Introduction
Gastric cancer is a highly prevalent malignant tumor
type; according to the Global Cancer Epidemiology Statistics
(GLOBOCAN) (1), published in 2018,
gastric cancer is the fifth most common cancer type and the third
leading cause of cancer-related mortality globally. Notably,
>50% of cases occur in Asia, and the incidence in men is ~2
times that in women. Gastric cancer is highly heterogeneous and
typically asymptomatic during the early stages. The majority of
patients are diagnosed in the advanced stage leading to a low
5-year survival rate of 20–25% (2).
Gastric cancer is also highly invasive and often results in distant
metastasis. Currently, an individualized chemotherapy regime is the
most common therapeutic method in patients with recurrent and
unresectable advanced gastric cancer; first-line treatment consists
of fluorouracil chemotherapy, combined with platinum and/or
paclitaxel to form a two- or three-drug regimen. However, the
median overall survival (OS) time in patients with advanced gastric
cancer is just 10–15 months (3).
Therefore, it is important to improve the therapeutic efficacy of
treatment options, and to study the mechanisms of tumorigenesis and
progression in gastric cancer.
Previous studies have determined that the occurrence
and development of human tumors are associated with epigenetic
alterations, which refers to reversible genetic phenotypic changes
that are not caused by changes to the DNA sequence, but remain
relatively stable during cell division. These heritable alterations
provide an outer transcriptional control model used for the
regulation of gene expression, and are implicated in tumor
development and progression (4). DNA
methylation plays an important role in epigenetics, and abnormal
DNA methylation patterns may lead to transcriptional repression,
cell cycle disorder, abnormal activation or inactivation of
signaling pathways, abnormal apoptotic mechanisms, activation of
proto-oncogenes and tumorigenesis (5–8).
Immunotherapy has gained traction as a viable
treatment option for multiple cancer types. Programmed death-1
(PD-1), a member of the CD28 superfamily, is an important
immunosuppressive molecule. Immunoregulatory targeting of PD-1
exhibits significant potential in tumor therapy; its ligand PD-L1
(programmed cell death-Ligand 1) is a transmembrane protein (40
kDa) that can be targeted using antibodies. Typically, the immune
system responds to foreign antigens that accumulate in the lymph
nodes or spleen by promoting T-cell proliferation, with antigen
specificity. Tumor cells evade destruction by T-cells by expressing
PD-L1 on their surface. When T-cell PD-1 recognizes its ligand
(PD-L1) it transmits inhibitory signals, and hence prevents T-cell
activation. PD-1/PD-L1 inhibitors block the binding of PD-1 to
PD-L1, preventing negative regulatory signals and restoring T-cell
activity, thereby enhancing the immune response (9,10).
Currently, immunological inhibitors of PD-1/PD-L1 signaling pathway
checkpoints are effective in the treatment of malignant tumors,
such as melanoma, non-small cell lung cancer (NSCLC) and lymphoma
(11–13). However, the potential of
immunotherapy as a treatment option for patients with gastric
cancer remains to be elucidated.
The purpose of the present study was to investigate
the methylation status of the PD-L1 gene in the cancerous and
adjacent tissues of patients with gastric cancer, alongside
immunohistochemical analysis of the PD-L1 protein. The association
between PD-L1 methylation patterns and clinical characteristics,
chemotherapy efficacy, progression-free survival (PFS) and OS times
in patients with advanced gastric cancer was evaluated.
Materials and methods
Patients and sample collection
A total of 70 paraffin-embedded tissue samples were
collected from patients with advanced gastric cancer, comprised of
49 men (30–83 years) and 21 women (43–79 years), who were
hospitalized at the Second Affiliated Hospital of Dalian Medical
University (Liaoning, China) between January 2010 and August 2017,
and were retrospectively analyzed in the present study. A total of
20/70 patients with gastric cancer were selected, and the adjacent
tissues of these 20 patients were used as controls. The inclusion
criteria were as follows: i) Recurrence after radical gastrectomy
or palliative surgery was histopathologically confirmed as gastric
cancer; ii) full follow-up data were available; iii) patients had
received ≥2 cycles of chemotherapy at the Second Affiliated
Hospital of Dalian Medical University (Liaoning, China); iv)
lesions were measured using imaging machines such as CT or MRI; and
v) the Eastern Cooperative Oncology Group score was ≤2 points. The
exclusion criteria included: i) Patients who had previously
received chemotherapy, radiotherapy and/or biological treatment at
another institution; ii) patients with abnormal liver, kidney or
bone marrow function; and iii) patients with other organ diseases,
immune dysfunction or malignant tumors. All 70 patients were
followed up via clinical visits or telephone calls; the final
follow-up was conducted in January 2019 and the median follow-up
time was 10.55 months. Tumor stage was classified according to the
American Joint Committee on Cancer TNM staging system (7th
edition). The present study was approved by the Ethics Committee of
the medical university and the patients provided written informed
consent.
Efficacy evaluation criteria
The majority of patients received two-drug
combination chemotherapy (n=49), with fewer patients receiving
single-agent (n=12) or three-drug combination treatment (n=9).
Patients that received two-drug therapy were divided into
paclitaxel/fluorouracil and platinum/fluorouracil groups.
Evaluation of treatment efficacy was calculated for all patients
following 2–3 cycles of chemotherapy, according to the Response
Evaluation Criteria in Solid Tumors 1.1 (RECIST1.1). The
chemotherapy efficacy and disease control rates were calculated as
[complete response (CR) + partial response (PR)]/total cases ×100%,
and [CR + PR + stable disease (SD)]/total cases ×100%,
respectively.
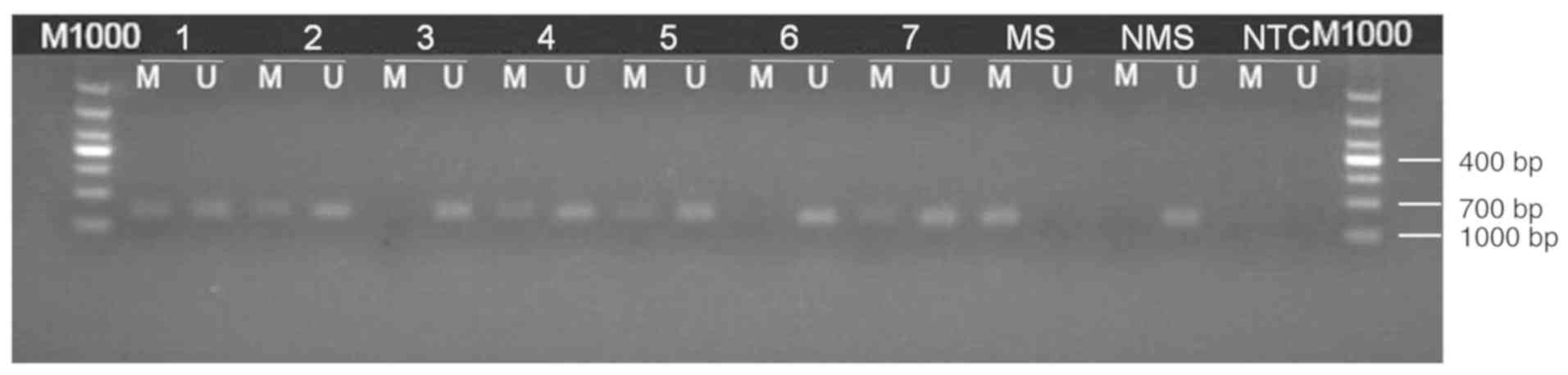
Methylation-specific PCR (MSP)
PD-L1 gene promoter methylation was detected using
MSP. Genomic DNA isolation was performed on five 10-µM
paraffin-fixed sections using a genomic DNA extraction kit, and
bisulfite-mediated DNA modification was performed using the EZ DNA
Methylation Kit (both Zymo Research Corp.) according to the
manufacturers' protocols. Methylation-specific primers were
designed using Sequenom Assay Design 3.1 software (Sequenom) and
the sequences are listed in Table I.
The MSP reaction (10 µl) included 3.5 µl ddH2O, 4 µl
modified DNA, 1 µl 10X Buffer I, 0.1 µl HsTaq DNA polymerase
mixture and 0.6 µl methylation-specific or non-methylation-specific
primers.
 | Table I.Sequences and amplicon sizes of
primers used for programmed cell death-Ligand 1
methylation-specific PCR. |
Table I.
Sequences and amplicon sizes of
primers used for programmed cell death-Ligand 1
methylation-specific PCR.
| Primer | Sequence | PCR product size,
bp |
|---|
| Forward
methylation-specific primer |
5′-ATGTTAGGTTGGAGGTTTGGATAC-3′ | 141 |
| Forward
non-methylation-specific primer |
5′-ATGTTAGGTTGGAGGTTTGGATAT-3′ | 141 |
| Universal reverse
primer |
5′-TTCC(G/A)TTCAAAAATCCTAAACCTAC-3′ | N/A |
PCR was performed using the following thermocycling
conditions: 95°C for 5 min; 35 cycles of 95°C for 30 sec, 63°C for
30 sec and 72°C for 30 sec; followed by extension at 72°C for 10
min and storage at 4°C. PCR products were extracted after gel
electrophoresis and subsequently sequenced (Invitrogen; Thermo
Fisher Scientific, Inc.). Amplification using methylation-specific
primers, or a lack of amplification using non-methylation-specific
primers was considered to indicate a positive result for
methylation. Additionally, amplifications using
methylation-specific and non-methylation-specific primers that also
exhibited partial methylation were recorded as positive
methylation. Results indicating negative methylation were reported
when no amplification was observed using methylation-specific
primers, or amplification was observed using
non-methylation-specific primers (Fig.
1).
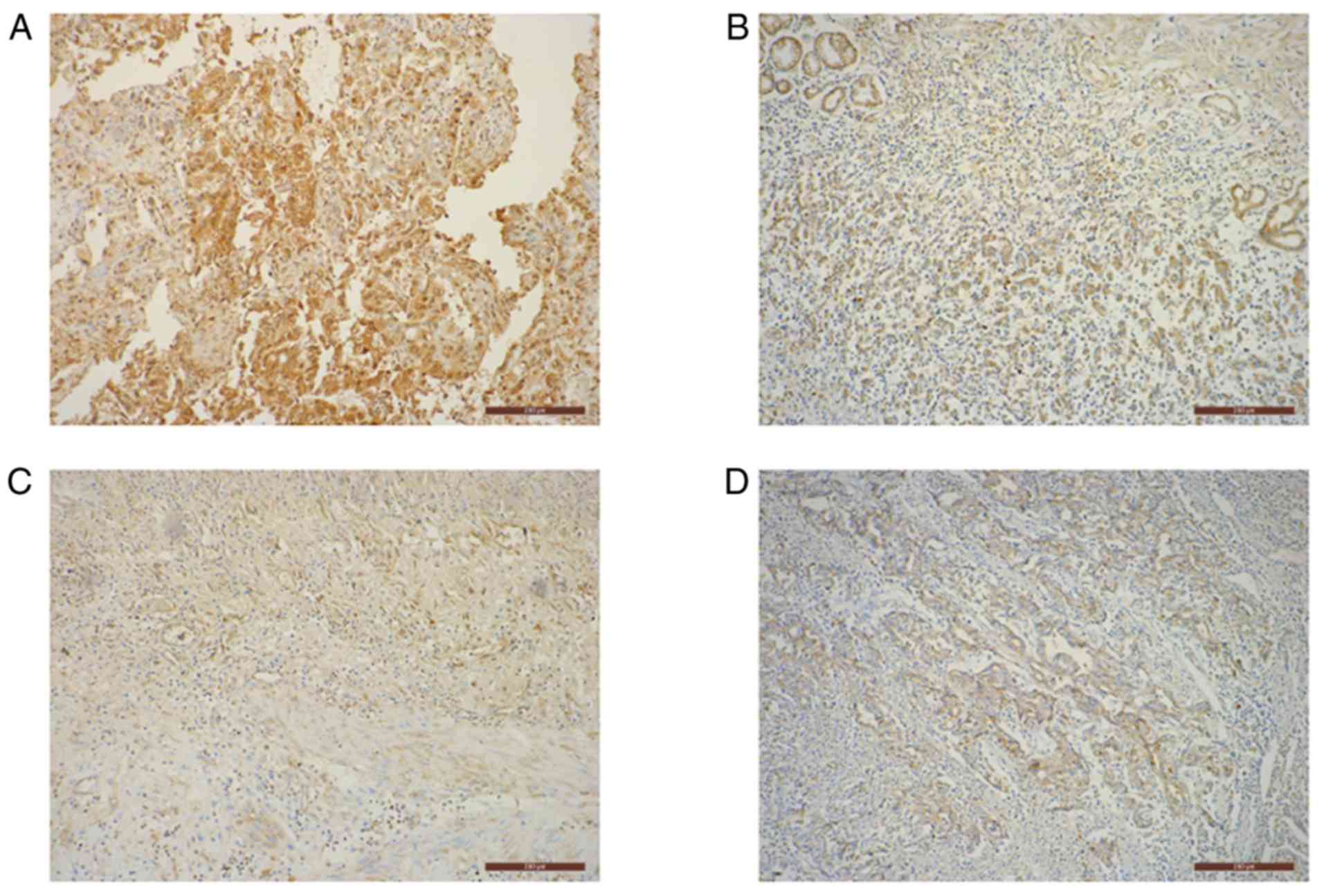
Immunohistochemistry (IHC)
Immunohistochemical staining of PD-L1 was performed
on 6-µm formalin-fixed (at 4°C, overnight), paraffin-embedded
tissue sections using the Immunohistochemical
Streptavidin-Peroxidase kit (OriGene Technologies, Inc.), according
to the manufacturer's protocol. Sections were then incubated with a
primary anti-PD-L1 rabbit polyclonal antibody (1:50; ProteinTech
Group, Inc.; cat. no. 17952-1-AP) at 4°C overnight, followed by
peroxidase-labeled secondary antibody (SPlink Detection Kits;
pre-diluted; cat. no. SP-9001; ZSGB-BIO) staining at 37°C for 1 h.
Immune complexes were stained using 3,3′-diamino-benzidine
tetrahydrochloride (DAB) substrate at room temperature for 1 min.
Subsequently, the slides were counterstained using hematoxylin
(Hematoxylin and Eosin Staining kit; cat. no. C0105) at room
temperature for 1 min and treated with neutral balsam, according
the manufacturer's protocol. Phosphate-buffered saline was used
instead of the primary antibody as a negative control.
The cytoplasmic presence of pale yellow to moderate
brown granules was considered to represent positive PD-L1 staining.
The staining was scored and averaged by three independent clinical
pathologists according to a predefined scoring system (14). Five random fields were imaged from
each slide using a BX41 light microscope (Olympus Corp.) at a
magnification of ×100. Staining was graded based on the intensity
of the majority of the positively stained cells: 0, no staining; 1,
pale yellow; 2, moderate brown; and 3, dark brown. Additionally, a
score was assigned based on the average percentage of positively
stained tumor cells from all five fields: 0, ≤25; 1, 26–50; 2,
51–75; and 3, >75%. The final score was obtained by adding the
percentage score and intensity grade, and stratified as: ‘−’ =0;
‘+’ =1–2; ‘++’ =3–4; and ‘+++’ =5–6. The ‘++’ and ‘+++’ groups
denoted positive expression, whilst the ‘−’ and ‘+’ groups
represented negative expression.
Statistical analysis
SPSS 22.0 (IBM Corp.) was used to perform
statistical analyses. The χ2 test was used to compare
PD-L1 methylation patterns between cancer and adjacent tissues, and
the Fisher's exact test was used to determine the association of
PD-L1 methylation in cancer tissues with certain clinical
characteristics and chemotherapeutic efficacy. The logistic
regression model was used to find predictors of chemotherapy
efficacy. The log-rank test was used to compare survival times and
Kaplan-Meier analysis was used to construct survival curves.
Prognostic factors were analyzed by Cox's regression. P<0.05 was
considered to indicate a statistically significant difference.
Results
PD-L1 promoter methylation levels
The methylation rate of the PD-L1 gene promoter was
significantly higher in gastric cancer tissue samples, compared
with the adjacent tissues [37.1% (26/70) vs. 10% (2/20);
χ2=5.374; P=0.021; Table
II).
 | Table II.Methylation status of PD-L1 in 70
gastric cancer and adjacent tissues. |
Table II.
Methylation status of PD-L1 in 70
gastric cancer and adjacent tissues.
|
| PD-L1 promoter
methylation |
|
|
|---|
|
|
|
|
|
|---|
| Tissue type | Yes | No | P-value | χ2
value |
|---|
| Gastric cancer | 26 | 44 | 0.021a | 5.374 |
| Adjacent
tissues | 2 | 18 |
|
|
Association of PD-L1 methylation
levels with clinical characteristics
PD-L1 methylation was significantly associated with
lymph node staging (Table III): In
the gastric cancer tissue group, a high methylation rate of the
PD-L1 promoter region was significantly associated with the N3
stage, but not with patients from the N0-N2 group (P=0.049).
However, no association was observed between PD-L1 methylation and
the other clinical characteristics investigated.
 | Table III.Correlation between PD-L1 promoter
methylation and the clinicopathological characteristics. |
Table III.
Correlation between PD-L1 promoter
methylation and the clinicopathological characteristics.
|
| PD-L1 promoter
methylation |
|
|---|
|
|
|
|
|---|
|
|
| Methylation |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Patients, n | Yes | No | P-value |
|---|
| Sex |
|
|
|
|
|
Male | 49 | 18 | 31 | 0.914 |
|
Female | 21 | 8 | 13 |
|
| Age, years |
|
|
|
|
|
≤60 | 31 | 12 | 19 | 0.809 |
|
>60 | 39 | 14 | 25 |
|
|
Recurrence/Palliative surgery |
|
|
|
|
|
Recurrence | 32 | 12 | 20 | 0.955 |
|
Palliative surgery | 38 | 14 | 24 |
|
| Tumor size |
|
|
|
|
| >5
cm | 32 | 10 | 22 | 0.326 |
| ≤5
cm | 35 | 15 | 20 |
|
| Lymph node
staging |
|
|
|
|
|
N0-N2 | 34 | 9 | 25 | 0.049a |
| N3 | 32 | 16 | 16 |
|
| Vessel carcinoma
embolus |
|
|
|
|
|
Positive | 35 | 14 | 21 | 0.653 |
|
Negative | 2 | 1 | 1 |
|
| Perineural
invasion |
|
|
|
|
|
Positive | 15 | 5 | 10 | 0.347 |
|
Negative | 5 | 3 | 2 |
|
| Degree of
differentiation |
|
|
|
|
|
Moderate or well | 32 | 10 | 22 | 0.326 |
|
Poor | 35 | 15 | 20 |
|
| Pathological
type |
|
|
|
|
| Simple
adenocarcinoma | 40 | 16 | 24 | 0.641 |
|
Other | 29 | 10 | 19 |
|
PD-L1 promoter methylation is
associated with increased protein expression
Of the 26 advanced gastric cancer tissues that were
identified as positive for PD-L1 promoter methylation, 73.1% were
also positive for PD-L1 protein expression (Fig. 2). Additionally, out of the 44 cancer
tissues negative for PD-L1 promoter methylation, 47.7% were
positive for PD-L1 protein expression (Fig. 2). Furthermore, a significantly
positive association between PD-L1 promoter methylation and protein
expression was identified (P=0.038; Table IV).
 | Table IV.Association between PD-L1 promoter
methylation and protein expression in advanced gastric cancer
tissues. |
Table IV.
Association between PD-L1 promoter
methylation and protein expression in advanced gastric cancer
tissues.
|
| PD-L1 protein
expression |
|
|
|
|---|
|
|
|
|
|
|
|---|
| PD-L1 promoter
methylation | + | − | Total | P-value | χ2
value |
|---|
| Methylation | 19 | 7 | 26 | 0.038a | 4.288 |
| No methylation | 21 | 23 | 44 |
|
|
| Total | 40 | 30 | 70 |
|
|
Increased PD-L1 methylation predicts
poor chemotherapeutic efficacy
In the population analyzed, chemotherapy was
significantly more effective in patients with a non-methylated
PD-L1 gene promoter (P=0.037) and lower lymph node stage (P=0.009).
The rate of chemotherapeutic efficacy (P=0.038) and disease control
(P=0.024) in patients with recurrence following radical gastrectomy
was higher than that of patients who underwent palliative surgery.
Consequently, these factors can be considered as predictors of
chemotherapeutic efficacy in patients with gastric cancer. Other
clinicopathological characteristics and protein expression levels
were not found to be significantly associated with chemotherapeutic
efficacy (Table V).
 | Table V.Correlation between
clinicopathological characteristics and chemotherapeutic
efficacy. |
Table V.
Correlation between
clinicopathological characteristics and chemotherapeutic
efficacy.
|
|
| Chemotherapy
effective |
| Disease
control |
|
|---|
|
|
|
|
|
|
|
|---|
| Characteristic | Patients, n | SD + PD (%) | CR + PR (%) | P-value | PD (%) | CR+PR+SD (%) | P-value |
|---|
| Total, n | 70 | 52 (74.3) | 18 (25.7) |
| 15 (21.4) | 55 (78.6) |
|
| Sex |
|
|
|
|
|
|
|
|
Male | 49 | 35 (71.4) | 14 (28.6) | 0.403 | 10 (20.4) | 39 (79.6) | 0.990 |
|
Female | 21 | 17 (81.0) | 4 (19.0) |
| 5 (23.8) | 16 (76.2) |
|
| Age, years |
|
|
|
|
|
|
|
|
≤60 | 31 | 24 (77.4) | 7 (22.6) | 0.593 | 6 (19.3) | 25 (80.7) | 0.706 |
|
>60 | 39 | 28 (71.8) | 11 (28.2) |
| 9 (23.1) | 30 (76.9) |
|
| PD-L1 promoter
methylation |
|
|
|
|
|
|
|
|
Methylation | 26 | 23 (88.5) | 3 (11.5) | 0.037a | 6 (23.1) | 20 (76.9) | 0.796 |
| No
methylation | 44 | 29 (65.9) | 15 (34.1) |
| 9 (20.5) | 35 (79.5) |
|
|
Recurrence/Palliative surgery |
|
|
|
|
|
|
|
|
Recurrence | 32 | 20 (62.5) | 12 (37.5) | 0.038a | 3 (9.4) | 29 (90.6) | 0.024a |
|
Palliative surgery | 38 | 32 (84.2) | 6 (15.8) |
| 12 (31.6) | 26 (68.4) |
|
| Lymph node
stage |
|
|
|
|
|
|
|
|
N0-N2 | 34 | 20 (58.8) | 14 (41.2) | 0.009a | 5 (14.7) | 29 (85.3) | 0.293 |
| N3 | 32 | 28 (87.5) | 4 (12.5) |
| 8 (25.0) | 24 (75.0) |
|
| Vessel carcinoma
embolus |
|
|
|
|
|
|
|
|
Positive | 35 | 27 (77.1) | 8 (22.9) | 0.432 | 7 (20.0) | 8 (80.0) | 0.990 |
|
Negative | 2 | 1 (50.0) | 1 (50.0) |
| 0 (0) | 2 (100) |
|
| Perineural
invasion |
|
|
|
|
|
|
|
|
Positive | 15 | 10 (66.6) | 5 (33.3) | 0.990 | 1 (6.7) | 14 (93.3) | 0.140 |
|
Negative | 5 | 4 (80) | 1 (20) |
| 2 (40) | 3 (60) |
|
| Degree of
differentiation |
|
|
|
|
|
|
|
|
Moderate or well | 32 | 22 (68.8) | 10 (31.2) | 0.290 | 4 (12.5) | 28 (87.5) | 0.063 |
|
Poor | 35 | 28 (80.0) | 7 (20.0) |
| 11 (31.4) | 24 (68.6) |
|
| Pathological
type |
|
|
|
|
|
|
|
| Simple
adenocarcinoma | 40 | 31 (77.5) | 9 (22.5) | 0.426 | 10 (25.0) | 30 (75.0) | 0.441 |
|
Other | 29 | 20 (69.0) | 9 (31.0) |
| 5 (17.2) | 24 (82.8) |
|
| Tumor size |
|
|
|
|
|
|
|
| >5
cm | 32 | 25 (78.1) | 7 (21.9) | 0.378 | 6 (18.75) | 26 (81.25) | 0.680 |
| ≤5
cm | 35 | 24 (68.6) | 11 (31.4) |
| 8 (22.9) | 27 (77.1) |
|
| Therapy |
|
|
|
|
|
|
|
|
Multi-drug combination | 58 | 40 (69.0) | 18 (31.0) | 0.061 | 10 (17.2) | 48 (82.8) | 0.136 |
|
Monotherapy | 12 | 12 (100) | 0 (0) |
| 5 (41.7) | 7 (58.3) |
|
| Chemotherapy
regimen |
|
|
|
|
|
|
|
|
Paclitaxel/Fluorouracil | 24 | 17 (70.8) | 7 (29.2) | 0.749 | 6 (25.0) | 18 (75.0) | 0.181 |
|
Platinum/Fluorouracil | 27 | 18 (66.7) | 9 (33.3) |
| 2 (7.4) | 25 (92.6) |
|
| PD-L1 protein
expression |
|
|
|
|
|
|
|
| + | 40 | 30 (70.8) | 10 (55.6) | 0.875 | 7 (46.7) | 33 (60.0) | 0.355 |
| − | 30 | 22 (66.7) | 8 (44.4) |
| 8 (53.3) | 22 (40.0) |
|
The results of the multivariate logistic regression
analysis are exhibited in Table VI.
Certain pre-selected patient characteristics (number of lymph node
metastases, recurrence after radical gastrectomy or palliative
surgery, monotherapy or multi-drug combination therapy) and the
degree of methylation were independent variables, and the effective
rate of chemotherapy and disease control rate of patients were
selected as the dependent variables. Multivariate logistic
regression analysis demonstrated that PD-L1 methylation was able to
independently predict chemotherapeutic efficacy in patients with
gastric cancer. Patients with recurrence after radical gastrectomy
exhibit improved chemotherapeutic efficacy and disease control rate
compared with palliative surgery patients (Table VI).
 | Table VI.Multivariate logistic regression
analysis. |
Table VI.
Multivariate logistic regression
analysis.
|
| Chemotherapy
effective | Disease
control |
|---|
|
|
|
|
|---|
| Characteristic | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| PD-L1 promoter
methylation | 6.784 | 1.383–33.280 | 0.018a |
|
|
|
|
Recurrence/palliative surgery | 4.252 | 1.036–17.456 | 0.045a | 6.350 | 1.377–29.274 | 0.018a |
| Lymph node
staging | 0.235 | 0.057–0.965 | 0.082 | 5.588 | 1.175–26.568 | 0.031a |
|
Monotherapy/multi-drug combination | – | 0.000 | 0.998 |
|
|
|
PFS and OS analysis
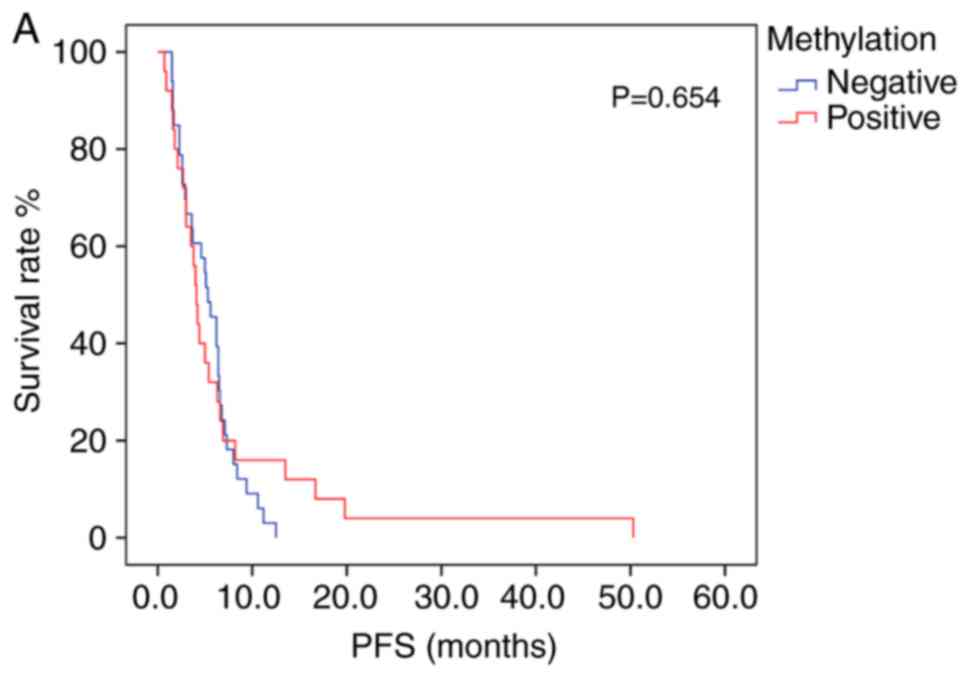
Patients exhibiting positive PD-L1 methylation had
lower mPFS and mOS times (log-rank test, Table VII) compared with patients with
negative PD-L1 methylation; however, the difference between groups
was not statistically significant (Fig.
3A and B). The association between survival time and PD-L1
protein expression was also not statistically significant (Table VII). Patients receiving
platinum/fluorouracil chemotherapy had a longer mPFS, suggesting an
overall improvement in PFS time in patients receiving this
treatment type. A Cox proportional hazards regression model was
constructed, in which multivariate survival analyses were applied
to assess the association between mPFS and several clinical
characteristics; however, no significant associations were
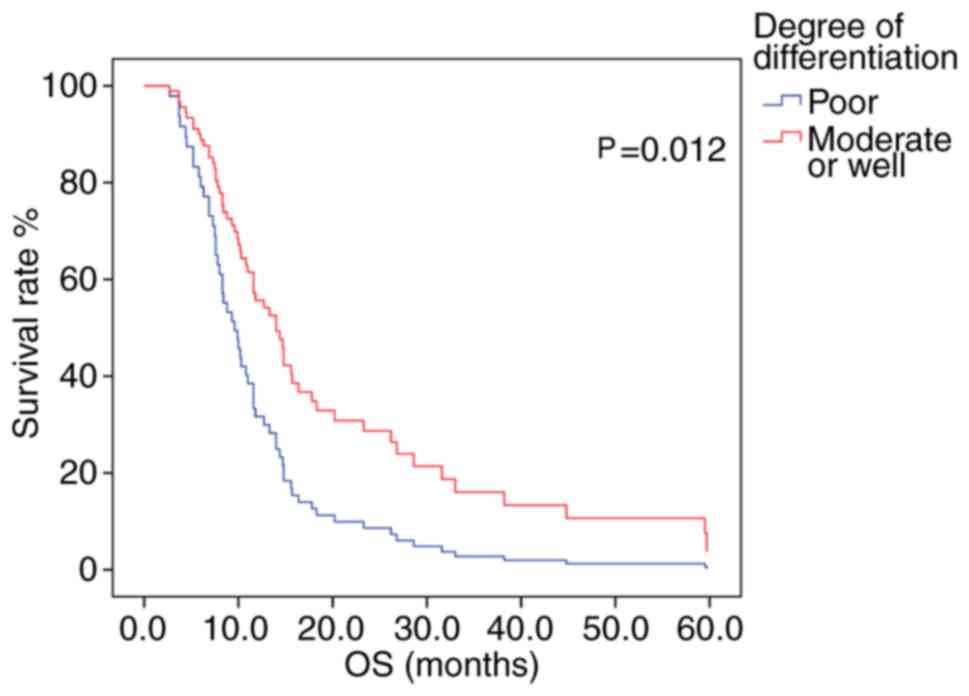
determined. The mOS time was greater in patients with moderately-
or well-differentiated tumors, compared with that in those with
poorly-differentiated tumors (P=0.012; log-rank test; Fig. 4).
 | Table VII.Survival outcome of 70 gastric
patients. |
Table VII.
Survival outcome of 70 gastric
patients.
|
| PFS |
| OS, months |
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | mPFS, months | χ2
value | P-value | Patients, n | mOS, months | χ2
value | P-value | Patients, n |
|---|
| Sex |
|
|
|
|
|
|
|
|
|
Male | 5.0 | 0.240 | 0.624 | 42 | 10.3 | 0.083 | 0.773 | 47 |
|
Female | 4.2 |
|
| 16 | 11.0 |
|
| 20 |
| Age, years |
|
|
|
|
|
|
|
|
|
≤60 | 5.6 | 3.630 | 0.057 | 27 | 10.2 | 0.011 | 0.916 | 29 |
|
>60 | 4.4 |
|
| 31 | 11.0 |
|
| 38 |
| PD-L1 promoter
methylation |
|
|
|
|
|
|
|
|
|
Methylation | 4.1 | 0.201 | 0.654 | 25 | 9.5 | 0.409 | 0.523 | 26 |
| No
methylation | 5.3 |
|
| 33 | 10.8 |
|
| 41 |
| Chemotherapy
regimen |
|
|
|
|
|
|
|
|
|
Platinum/Fluorouracil | 5.6 | 3.869 | 0.049a | 19 | 11.6 | 0.997 | 0.318 | 26 |
|
Paclitaxel/Fluorouracil | 4.2 |
|
| 23 | 10.3 |
|
| 24 |
|
Recurrence/Palliative surgery |
|
|
|
|
|
|
|
|
|
Recurrence | 5 | 1.455 | 0.228 | 25 | 11.6 | 3.141 | 0.076 | 30 |
|
Palliative operation | 3.8 |
|
| 33 | 9.5 |
|
| 37 |
| Degree of
differentiation |
|
|
|
|
|
|
|
|
|
Moderate or well | 5.1 | 0.237 | 0.626 | 23 | 14.0 | 7.574 | 0.006a | 29 |
|
Poor | 3.8 |
|
| 32 | 8.4 |
|
| 35 |
| Pathological
type |
|
|
|
|
|
|
|
|
| Simple
adenocarcinoma | 4.0 | 0.959 | 0.328 | 31 | 11.6 | 0.801 | 0.371 | 39 |
|
Other | 5.3 |
|
| 26 | 8.8 |
|
| 27 |
| Tumor size |
|
|
|
|
|
|
|
|
| >5
cm | 5.0 | 0.348 | 0.555 | 26 | 10.0 | 1.834 | 0.176 | 31 |
| ≤5
cm | 4.4 |
|
| 29 | 10.8 |
|
| 33 |
| Lymph node
staging |
|
|
|
|
|
|
|
|
|
N0-N2 | 5.0 | 0.064 | 0.801 | 25 | 11.6 | 2.602 | 0.107 | 32 |
| N3 | 5.4 |
|
| 29 | 9.9 |
|
| 31 |
| PD-L1 protein
expression |
|
|
|
|
|
|
|
|
| + | 5.6 | 2.480 | 0.115 | 35 | 11.6 | 0.461 | 0.479 | 40 |
| − | 3.7 |
|
| 23 | 8.6 |
|
| 30 |
Prognostic significance of PD-L1
methylation and clinical characteristics
In the Cox proportional hazards regression model,
univariate and multivariate survival analyses were performed to
assess the association between PD-L1 methylation and several
clinical characteristics. Univariate analyses demonstrated that
tumor differentiation was associated with mOS time only.
Multivariate analysis revealed that the degree of tumor
differentiation (P=0.012; HR=1.965) was able to independently
predict patient prognosis (Table
VIII).
 | Table VIII.Cox proportional hazards assessment
of prognostic factors in 70 gastric cancer patients. |
Table VIII.
Cox proportional hazards assessment
of prognostic factors in 70 gastric cancer patients.
|
|
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|
|---|
| Factor | β | SE | Wald | P-value | HR | Lower | Upper |
|---|
| Degree of
differentiation | 0.675 | 0.270 | 6.255 | 0.012 | 1.965 | 1.157 | 3.336 |
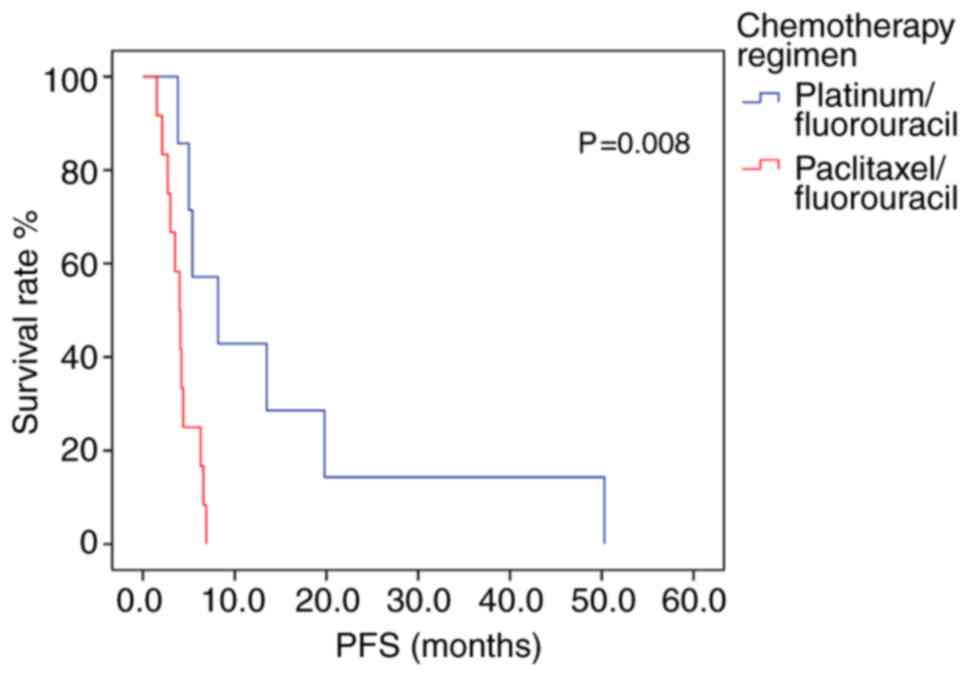
Of the 26 patients exhibiting a methylated PD-L1
promoter region, those receiving platinum/fluorouracil combination
therapy had an mPFS 4.2 months longer than patients who received
paclitaxel/fluorouracil chemotherapy (P=0.008; log-rank test;
Fig. 5; Table IX). Multivariate analysis included
age and chemotherapy regimen as independent variables, but the
model constructed indicated that age did not produce a significant
result, and it contained only chemotherapy regimen variables. The
risk of disease progression in patients receiving
paclitaxel/fluorouracil chemotherapy was 5.009 times higher than in
those who had received platinum/fluorouracil chemotherapy.
Therefore, Platinum-containing first-line chemotherapy could
represent an independent prognostic factor for PFS time in patients
with advanced gastric cancer exhibiting methylated PD-L1 promoter
regions (P=0.015; Table X). In the
44 patients with a non-methylated PD-L1 promoter region, patients
with moderately- or well-differentiated tumors exhibited higher
mPFS and mOS times than patients with poorly-differentiated tumors
(P<0.05; Table SI) and there was
no other statistically significant difference in survival time
associated with with treatment regimen (Table SI).
 | Table IX.Survival outcome of 26 patients
exhibiting methylated PD-L1. |
Table IX.
Survival outcome of 26 patients
exhibiting methylated PD-L1.
|
| PFS |
| OS |
|
|
|
|
|
|
|
| Characteristic | mPFS, months | χ2
value | P-value | Patients, n | mOS, months | χ2
value | P-value | Patients, n |
|---|
| Sex |
|
|
|
|
|
|
|
|
|
Male | 3.8 | 0.335 | 0.563 | 18 | 11.6 | 0.005 | 0.946 | 18 |
|
Female | 4.2 |
|
| 7 | 8.4 |
|
| 8 |
| Age (years) |
|
|
|
|
|
|
|
|
|
≤60 | 6.3 | 6.132 | 0.013a | 12 | 9.5 | 2.825 | 0.093 | 12 |
|
>60 | 4.0 |
|
| 13 | 9.3 |
|
| 14 |
| Chemotherapy
regimen |
|
|
|
|
|
|
|
|
|
Platinum/Fluorouracil | 8.2 | 7.115 | 0.008a | 7 | 11.7 | 2.994 | 0.084 | 8 |
|
Paclitaxel/Fluorouracil | 4.0 |
|
| 12 | 9.3 |
|
| 12 |
|
Recurrence/Palliative surgery |
|
|
|
|
|
|
|
|
|
Recurrence | 4.2 | 1.926 | 0.165 | 11 | 11.6 | 3.474 | 0.062 | 12 |
|
Palliative surgery | 3.5 |
|
| 14 | 7.3 |
|
| 14 |
| Degree of
differentiation |
|
|
|
|
|
|
|
|
|
Moderate or well | 3.5 | 1.434 | 0.231 | 9 | 9.3 | 0.206 | 0.65 | 10 |
|
Poor | 5.0 |
|
| 15 | 11.6 |
|
| 15 |
| Pathological
type |
|
|
|
|
|
|
|
|
| Simple
adenocarcinoma | 3.5 | 2.790 | 0.095 | 15 | 9.5 | 0.353 | 0.553 | 16 |
|
Other | 5.4 |
|
| 10 | 8.3 |
|
| 10 |
| Tumor size |
|
|
|
|
|
|
|
|
| >5
cm | 3.8 | 0.223 | 0.637 | 10 | 9.5 | 0.081 | 0.776 | 10 |
| ≤5
cm | 4.0 |
|
| 14 | 8.4 |
|
| 15 |
| Lymph node
staging |
|
|
|
|
|
|
|
|
|
N0-N2 | 4.2 | 0.036 | 0.849 | 9 | 9.3 | 0.033 | 0.855 | 9 |
| N3 | 4.0 |
|
| 15 | 9.9 |
|
| 16 |
 | Table X.Cox proportional hazard assessment of
prognostic factors in 26 patients exhibiting methylated Programmed
cell death-Ligand 1. |
Table X.
Cox proportional hazard assessment of
prognostic factors in 26 patients exhibiting methylated Programmed
cell death-Ligand 1.
|
|
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|
|---|
| Variable | B | SE | Wald | P-value | HR | Lower | Upper |
|---|
| Chemotherapy
regimen of Platinum/Fluorouracil | 1.611 | 0.661 | 5.939 | 0.015a | 5.009 | 1.371 | 18.304 |
Discussion
Numerous studies have suggested that the development
of gastric cancer is a complex, multi-factorial and multi-signal
biological process. Factors resulting in gene mutation and
epigenetic modifications also serve an important role in the
tumorigenesis and progression of gastric cancer (15). In multiple malignancies, aberrant DNA
methylation patterns are associated with transcriptional
repression, cell cycle disorder, abnormal activation or
inactivation of signaling pathways, increased cell invasion,
abnormal apoptotic mechanisms, activation of proto-oncogenes and
the promotion of tumorigenesis (16).
PD-L1 (also known as CD274 or B7-H1) is a type I
glycoprotein expressed in various cells, including T cells,
epithelial and endothelial cells, following the stimulation of
proinflammatory mediators such as interferon-γ (17). Various studies have demonstrated that
PD-L1 is differentially expressed in solid tumors, and that it
serves an important role in the development of numerous tumor
types, including NSCLC (18), colon
(19), breast (20) and ovarian cancers (21), and also in melanoma (22). A high expression level of PD-L1 is
frequently observed in tumor cells and is associated with the
promotion of metastasis and infiltration; it also facilitates tumor
cell escape from CD8+ T cells, thereby allowing them to
evade immune surveillance (23).
At present, the association between the expression
level of PD-L1 and disease prognosis in patients with gastric
cancer is inconclusive. Multiple studies have shown that the
expression of PD-L1 is upregulated in gastric cancer tissues
compared with little to no protein expression in adjacent tissues
(24–26). Therefore, in the present study, IHC
was performed to identify the different methylation states of the
PD-L1 gene in gastric cancer tissues. Previous studies have
illustrated that PD-L1 protein expression in gastric cancer is
associated with tumor size, lymph node metastasis and the depth of
tumor invasion. Moreover, patients with higher PD-L1 expression
levels exhibited a short OS period, indicating that PD-L1
expression is an important prognostic factor in gastric cancer
(24–26). However, a study in South Korea
(27) determined that high
expression levels of PD-L1 in gastric cancer cells predict
favorable clinicopathological features and prognosis. At present,
the majority of research on the PD-L1 gene in gastric cancer is
focused on protein and mRNA expression, and not on methylation
patterns. Therefore, in an attempt to elucidate its potential for
the prediction of disease prognosis, the present study was
conducted to explore the influence of PD-L1 gene methylation and
protein expression in patients with advanced gastric cancer, and to
characterize its association with various clinicopathological
characteristics.
HMSP was used to detect the extent of PD-L1
methylation in gastric cancer tissues (37.1%) compared with
adjacent tissues (10%). Previously, studies have concluded that
methylation of the PD-L1 promoter in prostate cancer tissues is
higher than in normal prostate tissue (28), corroborating the conclusions of the
present study. Notably, certain studies have elucidated that in
human melanoma, PD-L1 expression may be altered by DNA
hypomethylating agents (29).
Moreover, the expression level of PD-L1 can be regulated using DNA
methylation, in response to NF-κB or transforming growth factor-β
signaling in NSCLC (30). In the
current study, the PD-L1 methylation status correlated with PD-L1
protein expression and the number of lymph node metastases,
implying that methylation of the PD-L1 promoter may affect tumor
progression in advanced gastric cancer tissues, via the regulation
of protein expression. The results of the present study support the
hypothesis that methylation of the PD-L1 promoter promotes lymph
node metastasis, and that this may be due to changes in the tumor
microenvironment resulting from PD-L1 hypermethylation. Further
studies investigating the mechanism behind this process may offer
insights into potential therapeutic targets or prognostic
biomarkers. In summary, PD-L1 methylation regulates PD-L1
expression and may therefore, represent an optimal biomarker for
the indication of tumor progression in patients with advanced
gastric cancer.
It has previously been demonstrated that methylation
of the PD-L1 promoter may influence prognosis in numerous cancer
types. For example, increased methylation of PD-L1 was
significantly associated with shorter recurrence-free survival and
OS times in patients with colorectal cancer (31). Similarly, in prostate cancer, high
levels of PD-L1 methylation correlated with an increased risk of
recurrence (28). Micevic et
al (29) demonstrated that in
melanoma, PD-L1 hypermethylation was associated with poor OS, and
was also considered an independent prognostic factor. By contrast,
increased PD-L1 methylation was significantly associated with the
reduced risk of relapse and prolonged OS times in patients with
acute myelocytic leukemia (32). In
the present study, chemotherapy was less effective in patients with
methylated PD-L1 compared with those with no methylation (11.5 vs.
34.1%; n=70). The results also indicated that methylation of the
PD-L1 promoter may represent an independent prognostic factor for
chemotherapeutic efficacy in the treatment of advanced gastric
cancer. Furthermore, patients with methylated PD-L1 promoters
exhibited a shorter PFS and OS times than those without. The
results of the current study also indicated a correlation between
the methylation status of PD-L1 in the promoter region and OS time;
however this result was not statistical significant, which may be
due to the insufficient population size. Thus in the future,
further studies should be conducted on larger populations to
increase the validity of the conclusions drawn.
In the present study, the log-rank test was used to
compare the OS times, and to determine the association between,
PD-L1 protein expression and prognosis. However, in contrast to
previous studies, a significant association between PD-L1
expression and prognosis was not determined, perhaps due to the
fact that protein expression is not solely regulated by DNA
methylation, but also by other upstream factors. Other potential
explanations for this inconsistency may be differences in sample
size, methods of tissue preservation (fresh frozen tissue vs.
paraffin-embedded tissue), detection platforms and antibodies used,
and different thresholds selected.
The current study demonstrated that PD-L1
methylation is positively correlated with PD-L1 protein expression,
indicating that PD-L1 expression may be regulated by promoter
methylation in gastric cancer. Previous research has reported that
PD-L1 methylation is inversely correlated with PD-L1 mRNA
expression (31). Perhaps, PD-L1
methylation regulates protein expression at the mRNA level. A lack
of data regarding PD-L1 mRNA expression meant that this was a
limitation of the present study, thus future research should
investigate the associations between PD-L1 promoter methylation,
mRNA and protein expression in gastric cancer.
At present, first-line chemotherapy for advanced
gastric cancer consists of fluorouracil, which is typically
combined with platinum and/or paclitaxel to form a two- or
three-drug regimen (33). Since
there were fewer patients in the single-agent and three-drug
combination chemotherapy groups, the patients with double-drug
combination chemotherapy were further analyzed. According to the
chemotherapy regimen, patients were divided into
paclitaxel/fluorouracil or platinum/fluorouracil chemotherapy
groups and it was discovered that the PFS time of the patients
receiving a first-line chemotherapy regimen of platinum combined
with fluorouracil was 5.6 months, which was longer than that of the
patients receiving paclitaxel combined with fluorouracil (4.2
months). Therefore, platinum/fluorouracil combination treatment
confers a longer PFS time than paclitaxel/fluorouracil, in patients
with advanced gastric cancer.
Further investigation of the association between
PD-L1 promoter methylation and first-line chemotherapeutic efficacy
for advanced gastric cancer revealed that, in 26 patients
exhibiting methylated PD-L1, the mPFS time (8.2 months) of patients
receiving platinum/fluorouracil chemotherapy was significantly
longer than in patients receiving paclitaxel/fluorouracil (4.0
months). Furthermore, the risk of disease progression in patients
treated with paclitaxel/fluorouracil chemotherapy was 5.009 times
higher compared with patients receiving platinum/fluorouracil
chemotherapy. Recent research has determined that the breast cancer
1, early onset gene expression level is correlated with the
treatment response to cisplatin and oxaliplatin in patients with
gastric cancer (34). Phosphatase
and tensin homolog gene deficiency was observed in BRCA1 mutation
cancers (35,36). Loss of PTEN has been shown to
increase PD-L1 expression via the PI3K pathway (37,38). In
the current study, methylation status correlated with PD-L1 protein
expression. It was hypothesized that patients exhibiting a
methylated PD-L1 promoter region may also possess BRCA1 gene
mutations, and would see the greatest degree of improvement from
platinum-based chemotherapy, potentially resulting in longer PFS
times. Thus, detecting the methylation status of the PD-L1 promoter
region may offer guidance regarding clinical decision-making.
Enhancements in immunotherapy have greatly improved
the prognosis of patients with numerous cancer types. The
PD-1/PD-L1 axis is an inhibitory signaling pathway associated with
T-cell inactivation and exhaustion, which prevents excessive
inflammatory responses (39). Immune
checkpoint inhibitors significantly enhance T-cell function and
therefore exert antitumor activity (40). Results from the phase II KEYNOTE-059
study (41) indicated that the
monoclonal anti-PD-1 antibody pembrolizumab provided an objective
response rate of 60% in previously untreated advanced
gastric/gastroesophageal junction adenocarcinoma. Pembrolizumab
monotherapy showed improved efficacy and manageable safety in
patients with advanced gastric or gastroesophageal cancer who had
previously received ≥2 lines of treatment (42). Further studies are needed to
determine whether PD-L1 promoter methylation allows for survival
prediction in patients with advanced gastric cancer treated with
PD-1/PD-L1 antagonists.
In summary, the present study demonstrated that the
frequency of PD-L1 methylation was higher in gastric cancer tissues
compared with adjacent tissues, and that this correlated with both
protein expression of PD-L1 and the number of lymph node
metastases. This suggests that methylation frequency significantly
influences chemotherapeutic efficacy, and hence, may inform
clinical decisions regarding treatment. Therefore, the results of
the present study support the conclusion that methylation of PD-L1
in the promoter region may represent an independent prognostic
factor, affecting the efficacy of chemotherapy in advanced gastric
cancer via the regulation of protein expression. It may therefore
be used as a novel biomarker for the prediction of first-line
chemotherapeutic efficacy and the prognosis of patients receiving
platinum-containing chemotherapy regimens for the treatment of
advanced gastric cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data that were generated or analyzed in the
present study are included in this manuscript.
Authors' contributions
DL, CX and TW conceived and designed the study. DL
and LC acquired the data. DL, LC, YZ, TW and NG analyzed and
interpreted the data. DL, TW and NG drafted the article. All
authors critically revised the article for important intellectual
content and approved the final version of the article to be
published.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Second Affiliated Hospital of Dalian Medical
University, and all patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PD-1
|
programmed death 1
|
|
PD-L1
|
programmed cell death-Ligand 1
|
|
MSP
|
methylation-specific PCR
|
|
NSCLC
|
non-small cell lung cancer
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meyer HJ and Wilke H: Treatment strategies
in gastric cancer. Dtsch Arztebl Int. 108:698–706. 2011.PubMed/NCBI
|
|
3
|
Shitara K: Chemotherapy for advanced
gastric cancer: Future perspective in Japan. Gastric Cancer. 20
(Suppl 1):S102–S110. 2017. View Article : Google Scholar
|
|
4
|
Herman JG and Baylin SB: Gene silencing in
cancer in association with promoter hypermethylation. N Engl J Med.
349:2042–2054. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilson AS, Power BE and Molloy PL: DNA
hypomethylation and human diseases. Biochim Biophys Acta.
1775:138–162. 2007.PubMed/NCBI
|
|
6
|
Nandakumar V, Vaid M and Katiyar SK:
(−)-Epigallocatechin-3-gallate reactivates silenced tumor
suppressor genes, Cip1/p21 and p16INK4a, by reducing DNA
methylation and increasing histones acetylation in human skin
cancer cells. Carcinogenesis. 32:537–544. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Farias N, Ho N, Butler S, Delaney L,
Morrison J, Shahrzad S and Coomber BL: The effects of folic acid on
global DNA methylation and colonosphere formation in colon cancer
cell lines. J Nutr Biochem. 26:818–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pan Y, Liu G, Zhou F, Su B and Li Y: DNA
methylation profiles in cancer diagnosis and therapeutics. Clin Exp
Med. 18:1–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Constantinidou A, Alifieris C and Trafalis
DT: Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): A
new era in cancer active immunotherapy. Pharmacol Ther. 194:84–106.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Dang F, Ren J and Wei W:
Biochemical aspects of PD-L1 regulation in cancer immunotherapy.
Trends Biochem Sci. 43:1014–1032. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pilon-Thomas S, Mackay A, Vohra N and Mulé
JJ: Blockade of programmed death ligand 1 enhances the therapeutic
efficacy of combination immunotherapy against melanoma. J Immunol.
184:3442–3449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rizvi NA, Hellmann MD, Brahmer JR,
Juergens RA, Borghaei H, Gettinger S, Chow LQ, Gerber DE, Laurie
SA, Goldman JW, et al: Nivolumab in combination with platinum-based
doublet chemotherapy for first-line treatment of advanced
non-small-cell lung cancer. J Clin Oncol. 34:2969–2979. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Wu L, Tian C and Zhang Y:
PD-1-PD-L1 immune-checkpoint blockade in malignant lymphomas. Ann
Hematol. 97:229–237. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fromowitz FB, Viola MV, Chao S, Oravez S,
Mishriki Y, Finkel G, Grimson R and Lundy J: Ras p21 expression in
the progression of breast cancer. Hum Pathol. 18:1268–1275. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Samadani AA, Noroollahi SE,
Mansour-Ghanaei F, Rashidy-Pour A, Joukar F and Bandegi AR:
Fluctuations of epigenetic regulations in human gastric
Adenocarcinoma: How does it affect? Biomed Pharmacother.
109:144–156. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Liang J and Hou P: Hypermethylation
in gastric cancer. Clin Chim Acta. 448:124–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Passiglia F, Bronte G, Bazan V, Natoli C,
Rizzo S, Galvano A, Listì A, Cicero G, Rolfo C, Santini D and Russo
A: PD-L1 expression as predictive biomarker in patients with NSCLC:
A pooled analysis. Oncotarget. 7:19738–19747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koganemaru S, Inoshita N, Miura Y, Miyama
Y, Fukui Y, Ozaki Y, Tomizawa K, Hanaoka Y, Toda S, Suyama K, et
al: Prognostic value of programmed death-ligand 1 expression in
patients with stage III colorectal cancer. Cancer Sci. 108:853–858.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang C, Zhu H, Zhou Y, Mao F, Lin Y, Pan
B, Zhang X, Xu Q, Huang X and Sun Q: Prognostic value of PD-L1 in
breast cancer: A meta-analysis. Breast J. 23:436–443. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Drakes ML, Mehrotra S, Aldulescu M, Potkul
RK, Liu Y, Grisoli A, Joyce C, O'Brien TE, Stack MS and Stiff PJ:
Stratification of ovarian tumor pathology by expression of
programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) in ovarian
cancer. J Ovarian Res. 11:432018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hino R, Kabashima K, Kato Y, Yagi H,
Nakamura M, Honjo T, Okazaki T and Tokura Y: Tumor cell expression
of programmed cell death-1 ligand 1 is a prognostic factor for
malignant melanoma. Cancer. 116:1757–1766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Larsen SK: Cellular immune responses
towards regulatory cells. Dan Med J. 63:B51882016.PubMed/NCBI
|
|
24
|
Sun J, Xu K, Wu C, Wang Y, Hu Y, Zhu Y,
Chen Y, Shi Q, Yu G and Zhang X: PD-L1 expression analysis in
gastric carcinoma tissue and blocking of tumor-associated PD-L1
signaling by two functional monoclonal antibodies. Tissue Antigens.
69:19–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang D, Xu YY, Li F, Xu B and Zhang XG:
The role of B7-H1 in gastric carcinoma: Clinical significance and
related mechanism. Med Oncol. 31:2682014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qing Y, Li Q, Ren T, Xia W, Peng Y, Liu
GL, Luo H, Yang YX, Dai XY, Zhou SF and Wang D: Upregulation of
PD-L1 and APE1 is associated with tumorigenesis and poor prognosis
of gastric cancer. Drug Des Devel Ther. 9:901–909. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim JW, Nam KH, Ahn SH, Park DJ, Kim HH,
Kim SH, Chang H, Lee JO, Kim YJ, Lee HS, et al: Prognostic
implications of immunosuppressive protein expression in tumors as
well as immune cell infiltration within the tumor microenvironment
in gastric cancer. Gastric Cancer. 19:42–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gevensleben H, Holmes EE, Goltz D,
Dietrich J, Sailer V, Ellinger J, Dietrich D and Kristiansen G:
PD-L1 promoter methylation is a prognostic biomarker for
biochemical recurrence-free survival in prostate cancer patients
following radical prostatectomy. Oncotarget. 7:79943–79955. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Micevic G, Thakral D, McGeary M and
Bosenberg M: PD-L1 methylation regulates PD-L1 expression and is
associated with melanoma survival. Pigment Cell Melanoma Res.
32:435–440. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Asgarova A, Asgarov K, Godet Y, Peixoto P,
Nadaradjane A, Boyer-Guittaut M, Galaine J, Guenat D, Mougey V,
Perrard J, et al: PD-L1 expression is regulated by both DNA
methylation and NF-kB during EMT signaling in non-small cell lung
carcinoma. Oncoimmunology. 7:e14231702018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goltz D, Gevensleben H, Dietrich J and
Dietrich D: PD-L1 (CD274) promoter methylation predicts survival in
colorectal cancer patients. Oncoimmunology. 6:e12574542016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goltz D, Gevensleben H, Grünen S, Dietrich
J, Kristiansen G, Landsberg J and Dietrich D: PD-L1 (CD274)
promoter methylation predicts survival in patients with acute
myeloid leukemia. Leukemia. 31:738–743. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang FH, Shen L, Li J, Zhou ZW, Liang H,
Zhang XT, Tang L, Xin Y, Jin J, Zhang YJ, et al: The Chinese
society of clinical oncology (CSCO): Clinical guidelines for the
diagnosis and treatment of gastric cancer. Cancer Commun (Lond).
39:102019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim G, Kim J, Han SY, Hwang IG, Kim HS and
Min H: The effects of BRCA1 expression on the chemosensitivity of
gastric cancer cells to platinum agents. Oncol Lett. 17:5023–5029.
2019.PubMed/NCBI
|
|
35
|
Saal LH, Gruvberger-Saal SK, Persson C,
Lövgren K, Jumppanen M, Staaf J, Jönsson G, Pires MM, Maurer M,
Holm K, et al: Recurrent gross mutations of the PTEN tumor
suppressor gene in breast cancers with deficient DSB repair. Nat
Genet. 40:102–107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Phuah SY, Looi LM, Hassan N, Rhodes A,
Dean S, Taib NA, Yip CH and Teo SH: Triple-negative breast cancer
and PTEN (phosphatase and tensin homologue) loss are predictors of
BRCA1 germline mutations in women with early-onset and familial
breast cancer, but not in women with isolated late-onset breast
cancer. Breast Cancer Res. 14:R1422012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mittendorf EA, Philips AV, Meric-Bernstam
F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM,
Akcakanat A, et al: PD-L1 expression in triple-negative breast
cancer. Cancer Immunol Res. 2:361–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Song M, Chen D, Lu B, Wang C, Zhang J,
Huang L, Wang X, Timmons CL, Hu J, Liu B, et al: PTEN loss
increases PD-L1 protein expression and affects the correlation
between PD-L1 expression and clinical parameters in colorectal
cancer. PLoS One. 8:e658212013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou Q, Munger ME, Highfill SL, Tolar J,
Weigel BJ, Riddle M, Sharpe AH, Vallera DA, Azuma M, Levine BL, et
al: Program death-1 signaling and regulatory T cells collaborate to
resist the function of adoptively transferred cytotoxic T
lymphocytes in advanced acute myeloid leukemia. Blood.
116:2484–2493. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bang YJ, Kang YK, Catenacci DV, Muro K,
Fuchs CS, Geva R, Hara H, Golan T, Garrido M, Jalal SI, et al:
Pembrolizumab alone or in combination with chemotherapy as
first-line therapy for patients with advanced gastric or
gastroesophageal junction adenocarcinoma: Results from the phase II
nonrandomized KEYNOTE-059 study. Gastric Cancer. 22:828–837. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fuchs CS, Doi T, Jang RW, Muro K, Satoh T,
Machado M, Sun W, Jalal SI, Shah MA, Metges JP, et al: Safety and
efficacy of pembrolizumab monotherapy in patients with previously
treated advanced gastric and gastroesophageal junction cancer:
Phase 2 Clinical KEYNOTE-059 trial. JAMA Oncol. 4:e1800132018.
View Article : Google Scholar : PubMed/NCBI
|