity in
several cancer cell lines (59–63). A
recent study by Kasukabe et al (15) demonstrated that, upon exposure to
CN-A and PEITC, the proliferation of both PANC-1 cells and
gemcitabine-resistant PANC-1 cells (PANC-1/GR) was inhibited by
increasing ROS levels. Antioxidants (N-acetylcysteine and trolox),
ferroptosis inhibitors (ferrostatin-1) and the iron chelator
deferoxamine reverse this process, causing CN-A- and PEITC-induced
ferroptosis in pancreatic cancer (15). Furthermore, it was also observed that
CN-A and PEITC synergistically trigger more ROS accumulation when
combined, compared with when used separately. These results suggest
that CN-A and PEITC synergistically account for more
ROS-ferroptosis pathway-mediated pancreatic cancer cell death
compared with treatment with PEITC or CN-A alone. However, the
molecular mechanisms underlying the interaction between CN-A and
PEITC have not yet been elucidated in detail (15). Another study regarding the treatment
of pancreatic cancer demonstrated similar evidence (58). Yamaguchi et al (58) reported that CN-A or sulfasalazine
(SSZ) enhanced ROS production, which induced ferroptotic cell death
in human pancreatic cancer. Above all, these findings provide a new
drug treatment option for pancreatic cancer, and demonstrate that
ROS are a vital hallmark of ferroptosis in pancreatic cancer.
GPX4 and ferroptosis in pancreatic
cancer
GPX4 degradation promotes ferroptosis
in pancreatic cancer
Recently a study revealed that baicalein negatively
regulates ferroptosis by inhibiting the degradation of GPX4
(13). Baicalein, as a class of
molecules present in certain traditional Chinese medical herbs,
exhibits potent anticancer activities (64). In the study by Xie et al
(13), it was demonstrated that,
when compared with other well-known ferroptosis inhibitors (e.g.,
ferrostatin-1, liproxstatin-1, deferoxamine mesylate and
β-mercaptoethanol), baicalein exhibited a greater level of
anticancer activity in erastin-induced pancreatic cancer cell
ferroptosis. Baicalein could inhibit ferroptosis by suppressing the
degradation of GPX4 and decreasing the accumulation of iron
(13). Furthermore, it was also
revealed that, upon exposure to baicalein, the process of degrading
erastin-induced nuclear factor (erythroid-derived 2)-like 2 (NRF2),
a transcription factor that positively regulates the critical
proteins of ferroptosis, such as GPX4, was inhibited (65). Qin et al (66) also demonstrated that baicalein
modulates the NRF2/Keap1 system in both Keap1-independent and
-dependent pathways to inhibit oxidative injury. Therefore, it can
be concluded that baicalein may selectively activate the NRF2
pathway in pancreatic cancer. However, additional studies are
required in order to clarify the precise molecular mechanisms
underlying this process.
HSPA5-GPX4 pathway regulates
ferroptosis in pancreatic cancer
According to their molecular mass, heat shock
proteins (HSPs) are grouped into six families, namely, HSP100,
HSP90, HSP70, HSP60, HSP40 and small HSPs. It is already known that
HSPβ-1 is a negative regulator of ferroptosis. Inhibition of heat
shock factor 1-dependent HSPB1 expression and HSPB1 phosphorylation
increased erastin-induced ferroptosis in human xenograft mouse
tumour models (67,68). Recently, Zhu et al (38) revealed that heat shock 70 kDa protein
5 (HSPA5) is a negative regulator of ferroptosis in pancreatic
cancer cells. HSPA5 is regulated by activating transcription factor
4 (ATF4), and both suppression and knockdown of ATF4 inhibit
erastin-induced HSPA5 protein expression in PANC-1 cells and CFPAC1
cells. Upon activation of ATF4, HSPA5 protein binding to GPX4
increases the stability of GPX4 to protect against ferroptosis in
pancreatic cancer. Following knockdown of HSPA5 or ATF4, MDA
production was increased in erastin-induced cell death,
demonstrating that ATF4-dependent HSPA5 expression inhibits
ferroptosis through lipid peroxidation. Knockdown of HSPA5 and GPX4
in pancreatic cancer cells promotes ferroptosis in response to
gemcitabine or pharmacological inhibition of the HSPA5-GPX4 pathway
by epigallocatechin gallate or SSZ in pancreatic cancer cells
rendered tumours more sensitive to gemcitabine both in vitro
and in vivo. Furthermore, ferroptosis inhibitors reversed
gemcitabine-induced cell death, which demonstrates that the
HSPA5-GPX4 pathway regulates ferroptosis in pancreatic cancer cells
(38). Above all, activation of the
ATF4-HSPA5-GPX4 pathway protects from ferroptosis in pancreatic
cancer cells and suggests a promising therapeutic strategy for
pancreatic cancer.
System Xc- and ferroptosis in pancreatic
cancer
Inhibiting system Xc-induces
ferroptosis in pancreatic cancer
Sorafenib, a multikinase inhibitor, is currently
recognized as the only anticancer drug in hepatocellular carcinoma
treatment. In the process of treating hepatocellular carcinoma,
ferroptosis plays a significant role in inducing hepatocellular
carcinoma cell death (69). However,
it is not yet certain whether sorafenib induces ferroptosis in
human cancer cells originating from various tumours. Lachaier et
al (70) demonstrated that
sorafenib and erastin induced ferroptosis not only in
hepatocellular carcinoma, but also in other types of cancer
originating from tissues other than those of the pancreatic cancer.
However, this process did not involve inhibition of RAF kinase by
sorafenib (70). Dixon et al
(71) demonstrated that erastin
treatment and silencing of SLC7A11 have similar inhibition of
glutamate release. Erastin is capable of inhibiting ferroptosis by
blocking cystine-glutamate exchange, as sorafenib does. Cystine is
depleted due to system Xc-inhibition, which accounts for
ferroptosis (71). Likewise, the
study by Song et al (72)
demonstrated that BECN1 plays a unique role in promoting
ferroptosis in pancreatic cancer. The phosphorylation of BECN1 at
Ser90/93/96 by AMP-activated protein kinase leads to the formation
of the BECN1-SLC7A11 complex, which inhibits the activity of system
Xc- and results in ferroptosis. In summary, inhibition of system
Xc- can trigger ferroptosis in pancreatic cancer.
Ferroptosis in other associated
digestive system cancers
Aside from pancreatic cancer, ferroptosis is also
associated with other types of cancer of the digestive system,
including hepatocellular carcinoma (30,36,69,70,73–78), and
colorectal cancer (79,80). For example, hepatocellular carcinoma
is the most common type of liver cancer, and sorafenib is the only
first-line drug to treat patients with hepatocellular carcinoma. A
number of studies have demonstrated that the p62-Keap1-NRF2 pathway
plays a vital role in sorafenib-induced ferroptosis in
hepatocellular carcinoma (36,81,82). p62
suppresses the degradation of NRF2 by inactivating Keap1 and
accounts for the accumulation of NRF2. Expression of NRF2 increases
the transcription of quinone oxidoreductase 1, haem oxygenase-1 and
ferritin heavy chain 1, bringing about resistance to ferroptosis
(36). On the one hand, the
retinoblastoma protein and NRF2 inhibit sorafenib-induced
ferroptosis; on the other hand, haloperidol promotes
sorafenib-induced ferroptosis, which provides a new therapeutic
approach for hepatocellular carcinoma. Furthermore, certain
ferroptosis regulators, for example, CDGSH iron sulfur domain 1,
low-density lipoprotein-docosahexaenoic acid and acyl-CoA
synthetase long-chain family member 4, also regulate ferroptosis in
hepatocellular carcinoma via lipid metabolism (30,74,77).
Table I presents studies to compare
ferroptosis in pancreatic cancer, hepatocellular carcinoma and
colorectal cancer.
 | Table I.Ferroptosis in associated types of
digestive system cancer. |
Table I.
Ferroptosis in associated types of
digestive system cancer.
| A, Pancreatic
cancer |
|---|
|
|---|
| Author, year | Compound | Target | Effect | (Refs.) |
|---|
| Eling et al,
2015 | Artesunate | Ferritinophagy | Induces
ferroptosis | (14) |
| Hou et al,
2016 | Nuclear receptor
coactivator 4 | Ferritinophagy | Induces
ferroptosis | (42) |
| Shintoku et
al, 2017 | ALOX15
activator | Lipid
oxidation | Induces
ferroptosis | (50) |
| Krainz et
al, 2016 | XJB-5-131,
JP4-039 | Mitochondrial lipid
oxidation | Inhibits
ferroptosis | (54) |
| Kasukabe et
al, 2016 | Cotylenin A,
phenethyl isothiocyanate | ROS
accumulation | Induces
ferroptosis | (15) |
| Yamaguchi et
al, 2018 | Piperlongumine | ROS
accumulation | Induces
ferroptosis | (58) |
| Wang et al,
2016 | Dopamine | GSH depletion and
Fe2+ reduction | Inhibits
ferroptosis | (57) |
| Xie et al,
2016 | Baicalein | GSH depletion and
Fe2+ reduction | Inhibits
ferroptosis | (13) |
| Zhu et al,
2017 | Heat shock 70 kDa
protein 5 | GPX4 pathway | Inhibits
ferroptosis | (38) |
| Lachaier et
al, 2014 | Sorafenib | System Xc- | Induces
ferroptosis | (70) |
| Song et al,
2018 | Beclin 1 | System Xc- | Induces
ferroptosis | (72) |
|
| B,
Hepatocellular carcinoma |
|
| Author,
year |
Compound | Target | Effect | (Refs.) |
|
| Yuan et al,
2016 | Acyl-CoA synthetase
long-chain family member 4 | Lipid
oxidation | Induces
ferroptosis | (30) |
| Bai et al,
2017 | Haloperidol | Lipid peroxidation
and Fe2+ accumulation | Induces
ferroptosis | (73) |
| Chang et al,
2018 | BAY 11e7085 | Nuclear factor
(erythroide-derived 2)-like 2 pathway | Induces
ferroptosis | (75) |
| Louandre et
al, 2015 | Retinoblastoma | Mitochondrial lipid
oxidation | Inhibits
ferroptosis | (76) |
| Ou et al,
2017 | Low-density
lipoprotein-docosahexaenoic acid nanoparticles | GSH depletion and
GPX4 degradation | Induces
ferroptosis | (77) |
| Yuan et al,
2016 | CDGSH iron sulfur
domain 1 | Mitochondrial lipid
oxidation | Inhibits
ferroptosis | (74) |
| Jennis et
al, 2016 | TP53 | System Xc- | Inhibits
ferroptosis | (78) |
|
| C, Colorectal
cancer |
|
| Author,
year |
Compound | Target | Effect | (Refs.) |
|
| Guo et al,
2018 | Cisplatin | GSH depletion and
GPX4 degradation | Induces
ferroptosis | (79) |
| Xie et al,
2017 | TP53 | System Xc-, lipid
oxidation | Inhibits
ferroptosis | (80) |
Summary and perspective
Recently, a new iron-dependent and ROS-dependent
non-apoptotic form of cell death, called ferroptosis, has been
reported. The present review discusses the significant role of
ferroptosis in pancreatic cancer. NCOA4-mediated ferritinophagy
promotes the degradation of ferritin in lysosomes, which provides a
new approach in the treatment of pancreatic cancer. In addition to
iron metabolism, ROS metabolism also plays a pivotal role in
ferroptosis. ALOX15-catalysed lipid hydroperoxide generation
promotes ferroptosis in pancreatic cancer. By contrast,
mitochondrial-targeted nitroxide negatively regulates ferroptosis
by preventing mitochondrial lipid oxidation. Non-oxidative dopamine
and baicalein interfere with both iron metabolism and ROS
metabolism and attenuate pancreatic cancer. Furthermore, HSPA5, as
a negative regulator, acts on GPX4 to inhibit ferroptosis in
pancreatic cancer (Fig. 1). Although
important discoveries have been revealed by these studies, there
are also some limitations. The mechanisms of interaction between
CN-A and PEITC have not yet been elucidated in detail. The
mechanism of the iron metabolism pathway and ROS pathway remains
insufficiently comprehensive and clear, and additional studies
should be performed in order to improve the current understanding.
For early pancreatic cancer, surgical resection is the most
effective treatment. Currently, the main treatment for advanced
pancreatic cancer is chemotherapy. However, traditional
chemotherapy drugs have limited effects on pancreatic cancer due to
the anti-apoptotic effect of pancreatic cancer. One future
direction for research is in regard to the molecules from
traditional Chinese herbs (e.g., CN-A, PEITC and SSZ), which
exhibit effects in resistant pancreatic cancer cells and could be
used as a new therapy to treat pancreatic cancer. Another direction
is to use gemcitabine, the first-line drug used in patients with
advanced PDAC. HSPA5 overcomes gemcitabine resistance and improves
the anticancer activity of gemcitabine by inducing ferroptosis. In
brief, targeting ferroptosis could provide a new strategy to treat
pancreatic cancer.
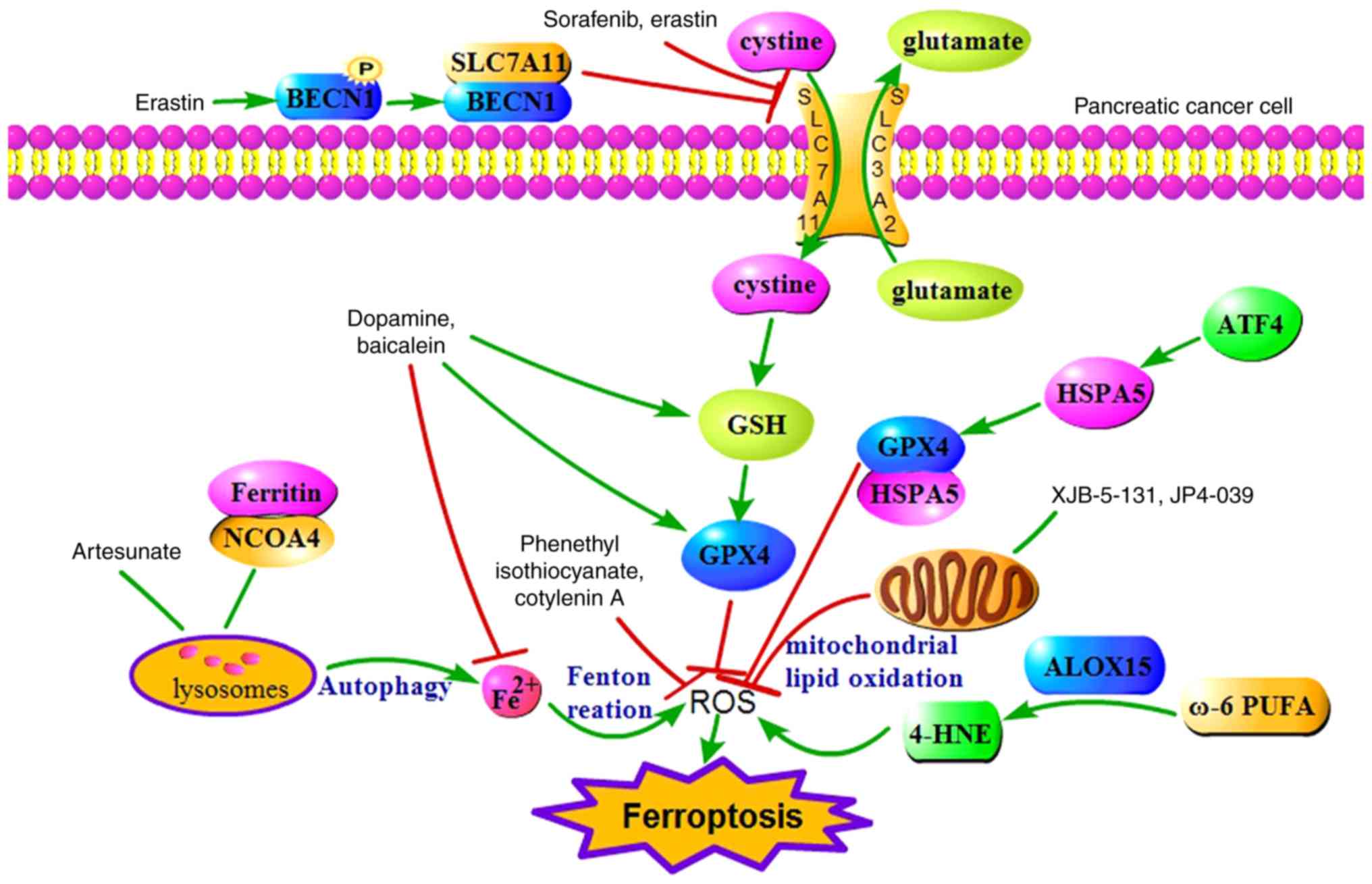 | Figure 1.Potential molecular mechanism
underlying ferroptosis in pancreatic cancer. Ferritinophagy, ROS
metabolism and core regulators are involved in the process of
ferroptosis. System Xc- and GPX4 are significant regulators of
ferroptosis. Erastin and sorafenib trigger ferroptosis by
inhibiting the function of system Xc-. Artesunate induces
ferroptosis via ferritinophagy. ALOX15 catalyses ω-6 PUFA to
produce more 4-HNE, contributing to ferroptosis. ATF4-dependent
HSPA5 expression inhibits ferroptosis through lipid peroxidation.
Dopamine and baicalein interfere with both iron metabolism and ROS
metabolism, and inhibit ferroptosis. Mitochondrially targeted
nitroxide XJB-5-131 and JP4-039 inhibit ferroptosis by suppressing
mitochondrial ROS accumulation. Co-treatment with cotylenin A and
phenethyl isothiocyanate inhibits ROS accumulation and suppresses
ferroptosis. ROS, reactive oxygen species; GPX4, glutathione
peroxidase 4; GSH, glutathione; ATF4, activating transcription
factor 4; HSPA5, heat shock 70 kDa protein 5; ALOX15, arachidonate
15-lipoxygenase; 4-HNE, 4-hydroxynonenal; NCOA4, nuclear receptor
coactivator 4; BECN1, beclin 1. |
Acknowledgements
Not applicable.
Funding
The review was supported by funding from the
National Science Foundation Grants of China (nos. 81160307 and
81560395), the Jiangxi Science & Technology Pillar Program and
the Science Foundation for Young Scholars of Jiangxi Province
(grant no. 2007GQY1167).
Availability of data and materials
Not applicable.
Authors' contributions
GC and GG wrote the manuscript. GC made the tables
and diagrams. XZ and HC put forward the concept, critically revised
the article for intellectual content, and were responsible for the
organization, revision and submission of the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ATF4
|
transcription factor 4
|
|
ART
|
artesunate
|
|
BECN1
|
Beclin 1
|
|
CN-A
|
Cotylenin A
|
|
Fe3+
|
ferric iron
|
|
Fe2+
|
ferrous iron
|
|
FtH
|
ferritin heavy chain
|
|
FtL
|
ferritin light chain
|
|
GSH
|
glutathione
|
|
GPX4
|
glutathione peroxidase 4
|
|
HSPA5
|
heat shock 70 kDa protein 5
|
|
HSPB1
|
heat shock protein β-1
|
|
LOX
|
lipoxygenases
|
|
MDA
|
malondialdehyde production
|
|
NOX
|
NADPH oxidase
|
|
NRF2
|
nuclear factor (erythroid
derived)-like 2
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
|
PEITC
|
phenethyl isothiocyanate
|
|
ROS
|
reactive oxygen species
|
|
RCD
|
regulated cell death
|
|
VDAC
|
mitochondrial voltage-dependent anion
channel
|
References
|
1
|
Raimondi S, Maisonneuve P and Lowenfels
AB: Epidemiology of pancreatic cancer: An overview. Nat Rev
Gastroenterol Hepatol. 6:699–708. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Colombet M, Soerjomataram I,
Dyba T, Randi G, Bettio M, Gavin A, Visser O and Bray F: Cancer
incidence and mortality patterns in europe: Estimates for 40
countries and 25 major cancers in 2018. Eur J Cancer. 103:356–387.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bosetti C, Bertuccio P, Negri E, La
Vecchia C, Zeegers MP and Boffetta P: Pancreatic cancer: Overview
of descriptive epidemiology. Mol Carcinog. 51:3–13. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamisawa T, Wood LD, Itoi T and Takaori K:
Pancreatic cancer. Lancet. 388:73–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim D, Zhu H, Nassri A, Mokdad A, Kukreja
S, Polanco P, Huerta S and Ramzan Z: Survival analysis of veteran
patients with pancreatic cancer. J Dig Dis. 17:399–407. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lakatos G, Balázs A, Kui B, Gódi S, Szücs
Á, Szentesi A, Szentkereszty Z, Szmola R, Kelemen D, Papp R, et al:
Pancreatic cancer: Multicenter prospective data collection and
analysis by the hungarian pancreatic study group. J Gastrointestin
Liver Dis. 25:219–225. 2016.PubMed/NCBI
|
|
8
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsuda T and Matsuda A: Five-year
relative survival rate of pancreas cancer in the USA, Europe and
Japan. Jpn J Clin Oncol. 44:398–399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Do Van B, Gouel F, Jonneaux A, Timmerman
K, Gelé P, Pétrault M, Bastide M, Laloux C, Moreau C, Bordet R, et
al: Ferroptosis, a newly characterized form of cell death in
parkinson's disease that is regulated by PKC. Neurobiol Dis.
94:169–178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu B, Chen XB, Ying MD, He QJ, Cao J and
Yang B: The role of ferroptosis in cancer development and treatment
response. Front Pharmacol. 8:9922017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie Y, Song X, Sun X, Huang J, Zhong M,
Lotze MT, Zeh HJ Rd, Kang R and Tang D: Identification of baicalein
as a ferroptosis inhibitor by natural product library screening.
Biochem Biophys Res Commun. 473:775–780. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eling N, Reuter L, Hazin J, Hamacher-Brady
A and Brady NR: Identification of artesunate as a specific
activator of ferroptosis in pancreatic cancer cells. Oncoscience.
2:517–532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kasukabe T, Honma Y, Okabe-Kado J, Higuchi
Y, Kato N and Kumakura S: Combined treatment with cotylenin a and
phenethyl isothiocyanate induces strong antitumor activity mainly
through the induction of ferroptotic cell death in human pancreatic
cancer cells. Oncol Rep. 36:968–976. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun
X, Kang R and Tang D: Ferroptosis: Process and function. Cell Death
Differ. 23:369–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Latunde-Dada GO: Ferroptosis: Role of
lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta
Gen Subj. 1861:1893–1900. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dixon SJ and Stockwell BR: The role of
iron and reactive oxygen species in cell death. Nat Chem Biol.
10:9–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Torti SV and Torti FM: Cellular iron
metabolism in prognosis and therapy of breast cancer. Crit Rev
Oncog. 18:435–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kazan HH, Urfali-Mamatoglu C and Gunduz U:
Iron metabolism and drug resistance in cancer. Biometals.
30:629–641. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Torti SV and Torti FM: Iron and cancer:
More ore to be mined. Nat Rev Cancer. 13:342–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao JY and Dixon SJ: Mechanisms of
ferroptosis. Cell Mol Life Sci. 73:2195–2209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yagoda N, von Rechenberg M, Zaganjor E,
Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I, Peltier JM,
Boniface JJ, et al: RAS-RAF-MEK-dependent oxidative cell death
involving voltage-dependent anion channels. Nature. 447:864–868.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lemasters JJ: Evolution of
voltage-dependent anion channel function: From molecular sieve to
governator to actuator of ferroptosis. Front Oncol. 19:3032017.
View Article : Google Scholar
|
|
26
|
Thomas T and Thomas TJ: Polyamine
metabolism and cancer. J Cell Mol Med. 7:113–126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu W, Zhang C, Wu R, Sun Y, Levine A and
Feng Z: Glutaminase 2, a novel p53 target gene regulating energy
metabolism and antioxidant function. Proc Natl Acad Sci USA.
107:7455–7460. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang R, Kroemer G and Tang D: The tumor
suppressor protein p53 and the ferroptosis network. Free Radic Biol
Med. 133:162–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Linkermann A, Skouta R, Himmerkus N, Mulay
SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz
PS, et al: Synchronized renal tubular cell death involves
ferroptosis. Proc Natl Acad Sci USA. 111:16836–16841. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuan H, Li X, Zhang X, Kang R and Tang D:
Identification of ACSL4 as a biomarker and contributor of
ferroptosis. Biochem Biophys Res Commun. 478:1338–1343. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao M, Monian P, Quadri N, Ramasamy R and
Jiang X: Glutaminolysis and transferrin regulate ferroptosis. Mol
Cell. 59:298–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lőrincz T, Jemnitz K, Kardon T, Mandl J
and Szarka A: Ferroptosis is involved in acetaminophen induced cell
death. Pathol Oncol Res. 21:1115–1121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kang Y, Tiziani S, Park G, Kaul M and
Paternostro G: Cellular protection using Flt3 and PI3Kα inhibitors
demonstrates multiple mechanisms of oxidative glutamate toxicity.
Nat Commun. 5:52014. View Article : Google Scholar
|
|
34
|
Ma S, Henson ES, Chen Y and Gibson SB:
Ferroptosis is induced following siramesine and lapatinib treatment
of breast cancer cells. Cell Death Dis. 7:e23072016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roh JL, Kim EH, Jang H and Shin D: Nrf2
inhibition reverses the resistance of cisplatin-resistant head and
neck cancer cells to artesunate-induced ferroptosis. Redox Biol.
11:254–262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R
and Tang D: Activation of the p62-Keap1-NRF2 pathway protects
against ferroptosis in hepatocellular carcinoma cells. Hepatology.
63:173–184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Greenshields AL, Shepherd TG and Hoskin
DW: Contribution of reactive oxygen species to ovarian cancer cell
growth arrest and killing by the anti-malarial drug artesunate. Mol
Carcinog. 56:75–93. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu S, Zhang Q, Sun X, Zeh HJ III, Lotze
MT, Kang R and Tang D: HSPA5 regulates ferroptotic cell death in
cancer cells. Cancer Res. 77:2064–2077. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gao M, Monian P, Pan Q, Zhang W, Xiang J
and Jiang X: Ferroptosis is an autophagic cell death process. Cell
Res. 26:1021–1032. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tang M, Chen Z, Wu D and Chen L:
Ferritinophagy/ferroptosis: Iron-related newcomers in human
diseases. J Cell Physiol. 233:9179–9190. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mancias JD, Wang X, Gygi SP, Harper JW and
Kimmelman AC: Quantitative proteomics identifies NCOA4 as the cargo
receptor mediating ferritinophagy. Nature. 509:105–109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh
HJ III, Kang R and Tang D: Autophagy promotes ferroptosis by
degradation of ferritin. Autophagy. 12:1425–1428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Theil EC: Iron, ferritin, and nutrition.
Ann Rev Nutr. 24:327–343. 2004. View Article : Google Scholar
|
|
44
|
Yang ND, Tan SH, Ng S, Shi Y, Zhou J, Tan
KS, Wong WS and Shen HM: Artesunate induces cell death in human
cancer cells via enhancing lysosomal function and lysosomal
degradation of ferritin. J Biol Chem. 289:33425–33441. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ooko E, Saeed ME, Kadioglu O, Sarvi S,
Colak M, Elmasaoudi K, Janah R, Greten HJ and Efferth T:
Artemisinin derivatives induce iron-dependent cell death
(ferroptosis) in tumor cells. Phytomedicine. 22:1045–1054. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Torii S, Shintoku R, Kubota C, Yaegashi M,
Torii R, Sasaki M, Suzuki T, Mori M, Yoshimoto Y, Takeuchi T and
Yamada K: An essential role for functional lysosomes in ferroptosis
of cancer cells. Biochem J. 15:769–777. 2016. View Article : Google Scholar
|
|
47
|
Haeggström JZ and Funk CD: Lipoxygenase
and leukotriene pathways: Biochemistry, biology, and roles in
disease. Chem Rev. 111:5866–5898. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yin H, Xu L and Porter NA: Free radical
lipid peroxidation: Mechanisms and analysis. Chem Rev.
111:5944–5972. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shah R, Shchepinov MS and Pratt DA:
Resolving the role of lipoxygenases in the initiation and execution
of ferroptosis. ACS Cent Sci. 4:387–396. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shintoku R, Takigawa Y, Yamada K, Kubota
C, Yoshimoto Y, Takeuchi T, Koshiishi I and Torii S:
Lipoxygenase-mediated generation of lipid peroxides enhances
ferroptosis induced by erastin and RSL3. Cancer Sci. 108:2187–2194.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kuhn H, Banthiya S and van Leyen K:
Mammalian lipoxygenases and their biological relevance. Biochim
Biophys Acta. 1851:308–330. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wipf P, Xiao J, Jiang J, Belikova NA,
Tyurin VA, Fink MP and Kagan VE: Mitochondrial targeting of
selective electron scavengers: Synthesis and biological analysis of
hemigramicidin−TEMPO conjugates. J Am Chem Soc. 127:12460–12461.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ji J, Kline AE, Amoscato A, Samhan-Arias
AK, Sparvero LJ, Tyurin VA, Tyurina YY, Fink B, Manole MD, Puccio
AM, et al: Lipidomics identifies cardiolipin oxidation as a
mitochondrial target for redox therapy of brain injury. Nat
Neurosci. 15:1407–1413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Krainz T, Gaschler MM, Lim C, Sacher JR,
Stockwell BR and Wipf P: A mitochondrial-targeted nitroxide is a
potent inhibitor of ferroptosis. ACS Cent Sci. 2:653–659. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yen GC and Hsieh CL: Antioxidant effects
of dopamine and related compounds. Biosci Biotechnol Biochem.
61:1646–1649. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tarazi FI: Neuropharmacology of dopamine
receptors: Implications in neuropsychiatric diseases. J Sci Res Med
Sci. 3:93–104. 2001.PubMed/NCBI
|
|
57
|
Wang D, Peng Y, Xie Y, Zhou B, Sun X, Kang
R and Tang D: Antiferroptotic activity of non-oxidative dopamine.
Biochem Biophys Res Commun. 480:602–607. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yamaguchi Y, Kasukabe T and Kumakura S:
Piperlongumine rapidly induces the death of human pancreatic cancer
cells mainly through the induction of ferroptosis. Int J Oncol.
52:1011–1022. 2018.PubMed/NCBI
|
|
59
|
Trachootham D, Zhou Y, Zhang H, Demizu Y,
Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J and
Huang P: Selective killing of oncogenically transformed cells
through a ROS-mediated mechanism by β-phenylethyl isothiocyanate.
Cancer Cell. 10:241–252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Xiao D, Powolny AA, Moura MB, Kelley EE,
Bommareddy A, Kim SH, Hahm ER, Normolle D, Van Houten B and Singh
SV: Phenethyl isothiocyanate inhibits oxidative phosphorylation to
trigger reactive oxygen species-mediated death of human prostate
cancer cells. J Biol Chem. 285:26558–26569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Honma Y, Kasukabe T, Yamori T, Kato N and
Sassa T: Antitumor effect of cotylenin a plus interferon-α:
Possible therapeutic agents against ovary carcinoma. Gynecol Oncol.
99:680–688. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Honma Y, Ishii Y, Yamamoto-Yamaguchi Y,
Sassa T and Asahi K: Cotylenin A, a differentiation-inducing agent,
and IFN-alpha cooperatively induce apoptosis and have an antitumor
effect on human non-small cell lung carcinoma cells in nude mice.
Cancer Res. 63:3659–3666. 2003.PubMed/NCBI
|
|
63
|
Honma Y: Cotylenin A-A plant growth
regulator as a differentiation-inducing agent against myeloid
leukemia. Leuk Lymphoma. 43:1169–1178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li-Weber M: New therapeutic aspects of
flavones: The anticancer properties of Scutellaria and its main
active constituents wogonin, baicalein and baicalin. Cancer Treat
Rev. 35:57–68. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kurzatkowski DM and Trombetta LD: Maneb
causes pro-oxidant effects in the hippocampus of Nrf2 knockout
mice. Environ Toxicol Pharmacol. 36:427–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Qin S, Deng F, Wu W, Jiang L, Yamashiro T,
Yano S and Hou DX: Baicalein modulates Nrf2/Keap1 system in both
keap1-dependent and keap1-independent mechanisms. Arch Biochem
Biophys. 559:53–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sun X, Ou Z, Xie M, Kang R, Fan Y, Niu X,
Wang H, Cao L and Tang D: HSPB1 as a novel regulator of ferroptotic
cancer cell death. Oncogene. 34:5617–5625. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wu C: Heat shock transcription factors:
Structure and regulation. Annu Rev Cell Dev Biol. 11:441–469. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Louandre C, Ezzoukhry Z, Godin C, Barbare
JC, Mazière JC, Chauffert B and Galmiche A: Iron-dependent cell
death of hepatocellular carcinoma cells exposed to sorafenib. Int J
Cancer. 133:1732–1742. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lachaier E, Louandre C, Godin C, Saidak Z,
Baert M, Diouf M, Chauffert B and Galmiche A: Sorafenib induces
ferroptosis in human cancer cell lines originating from different
solid tumors. Anticancer Res. 34:6417–6422. 2014.PubMed/NCBI
|
|
71
|
Dixon SJ, Patel DN, Welsch M, Skouta R,
Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS
and Stockwell BR: Pharmacological inhibition of cystine-glutamate
exchange induces endoplasmic reticulum stress and ferroptosis.
Elife. 3:e025232014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Song X, Zhu S, Chen P, Hou W, Wen Q, Liu
J, Xie Y, Liu J, Klionsky DJ, Kroemer G, et al: AMPK-mediated BECN1
phosphorylation promotes ferroptosis by directly blocking system
Xc-activity. Curr Biol. 28:2388–2399. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bai T, Wang S, Zhao Y, Zhu R, Wang W and
Sun Y: Haloperidol, a sigma receptor 1 antagonist, promotes
ferroptosis in hepatocellular carcinoma cells. Biochem Biophys Res
Commun. 491:919–925. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yuan H, Li X, Zhang X, Kang R and Tang D:
CISD1 inhibits ferroptosis by protection against mitochondrial
lipid peroxidation. Biochem Biophys Res Commun. 478:838–844. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chang LC, Chiang SK, Chen SE, Yu YL, Chou
RH and Chang WC: Heme oxygenase-1 mediates BAY 11-7085 induced
ferroptosis. Cancer Lett. 416:124–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Louandre C, Marcq I, Bouhlal H, Lachaier
E, Godin C, Saidak Z, François C, Chatelain D, Debuysscher V,
Barbare JC, et al: The retinoblastoma (Rb) protein regulates
ferroptosis induced by sorafenib in human hepatocellular carcinoma
cells. Cancer Lett. 356:971–977. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ou W, Mulik RS, Anwar A, McDonald JG, He X
and Corbin IR: Low-density lipoprotein docosahexaenoic acid
nanoparticles induce ferroptotic cell death in hepatocellular
carcinoma. Free Radic Biol Med. 112:597–607. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Jennis M, Kung CP, Basu S, Budina-Kolomets
A, Leu JI, Khaku S, Scott JP, Cai KQ, Campbell MR, Porter DK, et
al: An african-specific polymorphism in the TP53 gene impairs p53
tumor suppressor function in a mouse model. Genes Dev. 30:918–930.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Guo J, Xu B, Han Q, Zhou H, Xia Y, Gong C,
Dai X, Li Z and Wu G: Ferroptosis: A novel anti-tumor action for
cisplatin. Cancer Res Treat. 50:445–460. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Xie Y, Zhu S, Song X, Sun X, Fan Y, Liu J,
Zhong M, Yuan H, Zhang L, Billiar TR, et al: The tumor suppressor
p53 limits ferroptosis by blocking DPP4 activity. Cell Rep.
20:1692–1704. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Komatsu M, Kurokawa H, Waguri S, Taguchi
K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, et
al: The selective autophagy substrate p62 activates the stress
responsive transcription factor Nrf2 through inactivation of keap1.
Nat Cell Biol. 12:213–223. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Suzuki T, Motohashi H and Yamamoto M:
Toward clinical application of the Keap1-Nrf2 pathway. Trends
Pharmacol Sci. 34:340–346. 2013. View Article : Google Scholar : PubMed/NCBI
|















