Introduction
Nasopharyngeal carcinoma (NPC) is a subtype of head
and neck malignancy that develops from the lining of the
nasopharynx (1). The incidence of
NPC varies a lot across different regions of the world, with a high
prevalence in Southeast Asia, North Africa and Southern China
(2). Patients with NPC are prone to
develop neck invasion and distant tumor metastases (3,4).
Although radiotherapy and chemotherapy have been used to treat
advanced NPC, effective therapeutic options remain limited
(5). Numerous risk factors,
including Epstein-Barr virus infections, have been identified as
risk factors of NPC (6); however,
the pathogenesis of NPC remains unclear, which limits the
development of novel therapeutic approaches. The 5-year survival
rate of patients with advanced NPC was <50% in 2016 worldwide
(1,2).
Phosphatase and tensin homolog (PTEN) is a tumor
suppressor that has been validated in the majority of mammals
(7). Mutations in the PTEN gene are
associated with numerous types of cancer, such as endometrial
cancer (7). PTEN regulates the cell
cycle and inhibits the accelerated proliferation of cancer cells by
inhibiting the PI3K/AKT survival pathway (8,9).
Activation of PTEN may therefore be a promising approach for cancer
prevention and treatment (10). It
has been reported that PTEN signaling in cancer is regulated by
long non-coding (lnc)RNAs (11),
which are defined as functional RNA transcripts >200 nucleotides
in length with no protein-coding capacity (12). LncRNAs participate in cancer biology
by regulating the expression of oncogenes or tumor suppressors to
affect cancer cell behaviors, such as proliferation and apoptosis
(12). A recent study by Zhang et
al (13) reported that the novel
oncogenic lncRNA, NR2F2-AS1, interacts with the microRNA (miR)
miR-320b by serving as its endogenous sponge in lung cancer.
Furthermore, miR-320b and PTEN can upregulate each other, affecting
cancer biology (14). The present
study therefore aimed to investigate whether NR2F2-AS1 could
interact with PTEN.
Materials and methods
Patients with NPC and follow-up
In the present study, 58 patients with NPC (22 men
and 18 women; age range, 32–66 years; mean age, 44±12.3 years) were
selected among the 101 patients with NPC who were admitted at the
Dongying Shengli Hospital between June 2010 and November 2013. Only
patients newly diagnosed with NPC and who completed the 5-year
follow-up (via phone call every month) following admission were
included. The exclusion criteria were as follows: i) Patients
diagnosed with clinical disorders other than NPC; ii) patients with
recurrent NPC; iii) patients who had started receiving therapy for
any clinical disorders; iv) patients who died of causes unrelated
to NPC during follow-up; and vi) patients with undetectable PTEN in
tumor tissues. Based on the clinical data, 10, 12, 18 and 18
patients had NPC at clinical stages I, II, III and IV,
respectively, according to the eighth edition of the American Joint
Committee on Cancer staging system (15). This study was approved by the Ethics
Committee of Dongying Shengli Hospital, and signed informed consent
was provided by all patients.
NPC tissues and cells
Patients were diagnosed by histopathological
analysis of tumor biopsy. During the biopsy, non-tumor tissues (2
cm adjacent to tumors) and NPC tissues (0.013–0.019 g per sample)
were collected from each patient with NPC. Samples were stored at
−80°C before use. All tissues were histopathologically confirmed.
The two NPC cell lines C666-1 and 13-9B (SHUNRAN) cells were used.
Cells were cultured in RPMI-1640 medium (Sigma-Aldrich; Merck KGaA)
supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich; Merck
KGaA) and placed at 37°C in a humidified incubator containing 5%
CO2.
Transient transfections
Negative control small interfering (si)RNA
(5′-AUUGUCGUACGUAGCUACGUA-3′), NR2F2-AS1 siRNA
(5′-GCUUCUCUCUUGAUUACAUUG-3′) and PTEN siRNA
(5′-AAAUCUAGGGCAUCUUGUGCC-3′) were provided by Sangon Biotech Co.,
Ltd. pcDNA3 vector expression PTEN and empty pcDNA3 vector were
provided by GenePharma Co. Ltd. C666-1 and 13-9B cells were
harvested when confluence reached 70–80%. A total of
1×105 cells were transfected with 10 nM negative control
siRNA (negative control, NC), 10 nM NR2F2-AS1 siRNA or PTEN siRNA,
12 nM pcDNA3 vector expression PTEN or 12 nM empty pcDNA3 vector
using Lipofectamine® 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). Untransfected cells were used as a
control (C) as well as cells transfected with the empty vector
(NC). Cells were transfected for 24 h before performing subsequent
experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
NPC and non-tumor frozen tissues were powdered by
grinding in liquid nitrogen. TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) was mixed with tissue
powder (0.5 ml per 0.01 g tissue) or C666-1 and 13-9B cells (0.5 ml
per 1×105 cells) to extract total RNAs. All RNA samples
were digested with DNase I to remove genomic DNA. cDNA was
generated using QuantiTect Reverse Transcription kit (Qiagen China
Co., Ltd.) according to the manufacturer's protocol. QuantiFast
SYBR Green PCR kit (Qiagen China Co., Ltd.) was used to prepare all
PCR mixtures, and 18S rRNA was used as an endogenous control. PCR
mixture (20 µl) contained 0.5 nM of each primer and 1 µl template
DNA. The thermocycling conditions of the RT-qPCR were as follows:
95°C for 30 sec; followed by 40 cycles of 95°C for 10 sec and 58°C
for 45 sec. Each sample was analyzed at least three times. The
relative expressions levels were normalized to endogenous control
and expression was calculated using the 2−ΔΔCq method
(16). The sample with the lowest
expression level was set to a value of ‘1’ and all other samples
were normalized to this sample. The sequences of the primers were
as follows: NR2F2-AS1 forward, 5′-TCAGCCGGAAAACTACAAGCTC-3′ and
reverse, 5′-CTTCGTGTAGCTGTTCCACC-3′; 18s forward,
5′-GTAACCCGTTGAACCCCATT-3′ and reverse, 5′-CCATCCAATCGGTAGTAGCG-3′;
and PTEN forward, 5′-TTGGCGGTGTCATAATGTC-3′ and reverse,
5′-CAGAAAGACTTGAAGGCGTA-3′.
Cell proliferation assay
At 24 h post-transfection, C666-1 and 13-9B cells
were harvested using 0.25% trypsin. A total of 4×104
cells were mixed with 1 ml RPMI-1640 medium containing 10% FBS to
prepare single-cell suspensions. Cells were seeded in 96-well
plates (0.1 ml per well) and cultured at 37°C and 5%
CO2. Subsequently, to detect cell proliferation, Cell
Counting Kit-8 solution (10 µl; Dojindo Molecular Technologies,
Inc.) was added in each well at 4 h before cell collection. Cells
were collected every 24 h until 96 h. Absorbance was measured at
450 nm using a microplate reader.
Cell apoptosis assay
At 24 h post-transfection, C666-1 and 13-9B cells
were harvested using 0.25% trypsin. A total of 4×104
cells were mixed with 1 ml serum-free RPMI-1640 medium to prepare
single-cell suspensions. Cells were seeded in 6-well plates (2 ml
per well) and cultured at 37°C and 5% CO2 for 48 h.
Cells were then harvested with 0.25% trypsin, mixed with fresh cell
culture medium and incubated with propidium iodide (cat. no.
AD01-02; Dojindo Molecular Technologies, Inc.) and annexin
V-fluorescein isothiocyanate (cat. no. AD02-05; Dojindo Molecular
Technologies, Inc.) at 4°C for 20 min in the dark. All steps were
performed according to manufacturer's instructions. Apoptotic cells
were subsequently analyzed using a flow cytometer using BD
FACSCalibur Flow Cytometer (BD Biosciences). Data were analyzed by
CytExpert v.2.3 flow cytometry software (Beckman Coulter,
Inc.).
Western blotting
At 24 h post-transfection, C666-1 and 13-9B cells
were harvested, and 1×105 cells were mixed with 1 ml
RIPA solution (Sangon Biotech Co., Ltd.) to extract total proteins.
The BCA method (Sigma-Aldrich; Merck KGaA) was used to measure
protein concentration. Proteins were incubated with sample buffer
and boiled for 5 min to denature the proteins. Protein samples (30
µg per lane) were subsequently loaded on a 12% gel, resolved using
SDS-PAGE and transferred onto PVDF membranes. Membranes were
blocked with PBS containing 5% skimmed milk at 22°C for 2 h.
Membranes were incubated with rabbit polyclonal primary antibodies
against PTEN (1:1,200; cat. no. ab31392; Abcam) or GAPDH antibody
(1:900; cat. no. ab9485; Abcam) at 4°C overnight, and subsequently
with horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin G secondary antibody (1:1,200; cat. no. MBS435036;
MyBioSource, Inc.) at 22°C for 2 h. Enhanced chemiluminescence
reagent (Sigma-Aldrich; Merck KGaA) was used to detect the signal
on the membrane. Densitometry analysis was performed using Image J
version 1.46 (National Institutes of Health). GAPDH was used as the
loading control.
Statistical analysis
Each experiment in this study was performed three
times. All data are expressed as the mean values ± SEM. GraphPad
Prism 6 (GraphPad Software, Inc.) was used for all data analysis.
Differences between non-tumor and NPC tissues were compared using a
Student's paired t-test. Differences in >3 groups were compared
using an ANOVA followed by a post hoc Tukey's test. Linear
regression was used for association analysis. The 58 patients with
NPC were grouped into high (n=27) and low (n=31) groups according
to NR2F2-AS1 expression levels in NPC tissues, and into high (n=28)
and low (n=30) according to PTEN expression levels in NPC tissues,
according to the Youden's index of receiver operating
characteristic curve. Survival curves were plotted using the
Kaplan-Meier method and compared by log-rank test. P<0.05 was
considered to indicate a statistically significant difference.
Results
NR2F2-AS1 and PTEN expression are
negatively associated with each other in NPC tissues
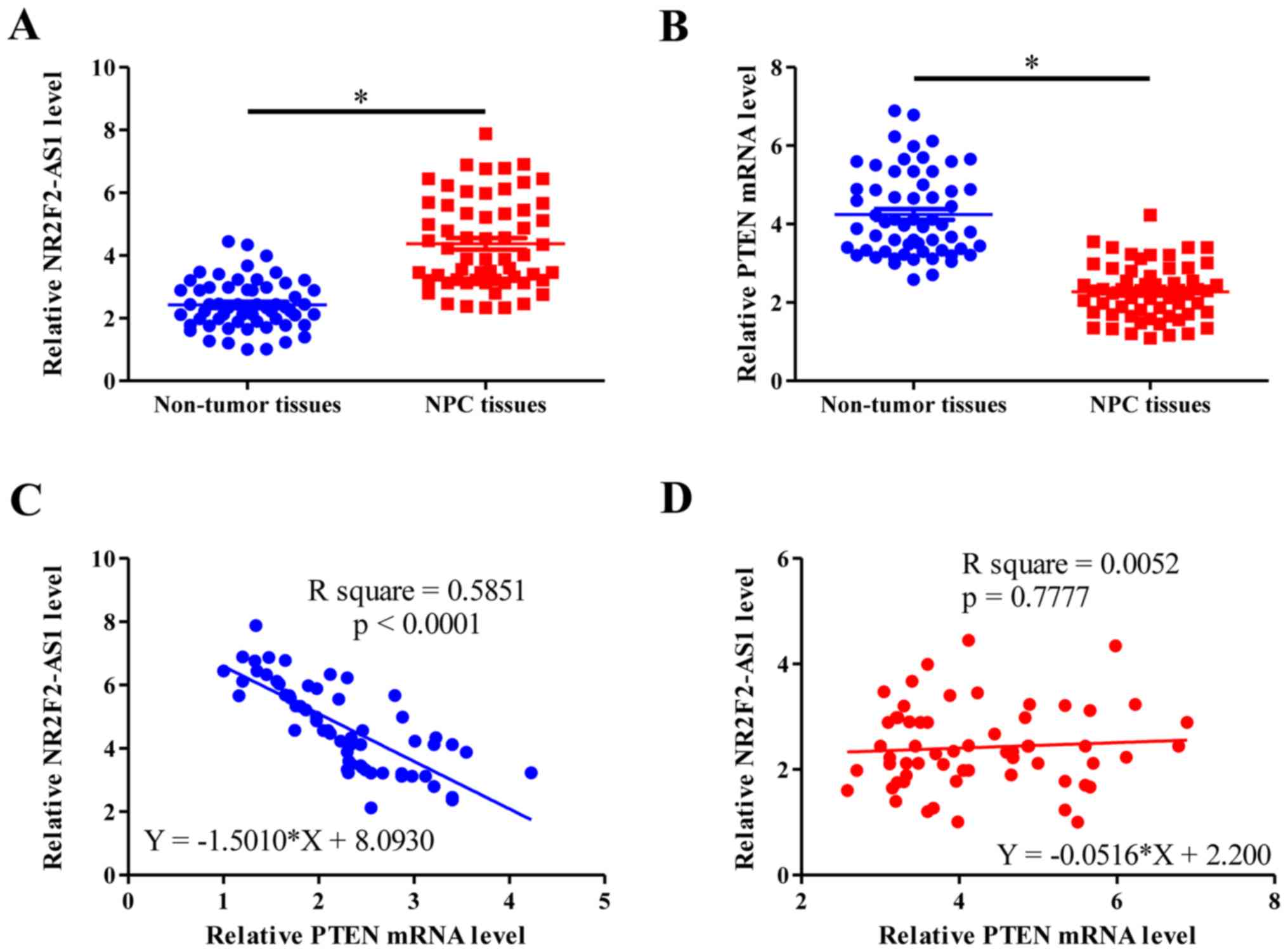
RT-qPCR was performed to measure the expression
levels of NR2F2-AS1 and PTEN in NPC and non-tumor tissues. Results
were compared using a paired t-test. NPC tissues with undetectable
PTEN expression were not included in this study. The results
demonstrated that NR2F2-AS1 and PTEN expression levels were
significantly higher (Fig. 1A) and
lower (Fig. 1B), respectively, in
NPC tissues compared with non-tumor tissues (P<0.05). Linear
regression was performed to analyze the association between
NR2F2-AS1 and PTEN. The results demonstrated that NR2F2-AS1 and
PTEN expression levels were significantly negatively associated in
NPC tissues (P<0.0001; Fig. 1C),
which was not the case in non-tumor tissues (P=0.7777; Fig. 1D).
NR2F2-AS1 and PTEN expression levels
can predict survival in patients with NPC
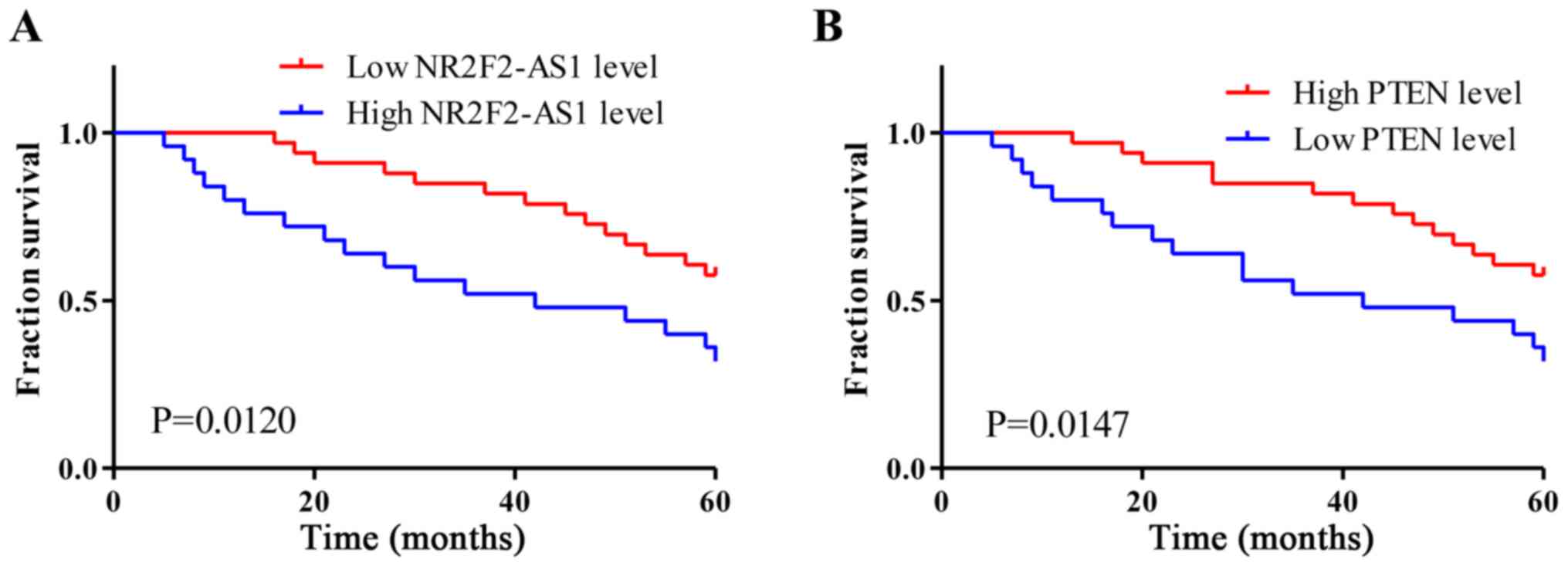
Survival curves were plotted using the Kaplan-Meier
method and compared using a log-rank test. As presented in Fig. 2A, patients with high NR2F2-AS1
expression levels had a significantly lower 5-year survival rate
compared with patients in the low NR2F2-AS1 expression level group
(P=0.0120). Conversely, the overall survival rate of patients in
the high PTEN expression level group was significantly higher
compared with patients in the low PTEN expression level group
(P=0.0147; Fig. 2B).
NR2F2-AS1 siRNA silencing leads to
PTEN upregulation in NPC cells
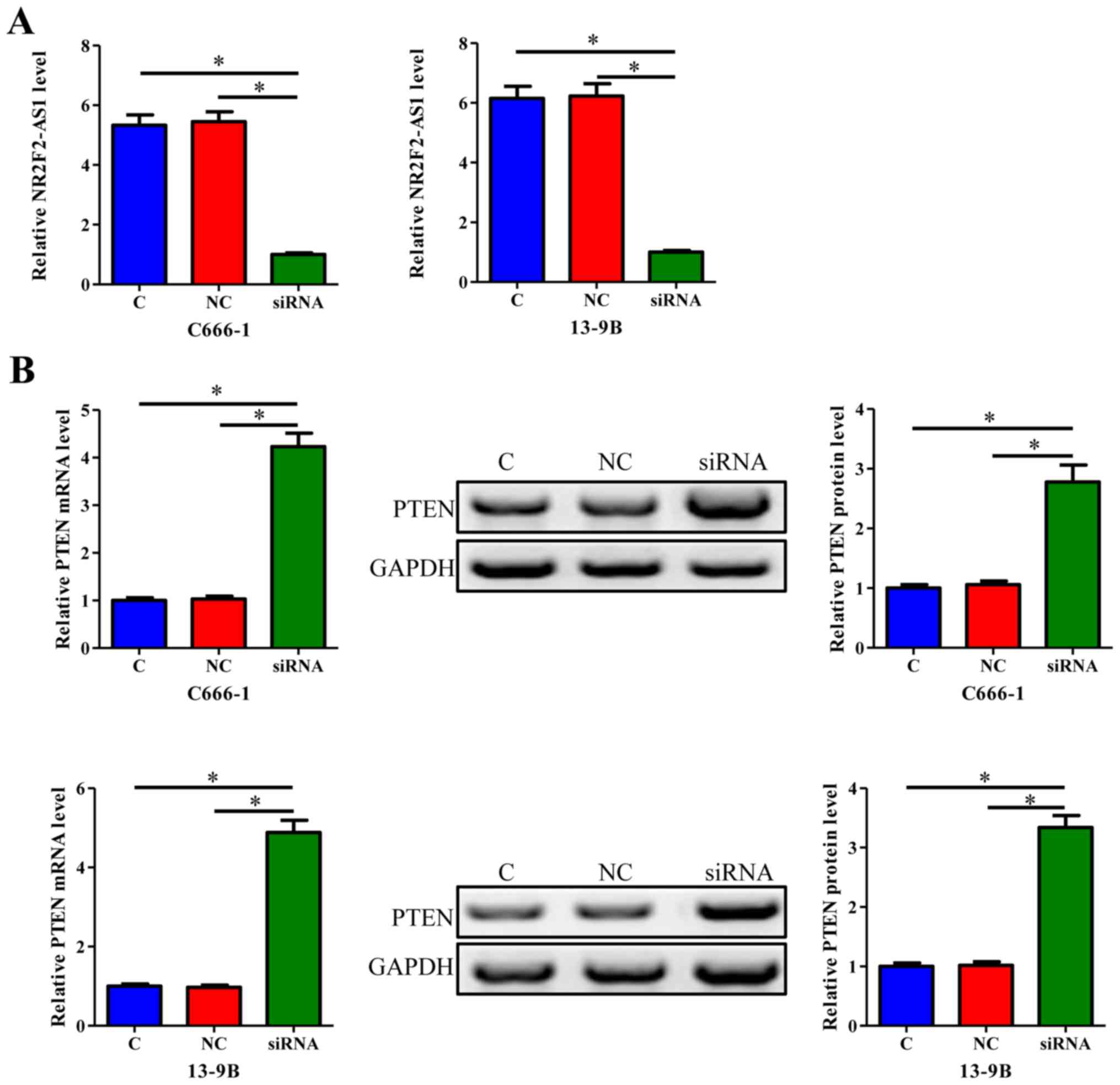
C666-1 and 13-9B cells were transfected with
NR2F2-AS1 siRNA. Compared with C and NC (negative control siRNA
transfection) groups, expression levels of NR2F2-AS1 were
significantly reduced 24 h post-transfection (Fig. 3A; P<0.05). Furthermore, cell
transfection with NR2F2-AS1 siRNA induced a significant increase in
PTEN expression at the mRNA and protein levels (Fig. 3B; P<0.05).
NR2F2-AS1 siRNA silencing and PTEN
overexpression results in altered proliferation and apoptosis of
NPC cells. Overexpression of PTEN in both C666-1 (Fig. S1A) and 13-9B (Fig. S1B) was confirmed by RT-qPCR
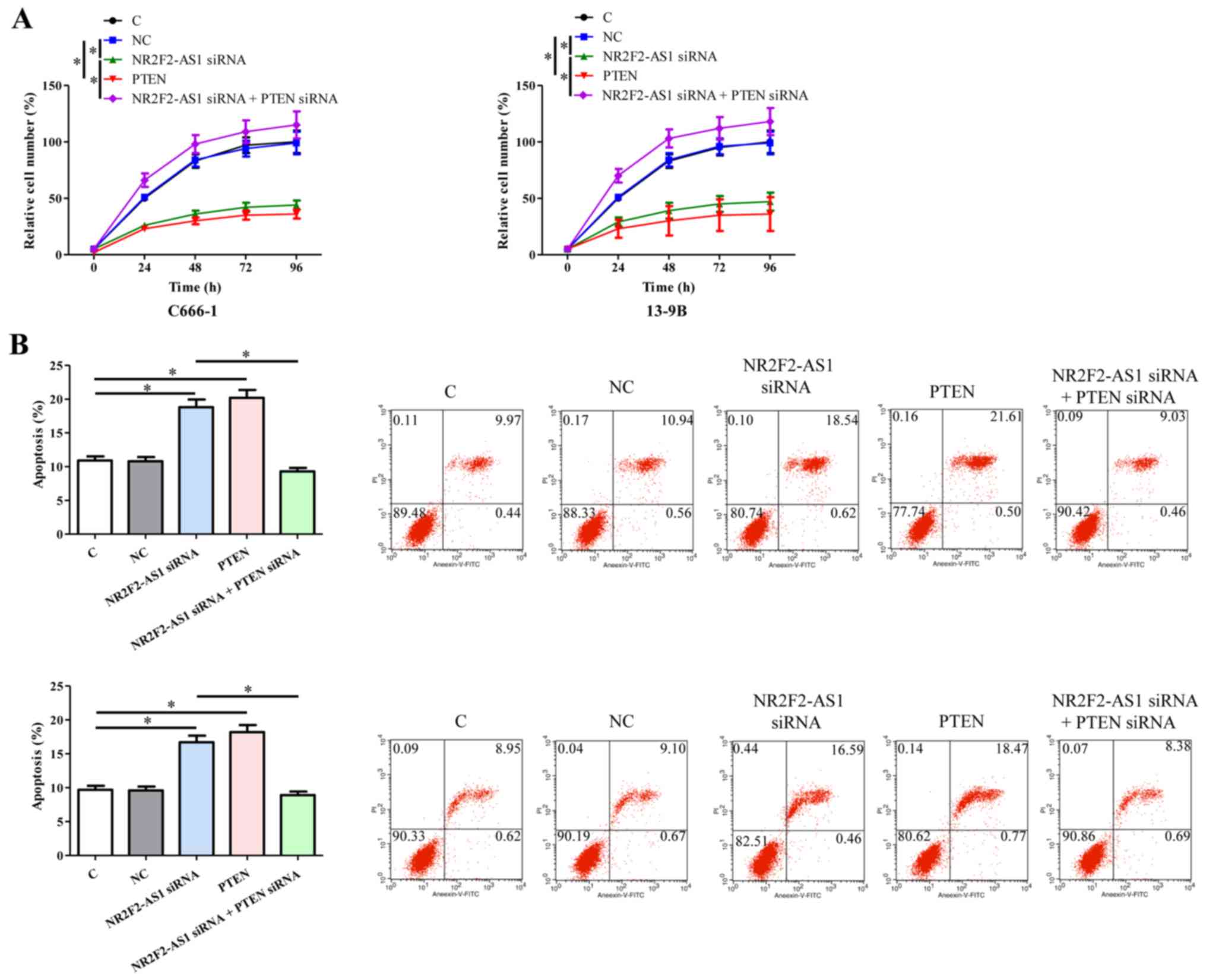
Cell proliferation and apoptosis were analyzed
following NR2F2-AS1 siRNA silencing and PTEN overexpression. The
results demonstrated that, compared with C and NC groups, NR2F2-AS1
siRNA silencing and PTEN overexpression resulted in significantly
decreased proliferation (Fig. 4A),
and increased apoptosis (Fig. 4B) of
NPC cells. In addition, PTEN siRNA silencing completely reversed
the effects of NR2F2-AS1 siRNA silencing on cell proliferation and
apoptosis (P<0.05).
Discussion
The present study investigated the role of NR2F2-AS1
and its prognostic value in NPC. The results demonstrated that
NR2F2-AS1 was upregulated in NPC. In addition, NR2F2-AS1 may affect
the expression of PTEN, which is known to regulate cancer cell
proliferation and apoptosis (7–9). The
present study also reported that NR2F2-AS1 and PTEN expression
levels may be considered as predictive biomarkers for the survival
of patients with NPC.
A previous study by Khew-Goodall et al
(14) reported a positive
correlation between the expression levels of PTEN and of miR-320
family members in the tumor stroma of breast cancer. Furthermore,
Bronisz et al (17)
demonstrated that PTEN can upregulate miR-320 expression to
reprogram the tumor microenvironment, by acting on the stromal
fibroblasts to inactivate oncogenic secretome. miR-320 is a
well-characterized tumor-suppressive miRNA with exhibits critical
functions in the regulation of cancer cell behaviors, such as
inhibiting cancer cell proliferation and inducing apoptosis
(18). As an oncogenic lncRNA,
NR2F2-AS1 in lung cancer may serve as a miRNA sponge and inhibit
miR-320b (13); however, this
conclusion was only made following bioinformatics analysis
(13).
In the present study, a negative association between
PTEN and NR2F2-AS1 expression levels in NPC tissues was observed,
which was not the case in non-tumor tissues. In addition, PTEN was
upregulated following NR2F2-AS1 knockdown. It has been demonstrated
that downregulated expression of miR-320 members, including
miR-320a, is observed in NPC (19);
however, PTEN is not a target of miR-320. Furthermore, no
alteration of miR-320b expression levels following NR2F2-AS1 siRNA
silencing was observed (data not shown). It has been reported that
PTEN activation can inhibit the PI3K/Akt pathway, which is the
primary cancer cell survival pathway, and subsequently induce
cancer cell apoptosis (8). NR2F2-AS1
may therefore upregulate PTEN expression, which subsequently
induces apoptosis of NPC cells. In the present study, a 5-year
follow-up was performed to analyze the association between
NR2F2-AS1 and PTEN expression levels and the survival of patients
with NPC. At present, early diagnosis of NPC remains limited, due
to the lack of sensitive and specific markers, and insufficiency of
suitable imaging techniques (20).
Precise prognosis would allow the choice of appropriate treatment
and care program, and therefore improve the survival of patients
with NPC. The data from this study suggested that patients with NPC
and high NR2F2-AS1 expression level or low PTEN expression levels
exhibited reduced survival times. Therefore, improved therapeutic
approaches may improve the survival times of these patients.
In conclusion, the present study demonstrated that
NR2F2-AS1 expression levels were upregulated in NPC and may
interact with PTEN in order to participate in NPC development.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HQ designed the study, performed the experiments,
analyzed the data and wrote the manuscript. CQ performed the
experiments, performed the literature search and reviewed the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Dongying Shengli Hospital. Signed informed consent was provided by
all patients prior to the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chua MLK, Wee JTS, Hui EP and Chan ATC:
Nasopharyngeal carcinoma. Lancet. 387:1012–1024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan AT, Teo PM and Johnson PJ:
Nasopharyngeal carcinoma. Ann Oncol. 13:1007–1015. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang Q, Zhou Y, Yang H, Li L, Deng X,
Cheng C, Xie Y, Luo X, Fang W and Liu Z: A directly negative
interaction of miR-203 and ZEB2 modulates tumor stemness and
chemotherapy resistance in nasopharyngeal carcinoma. Oncotarget.
7:67288–67301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Song P and Yin SC: Long non-coding RNA
EWSAT1 promotes human nasopharyngeal carcinoma cell growth in vitro
by targeting miR-326/-330-5p. Aging (Albany NY). 8:2948–2960. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee AW, Ma BB, Ng WT and Chan AT:
Management of nasopharyngeal carcinoma: Current practice and future
perspective. J Clin Oncol. 33:3356–3364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsang CM and Tsao SW: The role of
Epstein-Barr virus infection in the pathogenesis of nasopharyngeal
carcinoma. Virol Sin. 30:107–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Steck PA, Pershouse MA, Jasser SA, Yung
WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T,
et al: Identification of a candidate tumour suppressor gene, MMAC1,
at chromosome 10q23.3 that is mutated in multiple advanced cancers.
Nat Genet. 15:356–362. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Georgescu MM: PTEN tumor suppressor
network in PI3K-Akt pathway control. Genes Cancer. 1:1170–1177.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chu EC and Tarnawski AS: PTEN regulatory
functions in tumor suppression and cell biology. Med Sci Monit.
10:RA235–RA241. 2004.PubMed/NCBI
|
|
10
|
Dillon LM and Miller TW: Therapeutic
targeting of cancers with loss of PTEN function. Curr Drug Targets.
15:65–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liao Y, Shen L, Zhao H, Liu Q, Fu J, Guo
Y, Peng R and Cheng L: LncRNA CASC2 interacts with miR-181a to
modulate glioma growth and resistance to TMZ through PTEN pathway.
J Cell Biochem. 118:1889–1899. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang S, Zhang X, Sun Q, Zhuang C, Li G,
Sun L and Wang H: LncRNA NR2F2-AS1 promotes tumourigenesis through
modulating BMI1 expression by targeting miR-320b in non-small cell
lung cancer. J Cell Mol Med. 23:2001–2011. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khew-Goodall Y and Goodall GJ: Stromal
miR-320 keeps an oncogenic secretome in check. Nat Cell Biol.
14:124–125. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lydiatt WM, Patel SG, O'Sullivan B,
Brandwein MS, Ridge JA, Migliacci JC, Loomis AM and Shah JP: Head
and neck cancers-major changes in the American Joint Committee on
cancer eighth edition cancer staging manual. CA Cancer J Clin.
67:122–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bronisz A, Godlewski J, Wallace JA,
Merchant AS, Nowicki MO, Mathsyaraja H, Srinivasan R, Trimboli AJ,
Martin CK, Li F, et al: Reprogramming of the tumour
microenvironment by stromal PTEN-regulated miR-320. Nat Cell Biol.
14:159–167. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iorio MV and Croce CM: microRNA
involvement in human cancer. Carcinogenesis. 33:1126–1133. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi X, Li J, Zhou C, Lv C and Tian M:
MicroRNA-320a inhibits cell proliferation, migration and invasion
by targeting BMI-1 in nasopharyngeal carcinoma. FEBS Lett.
588:3732–3738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tabuchi K, Nakayama M, Nishimura B,
Hayashi K and Hara A: Early detection of nasopharyngeal carcinoma.
Int J Otolaryngol. 2011:6380582011. View Article : Google Scholar : PubMed/NCBI
|