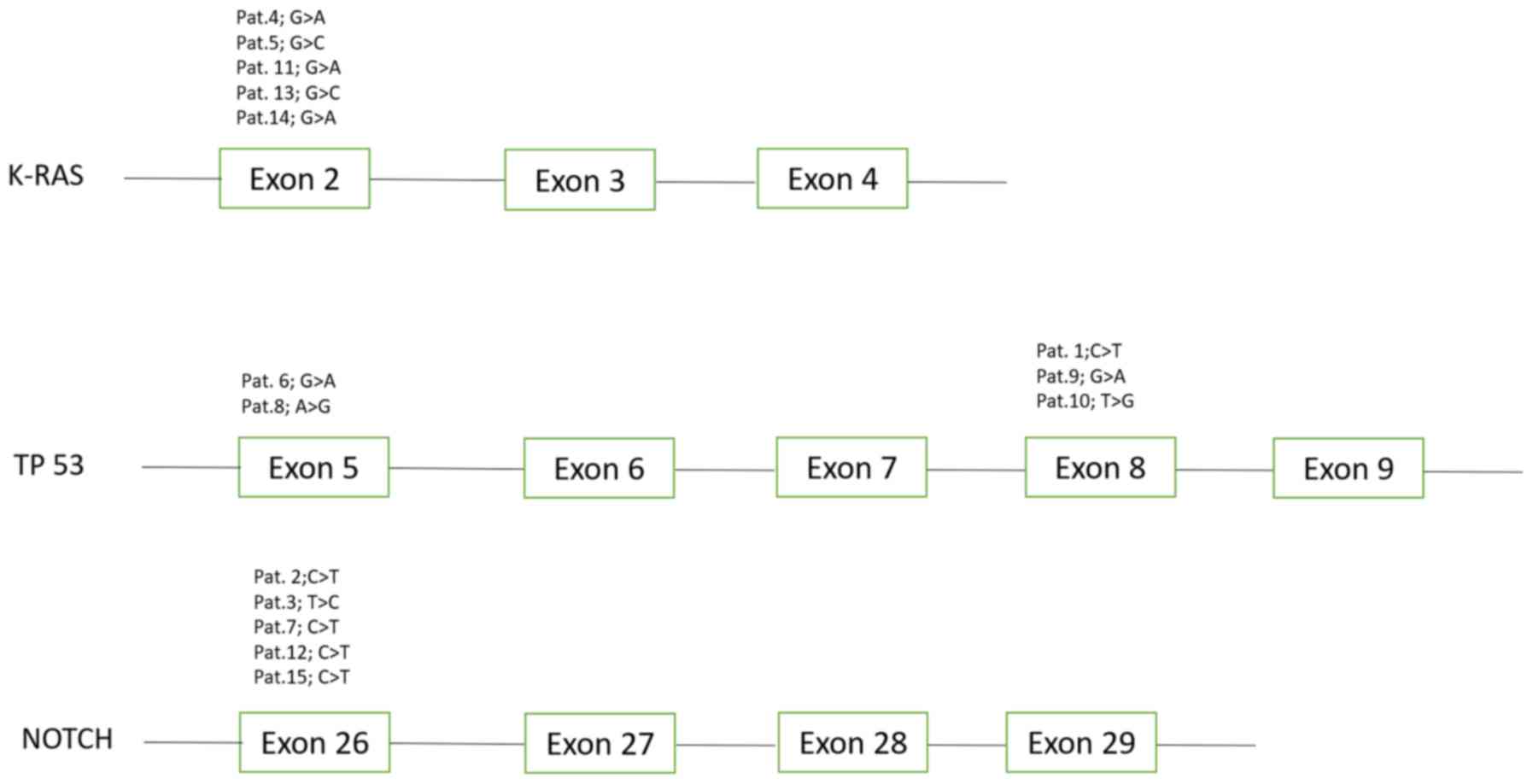
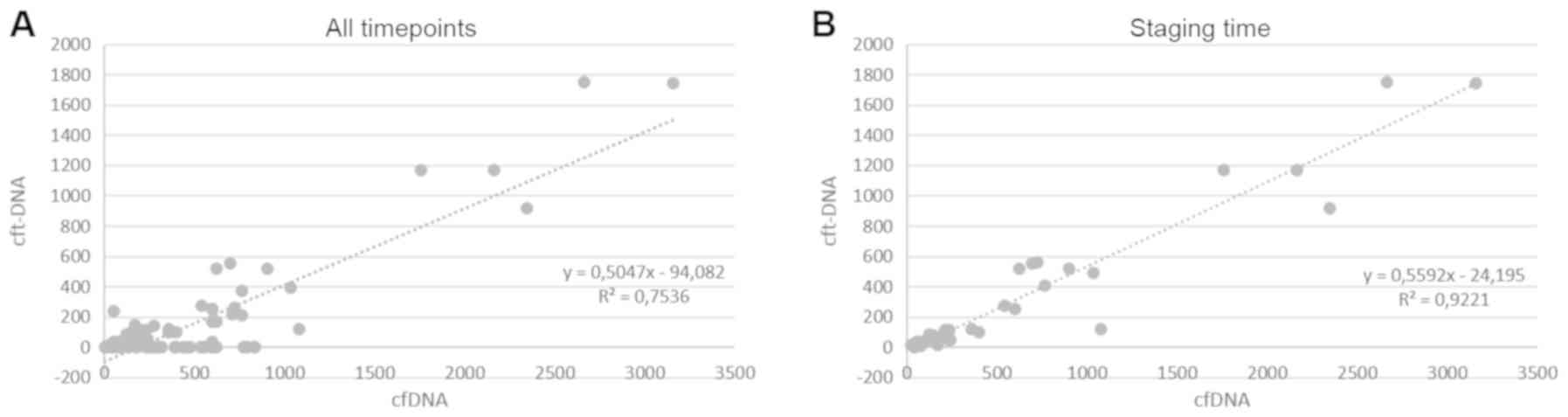
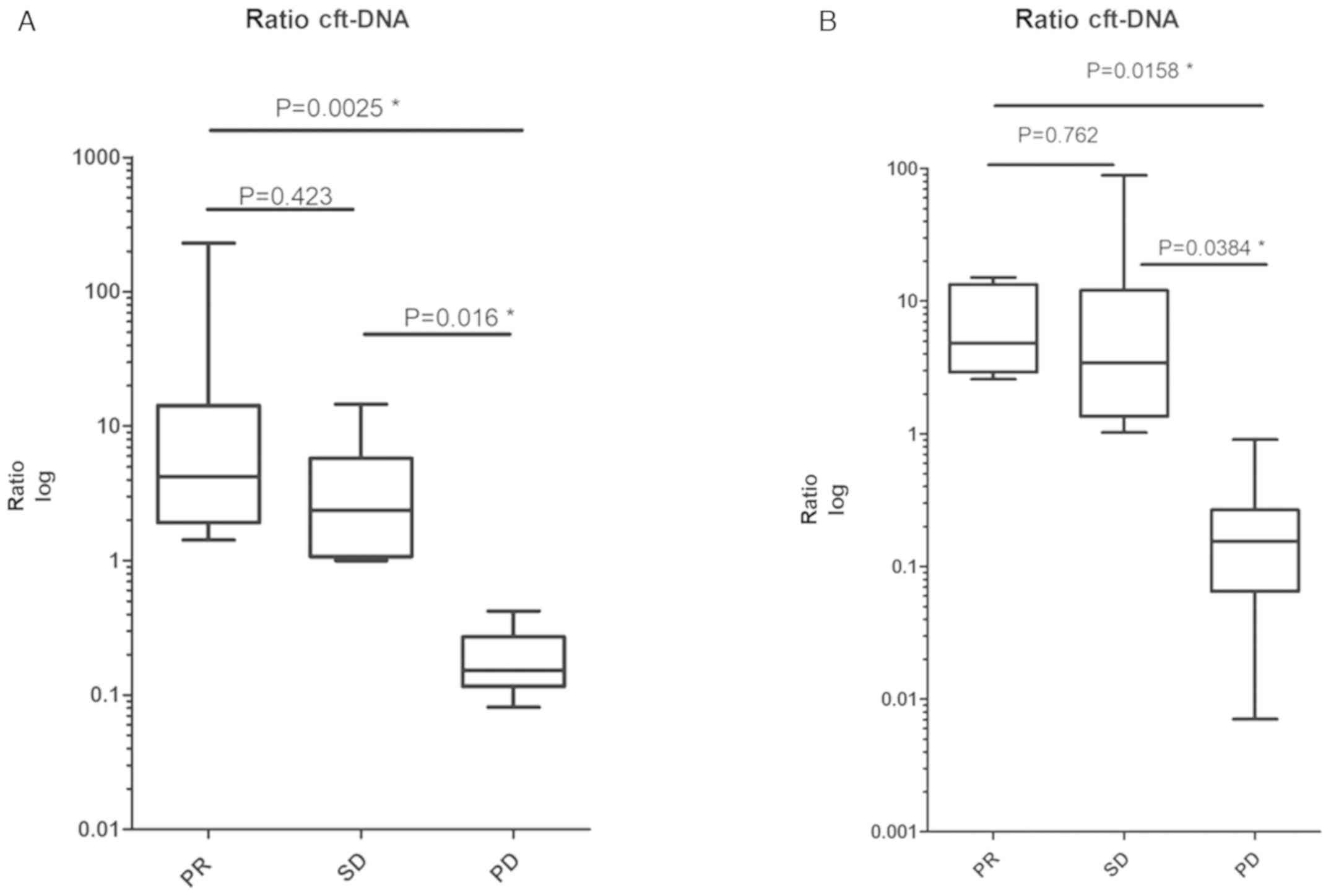
|
1
|
Ciombor KK, Haraldsdottir S and Goldberg
RM: How can next-generation sequencing (genomics) help us in
treating colorectal cancer? Curr Colorectal Cancer Rep. 10:372–379.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Diaz LA Jr and Bardelli A: Liquid
biopsies: Genotyping circulating tumor DNA. J Clin Oncol.
32:579–586. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi JH, Ahn MJ, Rhim HC, Kim JW, Lee GH,
Lee YY and Kim IS: Comparison of WHO and RECIST criteria for
response in metastatic colorectal carcinoma. Cancer Res Treat.
37:290–293. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Padhani AR and Ollivier L: The RECIST
(Response Evaluation Criteria in Solid Tumors) criteria:
Implications for diagnostic radiologists. Br J Radiol. 74:983–986.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Therasse P, Le Cesne A, Van Glabbeke M,
Verweij J and Judson I; EORTC Soft Tissue and Bone SarcomaGroup, :
RECIST vs. WHO: Prospective comparison of response criteria in an
EORTC phase II clinical trial investigating ET-743 in advanced soft
tissue sarcoma. Eur J Cancer. 41:1426–1430. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Watanabe H, Yamamoto S, Kunitoh H, Sekine
I, Yamamoto N, Ohe Y, Tamura T, Kodama T, Sugimura K and Saijo N:
Tumor response to chemotherapy: The validity and reproducibility of
RECIST guidelines in NSCLC patients. Cancer Sci. 94:1015–1020.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harris L, Fritsche H, Mennel R, Norton L,
Ravdin P, Taube S, Somerfield MR, Hayes DF and Bast RC Jr; American
Society of Clinical Oncology, : American Society of Clinical
Oncology 2007 update of recommendations for the use of tumor
markers in breast cancer. J Clin Oncol. 25:5287–5312. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schwartz LH, Seymour L, Litière S, Ford R,
Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, et
al: RECIST 1.1-Standardisation and disease-specific adaptations:
Perspectives from the RECIST Working Group. Eur J Cancer.
62:138–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arrieta O, Villarreal-Garza C,
Martínez-Barrera L, Morales M, Dorantes-Gallareta Y, Peña-Curiel O,
Contreras-Reyes S, Macedo-Pérez EO and Alatorre-Alexander J:
Usefulness of serum carcinoembryonic antigen (CEA) in evaluating
response to chemotherapy in patients with advanced non small-cell
lung cancer: A prospective cohort study. BMC Cancer. 13:2542013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ballehaninna UK and Chamberlain RS: The
clinical utility of serum CA 19-9 in the diagnosis, prognosis and
management of pancreatic adenocarcinoma: An evidence based
appraisal. J Gastrointest Oncol. 3:105–119. 2012.PubMed/NCBI
|
|
12
|
O'Connell M: PET-CT modification of RECIST
guidelines. J Natl Cancer Inst. 96:801–802; author reply 802. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watanabe S, Nakamura Y, Kariatsumari K,
Nagata T, Sakata R, Zinnouchi S and Date K: Pulmonary
paragonimiasis mimicking lung cancer on FDG-PET imaging. Anticancer
Res. 23:3437–3440. 2003.PubMed/NCBI
|
|
14
|
Solinas C, Porcu M, Hlavata Z, De Silva P,
Puzzoni M, Willard-Gallo K, Scartozzi M and Saba L: Critical
features and challenges associated with imaging in patients
undergoing cancer immunotherapy. Crit Rev Oncol Hematol. 120:13–21.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kidess E and Jeffrey SS: Circulating tumor
cells versus tumor-derived cell-free DNA: Rivals or partners in
cancer care in the era of single-cell analysis? Genome Med.
5:702013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alix-Panabières C and Pantel K: Challenges
in circulating tumour cell research. Nat Rev Cancer. 14:623–631.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dive C and Brady G: SnapShot: Circulating
tumor cells. Cell. 168:742–742.e1. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pantel K and Alix-Panabières C: Liquid
biopsy in 2016: Circulating tumour cells and cell-free DNA in
gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 14:73–74.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
González-Masiá JA, García-Olmo D and
García-Olmo DC: Circulating nucleic acids in plasma and serum
(CNAPS): Applications in oncology. Onco Targets Ther. 6:819–832.
2013.PubMed/NCBI
|
|
20
|
Circulating nucleic acids in plasma/serum
III and serum proteomics. Proceedings of the Third International
Symposium. November 9-12–2003, Santa Monica; California, USA: Ann
NY Acad Sci. 1022. pp. 1–322, 2004.
|
|
21
|
Anker P, Lyautey J, Lederrey C and Stroun
M: Circulating nucleic acids in plasma or serum. Clin Chim Acta.
313:143–146. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anker P, Mulcahy H and Stroun M:
Circulating nucleic acids in plasma and serum as a noninvasive
investigation for cancer: Time for large-scale clinical studies?
Int J Cancer. 103:149–152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Diehl F, Schmidt K, Choti MA, Romans K,
Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA, et al:
Circulating mutant DNA to assess tumor dynamics. Nat Med.
14:985–990. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fleischhacker M and Schmidt B: Free
circulating nucleic acids in plasma and serum (CNAPS)-useful for
the detection of lung cancer patients? Cancer Biomark. 6:211–219.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gahan PB: Circulating nucleic acids in
plasma and serum: Roles in diagnosis and prognosis in diabetes and
cancer. Infect Disord Drug Targets. 8:100–108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rolfo C, Castiglia M, Hong D, Alessandro
R, Mertens I, Baggerman G, Zwaenepoel K, Gil-Bazo I, Passiglia F,
Carreca AP, et al: Liquid biopsies in lung cancer: The new ambrosia
of researchers. Biochim Biophys Acta. 1846:539–546. 2014.PubMed/NCBI
|
|
27
|
Taback B and Hoon DS: Circulating nucleic
acids in plasma and serum: Past, present and future. Curr Opin Mol
Ther. 6:273–278. 2004.PubMed/NCBI
|
|
28
|
Tsang JC and Lo YM: Circulating nucleic
acids in plasma/serum. Pathology. 39:197–207. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yong E: Cancer biomarkers: Written in
blood. Nature. 511:524–526. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cervera Deval J: RECIST and the
radiologist. Radiologia. 56:193–205. 2014.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Krajewski KM, Nishino M, Ramaiya NH and
Choueiri TK: RECIST 1.1 compared with RECIST 1.0 in patients with
advanced renal cell carcinoma receiving vascular endothelial growth
factor-targeted therapy. AJR Am J Roentgenol. 204:W282–W288. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
van Persijn van Meerten EL, Gelderblom H
and Bloem JL: RECIST revised: Implications for the radiologist. A
review article on the modified RECIST guideline. Eur Radiol.
20:1456–1467. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lemoine NR: Molecular advances in
pancreatic cancer. Digestion. 58:550–556. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oden-Gangloff A, Di Fiore F, Bibeau F,
Lamy A, Bougeard G, Charbonnier F, Blanchard F, Tougeron D, Ychou
M, Boissière F, et al: TP53 mutations predict disease control in
metastatic colorectal cancer treated with cetuximab-based
chemotherapy. Br J Cancer. 100:1330–1335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Olivier M, Hollstein M and Hainaut P: TP53
mutations in human cancers: Origins, consequences, and clinical
use. Cold Spring Harb Perspect Biol. 2:a0010082010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shaw JA, Page K, Blighe K, Hava N, Guttery
D, Ward B, Brown J, Ruangpratheep C, Stebbing J, Payne R, et al:
Genomic analysis of circulating cell-free DNA infers breast cancer
dormancy. Genome Res. 22:220–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hufnagl C, Stöcher M, Moik M, Geisberger R
and Greil R: A modified Phenol-chloroform extraction method for
isolating circulating cell free DNA of tumor patients. J Nucleic
Acids Invest. 4:2013. View Article : Google Scholar
|
|
39
|
Paci M, Maramotti S, Bellesia E, Formisano
D, Albertazzi L, Ricchetti T, Ferrari G, Annessi V, Lasagni D,
Carbonelli C, et al: Circulating plasma DNA as diagnostic biomarker
in non-small cell lung cancer. Lung Cancer. 64:92–97. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sirera R, Bremnes RM, Cabrera A,
Jantus-Lewintre E, Sanmartín E, Blasco A, Del Pozo N, Rosell R,
Guijarro R, Galbis J, et al: Circulating DNA is a useful prognostic
factor in patients with advanced non-small cell lung cancer. J
Thorac Oncol. 6:286–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marten K, Auer F, Schmidt S, Rummeny EJ
and Engelke C: Automated CT volumetry of pulmonary metastases: The
effect of a reduced growth threshold and target lesion number on
the reliability of therapy response assessment using RECIST
criteria. Eur Radiol. 17:2561–2571. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ferrero A, Bernad B, Campos J, Perales E,
Velázquez JL and Martínez-Verdú FM: Color characterization of
coatings with diffraction pigments. J Opt Soc Am A Opt Image Sci
Vis. 33:1978–1988. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gordis L and Gold EB: Epidemiology of
pancreatic cancer. World J Surg. 8:808–821. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Maughan NJ and Quirke P: Genomics in
colorectal cancer: Godsend or gimmick? Scand J Gastroenterol.
(Suppl):26–29. 2003. View Article : Google Scholar
|
|
45
|
Michaud DS: Epidemiology of pancreatic
cancer. Minerva Chir. 59:99–111. 2004.PubMed/NCBI
|
|
46
|
Stein RG, Wollschläger D, Kreienberg R,
Janni W, Wischnewsky M, Diessner J, Stüber T, Bartmann C,
Krockenberger M, Wischhusen J, et al: The impact of breast cancer
biological subtyping on tumor size assessment by ultrasound and
mammography-a retrospective multicenter cohort study of 6543
primary breast cancer patients. BMC Cancer. 16:4592016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Anker P, Lefort F, Vasioukhin V, Lyautey
J, Lederrey C, Chen XQ, Stroun M, Mulcahy HE and Farthing MJ: K-ras
mutations are found in DNA extracted from the plasma of patients
with colorectal cancer. Gastroenterology. 112:1114–1120. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Boeck S, Jung A, Laubender RP, Neumann J,
Egg R, Goritschan C, Vehling-Kaiser U, Winkelmann C, Fischer von
Weikersthal L, Clemens MR, et al: EGFR pathway biomarkers in
erlotinib-treated patients with advanced pancreatic cancer:
Translational results from the randomised, crossover phase 3 trial
AIO-PK0104. Br J Cancer. 108:469–476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chappell WH, Steelman LS, Long JM, Kempf
RC, Abrams SL, Franklin RA, Bäsecke J, Stivala F, Donia M, Fagone
P, et al: Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors:
Rationale and importance to inhibiting these pathways in human
health. Oncotarget. 2:135–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
De Luca A, Maiello MR, D'Alessio A,
Pergameno M and Normanno N: The RAS/RAF/MEK/ERK and the PI3K/AKT
signalling pathways: Role in cancer pathogenesis and implications
for therapeutic approaches. Expert Opin Ther Targets. 16 (Suppl
2):S17–S27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Deramaudt T and Rustgi AK: Mutant KRAS in
the initiation of pancreatic cancer. Biochim Biophys Acta.
1756:97–101. 2005.PubMed/NCBI
|
|
52
|
Lui VW, Hedberg ML, Li H, Vangara BS,
Pendleton K, Zeng Y, Lu Y, Zhang Q, Du Y, Gilbert BR, et al:
Frequent mutation of the PI3K pathway in head and neck cancer
defines predictive biomarkers. Cancer Discov. 3:761–769. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rao SS, O'Neil J, Liberator CD, Hardwick
JS, Dai X, Zhang T, Tyminski E, Yuan J, Kohl NE, Richon VM, et al:
Inhibition of NOTCH signaling by gamma secretase inhibitor engages
the RB pathway and elicits cell cycle exit in T-cell acute
lymphoblastic leukemia cells. Cancer Res. 69:3060–3068. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
van Krieken JH, Jung A, Kirchner T,
Carneiro F, Seruca R, Bosman FT, Quirke P, Fléjou JF, Plato Hansen
T, de Hertogh G, et al: KRAS mutation testing for predicting
response to anti-EGFR therapy for colorectal carcinoma: Proposal
for an European quality assurance program. Virchows Arch.
453:417–431. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Heitzer E, Auer M, Ulz P, Geigl JB and
Speicher MR: Circulating tumor cells and DNA as liquid biopsies.
Genome Med. 5:732013. View
Article : Google Scholar : PubMed/NCBI
|
|
56
|
Estakhri R, Ghahramanzade A, Vahedi A and
Nourazarian A: Serum levels of CA15-3, AFP, CA19-9 and CEA tumor
markers in cancer care and treatment of patients with impaired
renal function on hemodialysis. Asian Pac J Cancer Prev.
14:1597–1599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Guo Q, Kang M, Zhang B, Chen Y, Dong X and
Wu Y: Elevated levels of CA 19-9 and CEA in pancreatic
cancer-associated diabetes. J Cancer Res Clin Oncol. 136:1627–1631.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hegele A, Mecklenburg V, Varga Z, Olbert
P, Hofmann R and Barth P: CA19.9 and CEA in transitional cell
carcinoma of the bladder: Serological and immunohistochemical
findings. Anticancer Res. 30:5195–5200. 2010.PubMed/NCBI
|
|
59
|
Ince AT, Yıldız K, Baysal B, Danalıoğlu A,
Kocaman O, Tozlu M, Gangarapu V, Sarbay Kemik A, Uysal Ö and
Şentürk H: Roles of serum and biliary CEA, CA19-9, VEGFR3, and TAC
in differentiating between malignant and benign biliary
obstructions. Turk J Gastroenterol. 25:162–169. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Thomakos N, Rodolakis A, Zagouri F,
Zacharakis D, Sotiropoulou M, Akrivos N, Haidopoulos D,
Papadimitriou CA, Dimopoulos MA and Antsaklis A: Serum CA 125, CA
15-3, CEA, and CA 19-9: A prognostic factor for uterine
carcinosarcomas? Arch Gynecol Obstet. 287:97–102. 2013. View Article : Google Scholar : PubMed/NCBI
|