Introduction
Pancreatic cancer is the fifth most commonly
diagnosed cancer and the fourth leading cause of cancer-related
mortality worldwide (1). Because of
its characterizations of aggressive and early dissemination, the
overall 5-year survival rate of pancreatic cancer patients is a
dismal 3–5%, which increases to 15–25% among those who undergo
curative resection (2,3). However, the mortality rate remains high
and has not shown any obvious improvement in the past few decades.
A better understanding of the underlying molecular mechanisms of
this cancer might contribute to demarcate the patients into
different prognostic groups, as well as identify novel markers
associated with prognosis.
Hypoxia is one of the common features of human
cancers, and manifested histologically by necrosis and peritumoral
fibrosis (PF) (4–6). Tumor necrosis has been identified as a
marker of poor prognosis in renal, breast, lung, pancreatic and
colorectal cancers (7–10), whereas PF affects the outcome and
prognosis of inflammatory and hematopoietic disorders (11,12). No
study so far has analyzed the formation of PF and its potential
relationship to the clinicopathological parameters and prognosis of
pancreatic head cancer (PHC). The present study evaluated the
clinical significance and prognostic value of PF in PHC patients
after resection.
Patients and methods
Patients and tumor samples
Total of 143 samples from patients with PHC
resection between January 2007 and December 2011 at the Department
of Hepatobiliary Surgery, Yi Ji Shan Hospital of Wannan Medical
College were included in the present study. All patients with PHC
received routine preoperative work-up including a contrast-enhanced
computed tomography (CT) or magnetic resonance imaging (MRI). The
contraindications for curative resection included metastases,
complete occlusions of the superior-mesenteric/portal vein or
arterial infiltration (>180° circumference), except tumor
contact to the portal vein alone. All the patients with PHC
underwent the Kausch-Whipple procedure and standard lymphadenectomy
along the right side of the superior mesenteric artery, the
hepatoduodenal ligament, and the celiac trunk/upper pancreatic
margin. PHC was confirmed histopathologically and classified
according to the criteria of World Health Organization and the
American Joint Committee on Cancer (AJCC) (13,14).
Patients who underwent preoperative chemoradiation were excluded
since it affects the ratio of fibrosis to cancer cells (15).
The study was approved by the Ethics Committee of
The First Affiliated Hospital of Wannan Medical College (Wuhu,
China). Patients who participated in this research had complete
clinical data. Signed informed consents were obtained from the
patients and/or the guardians.
Formalin-fixed and paraffin-embedded (FFPE) tumor
tissue blocks were retrieved from the database of the Department of
Pathology, Yijishan Hospital of Wannan Medical College, sectioned,
and stained with hematoxylin and eosin (H&E) for histological
analysis of the primary tumor stage and nodal status. The
clinicopathological characteristics of the patients with pancreatic
head cancer are summarized in Table
I. The patients were followed up after the operation by
telephone conversation and/or out-patient clinic interviews.
 | Table I.Relationship between
clinicopathological characteristics and presence of peritumoral
fibrosis. |
Table I.
Relationship between
clinicopathological characteristics and presence of peritumoral
fibrosis.
|
|
| Peritumoral
fibrosis |
|
|---|
|
|
|
|
|
|---|
| Characteristics | No. of patients | Presence | Absence | P-value |
|---|
| Age (years) |
|
|
| 0.404 |
|
<70 | 104 | 64 | 40 |
|
| ≥70 | 39 | 21 | 18 |
|
| Sex |
|
|
| 0.663 |
|
Female | 61 | 43 | 18 |
|
| Male | 82 | 55 | 27 |
|
| Intraoperative blood
transfusion |
|
|
| 0.918 |
| Yes | 42 | 30 | 12 |
|
| No | 101 | 73 | 28 |
|
| Vein resection |
|
|
| 0.820 |
| Yes | 25 | 14 | 11 |
|
| No | 118 | 69 | 49 |
|
| Grading |
|
|
| 0.814 |
| G1/2 | 114 | 72 | 42 |
|
| G3/4 | 29 | 19 | 10 |
|
| T stage |
|
|
| 0.067 |
| T1/2 | 21 | 14 | 7 |
|
| T3/4 | 122 | 102 | 20 |
|
| Resection margin |
|
|
| 0.140 |
|
Negative | 105 | 72 | 33 |
|
|
Positive | 38 | 21 | 17 |
|
| Nodal status |
|
|
| 0.678 |
|
Negative | 82 | 51 | 31 |
|
|
Positive | 61 | 40 | 21 |
|
| Preoperative CA19-9
(U/ml) |
|
|
| 0.842 |
|
<37 | 21 | 14 | 7 |
|
| ≥37 | 122 | 84 | 38 |
|
| No. of examined
nodes |
|
|
| 0.327 |
|
<12 | 52 | 23 | 29 |
|
| ≥12 | 91 | 48 | 43 |
|
| Complications |
|
|
| 0.339 |
|
Yes | 70 | 28 | 42 |
|
| No | 73 | 35 | 38 |
|
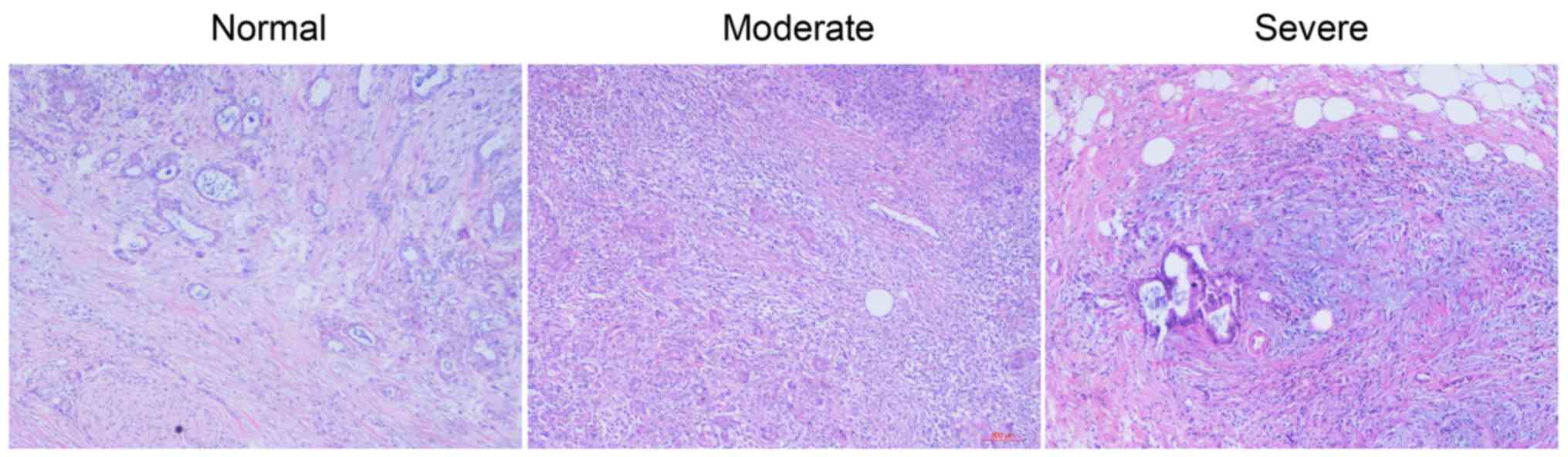
Classification of PF
The tumor specimens were classified into 3
categories according to the degree of PF: negative (<10%
fibrotic change), moderate (11–30%) and severe (>30%).
Statistical analysis
All statistical analyses were performed using the
R3.1.3 program (http://www.R-project.org). Pearson's Chi-square test
and Fisher exact probability test were performed to analyze the
correlation between different parameters. Univariate Kaplan-Meier
analysis was performed to assess the prognostic factors for
survival, as well as compared using the two-sided log-rank test.
The Cox proportional hazard model (forward selection strategy using
a likelihood ratio statistic; inclusion P=0.05) was performed by
multivariate survival analysis, including hazard ratios and their
95% confidence interval. P-values <0.05 were considered
statistically significant.
Results
Clinicopathological characteristics of
the patients
The clinicopathological features of patients with
PHC who underwent pancreatic tumor resection are summarized in
Table I. The median age of patients
was 64 years (range 32–85 years). PF was not significantly
correlated with any of the clinicopathological factors.
Univariate survival analysis
No patient died during the postoperative course. The
median follow-up duration of the entire cohort was 28.7 months
(range 5.3–60 months), and the median survival was 1.95 years. The
cumulative 3- and 5-year survival rates were 31 and 19%,
respectively. Univariate analysis (Table II) showed that vein resection,
resection margin, grading, nodal status, preoperative values of
CA19-9 and PF were significantly associated with survival. Patients
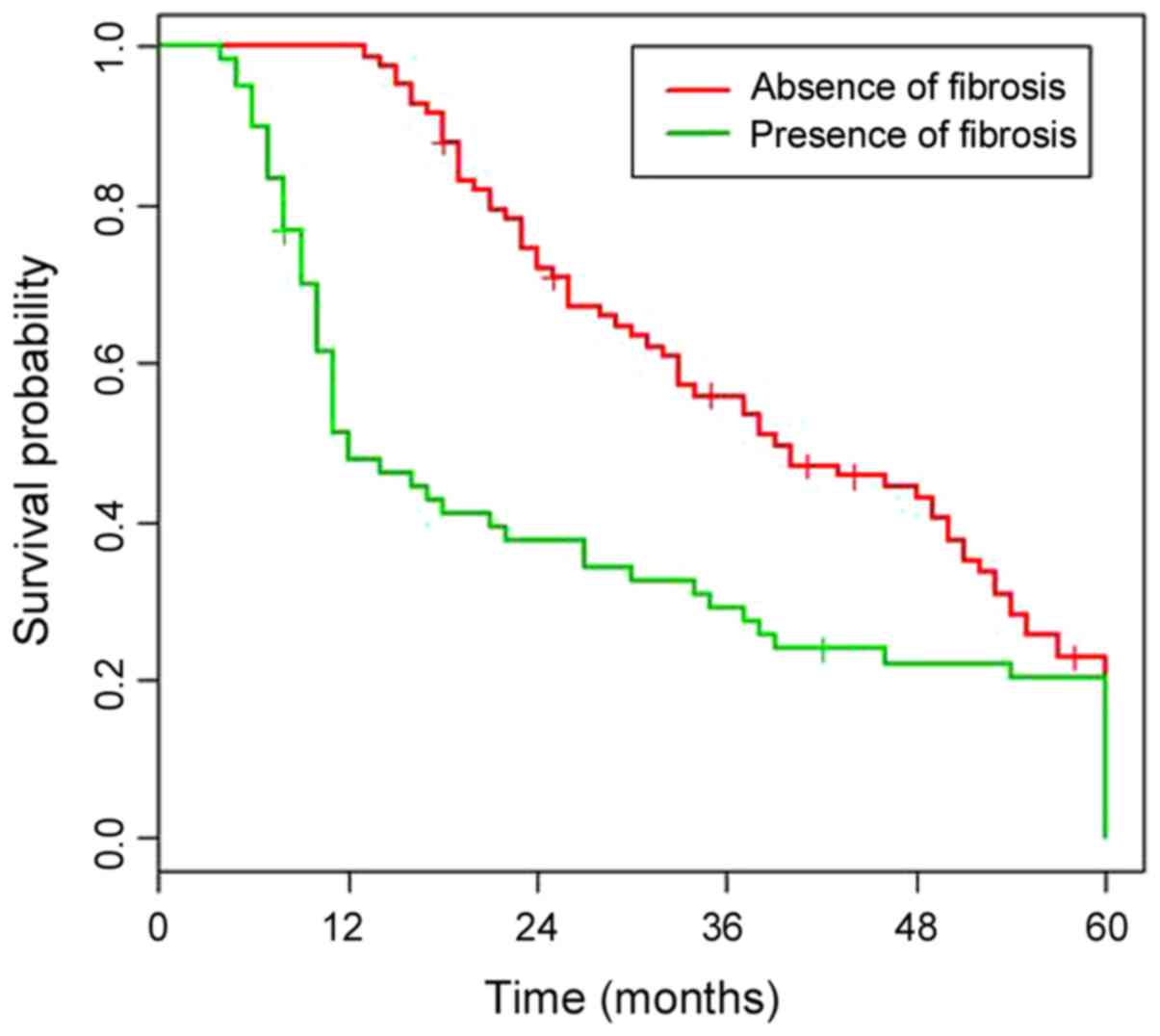
with PF had significantly worse survival compared to those without
(HR 3.079; P<0.001) (Fig. 1). The
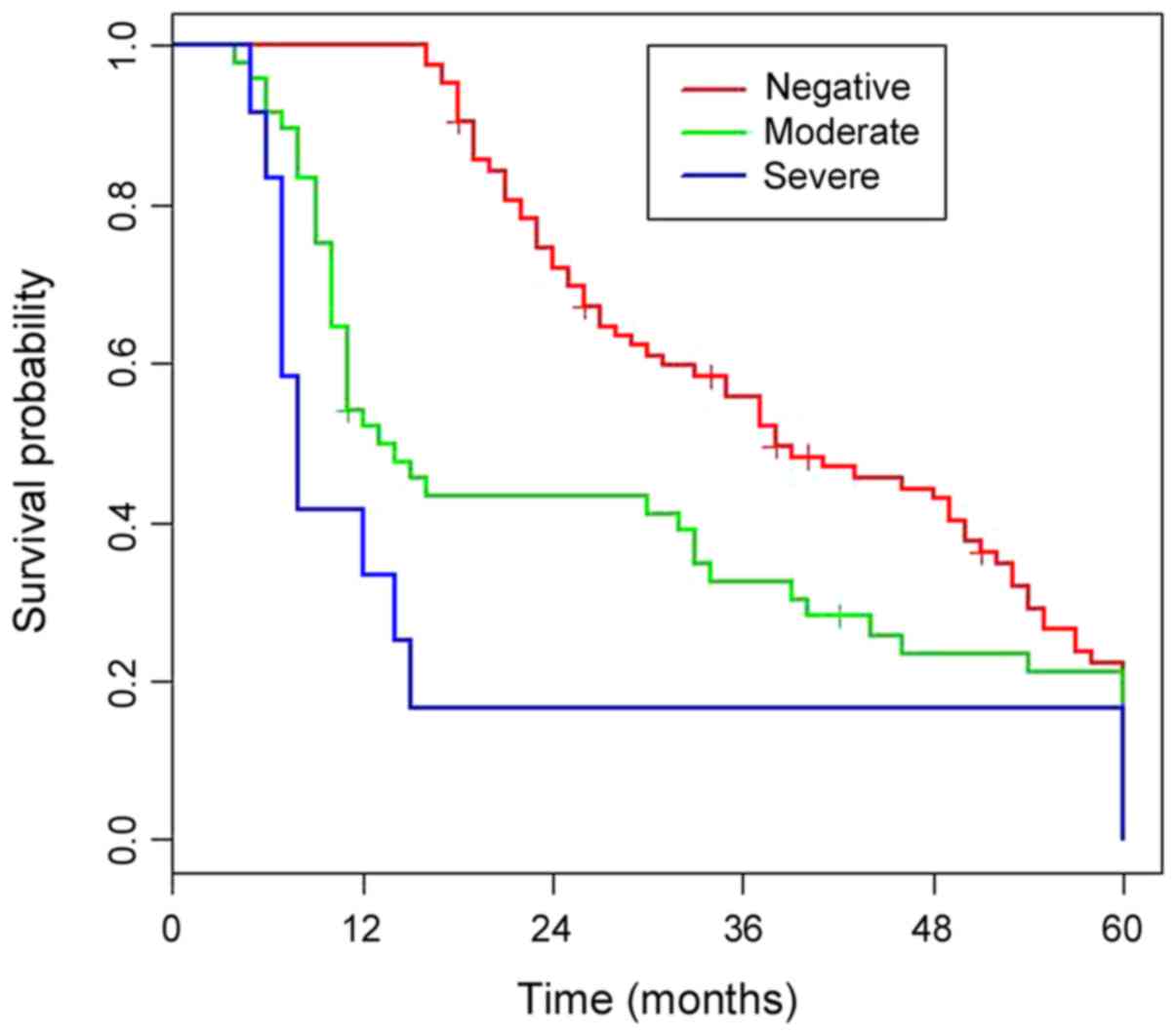
overall survival (OS) of patients with mild and severe PF is shown
in Fig. 2 (P=0.02).
 | Table II.Univariate survival analysis after
resection of pancreatic head cancer. |
Table II.
Univariate survival analysis after
resection of pancreatic head cancer.
|
Characteristics | No. of
patients | HR | 95% CI | P-value |
|---|
| Age (years) |
|
<70 | 104 | 0.923 | 0.611–1.394 | 0.704 |
|
≥70 | 39 | 1 |
|
|
| Sex |
|
Female | 61 | 1.051 | 0.746–1.480 | 0.777 |
|
Male | 82 | 1 |
|
|
| Intraoperative
blood transfusion |
|
Yes | 42 | 1.182 | 0.790–1.767 | 0.790 |
| No | 101 | 1 |
|
|
| Vein resection |
|
Yes | 25 | 1.646 | 1.048–2.588 | 0.031 |
| No | 118 | 1 |
|
|
| Grading |
|
G1/2 | 114 | 1 |
|
|
|
G3/4 | 29 | 1.843 | 1.135–2.993 | 0.013 |
| T stage |
|
T1/2 | 21 | 0.683 | 0.414–1.128 | 0.136 |
|
T3/4 | 122 | 1 |
|
|
| Resection
margin |
|
Negative | 105 | 1 |
|
|
|
Positive | 38 | 1.542 | 1.022–2.324 | 0.039 |
| Nodal status |
|
Negative | 82 | 1 |
|
|
|
Positive | 61 | 1.790 | 1.257–2.556 | 0.001 |
| Preoperative CA19-9
(U/ml) |
|
<37 | 21 | 1.783 | 1.070–2.972 | 0.026 |
|
≥37 | 122 | 1 |
|
|
| No. of examined
nodes |
|
<12 | 52 | 0.883 | 0.575–1.178 | 0.288 |
|
≥12 | 91 | 1 |
|
|
| Peritumoral
fibrosis |
|
Presence | 85 | 3.079 | 1.975 −4.844 |
<0.001 |
|
Absence | 58 | 1 |
|
|
| Complications |
|
Yes | 70 | 0.885 | 0.624–1.255 | 0.493 |
| No | 73 | 1 |
|
|
Multivariate survival analysis
Multivariate analysis (Table III) indicated that resection
margin, vein resection, grading, preoperative values of CA19-9,
nodal status and PF were all independent predictive factors of
survival. The survival of patients with PF was significantly worse
than those without (HR 1.392; P=0.027).
 | Table III.Multivariate survival analysis after
resection of pancreatic head cancer. |
Table III.
Multivariate survival analysis after
resection of pancreatic head cancer.
|
Characteristics | No. of
patients | HR | 95% CI | P-value |
|---|
| Vein resection |
|
Yes | 25 | 2.251 | 1.348–3.758 | 0.002 |
| No | 118 | 1 |
|
|
| Grading |
|
G1/2 | 114 | 1 |
|
|
|
G3/4 | 29 | 1.856 | 1.145–3.009 | 0.012 |
| Resection
margin |
|
Negative | 105 | 1 |
|
|
|
Positive | 38 | 1.977 | 1.212–3.225 | 0.006 |
| Nodal status |
|
Negative | 82 | 1 |
|
|
|
Positive | 61 | 2.973 | 1.947–4.540 |
<0.001 |
| Preoperative CA19-9
(U/ml) |
|
<37 | 21 | 2.398 | 1.166–4.926 | 0.017 |
|
≥37 | 122 | 1 |
|
|
| Peritumoral
fibrosis |
|
Presence | 85 | 1.392 | 1.038–1.869 | 0.027 |
|
Absence | 58 | 1 |
|
|
Discussion
Correlation between the presence of PF and various
clinicopathological parameters were evaluated in 143 pancreatic
head cancer patients who underwent tumor resection. This is the
first study to show the association between PF and poor
post-resection overall survival in pancreatic cancer patients, and
identify it as an independent negative prognostic factor (Fig. 3).
Scarce data is available on the association between
PF and the clinico-pathological characteristics of pancreatic
cancers. A recent study indicated the diagnostic importance of
histological PF in PHCs (16).
Consistent with this, we found that the presence of PF, as well as
the severity of necrosis, was associated with significantly
decreased OS. In addition, PF was also identified as independent
prognostic factor of post-resection outcome. Based on our results,
we hypothesize a diagnostic value of PF in evaluating the
post-resection outcome in PHC patients. A rational clinical
translation of these results suggests standardized utilization of
PF as a prognostic tool in the scope of pathological evaluation of
resected specimens from patients suffering from pancreatic head
cancer.
The cause of fibrosis in PHC and the mechanisms
underlying the poor clinical outcome in patients with PF remain
largely unknown. One hypothesis is that inflammation, which has
been recently described as the seventh hallmark of cancer (17,18),
likely plays a role in the formation of fibrotic masses as well.
Furthermore, there is evidence indicating that pancreatic stellate
cells trigger fibrosis through various stromal interactions and
allow wound healing, thereby promoting cancer cell invasion and
dissemination (19–21). Despite recent advances in our
understanding of the genetic and cellular basis of pancreatic head
cancer progression, its diagnostic and prognostic evaluation is
still mostly dependent on histopathological assessment. The
histopathological parameters such as tumor grading and PF are easy
to evaluate, and can allow individualized risk assessment and
identify patients at high risk of poor outcome.
There are several limitations to our study,
including those inherent to retrospective analyses. In addition,
the surgical resection was performed by multiple surgeons, and
reliable histological evaluation was only possible with the
resected tumor specimens. This can be circumvented in future with
high-resolution magnetic resonance imaging (MRI), which can allow
non-invasive in vivo visualization at a 3D spatial
resolution of up to 50 µm (22–24).
Despite these limitations, our data suggest that PF is a simple
diagnostic tool that can evaluate patients' outcome after resection
of PHC.
In conclusion, PF is an independent prognostic
factor of PHC and predictive of poor survival after resection. This
indicates its potential role in pancreatic cancer progression as
well as its diagnostic utility. Future prospective trials are
needed to assess the value of PF as a criterion for adjuvant
treatment.
Acknowledgements
We are grateful to Professor Entao Sun from the
Institute of Testing, Wannan Medical College for helping with our
research.
Funding
This study was supported by Anhui Provincial
Centralized Local Science and Technology Development Special
Project (YDZX20183400004899); Anhui Science and Technology Research
Fund Project (1501041156).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PC wrote the manuscript and was responsible for the
collection and classification of tumor samples. YW and XF
interpreted and analyzed the data. XW designed the study and
performed the experiments. GW was responsible for the analysis and
discussion of the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The First Affiliated Hospital of Wannan Medical College (Wuhu,
China). Patients who participated in this research had complete
clinical data. Signed informed consents were obtained from the
patients and/or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu Z, Luo G, Guo M, Jin K, Xiao Z, Liu L,
Liu C, Xu J, Ni Q, Long J, et al: Lymph node status predicts the
benefit of adjuvant chemoradiotherapy for patients with resected
pancreatic cancer. Pancreatology. 15:253–258. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lim JE, Chien MW and Earle CC: Prognostic
factors following curative resection for pancreatic adenocarcinoma:
A population-based, linked database analysis of 396 patients. Ann
Surg. 237:74–85. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vaupel P and Mayer A: Hypoxia in cancer:
Significance and impact on clinical outcome. Cancer Metastasis Rev.
26:225–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bristow RG and Hill RP: Hypoxia and
metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev
Cancer. 8:180–192. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frank I, Blute ML, Cheville JC, Lohse CM,
Weaver AL and Zincke H: An outcome prediction model for patients
with clear cell renal cell carcinoma treated with radical
nephrectomy based on tumor stage, size, grade and necrosis: The
SSIGN score. J Urol. 168:2395–2400. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fisher ER, Palekar AS, Gregorio RM,
Redmond C and Fisher B: Pathological findings from the national
surgical adjuvant breast project (Protocol No. 4). IV. Significance
of tumor necrosis. Hum Pathol. 9:523–530. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Swinson DE, Jones JL, Richardson D, Cox G,
Edwards JG and O'Byrne KJ: Tumour necrosis is an independent
prognostic marker in non-small cell lung cancer: Correlation with
biological variables. Lung Cancer. 37:235–240. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hiraoka N, Ino Y, Sekine S, Tsuda H,
Shimada K, Kosuge T, Zavada J, Yoshida M, Yamada K, Koyama T, et
al: Tumour necrosis is a postoperative prognostic marker for
pancreatic cancer patients with a high interobserver
reproducibility in histological evaluation. Br J Cancer.
103:1057–1065. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pearlman BL and Traub N: Sustained
virologic response to antiviral therapy for chronic hepatitis C
virus infection: A cure and so much more. Clin Infect Dis.
52:889–900. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Valent P, Orazi A, Büsche G, Schmitt-Gräff
A, George TI, Sotlar K, Streubel B, Beham-Schmid C, Cerny-Reiterer
S, Krieger O, et al: Standards and impact of hematopathology in
myelodysplastic syndromes (MDS). Oncotarget. 1:483–496. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kwon W, He J, Higuchi R, Son D, Lee SY,
Kim J, Kim H, Kim SW, Wolfgang CL, Cameron JL, et al: Multinational
validation of the American Joint Committee on Cancer 8th edition
pancreatic cancer staging system in a pancreas head cancer cohort.
J Hepatobiliary Pancreat Sci. 25:418–427. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu R, Wakabayashi G, Kim HJ, Choi GH,
Yiengpruksawan A, Fong Y, He J, Boggi U, Troisi RI, Efanov M, et
al: International consensus statement on robotic hepatectomy
surgery in 2018. World J Gastroenterol. 25:1432–1444. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chun YS, Cooper HS, Cohen SJ, Konski A,
Burtness B, Denlinger CS, Astsaturov I, Hall MJ and Hoffman JP:
Significance of pathologic response to preoperative therapy in
pancreatic cancer. Ann Surg Oncol. 18:3601–3607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tomita Y, Azuma K, Nonaka Y, Kamada Y,
Tomoeda M, Kishida M, Tanemura M and Miyoshi E: Pancreatic fatty
degeneration and fibrosis as predisposing factors for the
development of pancreatic ductal adenocarcinoma. Pancreas.
43:1032–1041. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vakkila J and Lotze MT: Inflammation and
necrosis promote tumour growth. Nat Rev Immunol. 4:641–648. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bachem MG, Schünemann M, Ramadani M, Siech
M, Beger H, Buck A, Zhou S, Schmid-Kotsas A and Adler G: Pancreatic
carcinoma cells induce fibrosis by stimulating proliferation and
matrix synthesis of stellate cells. Gastroenterology. 128:907–921.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial- mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vonlaufen A, Joshi S, Qu C, Phillips PA,
Xu Z, Parker NR, Toi CS, Pirola RC, Wilson JS, Goldstein D, et al:
Pancreatic stellate cells: Partners in crime with pancreatic cancer
cells. Cancer Res. 68:2085–2093. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jacoby C, Borg N, Heusch P, Sauter M,
Bönner F, Kandolf R, Klingel K, Schrader J and Flögel U:
Visualization of immune cell infiltration in experimental viral
myocarditis by (19)F MRI in vivo. MAGMA. 27:101–106. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van Heeswijk RB, De Blois J, Kania G,
Gonzales C, Blyszczuk P, Stuber M, Eriksson U and Schwitter J:
Selective in vivo visualization of immune-cell infiltration in a
mouse model of autoimmune myocarditis by fluorine-19 cardiac
magnetic resonance. Circ Cardiovasc Imaging. 6:277–284. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Figueiredo S, Cutrin JC, Rizzitelli S, De
Luca E, Moreira JN, Geraldes CF, Aime S and Terreno E: MRI tracking
of macrophages labeled with glucan particles entrapping a water
insoluble paramagnetic Gd-based agent. Mol Imaging Biol.
15:307–315. 2013. View Article : Google Scholar : PubMed/NCBI
|