Introduction
Breast cancer is one of the leading causes of death
among women (1). The high
heterogeneity of breast tumor cells prevents traditional treatment
options from satisfying the individualized treatment needs of
patients with breast cancer. Genetic alteration is a feature of
breast cancer; if molecular-targeted therapy was developed based on
different genetic abnormalities in patients, the mortality rates
could be decreased (2). With the
development of gene sequencing and bioinformatics, the association
between the regulation of non-coding RNA sequences in the cancer
genome and the complex biological characteristics of tumors has
become a hot topic of research. Our previous study demonstrated
that the long noncoding RNA (lncRNA) LINC00365 and the secreted
protein secretoglobin family 2A member 1 (SCGB2A1; also termed
mammaglobin B are expressed at low levels in gastric cancer, as
revealed by bioinformatics analysis, and that there is a regulatory
relationship between them (3).
Expression of SCGB2A1 is associated with chemotherapy and radiation
resistance and cancer cell stemness (4). Therefore, the present study aims to
determine the association between LINC00365 and SCGB2A1 in breast
cancer and to develop new targets for breast cancer treatment.
SCGB2A1 is a small secreted protein that is a member
of the superfamily of uterine proteins (5). The gene encoding this protein is
located on chromosome 11q12.2 (6).
SCGB2A1 was first isolated by Becker et al in 1998 (7); it has been identified in epithelial
cells in the lung, breast, salivary gland, sweat gland and prostate
and is closely related to cell secretion, inflammation, tissue
repair and tumorigenesis (8).
SCGB2A1 is considered a candidate marker for detecting certain
minimal cancers in lymph nodes and for diagnosing tumor cells
hidden in the exudate of patients with various malignancies
(4). In addition, gene expression
profiling identified SCGB2A1 as a highly expressed gene in all
histological types of ovarian cancer (9). Previous studies have reported that
SCGB2A1 is expressed at a low level in luminal breast cancer
compared with that in normal tissue (10,11), but
the specific mechanism behind its involvement in this disease
remains unclear. Long non-coding RNAs (lncRNAs) are a group of
non-protein-coding RNAs that are >200 nucleotides long (12,13). Due
to their complex spatial structure, the mechanisms involved in
regulating their expression are particularly diverse and complex.
Characterization of the functional mechanisms of lncRNA effects in
tumors not only contributes to the application of clinical
biomarkers, but also promotes the development of new cancer
therapeutic targets (14). A number
of lncRNAs have been demonstrated to regulate important
cancer-related processes (15),
including apoptosis, viability, metastasis, metabolism and
chemotherapy resistance (16,17).
LINC00365 is one of the lncRNAs encoded by a gene with a
chromosomal location 13q12.3 (18).
Our previous study revealed that LINC00365 exhibits significantly
different expression levels in gastric cancer compared with those
in normal tissue (3). In addition,
studies using bioinformatics approaches have predicted that SCGB2A1
secreted into the blood and urine is a potential target for
LINC00365 (3).
The activation of nuclear transcription factor κB
(NF-κB) is involved in the transcriptional regulation of many genes
(19). The role of the
NF-κB-mediated cell signal transduction pathway in cell viability
and apoptosis has been a focus of intensive research globally
(20–22). NF-κB suppresses apoptosis by inducing
the expression of apoptosis-inhibitory genes, including inhibitors
of apoptosis proteins (IAPs), cellular FLICE-like inhibitory
protein, TNF receptor-associated factor 1 (TRAF1) and TRAF2
(22–25). Two typical pro-survival NF-κB targets
are X-linked inhibitor of apoptosis and Bcl2-like 1 (Bcl-xl), which
can block apoptosis at multiple steps (26,27).
Similarly, NF-κB can promote tumor cell viability by regulating
TNF-α, chemokines, adhesion factors, transforming growth factors
and other molecules involved in various stages of the inflammatory
response (28). Previous findings
have demonstrated that NF-κB is overexpressed in multiple types of
breast cancer cells (29), but the
specific mechanism associated with this process remains to be
identified (30). Based on a
literature review and previous studies, it is hypothesized in the
present study that LINC00365 and SCGB2A1 may affect the activity of
breast cancer cells by affecting the transcriptional activity of
NF-κB.
The present study aimed to investigate the
underlying mechanism of LINC00365 and SCGB2A1 in breast cancer. In
addition, the LINC00365-SCGB2A1 axis was demonstrated to
participate in the viability and apoptosis of breast cancer cells
by regulating the NF-κB signaling pathway. The results of the
present study suggested that LINC00365 and SCGB2A1 may become
promising targets for breast cancer treatment.
Materials and methods
Tissue collection
Paired breast cancer and paracancerous (3–5 cm
distal from the cancer tissue) tissues were collected from 30
female patients (age range, 35–70 years) who underwent surgical
resection at the China-Japan Union Hospital of Jilin University
(Table I). Approval for this study
was provided by the Ethics Committee of the China-Japan Union
Hospital of Jilin University. The patients were not treated locally
or systemically prior to surgery. All tissues were washed with
sterile phosphate-buffered saline, snap-frozen in liquid nitrogen
and stored at −80°C until RNA extraction.
 | Table I.Patient information. |
Table I.
Patient information.
| No. | Sex | Age, years | ER | PR | HER-2 | Type |
|---|
| 1 | Female | 52 | + | + | − | Luminal B |
| 2 | Female | 47 | + | + | + | Luminal B |
| 3 | Female | 68 | − | − | + |
HER2+ |
| 4 | Female | 39 | + | + | + | Luminal B |
| 5 | Female | 62 | + | + | + | Luminal B |
| 6 | Female | 64 | + | + | + | Luminal B |
| 7 | Female | 58 | + | + | − | Luminal B |
| 8 | Female | 48 | − | − | − | Basal-like |
| 9 | Female | 65 | + | 2+ | + | Luminal A |
| 10 | Female | 64 | − | − | + |
HER2+ |
| 11 | Female | 46 | + | + | + | Luminal B |
| 12 | Female | 40 | + | + | − | Luminal B |
| 13 | Female | 54 | − | − | + |
HER2+ |
| 14 | Female | 50 | + | + | − | Luminal B |
| 15 | Female | 35 | + | + | + | Luminal B |
| 16 | Female | 47 | + | 2+ | + | Luminal B |
| 17 | Female | 45 | + | + | + | Luminal B |
| 18 | Female | 69 | + | + | + | Luminal B |
| 19 | Female | 69 | + | 2+ | + | Luminal B |
| 20 | Female | 65 | + | + | + | Luminal B |
| 21 | Female | 63 | + | + | + | Luminal B |
| 22 | Female | 43 | + | + | − | Luminal B |
| 23 | Female | 70 | + | + | − | Luminal B |
| 24 | Female | 60 | + | + | + | Luminal B |
| 25 | Female | 52 | − | − | + |
HER2+ |
| 26 | Female | 38 | − | − | + |
HER2+ |
| 27 | Female | 62 | + | + | − | Luminal B |
| 28 | Female | 59 | + | 2+ | − | Luminal A |
| 29 | Female | 56 | + | + | + | Luminal B |
| 30 | Female | 43 | + | + | + | Luminal B |
Cell lines and culture
Human invasive ductal carcinoma cell lines MCF-7 and
T47D were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS)
(Gibco; Thermo Fisher Scientific, Inc.), 100 IU/ml penicillin and
100 mg/ml streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.). Normal human breast epithelial MCF-10A cells were cultured
in F12 Ham's Mixture (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 5% FBS, epidermal growth factor, insulin,
hydrocortisone, cholera toxin, 100 IU/ml penicillin and 100 mg/ml
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.). All
cells were incubated at 37°C in a humidified atmosphere with 5%
CO2.
Expression of LINC00365 and
SCGB2A1
Short hairpin RNA (shRNA) sequences were constructed
by Shanghai Genechem Co., Ltd. Full-length human LINC00365 and
SCGB2A1 expression vectors were constructed by subcloning
full-length LINC00365 or SCGB2A1 cDNA fragments into pcDNA3.1
vectors (Shanghai Genechem Co., Ltd.). Transfections were performed
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Briefly, MCF-7 and T47D cells were seeded into 6-well plates at the
density of 6×105 cells per well and transfected with 4
µg LINC00365 or SCGB2A1 expression vector for 6 h with 10 µl (1
µg/µl) Lipofectamine® 2000 per well. After 48 h, the
cells were harvested for subsequent experiments.
Cell viability assay
Cell viability was determined by real-time unlabeled
cell assay (RTCA). MCF-7 and T47D cells transfected with an empty
vector, LINC00365 or SCGB2A1 overexpression vectors were inoculated
into an E-Plate assay plate at a density of 1.2×104
cells/well and subsequently maintained at room temperature for 30
min. The E-Plate detection was placed on a test stand, and a cell
viability curve was created following real-time dynamic detection
of cell viability.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR) analysis
Total RNA was extracted from tissues or cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and reverse-transcribed into cDNA according to the
manufacturer's instructions, by using the EasyScript First-Strand
cDNA Synthesis (Beijing Transgen Biotech Co., Ltd.). qPCR was
performed using an MX300P instrument (Agilent Technologies, Inc.),
followed by a three-step PCR protocol. cDNA was used as template
and cycling parameters were as follows: 95°C for 2 min, followed by
40 cycles of 95°C for 10 sec, 60°C for 30 sec and 72°C for 30 sec.
Relative expression was calculated by 2−ΔΔCq among
different experimental groups with normalization to GAPDH
expression (31). The primer
sequences were as follows: LINC00365 forward,
5-TGCTATTCTATGGCTGGGCTTC-3′ and reverse,
5′-ACTGTTGAATTTCCTGGATGTGTC-3′; SCGB2A1 forward,
5′-AAACTCCTGGAGGACATGGTT-3′ and reverse,
5′-ACTGCTTGAATTTCCCCATAGC-3′; C-X-C motif chemokine ligand 8
(CXCL8) forward, 5′-ATGACTTCCAAGCTGGCCGTGGCT-3′ and reverse,
5′-TCTCAGCCCTCTTCAAAAACTTCTC-3′; transforming growth factor β 1
(TGFβ1) forward, 5′-GGATAACACACTGCAAGTGG-3′ and reverse,
5′-GAGCTGAAGCAATAGTTGGTG-3′; c-IAP1 forward,
5′-GTTCAGTGGTTCTTACTCCAGC-3′, reverse,
5′-ACTGTAGGGGTTAGTCCTCGAT-3′; and GAPDH forward.
5′-AGAGGCAGGGATGATGTTCTG-3′ and reverse,
5′-GACTCATGACCACAGTCCATGC-3′.
Clone formation assay
Following transfection with LINC00365- and
SCGB2A1-overexpressing vectors for 12 h, MCF-7 and T47D cells were
collected, counted and seeded in a 6-well plate at 500 cells/well.
Negative control (NC) and LINC00365- and SCGB2A1-overexpressing
groups were established, and experiments were performed in
triplicate for each group. Following 1-week incubation with fresh
medium, the cells were fixed with 4% paraformaldehyde for 15 min at
37°C, in a humidified atmosphere with 5% CO2, washed
once with PBS and stained with 0.1% crystal violet for 30 min at
room temperature. The number of cell clones was counted using a
light microscope (OLYMPUS IX-71; Olympus Corporation;
magnification, ×10).
Flow cytometric analysis
MCF-7 and T47D breast cancer cells were seeded in
6-well plates and transfected with empty, LINC00365- or
SCGB2A1-overexpressing vectors for 48 h. Attaching cells and cells
in the supernatant were collected. Each group of cells was stained
with Annexin-V FITC/propidium iodide (Annexin V Apoptosis Detection
Kit II; BD Biosciences). Samples were analyzed using a BD Accuri C6
flow cytometer (Becton, Dickinson and Company).
Western blot assay
MCF-7 and T47D cells were lysed with a protein
extraction reagent buffer (RIPA; Beyotime Institute of
Biotechnology) containing a protease inhibitor cocktail and
phenylmethylsufonyl fluoride. Protein concentration was measured
using the Coomassie G250 assay (Beyotime Institute of
Biotechnology). Proteins (70 µg) were separated by 10% SDS-PAGE and
transferred to a PVDF membrane. The membrane was blocked with 10%
milk and washed with TBS + 0.1% Tween-20. Subsequently, the
membrane was incubated with a primary antibody (ProteinTech Group,
Inc.; BCL2, cat. no. 12789-1-AP, Bcl-XL, cat. no. 26967-1-AP,
Caspase 3, cat. no. 19677-1-AP, BAX, cat. no. 50599-2-Ig10268-1-AP,
β-actin, cat. no. 23660-1-AP) diluted 1:1,000 overnight at 4°C,
followed by incubation with the secondary antibody (ProteinTech
Group, Inc.; anti-mouse, cat. no. SA00001-1; anti-rabbit, cat. no.
SA00001-2) diluted 1:1,000 at room temperature for 1–2 h.
Immunodetection was performed using ECL reagent (Thermo Fisher
Scientific, Inc.) and visualized by Syngene Bio Imaging (Synoptics
Ltd.). ImageJ (×64) software (National Institutes of Health) was
used to quantify the results.
5-Ethynyl-2′-deoxyuridine (EdU) cell
viability assay
MCF-7 and T47D cells transfected with empty,
LINC00365- or SCGB2A-overexpressing vectors were inoculated into
96-well plates and fixed with 4% paraformaldehyde for 20 min at
room temperature. Diluted EdU (cat. no. C10310-1; Suzhou Ribo Life
Science Co. Ltd; 50 µl) was added per well according to the
manufacturer's instructions. The cells were washed with PBS,
followed by DNA staining with Hoechst-33342 at room temperature for
5 min, and the staining was observed using OLYMPUS IX-71
fluorescence microscope (magnification, ×20).
Immunofluorescence and confocal
microscopy
MCF-7 and T47D cells were seeded on glass slides,
transfected for 48 h, fixed with 4% paraformaldehyde for 25 min at
room temperature, washed with 0.01 M PBS, treated with 0.1%
Triton-PBS for 7 min and blocked with 5% FBS for 30 min at room
temperate. P50 primary antibody (ProteinTech Group, Inc.; NF-κB,
cat. no. 14220-1-AP; 1:200) was added and incubated overnight at
4°C, followed by washing with PBS and incubation with a fluorescent
secondary antibody (ProteinTech Group, Inc. FITC, cat. no.
SA00003-1; 1:200) for 30 min in the dark at room temperature.
Sections were fixed with glycerol at room temperature for 30 min
and imaged using an Olympus FV1000 confocal laser microscope
(magnification, ×20).
Luciferase reporter assay
MCF-7 and T47D cells were transfected with 2 µg/ml
NF-κB-Luc reporter plasmid and LINC00365/SCGB2A1 plasmid (Sangon
Biotech Co., Ltd.) used Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.), LINC00365 or SCGB2A1
overexpression vectors and the internal reference plasmid for 48 h,
Renilla luciferase assay substrate and firefly luciferase detection
reagent were added. Renilla luciferase was used as an internal
reference, and luciferase activity was measured using a dual
luciferase assay kit (Promega Corporation).
Statistical analysis
Data are expressed as the mean ± SD. SPSS 17.0
software (SPSS, Inc.) was used for statistical analysis. Pearson
correlation analysis was used to detect the correlation between
LINC00365 and SCGB2A1 transcription level. The differences among
multiple groups were analyzed using one-way ANOVA followed by
Dunnett's multiple comparison post hoc test to determine
statistical significance. P<0.05 was considered to indicate a
statistically significant difference.
Results
LINC00365 and SCGB2A1 exhibit low
expression and are positively correlated in clinical specimens of
breast cancer, as well as breast cancer cell lines
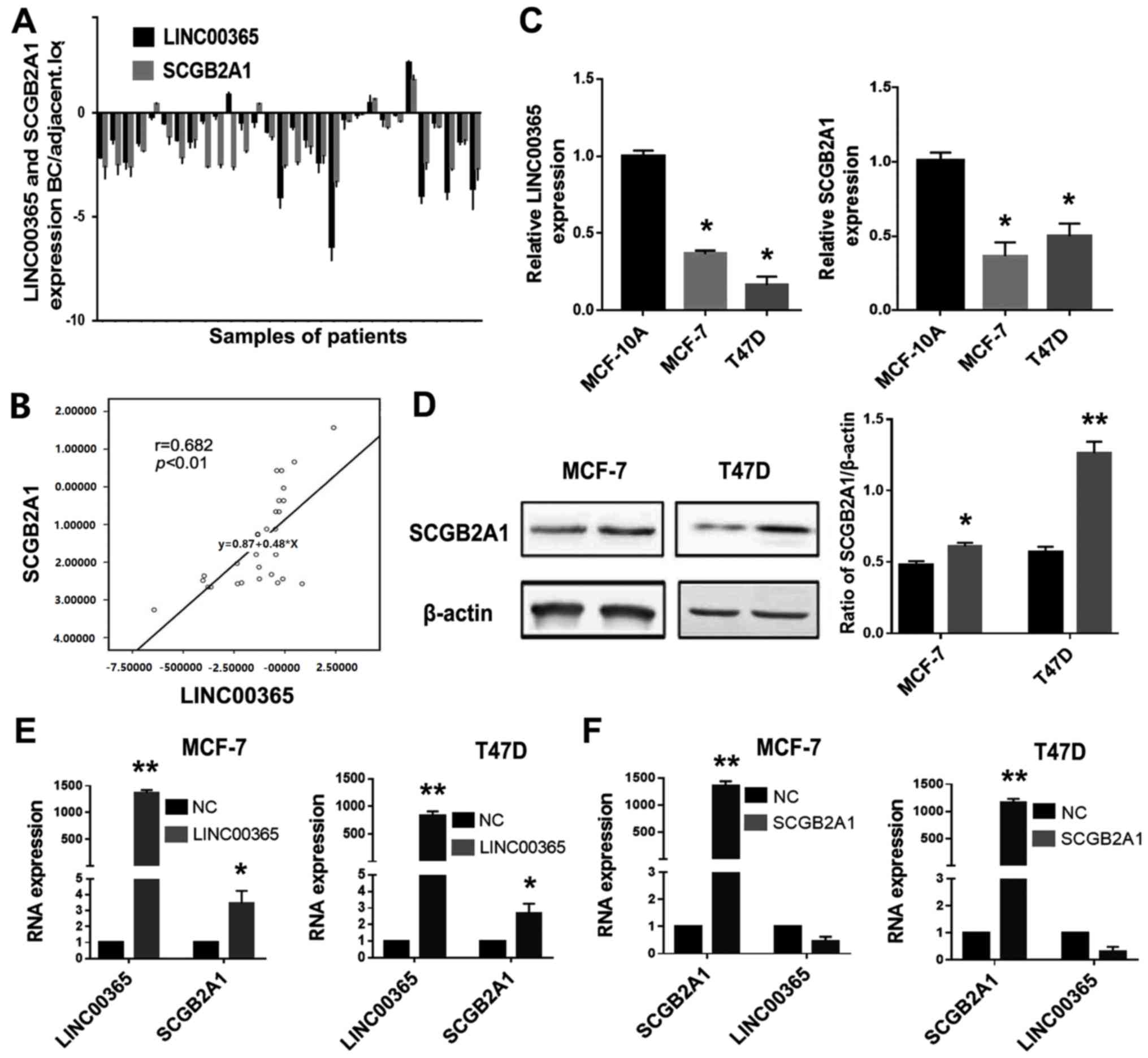
The expression levels of LINC00365 and SCGB2A1 mRNA
in 30 cancer and adjacent tissues from patients with different
types of breast cancer (Table I)
were determined by qPCR. The results demonstrated that the
expression levels of LNC00365 and SCGB2A1 were decreased in breast
cancer tissues compared with adjacent tissues in the majority of
patients (Fig. 1A). Pearson's
correlation analysis confirmed that LINC00365 expression was
positively correlated with SCGB2A1 (Fig.
1B). In addition, qPCR was used to determine the mRNA
expression levels of LINC00365 and SCGB2A1 in MCF-7 and T47D human
breast cancer cells, as well as MCF-10A normal breast cells.
Consistently with the ex vivo results, the expression levels
of LINC00365 and SCGB2A1 were lower in the two breast cancer cell
lines compared with those in MCF-10A cells (Fig. 1C).
To confirm the regulatory relationship between
LINC00365 and SCGB2A1, MCF-7 and T47D human breast cancer cells
were transfected with a LINC00365 overexpression vector. The
results demonstrated that SCGB2A1 expression was upregulated at the
mRNA and protein level compared with that in the negative control
group (NC) (Fig. 1D and E). However,
when SCGB2A1 was overexpressed in breast cancer cells, no
significant differences were observed in the change in LINC00365
expression compared with NC, which indicated that SCGB2A1 was
downstream of LINC00365 in breast cancer cells (Fig. 1F).
Overexpression of LINC00365 and
SCGB2A1 inhibits the viability of breast cancer cells
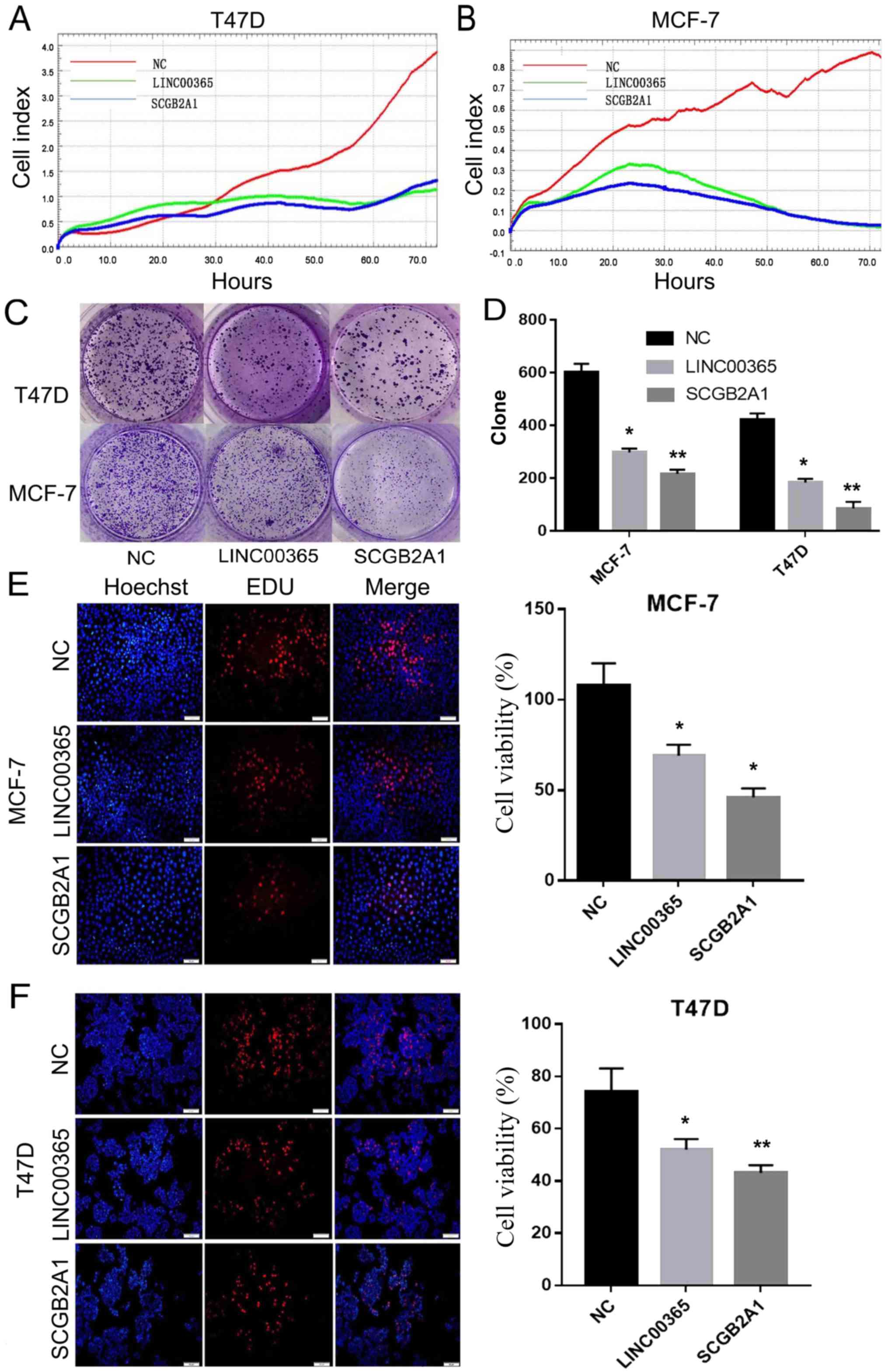
To examine the role of LINC00365, RTCA was used to
determine viability of MCF-7 and T47D breast cancer cells
transfected with LINC00365 for 72 h. The results demonstrated that
overexpression of LINC00365 inhibited breast cancer cell viability
by >50% compared with that in NC after 72 h. In addition, cells
transfected with SCGB2A1 exhibited similar results (Fig. 2A and B). Clone formation assay
confirmed that cell viability significantly decreased following
overexpression of LINC00365 or SCGB2A1 compared with that in NC
(Fig. 2C and D). EdU cell viability
assay results also demonstrated that overexpression of LINC00365
and SCGB2A1 significantly reduced the viability of MCF-7 and T47D
breast cancer cells compared with that in the NC group (Fig. 2E and F).
Overexpression of LINC00365 and
SCGB2A1 induces apoptosis in breast cancer cells
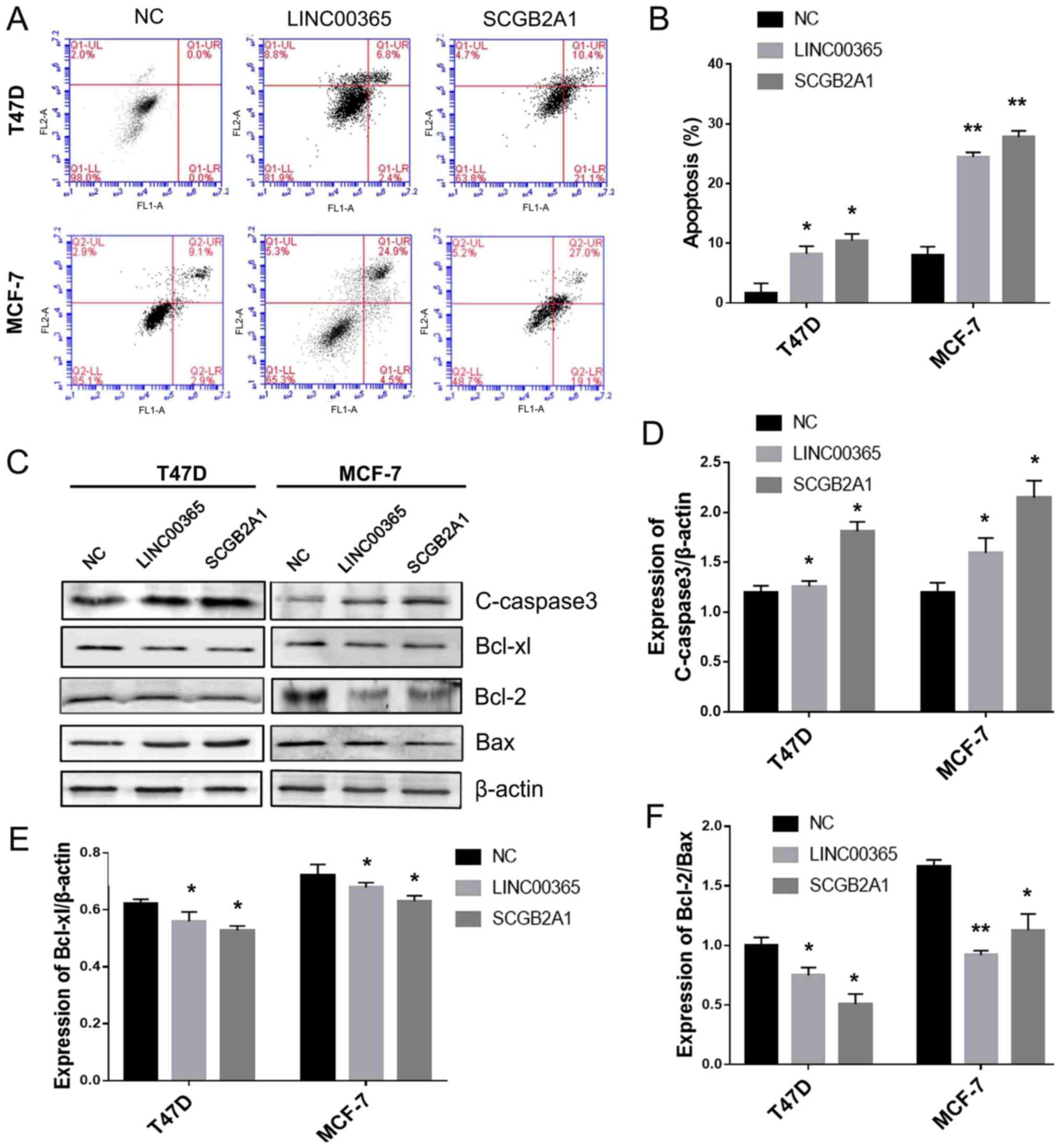
To assess the role of LINC00365 in cell death, flow
cytometry was used to evaluate apoptosis in cells transfected with
LINC00365 or SCGB2A1 overexpression vectors. The results
demonstrated that overexpression of LINC00365 or SCGB2A1 promoted
apoptosis in breast cancer cell lines MCF-7 and T47D (Fig. 3A and B). Western blotting was used to
detect the expression of cleaved-caspase 3 and Bcl-2 family
members, including the anti-apoptotic members Bcl-2 and Bcl-xl, as
well as the pro-apoptotic member Bax. The results revealed that
overexpression of LINC00365 and SCGB2A1 promoted the activation of
cleaved-caspase-3 in MCF-7 and T47D cells compared with that in the
NC group. In addition, the anti-apoptotic protein Bcl-xl was
downregulated in MCF-7 and T47D cells, whereas Bcl-2/Bax ratio was
decreased in MCF-7 and T47D cells compared with that in the NC
group, indicating that overexpression of LINC00365 or SCGB2A1
induced apoptosis in MCF-7 and T47D breast cancer cells (Fig. 3C-F).
LINC00365 inhibits cell viability by
suppressing the NF-κB signaling pathway
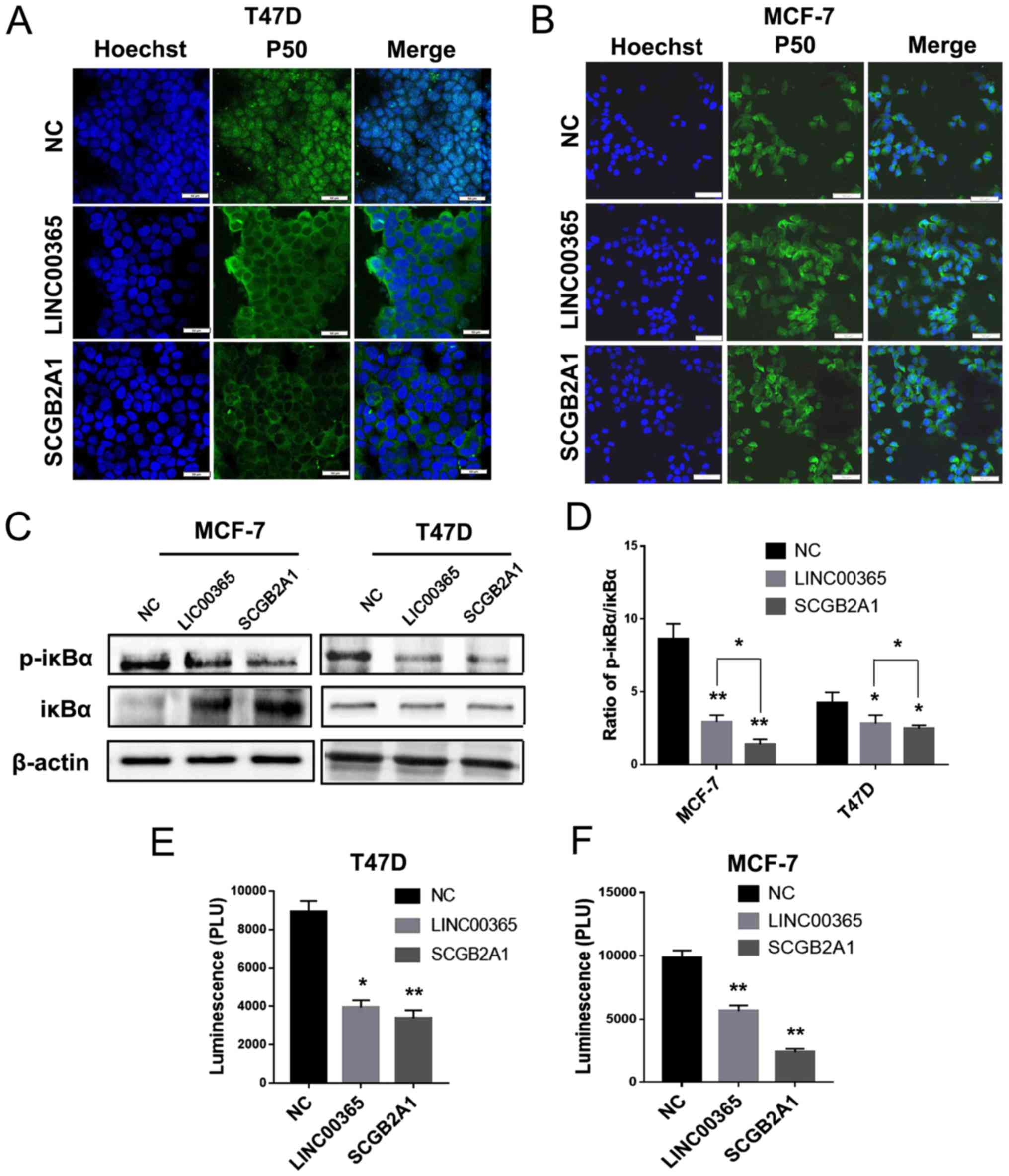
The levels of p-IκBα in breast cancer cells were
determined by western blotting; the results demonstrated that
LINC00365 or SCGB2A1 overexpression inhibited IκBα phosphorylation,
suggesting the potential involvement of NF-κB signaling in breast
cancer (Fig. 4C). The p-IκBα/IκBα
ratio revealed that there was at least a 2-fold difference between
the MCF-7 and T47D cell lines, which indicated that NF-κB signaling
in MCF-7 cells may be more sensitive to LINC00365 overexpression
compared with that in T47D cells (Fig.
4D). In addition, immunofluorescence was used to detect the
expression and localization of p50 follwoing LINC00365 or SCGB2A1
overexpression; the results demonstrated that, compared with that
in the NC group, the expression of p50 protein in the nucleus was
decreased following transfection with LINC00365 or SCGB2A1 in MCF-7
and T47D cells compared with NC (Fig. 4A
and B).
Dual luciferase reporter gene assay was performed to
confirm the effects of LINC00365 or SCGB2A1 overexpression on the
NF-κB signaling pathway. Consistent with results of the
immunofluorescence assay, transcriptional activity of NF-κB was
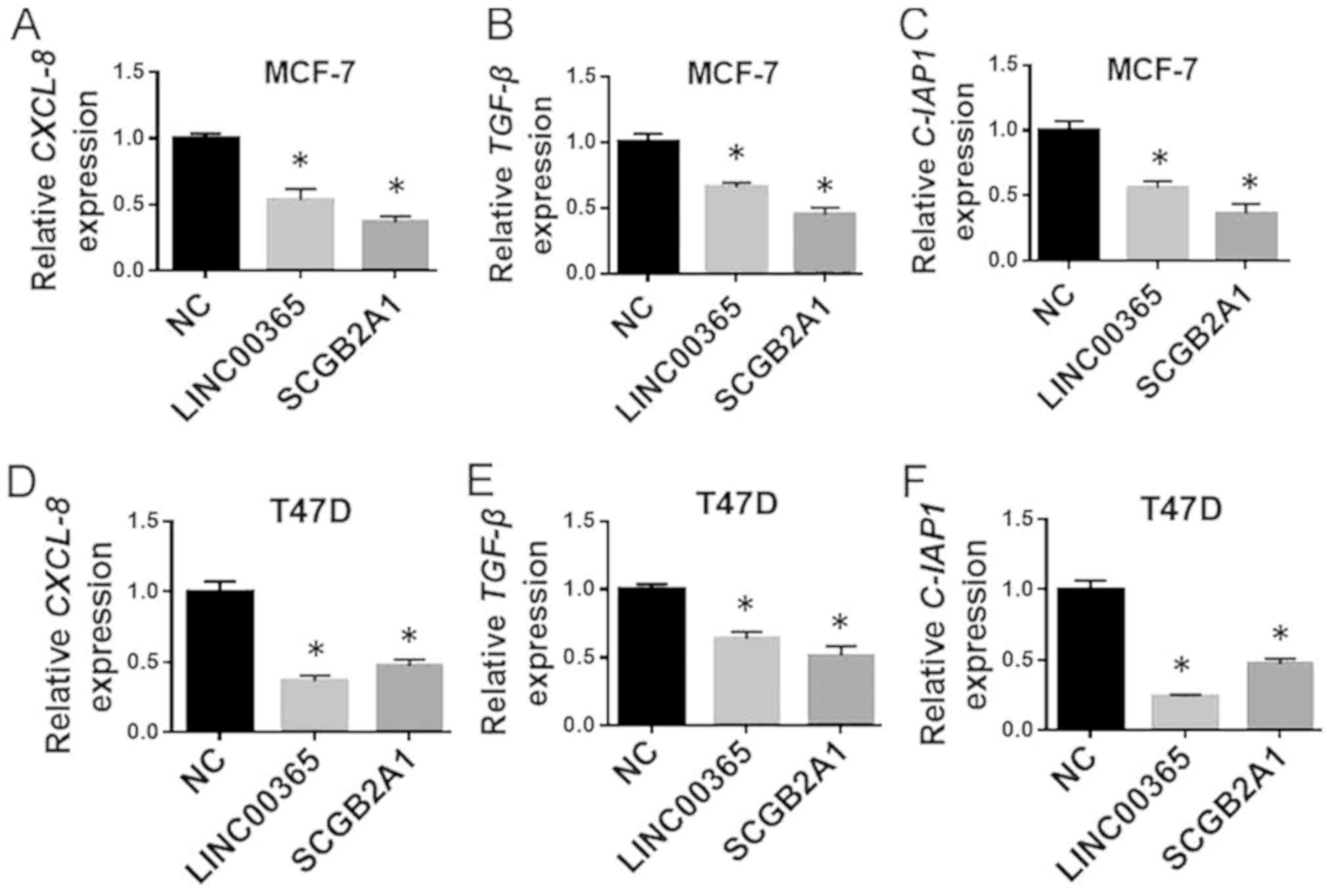
decreased in the transfected groups (Fig. 4E and F). In addition, qPCR was used
to detect apoptosis and viability-related genes regulated by NF-κB.
The results demonstrated that the levels of the anti-apoptotic gene
cellular inhibitor of apoptosis 1 and the pro-survival genes CXCL-8
and TGF-β were decreased in the transfected groups in the two cell
lines, which suggested that LINC00365 and SCGB2A1 may control cell
viability through the regulation of NF-κB signaling (Fig. 5A-F).
Discussion
Increasing evidence has demonstrated that lncRNAs
are involved in cell viability, apoptosis, migration and invasion
in breast cancer (32). Certain
lncRNAs have been identified as tumor-driving oncogenes, whereas
others are tumor suppressors (33,34).
Therefore, lncRNAs can be used as biomarkers for diagnosis and
prognosis of breast cancer and as potential therapeutic targets
(35). The results of the present
study demonstrated that overexpression of lncRNA LINC00365 resulted
in the upregulation of SCGB2A1. In addition, the LINC00365-SCGB2A1
axis inhibited the activation of the NF-κB signaling pathway in
breast cancer cells, subsequently inhibiting cell viability and
inducing apoptosis. Therefore, the LINC00365-SCGB2A1 axis may be a
potential therapeutic target in breast cancer (36).
Our previous study hypothesized that the level of
LINC00365 may correlate with that of the protein encoded by its
potential regulatory target gene SCGB2A1 in gastric cancer
(3). Recently, SCGB2A1 was detected
in the secretory mucosal epithelia of the breast, uterus and
lacrimal glands, and it is known to be expressed in the prostate
(10). Wong et al (5) also demonstrated that SCGB2A1 was
upregulated upon bisphenol A treatment in the rat prostate,
suggesting its involvement in prostate development. Other studies
have reported that both LINC00365 and SCGB2A1 are expressed at a
low level in primary breast cancer and occult breast metastatic
tissues (3,10). It may be speculated that LINC00365
and SCGB2A1 may be inhibited by cancer-related factors and
pathways, which would result in a trend for low expression. Based
on the analyses of clinical breast cancer specimens and breast
cancer cell lines MCF-7 and T47D, the results of the present study
demonstrated that SCGB2A1 may participate in tumor development by
affecting the activation of NF-κB signaling.
NF-κB is constitutively activated in a number of
tumors and is considered to be a key factor in cancer development
(37,38). NF-κB is involved in gene regulation,
the activation of which promotes cell viability and inhibits cell
death (39). Its normally localized
in cytoplasm in an inactive form and binds to unphosphorylated
IκBα. During its activation, IκB is phosphorylated and thus
dissociates from NF-κB; subsequently, NF-κB is transferred from the
cytoplasm to the nucleus, where it regulates gene expression
(40). The results of the present
study indicated that overexpression of either LINC00365 or SCGB2A1
downregulated the activity of the NF-κB signaling pathway.
Interestingly, compared with LINC00365 transfection, SCGB2A1
exhibited stronger effects on NF-κB signaling pathway inhibition.
Similarly, pro-apoptotic effects induced by SCGB2A1 overexpression
were stronger compared with those induced by LINC00365 in T47D and
MCF-7 breast cancer cells. These results indicated that LINC00365
may have other target genes that impair the effects caused by
SCGB2A1. In addition, following overexpression of LINC00365 or
SCGB2A1, the apoptotic rate of MCF-7 cells was notably higher
compared with that of T47D cells, which suggested that different
breast cancer cell lines may have different sensitivity to
LINC00365.
Apoptosis can be triggered in a cell through either
the caspase-mediated intrinsic or extrinsic pathway (41). The extrinsic apoptotic pathway (death
receptor-dependent) is initiated by the interaction of death
receptors exposed on the cell surface; the more intensely
characterized signaling systems of death receptor-ligands include
recombinant Human TNF Receptor Type 1-TNFα, factor associated
suicide (FAS) (CD95, APO-1)-FasL, TNF related apoptosis inducing
ligand related receptor 2 TRAILR2 (DR5)-TRAIL, and TRAILR1
(DR4)-TRAIL (41). By contrast, the
intrinsic apoptotic pathway (mitochondrion-dependent) is mediated
by intracellular signals that gather at the mitochondrial level in
response to stress. The intrinsic pathway is frequently regulated
by the Bcl-2 family of intracellular proteins (42). This protein family controls the
alteration of mitochondrial outer membrane permeabilization by
regulating the intrinsic pathways that promote and inhibit
apoptosis (43). Bax serves a key
role in the mitochondrial stress-induced apoptosis and can form a
dimer with Bcl-2; this dimer enhances the permeability of the
mitochondrial membrane and releases cytochrome C and
Ca2+, leading to caspase activation (44). In MCF-7 cells, the Bcl-2/Bax dimer
expression was decreased following overexpression of LINC00365
compared with that upon overexpression of SCGB2A1; however, the
overall apoptotic rate was higher, which indicated that
overexpression of LINC00365 may activate the extrinsic apoptotic
pathway. In addition, following transfection with LINC00365 or
SCGB2A1 overexpression vectors, the expression levels of Bcl-2
family proteins changed significantly, but no notably change was
observed in cleaved-caspase 3, which indicated that LINC00365 and
SCGB2A1 may regulate additional pathways to counteract apoptosis in
MCF-7 cells. In T47D cells, Bcl-2 family protein expression levels
exhibited little change, but cleaved-caspase 3 expression was
increased significantly, which indicated that LINC00365 and SCGB2A1
may regulate extrinsic apoptosis. Therefore, although the
LINC00365-SCGB2A1 axis may be used as a tumor marker or a
therapeutic target, it exhibits certain differences among breast
cancer cell lines. However, the LINC00365-SCGB2A1 axis may affect
biological characteristics of breast cancer by regulating the NF-κB
signaling pathway.
The results of the present study suggested that
LINC00365 may be a potential lncRNA biomarker for breast cancer
treatment. Further in vivo experiments and clinical studies
are needed to verify the activation of the LINC00365-SCGB2A1 axis.
Based on existing reports, LINC00365 may further regulate SCGB2A1
by chromatin modifications, which will be verified in our future
studies (45,46). In addition, in further studies,
high-throughput sequencing will be used to explore other regulatory
mechanisms.
In conclusion, the results of the present study
revealed the involvement of the LINC00365-SCGB2A1 axis in breast
cancer. This axis may inhibit breast cancer cell viability and
promote apoptosis through the suppression of the NF-κB signaling
pathway. The LINC00365-SCGB2A1 axis may become a novel target of
breast cancer therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81672948, 81772794
and 81501982), Jilin Provincial Health Special Project (grant no.
2018SCZ021), Jilin Provincial Industrial Innovation Project (grant
no. 2018C052-7) and Jilin University Bethune Plan B Projects (grant
no. 2015222).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ, XZ, and SY obtained the data and created the
first draft of the article. JS and XB obtained the clinical tissue
samples and designed the study. LX and RT analyzed the experimental
data. LZ, XY, and JS participated in the project design and article
revision. XB and XY revised the manuscript. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the China-Japan Union Hospital of Jilin University. Complete
clinical data were available for the patients participating in the
study, and written informed consent was obtained.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Harbeck N and Gnant M: Breast cancer.
Lancet. 389:1134–1150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gu G, Dustin D and Fuqua SA: Targeted
therapy for breast cancer and molecular mechanisms of resistance to
treatment. Curr Opin Pharmacol. 31:97–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, He Y, Han S and Liang Y:
Identification and functional inference for tumor-associated long
non-coding RNA. IEEE/ACM Trans Comput Biol Bioinform. 16:1288–1301.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Munakata K, Uemura M, Takemasa I, Ozaki M,
Konno M, Nishimura J, Hata T, Mizushima T, Haraguchi N, Noura S, et
al: SCGB2A1 is a novel prognostic marker for colorectal cancer
associated with chemoresistance and radioresistance. Int J Oncol.
44:1521–1528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wong RL, Wang Q, Trevino LS, Bosland MC,
Chen J, Medvedovic M, Prins GS, Kannan K, Ho SM and Walker CL:
Identification of secretaglobin Scgb2a1 as a target for
developmental reprogramming by BPA in the rat prostate.
Epigenetics. 10:127–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bellone S, Tassi R, Betti M, English D,
Cocco E, Gasparrini S, Bortolomai I, Black JD, Todeschini P, Romani
C, et al: Mammaglobin B (SCGB2A1) is a novel tumour antigen highly
differentially expressed in all major histological types of ovarian
cancer: Implications for ovarian cancer immunotherapy. Br J Cancer.
109:462–471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Becker RM, Darrow C, Zimonjic DB, Popescu
NC, Watson MA and Fleming TP: Identification of mammaglobin B, a
novel member of the uteroglobin gene family. Genomics. 54:70–78.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu X, Wang N, Long XB, You XJ, Cui YH and
Liu Z: The cytokine-driven regulation of secretoglobins in normal
human upper airway and their expression, particularly that of
uteroglobin-related protein 1, in chronic rhinosinusitis. Respir
Res. 12:282011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lacroix M: Significance, detection and
markers of disseminated breast cancer cells. Endocr Relat Cancer.
13:1033–1067. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zubor P, Hatok J, Moricova P, Kajo K,
Kapustova I, Mendelova A, Racay P and Danko J: Gene expression
abnormalities in histologically normal breast epithelium from
patients with luminal type of breast cancer. Mol Biol Rep.
42:977–988. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Claerhout S, Lim JY, Choi W, Park YY, Kim
K, Kim SB, Lee JS, Mills GB and Cho JY: Gene expression signature
analysis identifies vorinostat as a candidate therapy for gastric
cancer. PLoS One. 6:e246622011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Li Z, Zheng W, Li X, Wang Z, Cui Y
and Jiang X: LncRNA-ATB: An indispensable cancer-related long
noncoding RNA. Cell Prolif. 50:2017. View Article : Google Scholar
|
|
13
|
Yan B, Yao J, Liu JY, Li XM, Wang XQ, Li
YJ, Tao ZF, Song YC, Chen Q and Jiang Q: lncRNA-MIAT regulates
microvascular dysfunction by functioning as a competing endogenous
RNA. Circ Res. 116:1143–1156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huarte M: The emerging role of lncRNAs in
cancer. Nat Med. 21:1253–1261. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mao Z, Li H, Du B, Cui K, Xing Y, Zhao X
and Zai S: LncRNA DANCR promotes migration and invasion through
suppression of lncRNA-LET in gastric cancer cells. Biosci Rep.
37:BSR201710702017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Geng Z, Weng J, Shen L, Li M, Cai
X, Sun C and Chu M: Microarray analysis reveals a potential role of
LncRNAs expression in cardiac cell proliferation. BMC Dev Biol.
16:412016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan Q and Liu B: Identification of a
RNA-Seq based 8-long non-coding RNA signature predicting survival
in esophageal cancer. Med Sci Monit. 22:5163–5172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Zhang G, Zhang H, Karin M, Bai H
and Cai D: Hypothalamic IKKbeta/NF-kappaB and ER stress link
overnutrition to energy imbalance and obesity. Cell. 135:61–73.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Turco MC, Romano MF, Petrella A, Bisogni
R, Tassone P and Venuta S: NF-kappaB/Rel-mediated regulation of
apoptosis in hematologic malignancies and normal hematopoietic
progenitors. Leukemia. 18:11–17. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rogers C, Fernandes-Alnemri T, Mayes L,
Alnemri D, Cingolani G and Alnemri ES: Cleavage of DFNA5 by
caspase-3 during apoptosis mediates progression to secondary
necrotic/pyroptotic cell death. Nat Commun. 8:141282017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou A, Scoggin S, Gaynor RB and Williams
NS: Identification of NF-kappa B-regulated genes induced by
TNFalpha utilizing expression profiling and RNA interference.
Oncogene. 22:2054–2064. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kato T, Khanh VC, Sato K, Takeuchi K,
Carolina E, Yamashita T, Sugaya H, Yoshioka T, Mishima H and Ohneda
O: Corrigendum to ‘SDF-1 improves wound healing ability of
glucocorticoid-treated adipose tissue-derived mesenchymal stem
cells’ [Biochem. Biophys. Res. Commun. 493/2 (2017) 1010–1017].
Biochem Biophys Res Commun. 497:464–465. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park JH, Seo YH, Jang JH, Jeong CH, Lee S
and Park B: Asiatic acid attenuates methamphetamine-induced
neuroinflammation and neurotoxicity through blocking of
NF-kB/STAT3/ERK and mitochondria-mediated apoptosis pathway. J
Neuroinflammation. 14:2402017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan J, Xiang J, Lin Y, Ma J, Zhang J,
Zhang H, Sun J, Danial NN, Liu J and Lin A: Inactivation of BAD by
IKK inhibits TNFα-induced apoptosis independently of NF-βB
activation. Cell. 152:304–315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taniguchi F, Uegaki T, Nakamura K, Mon KY
and Harada T, Ohbayashi T and Harada T: Inhibition of IAP
(inhibitor of apoptosis) proteins represses inflammatory status via
nuclear factor-kappa B pathway in murine endometriosis lesions. Am
J Reprod Immunol. 79:2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Y, Decker KF, Zheng D, Matkovich SJ,
Jia L and Dorn GW 2nd: A nucleus-targeted alternately spliced
Nix/Bnip3L protein isoform modifies nuclear factor KB
(NFKB)-mediated cardiac transcription. J Biol Chem.
288:15455–15465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oeckinghaus A and Ghosh S: The NF-kappaB
family of transcription factors and its regulation. Cold Spring
Harb Perspect Biol. 1:a0000342009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pires BR, Mencalha AL, Ferreira GM, de
Souza WF, Morgado-Díaz JA, Maia AM, Corrêa S and Abdelhay ES:
NF-kappaB is involved in the regulation of EMT genes in breast
cancer cells. PLoS One. 12:e01696222017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang Y, Wang Y, Kiani MF and Wang B:
Classification, treatment strategy, and associated drug resistance
in breast cancer. Clin Breast Cancer. 16:335–343. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Soudyab M, Iranpour M and Ghafouri-Fard S:
The role of long non-coding RNAs in breast cancer. Arch Iran Med.
19:508–517. 2016.PubMed/NCBI
|
|
33
|
Xue X, Yang YA, Zhang A, Fong KW, Kim J,
Song B, Li S, Zhao JC and Yu J: LncRNA HOTAIR enhances ER signaling
and confers tamoxifen resistance in breast cancer. Oncogene.
35:2746–2755. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang Q, Wang J, Wu X, Ma R, Zhang T, Jin
S, Han Z, Tan R, Peng J, Liu G, et al: LncRNA2 target: A database
for differentially expressed genes after lncRNA knockdown or
overexpression. Nucleic Acids Res. 43:D193–D196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Boon RA, Jae N, Holdt L and Dimmeler S:
Long noncoding RNAs: From clinical genetics to therapeutic targets?
J Am Coll Cardiol. 67:1214–1226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen KS, Lim JWC, Richards LJ and Bunt J:
The convergent roles of the nuclear factor I transcription factors
in development and cancer. Cancer Lett. 410:124–138. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun SC: The non-canonical NF-kappaB
pathway in immunity and inflammation. Nat Rev Immunol. 17:545–558.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huxford T, Huang DB, Malek S and Ghosh G:
The crystal structure of the IkappaBalpha/NF-kappaB complex reveals
mechanisms of NF-kappaB inactivation. Cell. 95:759–770. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pistritto G, Trisciuoglio D, Ceci C,
Garufi A and D'Orazi G: Apoptosis as anticancer mechanism: Function
and dysfunction of its modulators and targeted therapeutic
strategies. Aging (Albany NY). 8:603–619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pena-Blanco A and Garcia-Saez AJ: Bax, Bak
and beyond - mitochondrial performance in apoptosis. FEBS J.
285:416–431. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ashkenazi A, Fairbrother WJ, Leverson JD
and Souers AJ: From basic apoptosis discoveries to advanced
selective BCL-2 family inhibitors. Nat Rev Drug Discov. 16:273–284.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bierhoff H: Analysis of lncRNA-protein
interactions by RNA-protein pull-down assays and RNA
immunoprecipitation (RIP). Methods Mol Biol. 1686:241–250. 2018.
View Article : Google Scholar : PubMed/NCBI
|