Introduction
Non-small cell lung cancer (NSCLC) represents the
most common malignancy and the leading cause of cancer death
(1,2). Systemic therapy with platinum doublets
with or without Radiation Therapy is the standard frontline
treatment for patients in advanced stage of disease (stage
IIIB-IV). Patients with driver mutations/rearrangements require
molecular targeted specific drugs against EGFR or EML-ALK (3–5) while
first line Pembrolizumab an immune checkpoint inhibitor (ICI) can
be offered to patients whose tumors have PD-1 ligand-1 (PDL1)
expression >50%. Recently, the clinical development of ICI mAbs
has offered new treatment opportunities for NSCLC patients in
different settings. In fact, salvage therapy with mAbs to
programmed cell death receptor-1 (PD-1) and PDL1 has improved the
survival of many of these patients by rescuing pre-existing
tumor-specific cytotoxic-T-cells (CTLs) in the tumor sites
(6–12). CTLs are the ultimate
immune-surveillance system effectors able to recognize and kill
tumour cells. PD-1/PDL1 axis is a terminal inflammatory-mediated
immune-checkpoint able to protect tumour target cells by delivering
an inhibitory signal to tumour-specific CTLs expressing the PD-1
receptor in the tumour site. Experimental evidence have shown that
PD-1/PDL1 blockade with specific mAbs restores the antitumor
activity of these tumour-antigen specific CTLs with consequent
therapeutic effects in cancer patients. Nivolumab, in particular is
a human immune-globulin to PD-1 approved in the treatment of a
number of different malignancies including NSCLC, kidney cancer,
urothelial carcinoma, and melanoma. Although its use yields a clear
benefit and a prolonged survival in a number of patients, this
treatment is hampered by high costs, which makes eagerly needed the
identification of predictive markers of response, that so far are
still undefined and PDL1 analysis has not a clear role in the
second line setting. Additionally, its mechanism of action, so
different by the conventional cytotoxic treatments or
radio/chemotherapy, makes very difficult the monitoring of the
patients with the conventional radiology as well as the prevention
of possible side effects. In order to identify possible predictive
markers of response, we took in consideration that PD-1/PDL1
blockade antitumor effects occur throughout the reactivation of a
pre-existing immune response, whose efficacy is strictly related to
the presence of necrosis, hypoxia, and inflammation in the tumour
sites. We considered that these biological events could be
potentially evaluated by specific imaging assessments with a
computed tomography (CT) texture analysis (TA) or radiomics.
Radiomics, in particular, is the extraction of
quantitative imaging features from medical images. These
quantitative values can be used to develop models for cancer
diagnosis, patient prognosis, or relative tumor heterogeneity that
can then guide clinical decisions (13–15).
This process can be conceptually similar to the
current application of tumor staging or genetic and biomolecular
information derived from tumor biopsy specimens for clinical
decision-making.
TA is performed by means of a computer
quantification of both gray-level intensity, position of pixels,
and its use is being investigated in several fields (16–23).
In recent years, numerous studies have examined the
potential clinical utility of radiomics features calculated from
computed tomography (CT) images of NSCLC, correlated with tumor
histology (24–26), staging (27), patient prognosis (19,28–30) and
genetic mutations (31–33).
On these bases, we have designed a retrospective
study to evaluate the potential use of CT TA to predict the OS of
pre-treated advanced NSCLC patients treated with PD-1/PDL1 ICI
Nivolumab.
Patients and methods
Patient series
We performed a retrospective analysis of pretreated
advanced NSCLC cancer patients treated with Nivolumab between
January 2015 and July 2017 at the Radiation Oncology Unit of Siena
University Hospital, at the Medical Oncology and Translational
Oncology Units of Catanzaro University Hospital and at Reggio
Calabria Grand Metropolitan Hospital in Italy.
In our analysis all patients with a complete setting
of diagnostic histological samples and a clinical-radiological
pre-treatment staging, including a total body CT scan with and
without contrast, and an intact (i.e., not previously treated with
surgery or radiotherapy) evaluable target lesion in the lung were
included.
Ethics approval
All the patients gave written consent to anonymous
use of their examinations for research scope. A study notification
was submitted to local ethical committee as established by national
laws. The University Hospital of Siena Institutional Review Board
at the pilot center authorized the retrospective analysis of the
data. All procedures were undertaken in compliance with the ethical
statements of the Helsinki Declaration (2008) of the World Medical
Association.
Nivolumab therapy
All the patients were staged as IIIB/IV and
progressed after systemic therapy with cis-platinum doublets. All
the patients received intravenous nivolumab (3 mg/kg in 60 min) on
biweekly bases. Treatment was given until progression, unacceptable
toxicity or death.
Computed tomography imaging
All CTs at Siena University Hospital were performed
using a 64-detector row CT scanner (Discovery 750 HD; GE
Healthcare, Milwaukee, WI, USA).
In all patients, CT of the chest was performed with
a spiral technique in tail-cranial direction (from the bases of the
lungs to a plane cutting through the upper thoracic outlet, with
the patient lying supine). Enhanced CT scans were obtained in the
portal venous phase (delay 65–80 sec) with an intravenous injection
of 2 ml/kg of non-ionic contrast material after a bolus injection
of non-ionic contrast material (Iopamidolo 370 mgI/ml; Iopamiro,
Bracco Diagnostics, Italy or Iopromide 370 mgI/ml, Ultravist 370
mgI/ml; Bayer HealthCare Pharmaceuticals, Italy), followed by 30 ml
of saline solution using a peristaltic semiautomated power injector
(3–4 ml/sec flow rate; SIAS 757, Bologna, Italy) with an 18-gauge
needle in the antecubital vein. The following technical parameters
were used: slice thickness 2.5 mm, beam pitch 1.375/0.937,
reconstruction interval 0.8 mm, 120–140 kVp and 250–500 mA. An
automatic current modulation tube was used to minimize radiation
exposure. Standard reconstruction algorithm was used. Patients were
instructed not to breathe during helical imaging in order to avoid
motion artefacts.
Follow-up
A CT-scan was repeated, in order to assess the
response to immunotherapy, every 3 months or in any case showing
clinical signs suggesting progressive disease (PD). General
examinations with recording of toxicity, blood cell counts and
chemistry and serum markers levels were evaluated every two
weeks.
Feature extraction and TA
The gross tumour volume (GTV) was contoured by a
radiation oncologist (VN) and confirmed by three expert
radiologists (EA, MAM, VR) on pre-contrast and post-contrast
sequences (Fig. 1). The impact of
variations on contouring was analysed performing two delineation on
each patient, and the TA parameters were evaluated for reliability
with Intraclass Coefficient Correlation method (ICC). All the
analyses for work have been accomplished with LifeX
Software© (34). TA
parameters were limited to features of gray-level co-occurrence
matrix (GLCM), sphericity and indices from the gray-level
histogram, to avoid an excessive number of parameters and
overfitting of models.
Preselection of variables
The reliability analysis performed with ICC showed
that the TA parameters that resulted significantly reproducible
(ICC>0.70, single measure) were, respectively, 12 out of 14 for
the post-contrast CT (85%), 13 out of 14 (93%) for the pre-contrast
CT (Table I).
 | Table I.Reliability analysis of TA
parameters. |
Table I.
Reliability analysis of TA
parameters.
| TA Parameter | Enhanced CT ICC
(single measure) | CT ICC (single
measure) |
|---|
| Volume (ml) | 0.998a | 0.996a |
| Volume | 0.996a | 0.994a |
| Skewness | 0.519 | 0.829a |
| Kurtosis | 0.386 | 0.616 |
| Entropy | 0.879a | 0.968a |
| Energy | 0.808a | 0.841a |
| Sphericity | 0.985a | 0.888a |
| Compacity | 0.994a | 0.989a |
|
GLCM-homogeneity | 0.862a | 0.963a |
| GLCM-energy | 0.787a | 0.917a |
| GLCM-contrast | 0.935a | 0.986a |
|
GLCM-correlation | 0.707a | 0.847a |
| GLCM-entropy | 0.863a | 0.973a |
|
GLCM-dissimilarity | 0.919a | 0.994a |
End-points and statistical
analysis
In order to perform a statistical correlation among
the reliable TA parameters and outcome, we used the software
X-Tile (35) to calculate for
each TA parameter a cut-off value that could be significant at
survival analysis (Kaplan Meier analysis), on the overall
population, after normalization of the parameters for the training
and validation population.
For each significant cut-off, we assigned the score
1 for the subgroup with the worse prognosis and 0 for the subgroup
with the better prognosis.
We then summed the scores to obtain a global texture
score that was finally partitioned into two subgroups. The clinical
variables of the two subgroups (sex, age, histology) were compared
with Chi-square test, in order to exclude biases due to clinical
factors not taken into account for the comparison of the
above-mentioned subsets.
To validate our performance model, our cohort of
patients were separated into two partitions, with the patients
treated in our Unit (35 patients) as the training dataset and the
patients treated in other Institutions (24 patients) as the
validation set.
A survival analysis of progression free survival
(PFS) and overall survival (OS) with Kaplan-Meier method was used
in the two subgroups to test the texture score.
PFS was calculated from the date of the patient's
beginning of immunotherapy to date of CT examination showing
progression of disease, or censored to last follow-up visit.
Conversely, OS was calculated from the date of the patient's
beginning of immunotherapy to the death of patients or censored to
last follow-up visit.
All the statistical analysis was conducted with SPSS
software v.23.0.
Results
Patients' features
This is a retrospective analysis performed on an
unmasked sample of fifty-nine consecutive patients with advanced
NSCLC cancer who had progressed standard frontline chemotherapy and
had received salvage therapy with Nivolumab, between January 2015
and July 2017. We have enrolled 48 (81%) males and 11 (19%) females
with a median age of 69 years (mean +-/ SD=67.4 +/- 9.7 years,
range 42–86 years). Thirty-six patients (61%) were diagnosed as
adenocarcinoma, 18 (30.5%) as squamous cell carcinoma and five as
undefined NSCLC histology. Forty-five patients were in stage IV
(76%), whereas 14 patients were in stage IIIB (24%). Τhe
description of parameters in the two datasets is presented in
Table II.
 | Table II.Patient characteristics. |
Table II.
Patient characteristics.
| Parameter | Training
dataset | Validation
dataset | Whole dataset |
|---|
| Sex |
|
|
|
|
Males | 29 (83%) | 19 (79%) | 48 (81%) |
|
Females | 6 (17%) | 5 (21%) | 11 (19%) |
| Age |
|
|
|
|
Median | 71 | 65 | 69 |
| Mean
+/- s.d. | 68 +/- 8 | 67 +/- 11 | 67 +/- 10 |
| Stage |
|
|
|
|
IIIB | 8 (23%) | 6 (25%) | 14 (24%) |
| IV | 27 (77%) | 18 (75%) | 45 (76%) |
| Histology |
|
|
|
|
Adenocarcinoma | 23 (66%) | 13 (54%) | 36 (61%) |
|
Squamous cell | 7 (20%) | 11 (46%) | 18 (30.5%) |
| Not
specified | 5 (14%) |
| 5 (8.5%) |
| PFS |
|
|
|
|
Progression | 22 (63%) | 15 (62%) | 37 (62%) |
| No
progression | 13 (37%) | 9 (38%) | 22 (38%) |
| OS |
|
|
|
|
Dead | 15 (43%) | 12 (50%) | 27 (45%) |
|
Alive | 20 (57%) | 12 (50%) | 32 (55%) |
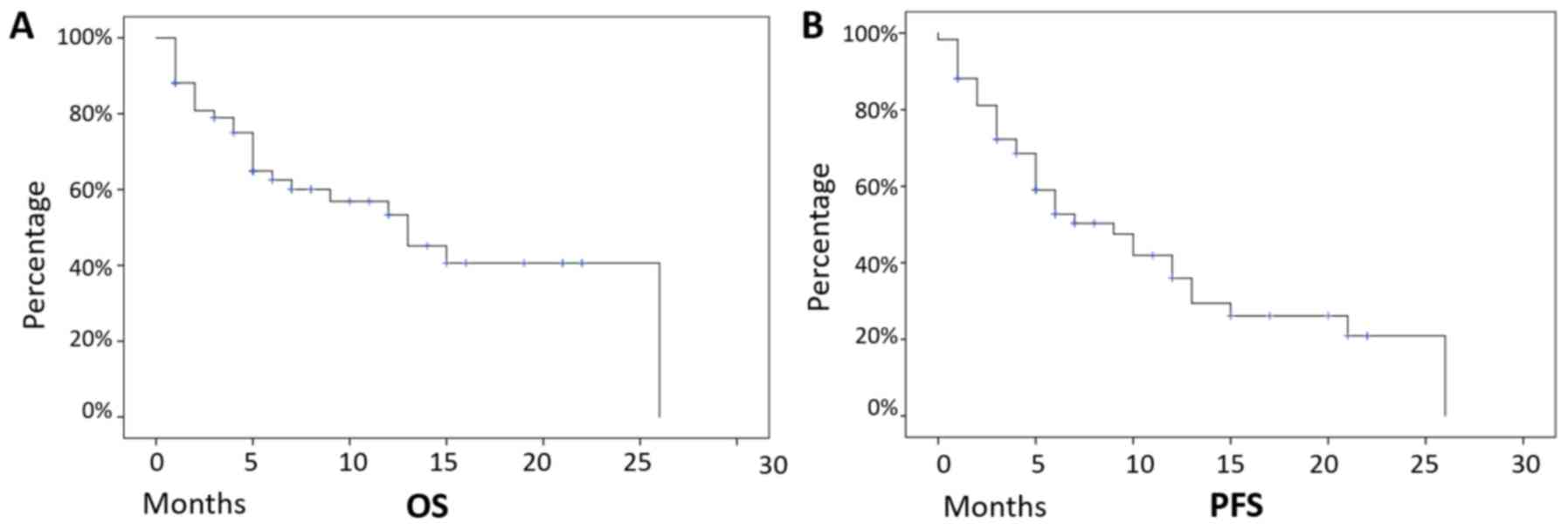
Median follow-up was 12 months and, on the overall,
these patients presented a median PFS of 9 months (mean +-/ SD=11
months +/- 1.3 months, 95% CI 8.3–13.7 months), and a median OS of
13 months (mean +-/ SD=14.5 months +/- 1.6 months, 95% CI 11.1–17.4
months), with 32 (54%) patients still alive at the last follow-up
examination (Fig. 1).
Median cycles of Nivolumab were 10 for the whole
population (mean 14, range 2–52 cycles). In our series, survival
analysis did not find any correlation with gender (male: Median OS
13 months, mean 14 +/- 1.8 months, 95% CI 10–18 months, vs. female:
Median OS 7 months, mean 10 +/- 2.7 months, 95% CI 5–15 months,
P-value: 0.58), with age (Cox-Regression P-value: 0.44), stage
(IIIB: Median OS 15 months, mean 14 +/- 2.4 months, 95% CI 10–19
months, vs. IV: Median OS 13 months, mean 12 +/- 1.7 months, 95% CI
9–15 months, P-value: 0.60) and with histology (squamous cell:
Median OS 15 months, mean 14 +/- 2.7 months, 95% CI 9–20 months,
vs. adenocarcinoma: Median OS 13 months, mean 12 +/- 1.6 months,
95% CI 9–15 months, P-value 0.88).
Survival analysis and texture
score
In order to calculate a reproducible cut-off for the
targeted TA parameters in CT images, we used the X-Tile Software as
described in the Methods section.
A valid parameter cut-off could be selected in the
whole population for: 1) volume (score 1 > 36 ml); 2) histogram
entropy (score 1 > 1.30), 3) compacity (score 1 < 3.4),
GLCM-entropy (score 1 > 1.8), 5) GLCM-dissimilarity (score 1
> 5.6) GLCM-correlation (score 1<0.5). These cut-off values
were applied to both training and validation set.
In our analysis, we have restricted the testing of
texture parameters describing morphological, histogram and GLCM
features, as these parameters have been successfully used and
correlated in lung cancer imaging (26), to avoid the risk of overfitting our
model using too many parameters (36).
The global texture score, as described in the
Methods section, allowed to classify the patients into two cohorts
(low risk: Score 0–1, and high risk: Score >1).
The clinical parameters of the two cohorts were then
tested with Chi-square analysis, that demonstrated no statistical
differences of the clinical variables in the two subsets, as
follows: Age (P=0.282), sex (P=0.843), histology (P=0.107), stage
(P=0.834) (Table II).
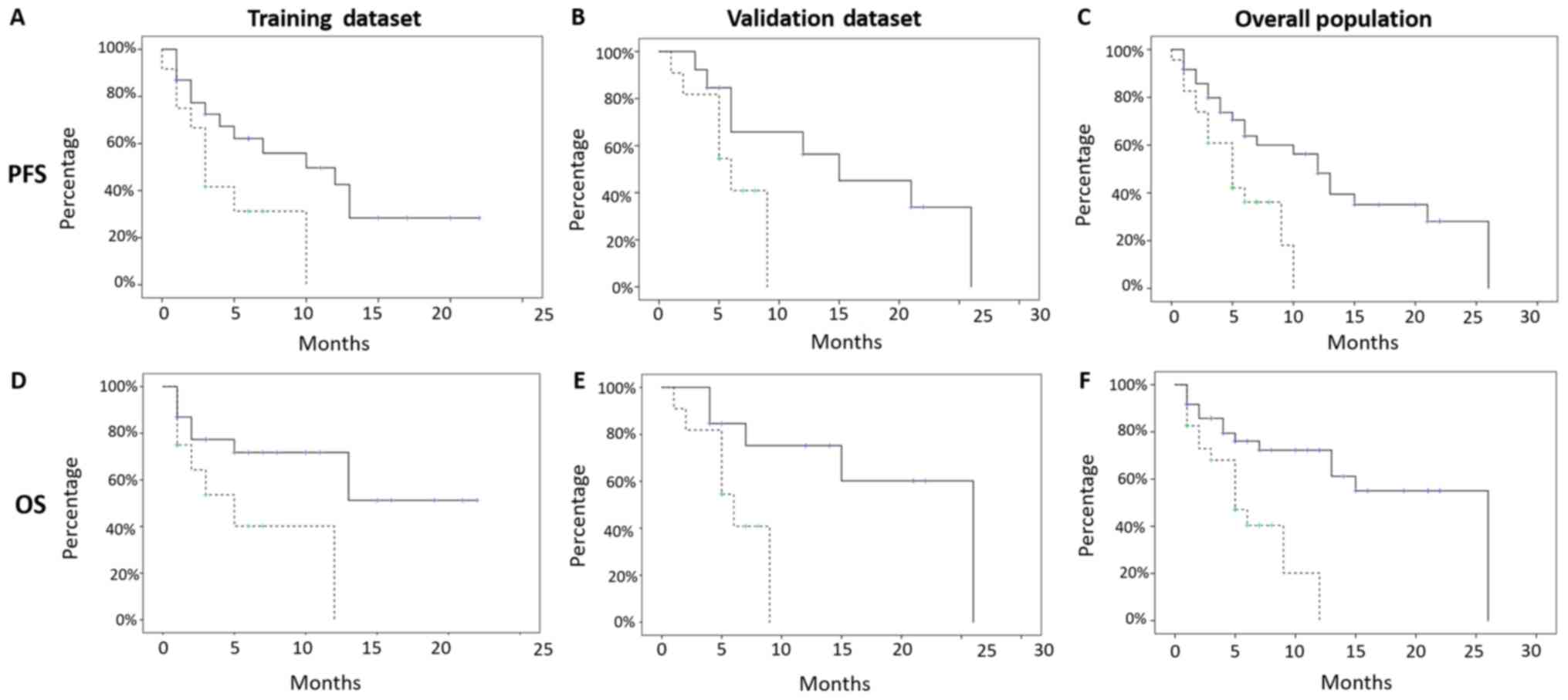
PFS resulted significant for both the subsets
TRAINING SUBSET (low risk median PFS 10 months, mean 10.9
+/- 1.8 months, 95% CI 7–14 months, vs. high risk median PFS
3 months, mean 4.7 +/- 1.1 months, 95% CI 2–6 months, P-value:
0.04) and VALIDATION SUBSET (low risk median PFS 15 months,
mean 15.5 +/- 2.9 months, 95% CI 9–21 months, vs. high risk
median PFS 6 months, mean 6 +/- 0.9 months, 95% CI 4–8 months,
P-value: 0.04; Fig. 2).
OS, also, resulted significant in both subsets
TRAINING SUBSET (low risk median OS: Not reached, mean 14.4
+/- 2 months, 95% CI 10–18 months, vs. high risk median OS:
5 months, mean 6.2 +/- 1.6 months, 95% CI 3–9 months, P-value:
0.02) and VALIDATION SUBSET (low risk median OS: 26 months,
mean 19 +/- 3 months, 95% CI 13–25 months, vs. high risk
median OS 6 months, mean 6.1 +/- 0.9 months, 95% CI 4–8 months,
P-value 0.03; Fig. 2).
The global texture score correlated with patients'
survival allowed to classify patients included into two cohorts at
low (Score 0–1, 36 patients, 61%) and high risk (Score> 1, 23
patients, 39%) of recurrence, respectively, presenting a median PFS
of 12 months (mean 13 +/- 1.7 months, 95% CI 9–16 months), vs. a
median PFS of 5 months (mean 5.4 +/- 0.7 months, 95% CI 4–7
months), with a P-value of 0.01, and a median OS of 26 months (mean
+/- SD: 17.5 +/- 1.98 months, 95% CI 14–21 months) and 5 months
(mean +/- SD: 6 +/- 0.99 months, 95% CI: 4–8 months), with a
P-value <0.01, in the low and high risk groups, respectively
(Fig. 2).
Number of cycles of Nivolumab, also, were different
among subgroups (P=0.026), with low risk subset showing a median of
12 cycles (mean 18.5 cycles, range 2–52 cyles) and high risk subset
showing a median of 10 cycles (mean 8.8 cycles, range 2–20
cycles).
Discussion
The recent clinical development of immune-biological
drugs such as PD-1/PDL1 inhibitor mAbs for the treatment of common
malignancies has clearly risen an extraordinary challenge in terms
of cost, adverse events and patients' monitoring. So far, no clear
biomarkers are presently able to select patients who may benefit by
treatment with Nivolumab in NSCLC, although some interesting
preliminary results (37).
Additionally, these drugs act by turning the immune-balance between
tumour and immune system in favour of the latter, thus providing a
clinical benefit and prolonging patients' survival even in the
presence of an apparent radiological progression. This fact makes
still more difficult the monitoring of these patients with
conventional imaging techniques. In fact, the latter have been
designed to evaluate the effects of classical
cytotoxic/cytoreductive strategies, as well as chemo-radiotherapy,
aimed to provide serial comparisons of tumour volume and number of
lesions. In this context, functional imaging techniques, such as
diffusion-weighted magnetic resonance imaging (MRI), perfusion
methods, positron emission tomography (PET), and radiomics, which
are also able to define more qualitative analysis of targeted
tumour lesions, are under investigation for providing useful
information for these patients (38,39).
These techniques are of interest in the search for surrogate
biomarkers to potentially define and monitor tumour specific
biological processes, including tumour cellularity, growth,
necrosis, tumour-associated inflammation and angiogenesis. All
these biological events can strictly reflect either tumour
progression or responsiveness to the new generation of biological
drugs including inhibitors of PD-1/PDL1 immune-checkpoint axis
alike Nivolumab, Pembrolizumab, or Atezolizumab mAbs (monoclonal
antibodies) (40–44).
In our retrospective multi-institutional analysis,
we have restricted the testing of texture parameters to NSCLC
patients undergone to salvage treatment with Nivolumab. We have
described morphological, histogram and GLCM matrix features
defining parameters which have been subsequently correlated in lung
cancer imaging, and in recent years with immunotherapy in NSCLC
(26,36,45–49).
Interestingly, within our sample of NSCLC patients, subjected to
Nivolumab treatment, we defined morphological features able to
identify a cohort of patients with a very poor prognosis. These
patients before the beginning of the immunological treatment had a
larger volume of the primary lung lesions associated to a lower
compactness, measured as compacity of volume. These combined
parameters reflect larger tumour cell burdens paralleled by central
hypoxia, necrosis, and invasion beyond the tumour and poor response
to cytotoxic treatments. These events additionally define a disease
with a very unbalanced antitumour immune response (19,50,51).
In our analysis, a higher histogram entropy was
associated with worse outcome. Several reports suggest that this
parameter reflects the aggressiveness of the disease as associated
to a rapid growth and invasiveness (27,30,52).
The GLCM-entropy, specifically measures the
randomness of gray-level voxel pairs, and has been significantly
associated, as above, with a worse prognosis in other works
(19,27,30).
The GLCM-dissimilarity, on the other hand, measures
the variation of gray-level voxel pairs, and can reflect
intra-tumour heterogeneity, whereas the GLCM-correlation measures
the linear dependence of gray-level in GLCM, and it has been
described an important feature in previous studies (19).
These radiomics parameters including higher
GLCM-entropy and -dissimilarity and lower GLCM-correlations were
strictly associated with a worse prognosis. These TA parameters
describe a poor immunogenic tumour pattern weakly responsive to
PD-1/PDL1 blockade. However, it has to be considered that our
results need to undergo critical consideration for many
methodological and technical refinements. In particular, our study
is retrospective, and the correlations between textural parameters
and clinical outcome requires additional investigations in order to
better understand the pathological bases of the TA parameters.
Moreover, the arbitrary choice of the cut-off can
lead to significant bias even if we have not unbalanced the
subgroups (35) and we have
validated our model with an external validation cohort. Conversely,
in our series, survival analysis did not show any correlation with
gender and age.
In this regard, we believe that the low number of
patients analyzed, as well as the low ratio of females (19%) and
the low standard deviation of the age (mean age 67.4 years +/- 9.7
years) can easily justify these discrepancies with the literature
(53). At the same time, the
specific subset of patients analyzed (NSCLC patients in progression
after first line chemotherapy) could also justify the
non-significance of clinical variables such as sex and age.
In conclusion, the predictive value of our TA
parameters seems to deserve consideration as a potential and
inexpensive biomarker to select NSCLC patients who may benefit by
Nivolumab treatment and deserves further development in prospective
Randomized trial.
Acknowledgements
Not applicable.
Funding
This study was supported by POR CAMPANIA FESR
2014/2020 Sensormircircolar
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors have substantially contributed to the
conception of the study. VN, PTi, PP, CB, AR, SFC, PC, VB, RG, GC,
CT, CG, PTas, PTag, SC, RC, AL, MC, AG, MAM and LP have acquired
the data, performed the analysis and interpretation of the data and
they have contributed to the drafting and revision of the different
versions of the manuscript. All authors have given final approval
of the version to be published and agree to be accountable for all
the aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved
Ethics approval and consent to
participate
All patients gave written consent for anonymous use
of their examinations for research scope. All procedures were
undertaken in compliance with the ethical statements of the
Helsinki Declaration (1964, amended most recently in 2008) of the
World Medical Association.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cetin K, Ettinger DS, Hei YJ and O'Malley
CD: Survival by histologic subtype in stage IV nonsmall cell lung
cancer based on data from the surveillance, epidemiology and end
results program. Clin Epidemiol. 3:139–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pilkington G, Boland A, Brown T, Oyee J,
Bagust A and Dickson R: A systematic review of the clinical
effectiveness of first-line chemotherapy for adult patients with
locally advanced or metastatic non-small cell lung cancer. Thorax.
70:359–367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, et al: Phase III study comparing cisplatin
plus gemcitabine with cisplatin plus pemetrexed in
chemotherapy-naive patients with advanced-stage non-small-cell lung
cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Noh JM, Kim JM, Ahn YC, Pyo H, Kim B, Oh
D, Ju SG, Kim JS, Shin JS, Hong CS, et al: Effect of radiation
therapy techniques on outcome in N3-positive IIIB non-small cell
lung cancer treated with concurrent chemoradiotherapy. Cancer Res
Treat. 48:106–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luke JJ and Ott PA: PD-1 pathway
inhibitors: The next generation of immunotherapy for advanced
melanoma. Oncotarget. 6:3479–3492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Creelan BC: Update on immune checkpoint
inhibitors in lung cancer. Cancer Control. 21:80–89. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Horn L, Spigel DR, Vokes EE, Holgado E,
Ready N, Steins M, Poddubskaya E, Borghaei H, Felip E, Paz-Ares L,
et al: Nivolumab versus docetaxel in previously treated patients
with advanced non-small-cell lung cancer: Two-year outcomes from
two randomized, open-label, phase III trials (CheckMate 017 and
CheckMate 057). J Clin Oncol. 35:3924–3933. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pastina P, Nardone V, Botta C, Croci S,
Tini P, Battaglia G, Ricci V, Cusi MG, Gandolfo C, Misso G, et al:
Radiotherapy prolongs the survival of advanced non-small-cell lung
cancer patients undergone to an immune-modulating treatment with
dose-fractioned cisplatin and metronomic etoposide and bevacizumab
(mPEBev). Oncotarget. 8:75904–75913. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pastina P, Nardone V, Croci S, Battaglia
G, Vanni F, Bellan C, Barbarino M, Ricci V, Costantini S, Capone F,
et al: Anti-cancer activity of dose-fractioned mPE +/- bevacizumab
regimen is paralleled by immune-modulation in advanced squamous
NSLC patients. J Thorac Dis. 9:3123–3131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nardone V, Pastina P, Giannicola R,
Agostino R, Croci S, Tini P, Pirtoli L, Giordano A, Tagliaferri P
and Correale P: How to increase the efficacy of immunotherapy in
NSCLC and HNSCC: Role of radiation therapy, chemotherapy, and other
strategies. Front Immunol. 9:29412018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ganeshan B, Panayiotou E, Burnand K,
Dizdarevic S and Miles K: Tumour heterogeneity in non-small cell
lung carcinoma assessed by CT texture analysis: A potential marker
of survival. Eur Radiol. 22:796–802. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ganeshan B, Abaleke S, Young RC, Chatwin
CR and Miles KA: Texture analysis of non-small cell lung cancer on
unenhanced computed tomography: Initial evidence for a relationship
with tumour glucose metabolism and stage. Cancer Imaging.
10:137–143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ganeshan B, Miles KA, Young RC and Chatwin
CR: Hepatic enhancement in colorectal cancer: Texture analysis
correlates with hepatic hemodynamics and patient survival. Acad
Radiol. 14:1520–1530. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alobaidli S, McQuaid S, South C, Prakash
V, Evans P and Nisbet A: The role of texture analysis in imaging as
an outcome predictor and potential tool in radiotherapy treatment
planning. Br J Radiol. 87:201403692014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mattonen SA, Tetar S, Palma DA, Louie AV,
Senan S and Ward AD: Imaging texture analysis for automated
prediction of lung cancer recurrence after stereotactic
radiotherapy. J Med Imaging (Bellingham). 2:0410102015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mattonen SA, Palma DA, Haasbeek CJ, Senan
S and Ward AD: Early prediction of tumor recurrence based on CT
texture changes after stereotactic ablative radiotherapy (SABR) for
lung cancer. Med Phys. 41:0335022014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Coroller TP, Agrawal V, Narayan V, Hou Y,
Grossmann P, Lee SW, Mak RH and Aerts HJ: Radiomic phenotype
features predict pathological response in non-small cell lung
cancer. Radiother Oncol. 119:480–486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nardone V, Tini P, Carbone SF, Grassi A,
Biondi M, Sebaste L, Carfagno T, Vanzi E, De Otto G, Battaglia G,
et al: Bone texture analysis using CT-simulation scans to
individuate risk parameters for radiation-induced insufficiency
fractures. Osteoporos Int. 28:1915–1923. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nardone V, Tini P, Nioche C, Biondi M,
Sebaste L, Mazzei MA, Banci Buonamici F and Pirtoli L: Texture
analysis of parotid gland as a predictive factor of radiation
induced xerostomia: A subset analysis. Radiother Oncol.
122:3212017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nardone V, Tini P, Croci S, Carbone SF,
Sebaste L, Carfagno T, Battaglia G, Pastina P, Rubino G, Mazzei MA
and Pirtoli L: 3D bone texture analysis as a potential predictor of
radiation-induced insufficiency fractures. Quant Imaging Med Surg.
8:14–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nardone V, Tini P, Nioche C, Mazzei MA,
Carfagno T, Battaglia G, Pastina P, Grassi R, Sebaste L and Pirtoli
L: Texture analysis as a predictor of radiation-induced xerostomia
in head and neck patients undergoing IMRT. Radiol Med. 123:415–423.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Liu S, Qu F, Li Q, Cheng R and Ye
Z: Tumor heterogeneity assessed by texture analysis on
contrast-enhanced CT in lung adenocarcinoma: Association with
pathologic grade. Oncotarget. 8:53664–53674. 2017.PubMed/NCBI
|
|
25
|
Bae JM, Jeong JY, Lee HY, Sohn I, Kim HS,
Son JY, Kwon OJ, Choi JY, Lee KS and Shim YM: Pathologic
stratification of operable lung adenocarcinoma using radiomics
features extracted from dual energy CT images. Oncotarget.
8:523–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee G, Lee HY, Park H, Schiebler ML, van
Beek EJR, Ohno Y, Seo JB and Leung A: Radiomics and its emerging
role in lung cancer research, imaging biomarkers and clinical
management: State of the art. Eur J Radiol. 86:297–307. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Craigie M, Squires J and Miles K: Can CT
measures of tumour heterogeneity stratify risk for nodal metastasis
in patients with non-small cell lung cancer? Clin Radiol.
72:899.e1–899.e7. 2017. View Article : Google Scholar
|
|
28
|
Fave X, Zhang L, Yang J, Mackin D, Balter
P, Gomez D, Followill D, Jones AK, Stingo F, Liao Z, et al:
Delta-radiomics features for the prediction of patient outcomes in
non-small cell lung cancer. Sci Rep. 7:5882017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miles KA: How to use CT texture analysis
for prognostication of non-small cell lung cancer. Cancer Imaging.
16:102016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sacconi B, Anzidei M, Leonardi A, Boni F,
Saba L, Scipione R, Anile M, Rengo M, Longo F, Bezzi M, et al:
Analysis of CT features and quantitative texture analysis in
patients with lung adenocarcinoma: A correlation with EGFR
mutations and survival rates. Clin Radiol. 72:443–450. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou M, Leung A, Echegaray S, Gentles A,
Shrager JB, Jensen KC, Berry GJ, Plevritis SK, Rubin DL, Napel S
and Gevaert O: Non-small cell lung cancer radiogenomics map
identifies relationships between molecular and imaging phenotypes
with prognostic implications. Radiology. 286:307–315. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Y, Kim J, Balagurunathan Y, Li Q,
Garcia AL, Stringfield O, Ye Z and Gillies RJ: Radiomic features
are associated with EGFR mutation status in lung adenocarcinomas.
Clin Lung Cancer. 17:441–448.e6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Halpenny DF, Plodkowski A, Riely G, Zheng
J, Litvak A, Moscowitz C and Ginsberg MS: Radiogenomic evaluation
of lung cancer-are there imaging characteristics associated with
lung adenocarcinomas harboring BRAF mutations? Clin Imaging.
42:147–151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nioche C, Orlhac F, Boughdad S, Reuze S,
Soussan M, Robert C, Barakat C and Buvat I: A freeware for tumor
heterogeneity characterization in PET, SPECT, CT, MRI and US to
accelerate advances in radiomics. J Nucl Med. 58 (Suppl
1):S13162017.
|
|
35
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: A new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
van der Schaaf A, Xu CJ, van Luijk P,
Van't Veld AA, Langendijk JA and Schilstra C: Multivariate modeling
of complications with data driven variable selection: Guarding
against overfitting and effects of data set size. Radiother Oncol.
105:115–121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Giannicola R, D'Arrigo G, Botta C,
Agostino R, Del Medico P, Falzea AC, Barbieri V, Staropoli N, Del
Giudice T, Pastina P, et al: Early blood rise in auto-antibodies to
nuclear and smooth muscle antigens is predictive of prolonged
survival and autoimmunity in metastatic-non-small cell lung cancer
patients treated with PD-1 immune-check point blockade by
nivolumab. Mol Clin Oncol. 11:81–90. 2019.PubMed/NCBI
|
|
38
|
Mazzei MA, Nardone V, Di Giacomo L,
Bagnacci G, Gentili F, Tini P, Marrelli D and Volterrani L: The
role of delta radiomics in gastric cancer. Quant Imaging Med Surg.
8:719–721. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nardone V, Reginelli A, Scala F, Carbone
SF, Mazzei MA, Sebaste L, Carfagno T, Battaglia G, Pastina P,
Correale P, et al: Magnetic-resonance-imaging texture analysis
predicts early progression in rectal cancer patients undergoing
neoadjuvant chemoradiation. Gastroenterol Res Pract.
2019:85057982019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee HY, Jeong JY, Lee KS, Yi CA, Kim BT,
Kang H, Kwon OJ, Shim YM and Han J: Histopathology of lung
adenocarcinoma based on new IASLC/ATS/ERS classification:
Prognostic stratification with functional and metabolic imaging
biomarkers. J Magn Reson Imaging. 38:905–913. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim YN, Lee HY, Lee KS, Seo JB, Chung MJ,
Ahn MJ, Park K, Kim TS and Yi CA: Dual-energy CT in patients
treated with anti-angiogenic agents for non-small cell lung cancer:
New method of monitoring tumor response? Korean J Radiol.
13:702–710. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
van Elmpt W, Zegers CML, Reymen B, Even
AJG, Dingemans AC, Oellers M, Wildberger JE, Mottaghy FM, Das M,
Troost EGC and Lambin P: Multiparametric imaging of patient and
tumour heterogeneity in non-small-cell lung cancer: Quantification
of tumour hypoxia, metabolism and perfusion. Eur J Nucl Med Mol
Imaging. 43:240–248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chi JT, Thrall DE, Jiang C, Snyder S, Fels
D, Landon C, McCall L, Lan L, Hauck M, MacFall JR, et al:
Comparison of genomics and functional imaging from canine sarcomas
treated with thermoradiotherapy predicts therapeutic response and
identifies combination therapeutics. Clin Cancer Res. 17:2549–2560.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tini P, Nardone V, Pastina P, Pirtoli L,
Correale P and Giordano A: The effects of radiotherapy on the
survival of patients with unresectable non-small cell lung cancer.
Expert Rev Anticancer Ther. 18:593–602. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Virginia BM, Laura F, Silvia R, Roberto F,
Francesco F, Eva H, Charles F, Samy A, Stefan M, Jean-Charles S, et
al: Prognostic value of histogram analysis in advanced non-small
cell lung cancer: A radiomic study. Oncotarget. 9:1906–1914. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bera K, Velcheti V and Madabhushi A: Novel
quantitative imaging for predicting response to therapy: Techniques
and clinical applications. Am Soc Clin Oncol Educ Book.
38:1008–1018. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Trebeschi S, Drago SG, Birkbak NJ,
Kurilova I, Cǎlin AM, Pizzi AD, Lalezari F, Lambregts DMJ, Rohaan
M, Parmar C, et al: Predicting response to cancer immunotherapy
using non-invasive radiomic biomarkers. Ann Oncol. Mar
21–2019.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Harmon S, Seder CW, Chen S, Traynor A,
Jeraj R and Blasberg JD: Quantitative FDG PET/CT may help
risk-stratify early-stage non-small cell lung cancer patients at
risk for recurrence following anatomic resection. J Thorac Dis.
11:1106–1116. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shi L, He Y, Yuan Z, Benedict S, Valicenti
R, Qiu J and Rong Y: Radiomics for response and outcome assessment
for non-small cell lung cancer. Technol Cancer Res Treat.
17:15330338187827882018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Einenkel J, Braumann UD, Horn LC, Pannicke
N, Kuska JP, Schütz A, Hentschel B and Höckel M: Evaluation of the
invasion front pattern of squamous cell cervical carcinoma by
measuring classical and discrete compactness. Comput Med Imaging
Graph. 31:428–435. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nardone V, Nanni S, Pastina P, Vinciguerra
C, Cerase A, Correale P, Guida C, Giordano A, Tini P, Reginelli A,
et al: Role of perilesional edema and tumor volume in the prognosis
of non-small cell lung cancer (NSCLC) undergoing radiosurgery (SRS)
for brain metastases. Strahlenther Onkol. 195:734–744. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Grove O, Berglund AE, Schabath MB, Aerts
HJ, Dekker A, Wang H, Velazquez ER, Lambin P, Gu Y, Balagurunathan
Y, et al: Quantitative computed tomographic descriptors associate
tumor shape complexity and intratumor heterogeneity with prognosis
in lung adenocarcinoma. PLoS One. 10:e01182612015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liao Y, Fan X and Wang X: Effects of
different metastasis patterns, surgery and other factors on the
prognosis of patients with stage IV non-small cell lung cancer: A
surveillance, epidemiology, and end results (SEER) linked database
analysis. Oncol Lett. 18:581–592. 2019.PubMed/NCBI
|