Introduction
Ovarian carcinoma is one of the most frequently
diagnosed gynecological cancers and is also the fourth leading
cause of cancer-associated mortality in females (1). Ovarian carcinoma usually causes a high
mortality rate due to the high prevalence of cancer metastasis by
the time of first diagnosis and the lack of radical treatment for
metastatic tumor (2). In addition,
the postoperative tumor recurrence rate is also high, leading to
the low overall cure rate (3). The
occurrence, development and progression of ovarian carcinoma
requires the involvement of multiple internal and external factors,
such as genetic, reproductive and dietary risk factors (4). However, the molecular mechanism of this
disease remains to be further elucidated (5).
Rho-associated protein kinase 2 (ROCK2) plays
pivotal roles in regulating cytokinesis, smooth muscle contraction
and formation of focal adhesions and actin stress fibers (6). A ROCK2 also participate in a number of
types of human malignancies including ovarian carcinoma (7) and inhibition of ROCK2 may help the
treatment of ovarian carcinoma (8).
ROCK signaling in some cases interacts with long noncoding
(lnc)RNAs to perform their roles (9), which are a group of non-protein-coding
transcripts that have essential roles in cancer development
(10). mi-R4435-2HG promotes lung
cancer (11), while its involvement
in other human cancers is unknown. It was demonstrated that
mi-R4435-2HG promoted proliferation and inhibited apoptosis of
cancer cells in ovarian carcinoma by upregulating ROCK2.
Materials and methods
Patients, specimens and cell line
A total of 63 patients (females) with ovarian
carcinoma were enrolled in the First Affiliated Hospital, School of
Medicine, Zhejiang University (Hangzhou, China) from March 2015 to
January 2018. Inclusion criteria: i) Ovarian carcinoma patients
diagnosed by pathological examinations; ii) Patients with a
complete medical record. Exclusion criteria: i) Other medical
disorders were observed; ii) therapies were performed before this
study. Tumor tissue and adjacent healthy tissue specimens were
obtained from each participant. Age of patients ranged from 38
years to 68 years (51.4±6.3 years). According to the American Joint
Committee on Cancer (AJCC) stage (12), there were 12, 18, 16 and 17 cases at
stage I–IV, respectively. This study passed the review of Ethics
Committee of the aforementioned hospital. All patients signed
informed consent.
Human ovarian carcinoma cell line UWB1.289 from the
American Type Culture Collection (ATCC; Manassas, VA, USA) and
immortalized human ovarian epithelial cell line SV40 from Applied
Biological Materials (Richmond, BC, Canada) were used. Cell culture
medium was 50% ATCC-formulated RPMI-1640 medium (ATCC) and 50%
mammary epithelial growth medium (ATCC) supplemented with 3% of
fetal bovine serum (Sangon, Shanghai, China). Cell culture
conditions were 37°C and 5% CO2.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
To detect the expression of mi-R4435-2HG and ROCK2,
RNAzol reagent (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) was
used to extract total RNA. Applied Biosystems™ High-Capacity cDNA
Reverse Transcription kit was used to perform reverse transcription
(25°C for 5 min, 55°C for 20 min and 75°C for 5 min) and
SYBR® Green Quantitative RT-qPCR kit (Sigma-Aldrich;
Merck KGaA) was used to prepare PCR reaction systems. Reaction
conditions were: 95°C for 1 min, 40 cycles of 95°C for 20 sec and
57°C for 50 sec. Primers of mi-R4435-2HG and ROCK2 as well as
endogenous control GAPDH were designed and synthesized by Shanghai
GenePharma Co., Ltd., (Shanghai, China). StepOnePlus real-time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) was
used to carry out all PCR reaction systems. Primer sequences were:
5′-GTAACCCGTTGAACCCCATT-3′ (forward) and 5′-CCATCCAATCGGTAGTAGCG-3′
(reverse) for 18S rRNA; 5′-GTGTAGGAGAGTCGGCCTTC-3′ (forward) and
5′-TTGGGCTGGGATAGTGTCT-3′ (reverse) for mi-R4435-2HG;
5′-TGAAGGTCGGAGTCAACGGATTTGGT3′ (forward) and
5′-CATGTGGGCCATGAGGTCCACCACforGAPDH; 5′-GTGTCGGCTCCTCTGATCTC-3′
(forward) and 5′-GGCATGTCTGGATGACCTCT-3′ (reverse) for ROCK2. Cq
values were normalized using 2−∆∆Cq method (13).
Cell transfection
UWB1.289 cells were cultivated overnight to reach
70–80% confluence. Vectors expressing mi-R4435-2HG (accession:
NR_015395.2) or ROCK2 as well as empty vectors were designed and
constructed by Sangon Biotech Co., Ltd., (Shanghai, China). ROCK2
small interfering (si)RNA (5′-CAGAAGCGTTGTCTTATGCAA-3′) and
negative control siRNA (5′-UUCUCCGAACGUGUCACGUdTdT-3′) were also
designed and constructed by Sangon Biotech Co., Ltd. Vectors (10
nM) or siRNAs (30 nM) were first mixed with lipofectamine 2000
reagent (Thermo Fisher Scientific, Inc.), followed by incubation
with cells for 5 h. Cells with no transfections were control cells
(C). Negative control (NC) was empty vector or NC siRNA
transfection. Overexpression rates of mi-R4435-2HG and ROCK2 above
200% and ROCK2 knockdown rate below 30% were confirmed by RT-qPCR
before subsequent experiments.
Cell proliferation assay
Cell Counting Kit-8 (CCK-8; Sigma-Aldrich; Merck
KGaA) was used to perform cell proliferation assay. Briefly, cells
were harvested, and single cell suspensions were prepared. Each
well of a 96-well plate was filled with 0.1 ml cell suspension,
followed by incubation at 37°C in a 5% CO2 incubator.
CCK-8 solution (10 µl) was added 24, 48, 72 and 96 h after the
beginning of cell culture. Optical density values at 450 nm were
measured after cell culture for an additional 4 h.
Cell apoptosis assay
Briefly, cells were used to make cell suspensions
with cell density of 3×104 cells/ml. Each well of a
6-well plate was filled with 10 ml cell suspension. Cells were
cultivated at 37°C in a 5% CO2 incubator. After that,
cells were harvested and subjected to 0.25% trypsin digestion,
followed by staining with Annexin V-FITC (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) and propidium iodide for 5 min
in the dark at 4°C. After that, a flow cytometer was used detect
apoptotic cells. Data were processed using FCS Express 6 Flow
Cytometry Software (version 6; De Novo Software, Glendale, CA,
USA).
Western-blotting
To detect the expression of ROCK2 at protein level,
total protein was extracted using RIPA solution (Genepharma) and
protein concentrations were measured using a BCA kit (Genepharma).
After protein denature in boiled water for 5 min, all proteins
samples were subjected to SDS-PAGE (10% gel) electrophoresis (30 µg
per lane), followed by gel transfer to PVDF membrane and blocking
in 5% non-fat milk (2 h at room temperature), membranes were
further incubated with primary antibodies of ROCK2 (1:1,500; rabbit
anti human, cat. no. ab71598; Abcam) and GAPDH (1:1,500; rabbit
anti human; cat. no. ab8245; Abcam) at 4°C overnight, followed by
incubation with goat anti-rabbit IgG-HRP (1:1,200; cat. no.
MBS435036; MyBioSource, Inc., San Diego, CA, USA) at 24°C for 2 h.
Immobilon ECL Ultra Western HRP Substrate (Sigma-Aldrich; Merck
KGaA) was used to develop signals and signals were normalized using
Image J software (version 1.48; National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
All experiments were performed in triplicate and
data were expressed as the mean ± standard deviation. Correlations
between mi-R4435-2HG and ROCK2 mRNA were analyzed by Pearson's
correlation coefficient. Comparisons of expression levels of
mi-R4435-2HG and ROCK2 mRNA between two types of tissues were
performed by a paired t test. Differences among multiple groups
were analyzed by one-way analysis of variance and Tukey test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
mi-R4435-2HG and ROCK2 are upregulated
in ovarian carcinoma
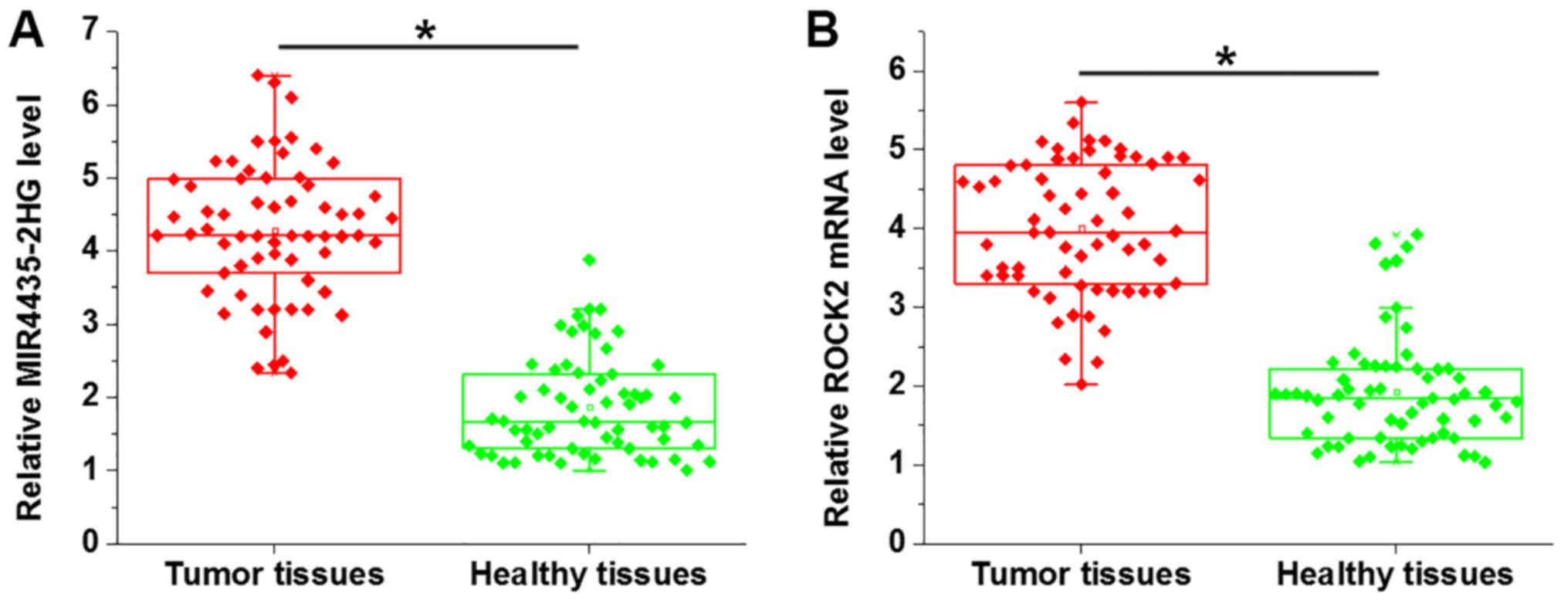
Expression of mi-R4435-2HG and ROCK2 in tumor
tissues and adjacent healthy tissues of ovarian carcinoma patients
were analyzed by RT-qPCR. Compared with healthy tissues,
mi-R4435-2HG (Fig. 1A) and ROCK2
(Fig. 1B) were both significantly
upregulated in tumor tissues (P<0.05).
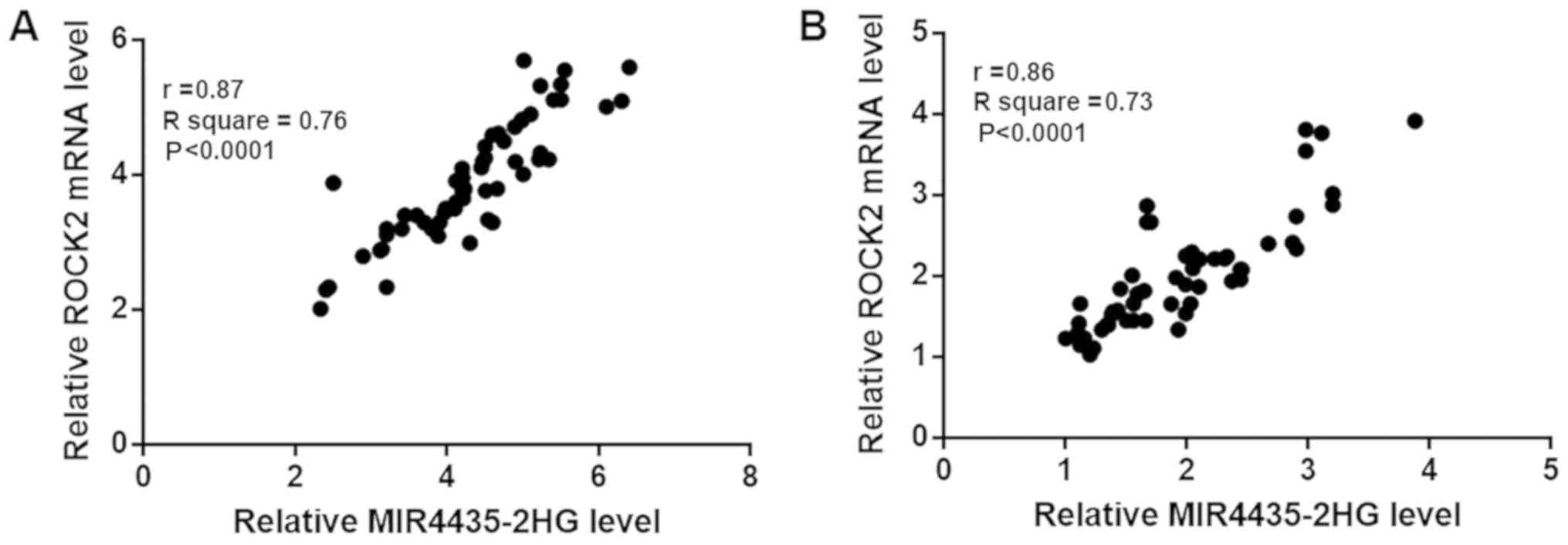
mi-R4435-2HG and ROCK2 are positively
correlated in both tumor and healthy tissues
Correlations between expression levels of
mi-R4435-2HG and ROCK2 mRNA were analyzed by Pearson's correlation
coefficient. As shown in Fig. 2A,
expression levels of mi-R4435-2HG and ROCK2 were significantly and
positively correlated in tumor tissues (P<0.0001; r=0.87). In
addition, expression levels of mi-R4435-2HG and ROCK2 were also
significantly and positively correlated in healthy tissues
(P<0.0001; Fig. 2B; r=0.85).
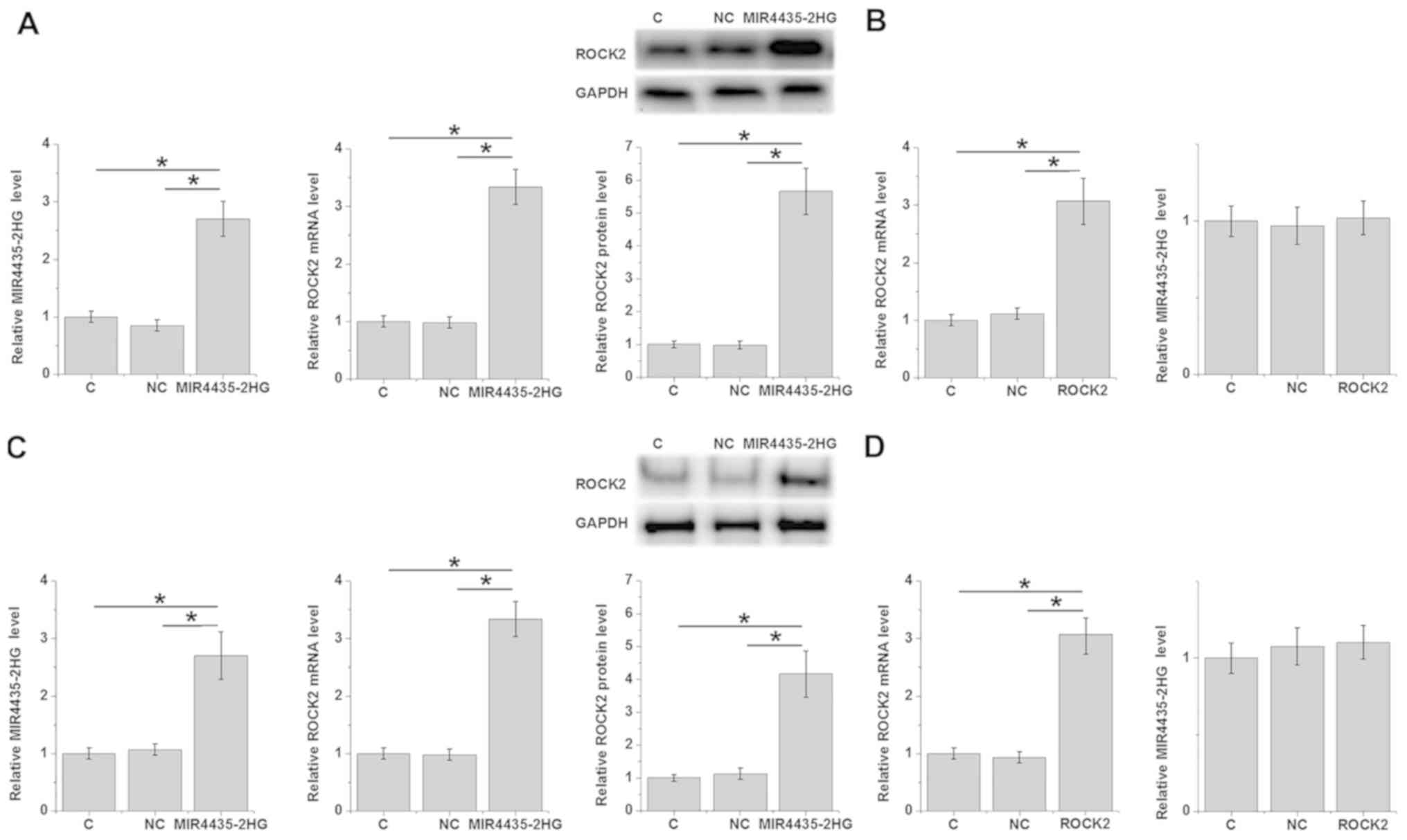
mi-R4435-2HG overexpression mediates
the upregulation of ROCK2
Overexpression of mi-R4435-2HG and ROCK2 in cells of
ovarian carcinoma cell line UWB1.289 were performed to further
explore the possible interactions between mi-R4435-2HG and ROCK2.
Compared with the C and NC groups, mi-R4435-2HG overexpression
mediated the significant upregulation of ROCK2 at both mRNA and
protein levels (P<0.05; Fig. 3A),
while overexpression of ROCK2 showed no significant effect on
expression of mi-R4435-2HG in UWB1.289 cells (Fig. 3B). In SV40 cells, similarly,
mi-R4435-2HG overexpression mediated the significant upregulation
of ROCK2 at both the mRNA and protein level (P<0.05; Fig. 3C), while overexpression of ROCK2
showed no significant effect on the expression of mi-R4435-2HG
(Fig. 3D).
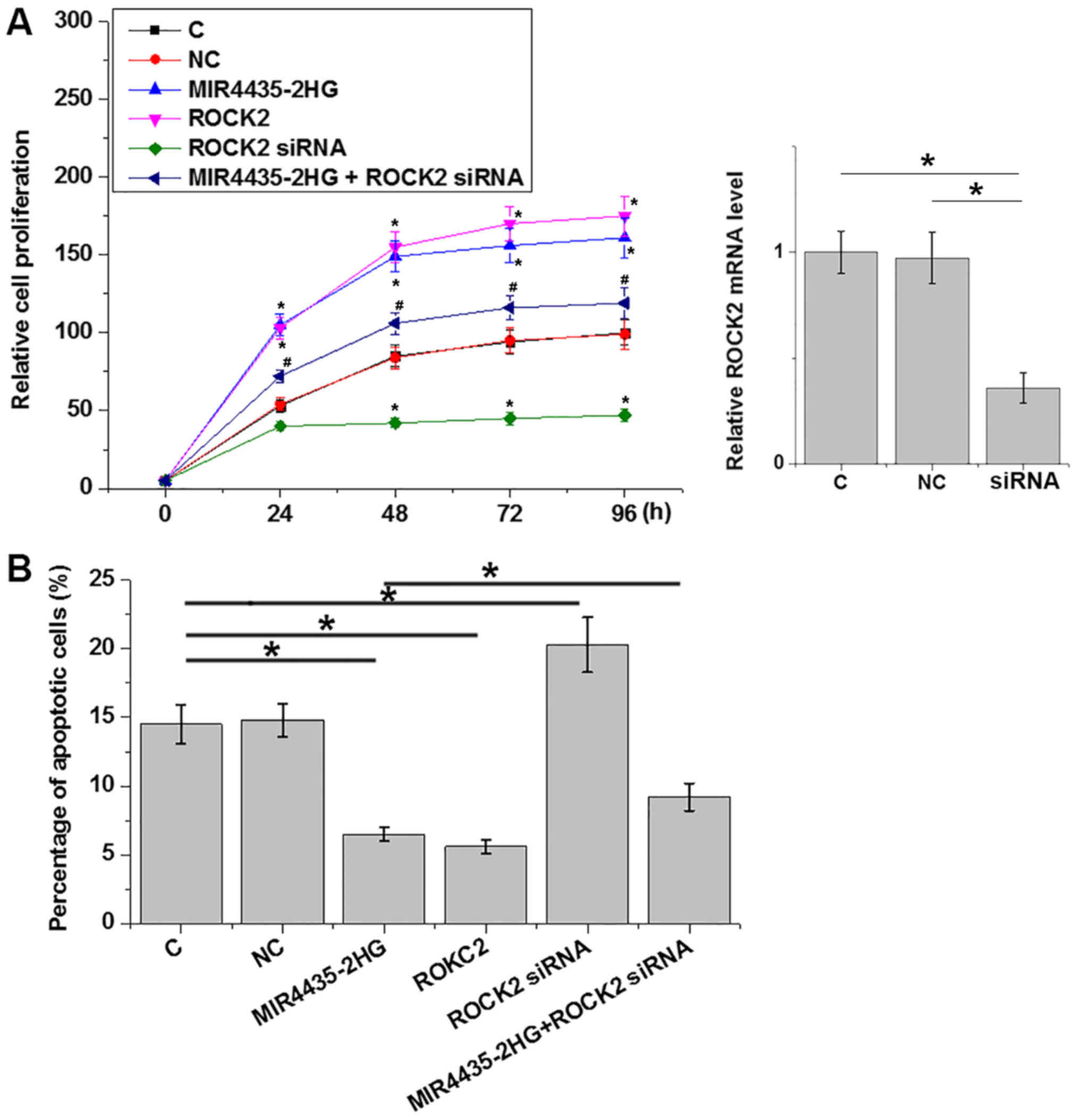
mi-R4435-2HG regulates ovarian
carcinoma cell proliferation and apoptosis through ROCK2
Compared with the C and NC groups, overexpression of
mi-R4435-2HG and ROCK2 led to significantly promoted proliferation
(P<0.05; Fig. 4A) and
significantly inhibited apoptosis (P<0.05; Fig. 4B) of cancer cells, while mi-R4435-2HG
and ROCK2 knockdown played an opposite role. In addition, ROCK2
knockdown significantly attenuated the effects of mi-R4435-2HG
overexpression on cancer cell proliferation and apoptosis
(P<0.05). However, mi-R4435-2HG and ROCK2 overexpression as well
as ROCK2 knockdown failed to affect the behaviors of SV40 cells
(data not shown).
Discussion
The oncogenic function of mi-R4435-2HG has only been
reported in lung cancer, while its roles in other human diseases
are unknown. The present study first reported, to the best of our
knowledge that mi-R4435-2HG was upregulated in ovarian carcinoma
and regulates cancer cell proliferation and apoptosis. The actions
of mi-R4435-2HG in ovarian carcinoma is at least partially achieved
through the interactions with ROCK2.
ROCK2 overexpression is frequently observed during
the development of different types of human cancers (14). It is generally believed that ROCK2
plays an oncogenic role in cancer biology (15). In ovarian carcinoma, ROCK2 promotes
cancer cell proliferation and invasion, and inhibition of ROCK2
inhibits cancer development (8). The
present study also observed upregulated expression of ROCK2 in
ovarian carcinoma tissues compared with in adjacent healthy
tissues. In addition, the regulatory role in cancer cell
proliferation, the present study showed that ROCK2 can also
regulate cancer cell apoptosis. The results of the present study
further confirmed the oncogenic role of ROCK2 in ovarian
carcinoma.
The development and progression of ovarian carcinoma
globally affects the expression of lncRNAs (16). Some lncRNAs, such as lncRNA Meg3 and
PCGEM1 have been proved to be players in the pathogenesis of
ovarian carcinoma (17,18). The present study, to the best of our
knowledge first proved that mi-R4435-2HG plays an oncogenic role in
ovarian carcinoma by promoting cancer cell proliferation and
inhibiting cancer cell apoptosis. The present study also proved
that the regulatory role of mi-R4435-2HG in ovarian carcinoma
cancer cell proliferation and apoptosis is likely achieved through
its role as an upstream activator of ROCK2. However, the mechanism
of the upregulation of ROCK2 by mi-R4435-2HG is still unknown.
mi-R4435-2HG promotes ROCK2 at both the mRNA and protein levels.
Therefore, mi-R4435-2HG may regulate ROCK2 at a transcription
level.
It is worth noting that ROCK2 regulates the invasion
of ovarian carcinoma cell invasion. However, mi-R4435-2HG failed to
significantly affect the migration and invasion of cells of ovarian
carcinoma cell line UWB1.289 (data not shown, revealed by Transwell
migration and invasion assays). This is possibly due to the
specific cell line used in this study. Another explanation is that
mi-R4435-2HG may interact with multiple downstream effectors to
achieve a fine regulation of cancer cell proliferation and
invasion. This hypothesis is supported by the observation that
ROCK2 siRNA silencing only partially attenuated the effects of
mi-R4435-2HG overexpression on cancer cell proliferation and
apoptosis. It is worth noting that only one cancer cell line was
used in present study. Future studies may include more cell lines
to further test the conclusion of this study.
In conclusion, mi-R4435-2HG plays an oncogenic role
in ovarian carcinoma by promoting cancer cell proliferation and
inhibiting cancer cell apoptosis through the upregulation of
ROCK2.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JH designed the experiments. JH, LW, WZ and YH
performed the experiments. ZW and HS collected and analyzed data.
JH drafted the manuscript and all authors approved this
manuscript.
Ethics approval and consent to
participate
The present study passed the review of Ethics
Committee of the First Affiliated Hospital, School of Medicine,
Zhejiang University. All patients provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jelovac D and Armstrong DK: Recent
progress in the diagnosis and treatment of ovarian cancer. CA
Cancer J Clin. 61:183–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruney L, Liu Y, Grisoli A, Ravosa MJ and
Stack MS: Integrin-linked kinase activity modulates the
pro-metastatic behavior of ovarian cancer cells. Oncotarget.
7:21968–21981. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Agarwal R and Kaye SB: Ovarian cancer:
Strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mori M, Harabuchi I, Miyake H, Casagrande
JT, Henderson BE and Ross RK: Reproductive, genetic, and dietary
risk factors for ovarian cancer. Am J Epidemiol. 128:771–777. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McCluggage WG: Morphological subtypes of
ovarian carcinoma: A review with emphasis on new developments and
pathogenesis. Pathology. 43:420–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Trauger JW, Lin FF, Turner MS, Stephens J
and LoGrasso PV: Kinetic mechanism for human Rho-Kinase II
(ROCK-II). Biochemistry. 41:8948–8953. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhong Y, Yang S, Wang W, Wei P, He S, Ma
H, Yang J, Wang Q, Cao L, Xiong W, et al: The interaction of
Lin28A/Rho associated coiled-coil containing protein kinase 2
accelerates the malignancy of ovarian cancer. Oncogene.
38:1381–1397. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Li J, Xu C and Zhang X:
MicroRNA-139-5p inhibits cell proliferation and invasion by
targeting RHO-associated coiled-coil-containing protein kinase 2 in
ovarian cancer. Oncol Res. 26:411–420. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang Y, He Y, Zhang P, Wang J, Fan C, Yang
L, Xiong F, Zhang S, Gong Z, Nie S, et al: LncRNAs regulate the
cytoskeleton and related Rho/ROCK signaling in cancer metastasis.
Mol Cancer. 17:772018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qian H, Chen L, Huang J, Wang X, Ma S, Cui
F, Luo L, Ling L, Luo K and Zheng G: The lncRNA mi-R4435-2HG
promotes lung cancer progression by activating β-catenin
signalling. J Mol Med (Berl). 96:753–764. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hagemann IS, Cole LL, Cosin JA, Gress DM,
Mutch DG and Olawaiye AB: Controversies in Gynecologic Cancer
Staging: An AJCC Cancer Staging Manual, Perspective. AJSP.
23:118–128. 2018.
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using quantitative PCR and the
2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li M, Ke J, Wang Q, Qian H, Yang L, Zhang
X, Xiao J, Ding H, Shan X, Liu Q, et al: Upregulation of ROCK2 in
gastric cancer cell promotes tumor cell proliferation, metastasis
and invasion. Clin Exp Med. 17:519–529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei L, Surma M, Shi S, Lambert-Cheatham N
and Shi J: Novel insights into the roles of Rho kinase in cancer.
Arch Immunol Ther Exp (Warsz). 64:259–278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lou Y, Jiang H, Cui Z, Wang X, Wang L and
Han Y: Gene microarray analysis of lncRNA and mRNA expression
profiles in patients with highgrade ovarian serous cancer. Int J
Mol Med. 42:91–104. 2018.PubMed/NCBI
|
|
17
|
Xiu YL, Sun KX, Chen X, Chen S, Zhao Y,
Guo QG and Zong ZH: Upregulation of the lncRNA Meg3 induces
autophagy to inhibit tumorigenesis and progression of epithelial
ovarian carcinoma by regulating activity of ATG3. Oncotarget.
8:31714–31725. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen S, Wang LL, Sun KX, Liu Y, Guan X,
Zong ZH and Zhao Y: LncRNA PCGEM1 induces ovarian carcinoma
tumorigenesis and progression through RhoA pathway. Cell Physiol
Biochem. 47:1578–1588. 2018. View Article : Google Scholar : PubMed/NCBI
|