Introduction
Recurrent gene mutations are found in the majority
of cancer types. Genotyping tumor tissue for somatic genetic
alterations, which leads to an accurate diagnosis of the tumor
type, guides treatment decisions and/or predicts the response to
therapy, has become common practice in medical oncology (1–3).
Currently, tissue samples obtained via surgery or biopsy are the
gold standard for use in this analysis.
For the treatment of patients with metastatic
cancer, knowledge of tumor mass dynamics and response to therapy
are important. Currently, imaging techniques, including CT and
positron emission tomography (PET)-CT scanning are most commonly
used for this purpose (4–7). Additionally, serum protein biomarkers,
which are often referred to as tumor markers, are used in clinical
practice to assess tumor dynamics and treatment response over time
(8,9). However, the currently used tumor
markers do not always accurately reflect the actual disease burden,
and false positive results are sometimes seen in benign conditions
such as inflammation (10,11). Therefore, these markers cannot be
solely relied upon when estimating actual tumor mass, and need to
be interpreted together with imaging results (12,13).
Furthermore, for a number of tumor types, no reliable serum tumor
marker has been identified.
There is an urgent clinical requirement for the
identification of reliable tumor-specific biomarkers for the
management of patients with metastatic cancer due to a number of
reasons: i) Repetitive imaging studies can lead to a relevant
radiation exposure; ii) the differentiation between residual viable
tumor tissue and fibrotic tissue following neoadjuvant chemo-
and/or radiationtherapy is often challenging, and iii) the
differentiation between actual tumor progression and
pseudoprogression, using current immunooncological approaches, can
also be challenging in daily clinical practice (14).
In recent years, circulating cell-free DNA (cfDNA)
has been indicated as a potential novel biomarker, largely due to
the progression of sequencing technologies, including next
generation sequencing and digital PCR (15). It has also been indicated that
fragments of ‘normal’ DNA and circulating cell-free tumor DNA
(cftDNA) enter the bloodstream via tumor-cells (16–18),
cells undergoing apoptosis or necrosis or via the active release of
DNA (19). Cell-free DNA can be
detected in small amounts in healthy human plasma (3,20,21).
However, higher concentrations of cfDNA are detected in the plasma
of patients with cancer, due to tumor cell necrosis, apoptosis or
its active release by tumor cells (3,22–24).
cftDNA can reflect the mutations located in the primary tumor,
including oncogene or tumor-suppressor gene mutations or
gene-rearrangements (21,25,26), and
can potentially be used to predict tumor burden more accurately
than the protein biomarkers currently used (8,27–29).
The purpose of the current study was to evaluate
cfDNA and cftDNA and the correlation with serum protein tumor
markers and imaging results during therapy of patients with
metastatic colorectal cancer (CRC), pancreatic cancer (PC) or
breast cancer (BC).
Materials and methods
Patients and sample acquisition
The current study was approved by the Local
Institutional Research Ethics Committee (415-E/1469/11-2013) and
all patients provided written informed consent prior to blood
sampling and tumor tissue analysis. Formalin-fixed
paraffin-embedded (FFPE) tissue samples were obtained during
surgery and analyzed at the Institute of Pathology, Paracelsus
Medical University (Salzburg).
Blood sampling was performed between April 2012 and
December 2013. Plasma samples were prospectively collected from 15
patients who were diagnosed and treated at the Department of
Internal Medicine III, Salzburg Cancer Research Institute,
Paracelsus Medical University Salzburg (Salzburg, Austria).
Patients were recruited consecutively within the
study period. The only inclusion criterion was the diagnosis of
metastatic CRC, PC or BC. Patients were considered for analysis if
they had an adequate amount of sampling time-points available. All
patients received at least one course of palliative systemic
therapy. Patients with CRC most commonly received 5-FU based
regimens in combination with Oxaliplatin or Irinotecan. Patients
with PC were most commonly treated with Gemcitabine based regimens
and patients with BC were mainly treated with Taxans (Paclitaxel or
Docetaxel).
The response to treatment was assessed using CT
scans that were performed at 8–12 weeks intervals as indicated by
the physician. The response was defined according to the Response
Evaluation Criteria in Solid Tumors (RECIST) (30–33).
Isolation of DNA from FFPE
tissues
Genomic DNA was extracted from 3–7 sequential
sections (10 µm) of the primary tumor FFPE specimens. A Proteinase
K tissue digestion was performed in a 1.5 ml micro centrifuge tube
containing 3–7 sections of paraffin-embedded tissue, and incubated
at 70°C overnight to dissolve the tissue. DNA was then extracted
using a Maxwell DNA LEV tissue DNA kit (Promega Corporation),
according to the manufacturer's protocol, and eluted using 50 µl
elution buffer. The Maxwell® 16 Instrument purifies DNA
using silica-clad paramagnetic particles, which provide a mobile
solid phase that optimizes the capture, washing and elution of the
target material. The quality of extracted DNA was examined using
agarose gel electrophoresis and ethidium bromide staining, and
concentrations were evaluated using photometry (NanoDrop 1000
Spectrophotometers; Thermo Scientific Inc.).
Direct sequencing of FFPE samples
Primary tumor samples were analysed using PCR
amplification and Sanger sequencing. A number of genes were
analysed, including KRAS, NRAS, BRAF, Tp53, NOTCH, EGFR, PTEN and
PI3K, which are commonly mutated in cancer (34–37).
BigDye® Sequencing Master Mix was used to perform the
sequencing reaction according to the manufacturer's protocol. The
samples were analysed on a capillary sequencer ABI 3100-Analyser
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Oncogenic
mutations were identified in all 15 FFPE samples.
Plasma samples and DNA
purification
Serial blood samples (8 ml each) were collected upon
and following diagnosis. Mandatory blood sampling was performed on
all patients at staging time-points and at intervals of 1–3 weeks
between these points (depending on the frequency of clinical
visits). All samples were processed within 30 min following blood
collection and centrifuged once for 10 min at 1,500 × g. The
resulting plasma sample was spun once more for 10 min at high speed
(2,000 × g) in order to purify plasma from any remaining blood
cells. The plasma was aliquoted and stored at −80°C.
Total nucleic acids were purified from 1 ml plasma
using a modified phenol-chloroform extraction method, as previously
described (38). A total of 108
serial plasma samples were obtained from 15 patients.
Identification of cfDNA somatic
alterations in plasma
The specific mutations indicated in the primary
analysis of FFPE samples (using Sanger sequencing) were used for
every specific patient as a target for ultra-deep cfDNA
sequencing.
By designing sequences that flank the target regions
of interest, the specific PCR-products for ultra-deep sequencing
were isolated. This process was used to prepare libraries for next
generation sequencing. The primer sets that target the regions of
interest included adapter sequences for amplicon-based NGS analysis
and had a mean coverage of 62,000×. The PCR-products were purified
using Wizard SC Gel and a PCR Clean-Up system (Promega
Corporation), according to the manufacturer's protocol for targeted
re-sequencing (GATC Biotech AG).
Quantification of cfDNA and cftDNA in
plasma
Human telomerase reverse transcriptase (hTERT)
genomic amplification was used to quantify the total amount of
cfDNA using reverse transcriptase-quantitative (RT-q)PCR. To
quantify cfDNA, RT-qPCR was used, based on hTERT as the target
(39,40). This system used two amplicon primers
and a fluorgenic hybridization probe for amplifying hTERT. RT-qPCR
was performed with a 20 µl volume on a 7500 ABI detection system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Each run
consisted of patient samples in duplicate, negative controls and a
dilution of a standard TaqMan controlled human genomic DNA (Roche
Diagnostics; 0.2 µg/µl). The amount of cftDNA was calculated by
multiplying the allelic fraction of the respective target gene with
the total amount of cfDNA.
Analysis of serum tumor markers
Carcinoembryonic antigen (CEA), Carcinoma antigen
15-3 (CA 15-3) and carcinoma antigen 19-9 (CA 19-9) levels were
analyzed during therapy at the same time-points as DNA acquisition.
Analysis was performed on a Modular-E170 (CEA Ref: 11731629, CA
19-9 Ref: 11776193, CA 15-3 Ref: 03045838; Elektro Chemilumineszenz
Immuno Assay; Roche Diagnostics) in cooperation with the University
Institute of Medical and Chemical Laboratory Diagnostics (Salzburg,
Austria).
cfDNA and cftDNA levels were correlated with CEA and
CA19-9 levels in patients with colorectal and pancreatic cancer,
and CA15-3 levels in breast cancer patients.
Volumetry of target lesions
In the current study, the tumor volume of two main
metastatic target lesions of 5 CRC patients was analysed in the
lung and liver. For segmentation, open-radART©
(open-radART ion-ORAion Software Suite) was used to draw the
boundaries of the tumor in each CT-slice. This segmentation
produced a visual 3D-image and was used to analyse exact
tumor-volume as described previously (41). Measurements of circulating cell free
DNA were subsequently matched with the results of
tumor-volumetry.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 6.01 (GraphPad Software, Inc.) and SPSS (IBM Corporation).
Correlations were analysed using the Spearmans rank test. ANOVA
tests followed by post hoc Tukey tests were used to compare
multiple groups. P<0.05 was considered to indicate a
statistically significant result. All error bars represent the mean
± standard deviation.
Results
Patient characteristics and DNA
isolation
A total of 15 patients were included in the current
study. A total of 6 patients had metastatic pancreatic cancer, 5
patients had metastatic colorectal cancer and 4 patients had
metastatic breast cancer. The median age at diagnosis was 70 years.
A total of 9 patients (6 patients with PC and 3 patients with CRC)
exhibited synchronous metastatic disease at diagnosis, and 6
patients developed metastasis during subsequent follow up. All
patients had a median of three prior lines of palliative systemic
therapy. The median overall patient survival was 93.1 weeks, from
first diagnosis of metastatic disease for the whole cohort, and
183.3, 84.4 and 22.1 weeks for BC, CRC and PC, respectively.
Patient characteristics are outlined in Table I.
 | Table I.Baseline patient characteristics. |
Table I.
Baseline patient characteristics.
| Characteristic | Number of patients
(n=15) | Percentage of
patients (%) |
|---|
| Sex |
|
Female | 8 | 53.3 |
|
Male | 7 | 46.7 |
| Median age at
diagnosis (years; range) | 70 (47–82) |
|
| Median follow up
(months; range) | 6 (2–8) |
|
| Cancer type |
| Breast
cancer | 4 | 26.6 |
|
Colorectal cancer | 5 | 33.3 |
|
Pancreatic cancer | 6 | 40.1 |
| Primary
metastatic disease | 9 | 60 |
| Median prior lines
of palliative therapy (range) | 3 (1–6) |
|
| Median overall
survival (range; weeks) | 93.1
(15.8–196.9) |
|
| Breast
cancer | 183.3
(149.9–196.2) |
|
|
Colorectal cancer | 84.4
(55.5–187.3) |
|
|
Pancreatic cancer | 22.1
(12.3–63.9) |
|
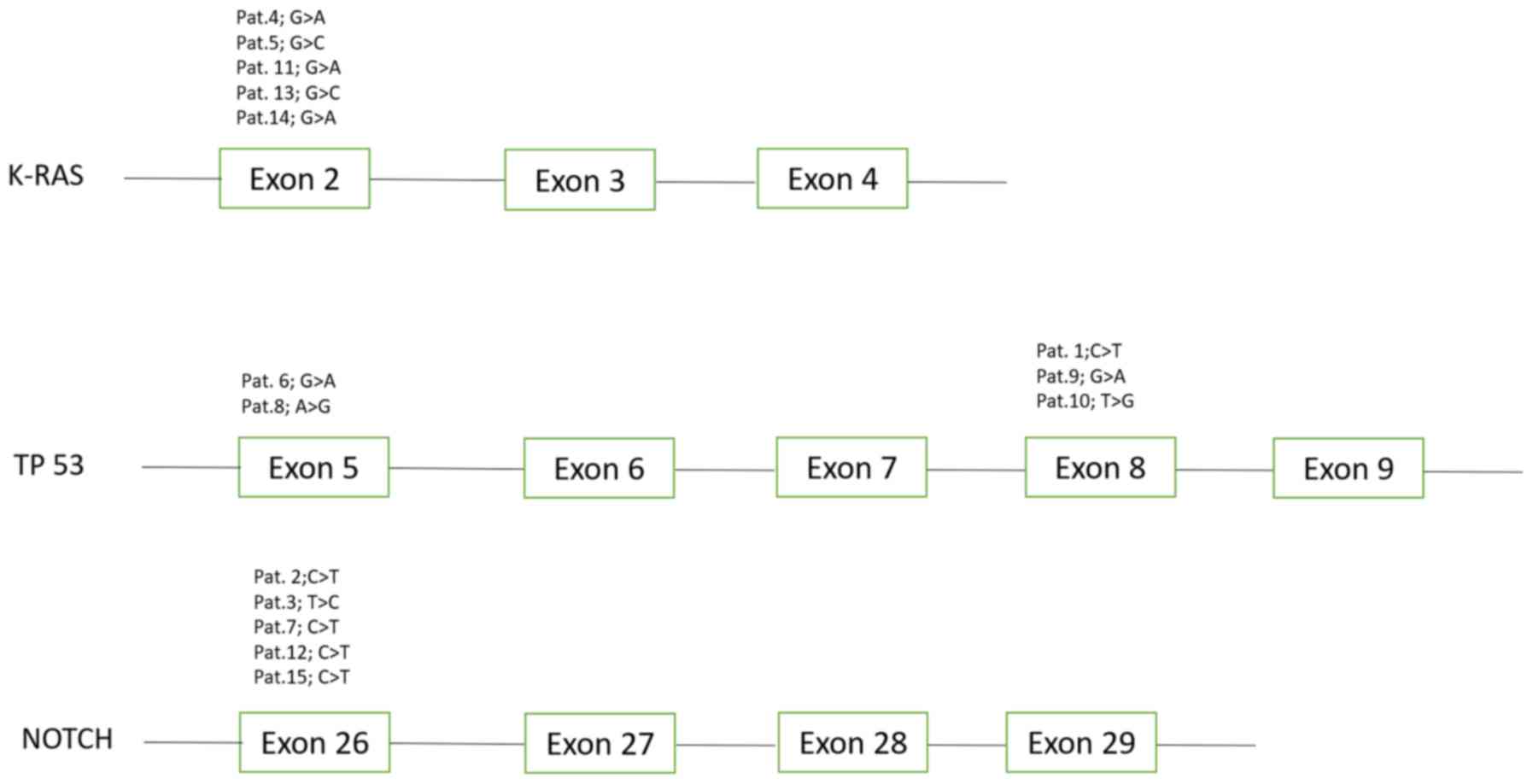
Analysis of KRAS, NRAS, BRAF, Tp53, NOTCH, EGFR,
PTEN and PI3K, revealed one common somatic mutation within Tp53,
KRAS and NOTCH1 in every primary tumor sample. Five distinct
mutations were revealed in KRAS, Tp53 and NOTCH1 (Fig. 1). These mutations were subsequently
detected and quantified, in the respective matched plasma samples,
using targeted re-sequencing. The median baseline plasma
concentration of cfDNA was 340.5 pg/µl (range, 31.8–3160.8 pg/µl).
The median concentration of cftDNA was 180.29 pg/µl (range, 0.011
pg/µl-1754.6 pg/µl).
Correlation of quantitative levels of
cfDNA, cftDNA and established tumor markers
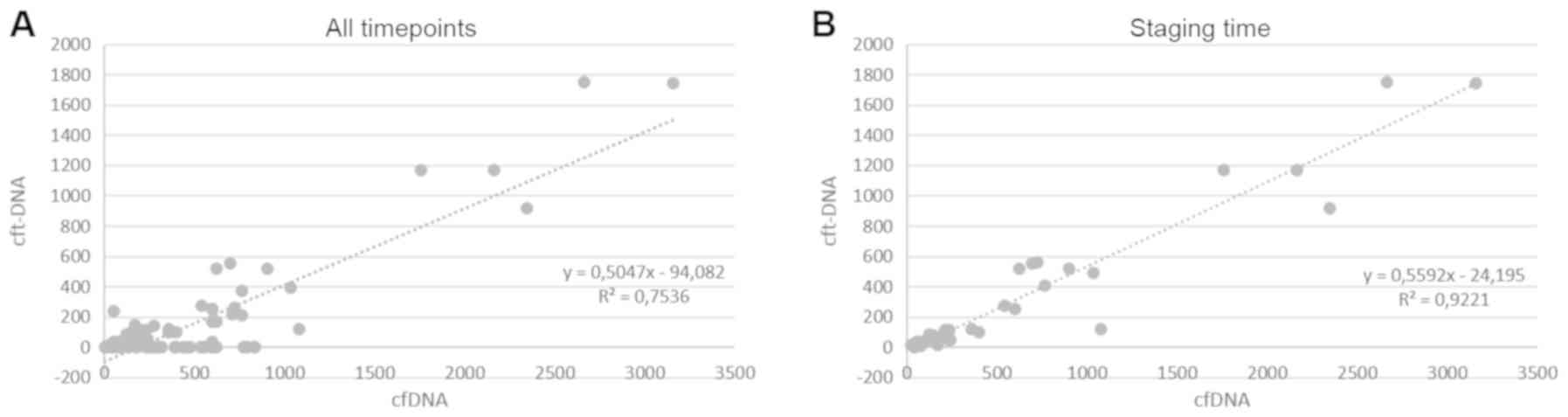
cfDNA and cftDNA concentrations were compared during
the course of treatment. Following analysis of all available plasma
samples (n=108), a modest overall correlation was observed between
the amount of cfDNA and cftDNA over time (Spearman correlation
coefficient 0.7536; P<0.001; Fig.
2A).
The results of the analysis indicated that a large
number of samples did not contain detectable amounts of cftDNA,
while exhibiting small amounts of cfDNA. It was suggested that this
may be due to a discordant expression in samples drawn in the days
following treatment. We therefore focused on samples drawn at
defined staging time-points.
When plasma samples, which were obtained at staging
time-points were analysed, the correlation between cfDNA and cftDNA
was strong (Spearman correlation coefficient 0.9221; P<0.0001;
Fig. 2B). No correlation was
observed between cfDNA and cftDNA in samples drawn in between
staging time-points (Spearman correlation coefficient 0.0325
P=0.2113; Fig. S1). This
correlation was also demonstrated when analysing the BC (Spearman
correlation coefficient 0.9335; P<0.001), PC (0.9158; P=0.002)
and CRC (0.563; P=0.004) subgroups separately (Fig. S2).
Whether the quantity of cfDNA levels of established
biomarkers and if cftDNA correlated with levels of established
biomarkers was assessed according to cancer subtype. In the
colorectal cancer group, a significant correlation was indicated
between cfDNA and CEA (0.8962, P=0.039) and cftDNA and CEA (0.9554;
P<0.001).
In the PC group, a significant correlation was
exhibited between cfDNA and CEA (0.8895; P=0.002; Fig. S3), but no significant correlation
was observed between cftDNA and CEA (0.7235; P=0.074; Fig. S3). No correlation was indicated
between cfDNA or cftDNA with CA 19-9 (P=0.192; P=0.724; Fig. S3).
In the BC group, no correlation was observed between
cfDNA and CA 15-3 (0.2526, P=0.527) or cftDNA and CA15-3 (0.4623;
P=0.702; Fig. S3).
Correlation between cfDNA, cftDNA and
tumor burden
CfDNA and cftDNA concentrations and serum tumor
markers were correlated with volumetric measurements of selected
metastatic target lesions in the liver and the lung of five
patients with metastatic CRC. A significant correlation was
demonstrated between tumor volume in the liver and cfDNA (P=0.016),
and tumor volume in the lung and cfDNA (P=0.003).
The results of cftDNA and tumor volume analysis
revealed a borderline significant correlation between tumor burden
in the liver (P=0.058), and no correlation in the lung
(P=0.383).
The results of the comparison of tumor marker levels
of CA 19-9 and CEA with tumor volume, no correlation was indicated
between tumor burden in the liver (P=0.104 for CA19-9; P=0.873 for
CEA) or the lung (P=0.789 for CA 19-9; P=0.052 for CEA).
cfDNA, cftDNA and clinical
response
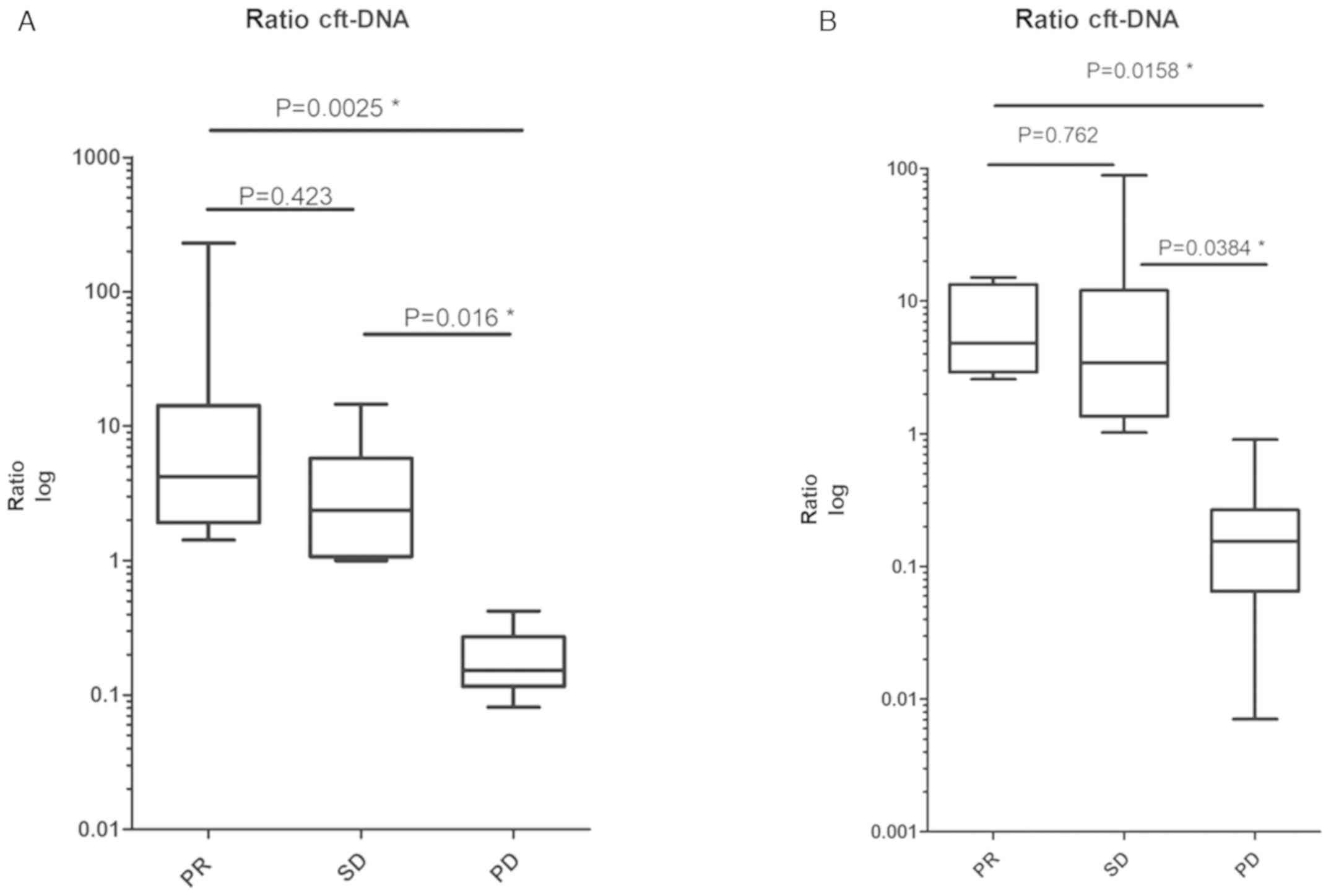
The current study investigated how changes in cfDNA
or cftDNA during treatment correlated with the response to therapy
(assessed via imaging), and how cfDNA and cftDNA performed compared
to currently used clinical biomarkers. Therefore, disease response
assessed by CT imaging [partial response (PR), stable disease (SD)
or progressive disease (PD) according to RECIST] was compared with
concentration changes of cfDNA and cftDNA and tumor markers over
time. The ratio of cf (t) DNA and tumor markers before and at the
time of the respective staging CT was measured (i.e.
cfDNAbefore staging
(pg/µl)/cfDNAstaging(pg/µl)), and this ratio was
correlated with the imaging result. A significant correlation was
exhibited between the response assessed via imaging, and cfDNA and
cftDNA (Fig. 3A). However, no
correlation was observed between imaging results and tumor marker
changes (Fig. 3B).
Subsequently, whether early changes in the ratio of
cfDNA to cftDNA could predict treatment response was assessed. The
ratio of cfDNA to cftDNA at time-points between treatment start and
the first restaging CT were compared. However, the results did not
demonstrate a significant correlation between treatment response
and changes to the cfDNA/cftDNA ratio before imaging (Fig. S4). However, there was a marked
trend, which was not statistically significant.
Discussion
In the current study, the potential role of cfDNA as
a quantitative monitoring tool during cancer therapy in daily
clinical practice was explored, and cfDNA was compared between
imaging techniques and classical tumor markers that are currently
used.
A total of 15 patients with three common tumor
subtypes were assessed. The majority of patients had metastatic
disease at diagnosis and the median OS observed in the respective
cancer subtypes was in line with previously published cohorts
(42–46).
Analyses were performed using mutations in commonly
mutated genes, which were indicated by previously published data
(21,36,47–54). The
results of Sanger sequencing showed the presence of mutations
within KRAS, Tp53 and NOTCH1 in the primary tumor sample. No other
mutations were investigated. The overall concentration of cfDNA in
our cohort was comparable to previously published reports (21,24,55).
A strong correlation was demonstrated between cfDNA
and cftDNA from plasma samples obtained at staging time-points,
compared to the correlation in all plasma samples or samples drawn
in-between staging time-points.
In our practice, restaging time-points were often
scheduled two to three weeks following the last application of
systemic therapy (prior to the next scheduled application).
Therefore, less fluctuations in cfDNA or cftDNA levels at these
time-points were expected, presumably due to less tumor cell
turnover. The data revealed that the time-point of sample
acquisition was important for the interpretation of cfDNA or cftDNA
levels, and should be further standardized in the future.
In contrast, it was observed that the changes in
ratio between cfDNA and cftDNA in-between staging CTs may be able
to predict treatment response. However, due to the small sample
size we were only able to see a trend, which was not statistically
significant and therefore needs further investigation in future
trials.
cfDNA was subsequently compared with tumor markers
in colorectal cancer, and a correlation was indicated between CEA,
but not CA 19-9. These results may be due to CEA being a more
specific tumor marker in CRC than CA 19-9 (56–60).
In the pancreatic cancer group, a correlation was
indicated between cfDNA and CEA, but not CA 19-9. A rise in cfDNA
was observed when patients were examined in more detail, which
correlated with disease progression upon imaging, but was not
reflected by a rise in CA 19-9. Likewise, no correlation was
demonstrated between cfDNA/cftDNA and CA 15-3 in the breast cancer
group. However, a rise in cfDNA/cftDNA correlated with disease
progression upon imaging, but was not reflected by a rise in CA
15-3. These observations support the potentially superior
reflection of tumor dynamics with the use of cfDNA and cftDNA
compared to classical biomarkers.
The results of the comparison of treatment response
upon imaging demonstrated a stronger correlation between clinical
staging and cfDNA and cftDNA than between classical tumor markers,
further highlighting the potential of this new biomarker.
In the current study, tumor volumetric measurements
were also compared during treatment with cfDNA/cftDNA, in
comparison with classical biomarkers. A total of 5 patients with
CRC who all had metastatic disease in the lung or the liver were
assessed. These 5 patients were focused on due to the fact that
volumetry of metastatic lesions can be performed more accurately in
the lung and liver because of the better contrast between tumor and
normal organ tissue. A strong correlation was observed between the
amount of cfDNA and volume of the metastatic lesions. This
correlation could not be demonstrated with classical biomarkers.
cfDNA indicated a stronger correlation with the metastatic tumor
burden than cftDNA. Possible explanations for this observation are
the molecular heterogeneity of the tumor, clonal evolution during
treatment or changes in the genetic background of the tumor, which
were not detected by targeted resequencing.
The sample size of 15 patients in this study is
relatively small, therefore further trials with higher patient
numbers are required to confirm the reported findings. However, the
correlation between cfDNA and cftDNA and the correlation between
clinical staging and cfDNA/cftDNA in our study is significant
despite the small patient number. Patients were selected with three
different tumor types, which allowed investigation across different
disease entities; however, this leads to a certain amount of
heterogeneity of the data. Tumor volumetry was only available for
the CRC group, so conclusions regarding the other two tumor types
could not be made. We were not able to perform a fragment analysis
of cfDNA due to the low concentration in the plasma samples. This
should be implemented in future studies.
Overall, the results of the current study indicated
that cfDNA and cftDNA outperformed currently used biomarkers in
predicting the response to therapy and quantifying tumor burden in
a small patient cohort. Standards for the optimal time-point of
sample acquisition for cfDNA analysis should be defined further in
the future.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Mrs Ulrike Seidl,
Mrs Manuela Glück and Mrs Maria Dreier, (IIIrd Medical Department
with Hematology and Medical Oncology, Oncologic Center, Paracelsus
Medical University Salzburg), for sample collection.
Funding
This work was supported by the Österreichische
Krebshilfe Salzburg.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CH, ML and AE: Data analysis, review and writing.
CH: Molecular analysis. CH, ML, LW and TMel: Data analysis, MM, DA,
RG, GR and DN: Statistical analysis and interpretation, PS and TMei
CT/PET-CT analysis, AE and RG supervised this work and assisted in
preparing the manuscript. All authors have read and approved the
final manuscript. All co-authors provided continuous intellectual
guidance, repeatedly reviewed and edited the manuscript, and gave
the final approval for submission.
Ethics approval and consent to
participate
The current study was approved by the local
institutional research ethics committee (permit no.
415-E/1469/11-2013) and all patients provided written informed
consent prior to blood or tissue sampling.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
cfDNA
|
circulating cell-free DNA
|
|
cftDNA
|
circulating cell-free tumor DNA
|
|
PET
|
Positron Emission Tomography
|
|
FFPE
|
formalin fixed with paraffin
embedded
|
|
CEA
|
Carcinoembryonic antigen
|
|
CA15-3
|
Carcinoma Antigen 15-3
|
|
CA19-9
|
Carcinoma Antigen 19-9
|
References
|
1
|
Ciombor KK, Haraldsdottir S and Goldberg
RM: How can next-generation sequencing (genomics) help us in
treating colorectal cancer? Curr Colorectal Cancer Rep. 10:372–379.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Diaz LA Jr and Bardelli A: Liquid
biopsies: Genotyping circulating tumor DNA. J Clin Oncol.
32:579–586. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi JH, Ahn MJ, Rhim HC, Kim JW, Lee GH,
Lee YY and Kim IS: Comparison of WHO and RECIST criteria for
response in metastatic colorectal carcinoma. Cancer Res Treat.
37:290–293. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Padhani AR and Ollivier L: The RECIST
(Response Evaluation Criteria in Solid Tumors) criteria:
Implications for diagnostic radiologists. Br J Radiol. 74:983–986.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Therasse P, Le Cesne A, Van Glabbeke M,
Verweij J and Judson I; EORTC Soft Tissue and Bone SarcomaGroup, :
RECIST vs. WHO: Prospective comparison of response criteria in an
EORTC phase II clinical trial investigating ET-743 in advanced soft
tissue sarcoma. Eur J Cancer. 41:1426–1430. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Watanabe H, Yamamoto S, Kunitoh H, Sekine
I, Yamamoto N, Ohe Y, Tamura T, Kodama T, Sugimura K and Saijo N:
Tumor response to chemotherapy: The validity and reproducibility of
RECIST guidelines in NSCLC patients. Cancer Sci. 94:1015–1020.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harris L, Fritsche H, Mennel R, Norton L,
Ravdin P, Taube S, Somerfield MR, Hayes DF and Bast RC Jr; American
Society of Clinical Oncology, : American Society of Clinical
Oncology 2007 update of recommendations for the use of tumor
markers in breast cancer. J Clin Oncol. 25:5287–5312. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schwartz LH, Seymour L, Litière S, Ford R,
Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, et
al: RECIST 1.1-Standardisation and disease-specific adaptations:
Perspectives from the RECIST Working Group. Eur J Cancer.
62:138–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arrieta O, Villarreal-Garza C,
Martínez-Barrera L, Morales M, Dorantes-Gallareta Y, Peña-Curiel O,
Contreras-Reyes S, Macedo-Pérez EO and Alatorre-Alexander J:
Usefulness of serum carcinoembryonic antigen (CEA) in evaluating
response to chemotherapy in patients with advanced non small-cell
lung cancer: A prospective cohort study. BMC Cancer. 13:2542013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ballehaninna UK and Chamberlain RS: The
clinical utility of serum CA 19-9 in the diagnosis, prognosis and
management of pancreatic adenocarcinoma: An evidence based
appraisal. J Gastrointest Oncol. 3:105–119. 2012.PubMed/NCBI
|
|
12
|
O'Connell M: PET-CT modification of RECIST
guidelines. J Natl Cancer Inst. 96:801–802; author reply 802. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watanabe S, Nakamura Y, Kariatsumari K,
Nagata T, Sakata R, Zinnouchi S and Date K: Pulmonary
paragonimiasis mimicking lung cancer on FDG-PET imaging. Anticancer
Res. 23:3437–3440. 2003.PubMed/NCBI
|
|
14
|
Solinas C, Porcu M, Hlavata Z, De Silva P,
Puzzoni M, Willard-Gallo K, Scartozzi M and Saba L: Critical
features and challenges associated with imaging in patients
undergoing cancer immunotherapy. Crit Rev Oncol Hematol. 120:13–21.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kidess E and Jeffrey SS: Circulating tumor
cells versus tumor-derived cell-free DNA: Rivals or partners in
cancer care in the era of single-cell analysis? Genome Med.
5:702013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alix-Panabières C and Pantel K: Challenges
in circulating tumour cell research. Nat Rev Cancer. 14:623–631.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dive C and Brady G: SnapShot: Circulating
tumor cells. Cell. 168:742–742.e1. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pantel K and Alix-Panabières C: Liquid
biopsy in 2016: Circulating tumour cells and cell-free DNA in
gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 14:73–74.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
González-Masiá JA, García-Olmo D and
García-Olmo DC: Circulating nucleic acids in plasma and serum
(CNAPS): Applications in oncology. Onco Targets Ther. 6:819–832.
2013.PubMed/NCBI
|
|
20
|
Circulating nucleic acids in plasma/serum
III and serum proteomics. Proceedings of the Third International
Symposium. November 9-12–2003, Santa Monica; California, USA: Ann
NY Acad Sci. 1022. pp. 1–322, 2004.
|
|
21
|
Anker P, Lyautey J, Lederrey C and Stroun
M: Circulating nucleic acids in plasma or serum. Clin Chim Acta.
313:143–146. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anker P, Mulcahy H and Stroun M:
Circulating nucleic acids in plasma and serum as a noninvasive
investigation for cancer: Time for large-scale clinical studies?
Int J Cancer. 103:149–152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Diehl F, Schmidt K, Choti MA, Romans K,
Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA, et al:
Circulating mutant DNA to assess tumor dynamics. Nat Med.
14:985–990. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fleischhacker M and Schmidt B: Free
circulating nucleic acids in plasma and serum (CNAPS)-useful for
the detection of lung cancer patients? Cancer Biomark. 6:211–219.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gahan PB: Circulating nucleic acids in
plasma and serum: Roles in diagnosis and prognosis in diabetes and
cancer. Infect Disord Drug Targets. 8:100–108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rolfo C, Castiglia M, Hong D, Alessandro
R, Mertens I, Baggerman G, Zwaenepoel K, Gil-Bazo I, Passiglia F,
Carreca AP, et al: Liquid biopsies in lung cancer: The new ambrosia
of researchers. Biochim Biophys Acta. 1846:539–546. 2014.PubMed/NCBI
|
|
27
|
Taback B and Hoon DS: Circulating nucleic
acids in plasma and serum: Past, present and future. Curr Opin Mol
Ther. 6:273–278. 2004.PubMed/NCBI
|
|
28
|
Tsang JC and Lo YM: Circulating nucleic
acids in plasma/serum. Pathology. 39:197–207. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yong E: Cancer biomarkers: Written in
blood. Nature. 511:524–526. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cervera Deval J: RECIST and the
radiologist. Radiologia. 56:193–205. 2014.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Krajewski KM, Nishino M, Ramaiya NH and
Choueiri TK: RECIST 1.1 compared with RECIST 1.0 in patients with
advanced renal cell carcinoma receiving vascular endothelial growth
factor-targeted therapy. AJR Am J Roentgenol. 204:W282–W288. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
van Persijn van Meerten EL, Gelderblom H
and Bloem JL: RECIST revised: Implications for the radiologist. A
review article on the modified RECIST guideline. Eur Radiol.
20:1456–1467. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lemoine NR: Molecular advances in
pancreatic cancer. Digestion. 58:550–556. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oden-Gangloff A, Di Fiore F, Bibeau F,
Lamy A, Bougeard G, Charbonnier F, Blanchard F, Tougeron D, Ychou
M, Boissière F, et al: TP53 mutations predict disease control in
metastatic colorectal cancer treated with cetuximab-based
chemotherapy. Br J Cancer. 100:1330–1335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Olivier M, Hollstein M and Hainaut P: TP53
mutations in human cancers: Origins, consequences, and clinical
use. Cold Spring Harb Perspect Biol. 2:a0010082010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shaw JA, Page K, Blighe K, Hava N, Guttery
D, Ward B, Brown J, Ruangpratheep C, Stebbing J, Payne R, et al:
Genomic analysis of circulating cell-free DNA infers breast cancer
dormancy. Genome Res. 22:220–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hufnagl C, Stöcher M, Moik M, Geisberger R
and Greil R: A modified Phenol-chloroform extraction method for
isolating circulating cell free DNA of tumor patients. J Nucleic
Acids Invest. 4:2013. View Article : Google Scholar
|
|
39
|
Paci M, Maramotti S, Bellesia E, Formisano
D, Albertazzi L, Ricchetti T, Ferrari G, Annessi V, Lasagni D,
Carbonelli C, et al: Circulating plasma DNA as diagnostic biomarker
in non-small cell lung cancer. Lung Cancer. 64:92–97. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sirera R, Bremnes RM, Cabrera A,
Jantus-Lewintre E, Sanmartín E, Blasco A, Del Pozo N, Rosell R,
Guijarro R, Galbis J, et al: Circulating DNA is a useful prognostic
factor in patients with advanced non-small cell lung cancer. J
Thorac Oncol. 6:286–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marten K, Auer F, Schmidt S, Rummeny EJ
and Engelke C: Automated CT volumetry of pulmonary metastases: The
effect of a reduced growth threshold and target lesion number on
the reliability of therapy response assessment using RECIST
criteria. Eur Radiol. 17:2561–2571. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ferrero A, Bernad B, Campos J, Perales E,
Velázquez JL and Martínez-Verdú FM: Color characterization of
coatings with diffraction pigments. J Opt Soc Am A Opt Image Sci
Vis. 33:1978–1988. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gordis L and Gold EB: Epidemiology of
pancreatic cancer. World J Surg. 8:808–821. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Maughan NJ and Quirke P: Genomics in
colorectal cancer: Godsend or gimmick? Scand J Gastroenterol.
(Suppl):26–29. 2003. View Article : Google Scholar
|
|
45
|
Michaud DS: Epidemiology of pancreatic
cancer. Minerva Chir. 59:99–111. 2004.PubMed/NCBI
|
|
46
|
Stein RG, Wollschläger D, Kreienberg R,
Janni W, Wischnewsky M, Diessner J, Stüber T, Bartmann C,
Krockenberger M, Wischhusen J, et al: The impact of breast cancer
biological subtyping on tumor size assessment by ultrasound and
mammography-a retrospective multicenter cohort study of 6543
primary breast cancer patients. BMC Cancer. 16:4592016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Anker P, Lefort F, Vasioukhin V, Lyautey
J, Lederrey C, Chen XQ, Stroun M, Mulcahy HE and Farthing MJ: K-ras
mutations are found in DNA extracted from the plasma of patients
with colorectal cancer. Gastroenterology. 112:1114–1120. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Boeck S, Jung A, Laubender RP, Neumann J,
Egg R, Goritschan C, Vehling-Kaiser U, Winkelmann C, Fischer von
Weikersthal L, Clemens MR, et al: EGFR pathway biomarkers in
erlotinib-treated patients with advanced pancreatic cancer:
Translational results from the randomised, crossover phase 3 trial
AIO-PK0104. Br J Cancer. 108:469–476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chappell WH, Steelman LS, Long JM, Kempf
RC, Abrams SL, Franklin RA, Bäsecke J, Stivala F, Donia M, Fagone
P, et al: Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors:
Rationale and importance to inhibiting these pathways in human
health. Oncotarget. 2:135–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
De Luca A, Maiello MR, D'Alessio A,
Pergameno M and Normanno N: The RAS/RAF/MEK/ERK and the PI3K/AKT
signalling pathways: Role in cancer pathogenesis and implications
for therapeutic approaches. Expert Opin Ther Targets. 16 (Suppl
2):S17–S27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Deramaudt T and Rustgi AK: Mutant KRAS in
the initiation of pancreatic cancer. Biochim Biophys Acta.
1756:97–101. 2005.PubMed/NCBI
|
|
52
|
Lui VW, Hedberg ML, Li H, Vangara BS,
Pendleton K, Zeng Y, Lu Y, Zhang Q, Du Y, Gilbert BR, et al:
Frequent mutation of the PI3K pathway in head and neck cancer
defines predictive biomarkers. Cancer Discov. 3:761–769. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rao SS, O'Neil J, Liberator CD, Hardwick
JS, Dai X, Zhang T, Tyminski E, Yuan J, Kohl NE, Richon VM, et al:
Inhibition of NOTCH signaling by gamma secretase inhibitor engages
the RB pathway and elicits cell cycle exit in T-cell acute
lymphoblastic leukemia cells. Cancer Res. 69:3060–3068. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
van Krieken JH, Jung A, Kirchner T,
Carneiro F, Seruca R, Bosman FT, Quirke P, Fléjou JF, Plato Hansen
T, de Hertogh G, et al: KRAS mutation testing for predicting
response to anti-EGFR therapy for colorectal carcinoma: Proposal
for an European quality assurance program. Virchows Arch.
453:417–431. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Heitzer E, Auer M, Ulz P, Geigl JB and
Speicher MR: Circulating tumor cells and DNA as liquid biopsies.
Genome Med. 5:732013. View
Article : Google Scholar : PubMed/NCBI
|
|
56
|
Estakhri R, Ghahramanzade A, Vahedi A and
Nourazarian A: Serum levels of CA15-3, AFP, CA19-9 and CEA tumor
markers in cancer care and treatment of patients with impaired
renal function on hemodialysis. Asian Pac J Cancer Prev.
14:1597–1599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Guo Q, Kang M, Zhang B, Chen Y, Dong X and
Wu Y: Elevated levels of CA 19-9 and CEA in pancreatic
cancer-associated diabetes. J Cancer Res Clin Oncol. 136:1627–1631.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hegele A, Mecklenburg V, Varga Z, Olbert
P, Hofmann R and Barth P: CA19.9 and CEA in transitional cell
carcinoma of the bladder: Serological and immunohistochemical
findings. Anticancer Res. 30:5195–5200. 2010.PubMed/NCBI
|
|
59
|
Ince AT, Yıldız K, Baysal B, Danalıoğlu A,
Kocaman O, Tozlu M, Gangarapu V, Sarbay Kemik A, Uysal Ö and
Şentürk H: Roles of serum and biliary CEA, CA19-9, VEGFR3, and TAC
in differentiating between malignant and benign biliary
obstructions. Turk J Gastroenterol. 25:162–169. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Thomakos N, Rodolakis A, Zagouri F,
Zacharakis D, Sotiropoulou M, Akrivos N, Haidopoulos D,
Papadimitriou CA, Dimopoulos MA and Antsaklis A: Serum CA 125, CA
15-3, CEA, and CA 19-9: A prognostic factor for uterine
carcinosarcomas? Arch Gynecol Obstet. 287:97–102. 2013. View Article : Google Scholar : PubMed/NCBI
|