Introduction
As a multifaceted neurodegenerative disorder,
Alzheimer's disease (AD) greatly impacts the health of the affected
elderly population (1). However, the
pathophysiology of AD has not been completely elucidated to date.
Cholinergic deficiency and β-amyloid (Aβ) deposition are widely
accepted as important pathological events in the progress of AD
(2). Previous studies have suggested
that overactivation of acetylcholinesterase (AChE) may be
responsible for the dysfunction of the cholinergic system, leading
to cognitive impairments in AD (3,4). In
addition, Aβ oligomer-induced neurotoxicity is considered as the
main cause of neuronal loss in AD (5). Due to the complexity of the pathology
of this disease, a one-molecule multi-target strategy may be useful
for identifying novel anti-AD drugs (6).
Scopolamine, a muscarinic cholinergic antagonist,
can induce short-term learning and memory impairments in mice
(7), while scopolamine-induced
amnesia is associated with deficits in cholinergic
neurotransmission, which is also observed in patients with AD
(8). Therefore, a
scopolamine-induced mouse model can be used for the evaluation of
cognition-enhancing drugs for treating AD (7,9).
In our previous studies,
6-bromo-N-propionyltryptamine from marine bacterium
Pseudoalteromonas rubra QD1-2 was synthesized (10), and it was observed that this compound
acts on 5-hydroxytryptamine (5-HT) receptors in the central nervous
system and may be used for treating neurological diseases,
including AD (11). In the present
study, another compound was further synthesized, namely
6-bromotryptamine A, which is a molecule with a similar structure
to that of 6-bromo-N-propionyltryptamine (11). Next, the effects of 6-bromotryptamine
A on scopolamine-induced short-term impairments in recognition and
spatial cognition was evaluated in mice. Furthermore, the study
examined whether 6-bromotryptamine A is able to directly inhibit
AChE activity and reduce the formation of Aβ oligomer.
Materials and methods
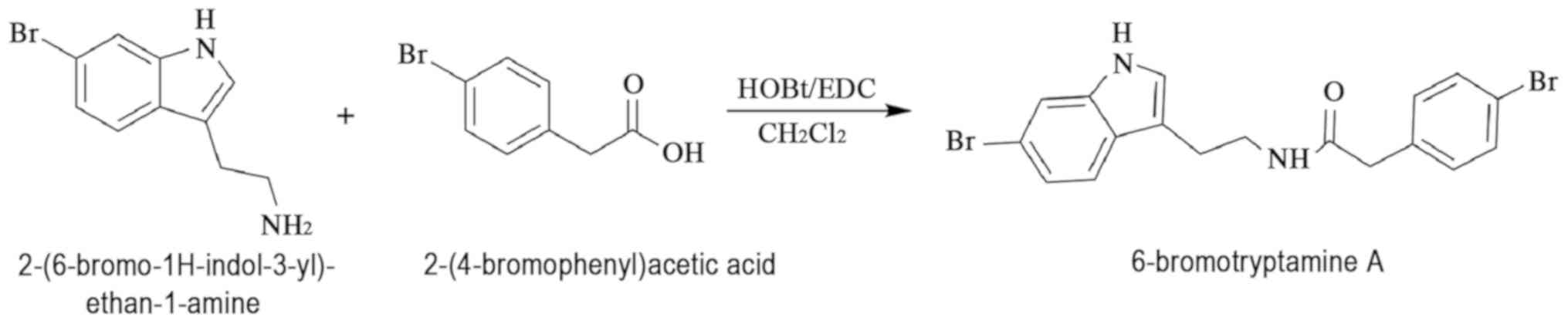
Synthesis of 6-bromotryptamine A
The molecule 6-bromotryptamine A was synthesized
through the condensation of 2-(6-bromo-1H-indol-3-yl)ethan-1-amine
and 2-(4-bromophenyl)acetic acid, as shown in Fig. 1. For this reaction,
hydroxybenzotriazole and
1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride in
dichloromethane solution were used at room temperature for 12 h.
The purity of 6-bromotryptamine A was greater than 99%.
Animals and drug treatment
Male ICR mice weighing 25–30 g were purchased from
Zhejiang Academy of Medical Sciences (Hangzhou, China). The animals
were maintained on a 12-h light/dark cycle under controlled
temperature (22±2°C) and humidity (50±10%), and given standard diet
and water access. The animals were allowed to acclimatize for 3
days before the experiments. All animal experiments followed the
guidelines of the National Institutes of Health Guide for the Care
and Use of Laboratory Animals (publication no. 80–23, revised
1996), and were approved by the Animal Ethics and Welfare Committee
of Ningbo University (Ningbo, China; approval no.
SYXK-2008-0110).
Prior to administration, 6-bromotryptamine A was
dissolved in sterile saline containing 0.1% DMSO, 0.5% Tween-20 and
1% ethanol, while scopolamine was dissolved in sterile saline.
Intraperitoneal (i.p.) injection of scopolamine at the dose of 1–5
mg/kg has previously been reported for the establishment of an
amnesia model and for evaluation of cognition-enhancing drugs
(12,13). Therefore, in the present study, mice
were intraperitoneally injected with 3 mg/kg scopolamine to
establish an AD animal model. Briefly, the mice were randomly
distributed into six groups of 8 animals each, and treated
accordingly, as follows: Control, 3 mg/kg scopolamine, and 3 mg/kg
scopolamine plus low (0.5 mg/kg), medium (1.5 mg/kg) or high (5.0
mg/kg) dose of 6-bromotryptamine A. Administration of
6-bromotryptamine A was conducted by i.p. injection at the same
time as scopolamine. All drugs were administered 30 min prior to
behavioral tests once a day for 10 successive days. The open-field
test was performed on the first day of the experiment, while the
novel object recognition (NOR) test was conducted for the following
three days. Subsequently, the Morris water maze task was performed
on day 5–10. After behavioral tests, the mice were sacrificed for
biochemical study. All the animals were given the last injection of
drugs 30 min prior to sacrifice.
Open-field test
Open-field test was used to analyze the activities
of exploration and locomotion (14).
In the present study, open-field test was performed according to
the protocols described previously, with certain modifications
(15). Briefly, the animals were
placed in the left rear quadrant of a 50×50×39 cm open field with
white plywood walls and a brown floor divided into four identical
squares of equal dimensions (25×25 cm). The mice were placed in
turns in the middle of the box and allowed to explore it for 5 min.
Stopwatches and hand-operated counters were used to score the
number of line crossing and the number of rearings (defined as the
number of times the animal stood on its hind legs), which were used
as indicators of the locomotor and exploratory activities,
respectively. The researcher performing the counting was blinded to
the drug status of the animals. To avoid perturbation of the
animals due to urine and feces, the apparatus was cleaned with 10%
ethanol solution and a dry cloth following each test.
NOR test
The NOR test is typically used to evaluate the
object recognition abilities of rodents (16). In the current study, the NOR test was
performed according to a previously described protocol, with
modifications (17). Briefly, an
open-field arena (30×30×30 cm) was built with polyvinyl chloride
plastic, plywood and transparent acrylic. The task was composed of
three sessions, including the habituation, familiarization and test
phases, which were performed over a period of three consecutive
days. On the first day of the experiment, the animals were
habituated to the experimental arena by allowing them to freely
explore the arena for 5 min. On the second day, the animals were
allowed to explore two identical objects for 5 min. On the third
day, one of the objects was changed to a novel one with a different
shape and color, and the animals were allowed to explore for 5 min.
The field was cleansed with 10% ethanol solution and dried
following occupancy by each mouse.
Exploration was defined as sniffing or touching the
objects with the nose and/or forepaws at a distance of <2 cm.
Sitting on or turning around the objects closely was not considered
as exploratory behavior. The exploratory track was manually
recorded using a video camera positioned over the arena by an
observer blinded to the testing conditions. The total exploration
time was defined as the amount of time spent exploring the two
objects. The object recognition ability of mice was expressed as
the ratio of the time spent exploring either of the two objects
(familiarization phase) or the novel object (test phase) over the
total exploration time.
Morris water maze task
The Morris water maze task was used to measure the
spatial learning and memory of animals (18), and performed as previously described
with minor modification (19). The
water maze apparatus consisted of a circular pool with a diameter
of 110 cm, which was filled with water at 23±2°C to cover a
platform. The platform was always laid in the center of the
northeast quadrant, except on the last day of the experiment. Each
mouse's swimming was observed by a video camera linked to a
computer-based image analyzer. The learning performance was
assessed for four consecutive days, beginning on day 5 after the
first injection of scopolamine (training trials). Each mouse was
trained to find the platform through four trials per day. In each
trial, the time required to escape onto the hidden platform was
recorded. On day 10, a probe trial was arranged by removing the
platform and allowing each mouse to swim for 90 sec in order to
find it. The swimming time in each of the four quadrants of the
pool was calculated. The tendency of mice to swim in the target
area indicated that they had acquired and remembered the spatial
task.
Measurement of AChE activity
A colorimetric method, which was adapted to
96-well-plates with a final volume of 200 µl, was used for the
detection of AChE activity (20). In
brief, following anesthesia by intraperitoneal injection of sodium
pentobarbital (50 mg/kg), mice were decapitated and brains were
immediately collected to examine the AChE activity. The brain was
weighted, and then a 10X volume of lysis buffer was added
(containing 1 mM EGTA, 10 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM EDTA
and 0.5% Triton X-100). Following vortexing on ice for 15 min,
tissue homogenization was achieved. The supernatant was then
obtained by centrifugation for 15 min at 956 × g at 4°C. The assay
medium included 5% supernatant as mentioned above, 10 mM DTNB, 1 mM
acetylthiolcholine iodide and 0.1 M Na2HPO4
(pH 7.5). The assay medium was incubated with 0.1 mM ethopropazine
hydrochloride for 5 min to inhibit the butyrylcholinesterase
activity. Subsequently, 6-bromotryptamine A was mixed with assay
medium and pre-incubated at 37°C for 15 min, and the AChE activity
was then determined by measuring the absorbance at 412 nm. The half
maximal inhibitory concentration (IC50) of
6-bromotryptamine A on AChE inhibition was automatically calculated
using GraphPad Prism (version 5.0; GraphPad Software. Inc).
Molecular docking analysis
Molecular docking analyses were conducted with the
SYBYL 2.0 (Tripos Inc.) software and associated programs. The
three-dimensional (3D) crystal structure of AChE was obtained from
the Protein Data Bank (PDB cod: 1EVE) (21). The three-dimensional structure of
6-bromotryptamine A was constructed using the geometric parameters
of SYBYL and then optimized by the Powell method (7). The Surflex-Dock program, which uses an
empirically-derived scoring function based on the binding
affinities of protein-ligand complexes, was then used to perform
docking analysis. As a flexible docking method, Surflex-Dock has
been proven to be efficient in analyzing a variety of receptors
(22). The active site of AChE was
further defined relative to the coordinates of donepezil. During
the simulations, the rotatable bonds of the ligands were defined,
whereas the receptor was kept rigid.
Preparation of Aβ1–42
oligomer
Soluble Aβ1–42 oligomer was obtained as
previously described (23). Briefly,
Aβ1–42 (GL Biochem) was added in hexafluoroisopropanol
(HFIP) to form the Aβ1–42 monomer, which was further
spin-vacuumed in 10% HFIP solution. HFIP was then evaporated to
obtain the Aβ1–42 solution. Next, the Aβ1–42
solution, with or without 6-bromotryptamine A, was incubated for 2
days at 25°C under stirring and centrifuged at 14,000 × g for 15
min at 4°C. The supernatant, which contained mainly soluble
Aβ1–42 oligomer, was subsequently collected and
quantified by a BCA assay (Thermo Fisher Scientific, Inc.).
Dot blot analysis
A nitrocellulose membrane was divided into equal
grids, and a 2-µl sample (Aβ1–42 oligomer treated with
or without 6-bromotryptamine A as previously described) was dotted
onto the membrane and then air-dried. The membrane was blocked in a
Tris-buffered saline/Tween-20 (TBST) solution (containing 50 mM
Tris, 150 mM NaCl and 0.1% Tween-20) with 10% milk at 25°C
overnight. Subsequently, the membrane was incubated with
anti-oligomer antibody A11 (1:1,000; cat. no. AHB0052, Thermo
Fisher Scientific, Inc.) or anti-Aβ1–17 antibody 6E10
(1:1,000; cat. no. MAB1560, Sigma-Aldrich, Merck KGaA) at 25°C for
1 h with gentle shaking. Following three washes with TBST, the
membrane was incubated with secondary antibodies (cat. no. 14708,
Cell Signaling Technology) at 25°C for 1 h and then developed with
an enhanced chemiluminescence plus kit (24). The optical density of each band was
further quantified by using ImageJ (1.50i; National Institutes of
Health).
Statistical analysis
The data are expressed as the mean ± standard error
of the mean. Statistically significant differences were determined
by one-way analysis of variance (ANOVA) followed by Tukey's or
Dunnett's test for post hoc multiple comparison, with the exception
of mean escape latency, which was analyzed using two-way
repeated-measures ANOVA followed by the least significant
difference post hoc test. Differences were considered as
statistically significant at P<0.05.
Results
6-bromotryptamine A does not affect
the locomotor activity of mice in the open-field test
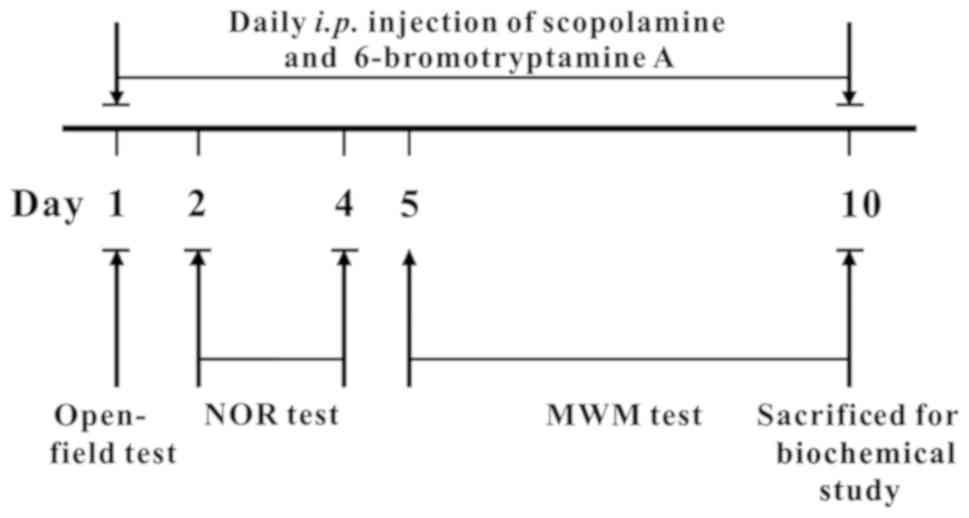
The protocol of the animal study and the tests
performed are displayed in detail in Fig. 2. The mice were treated with drugs 30
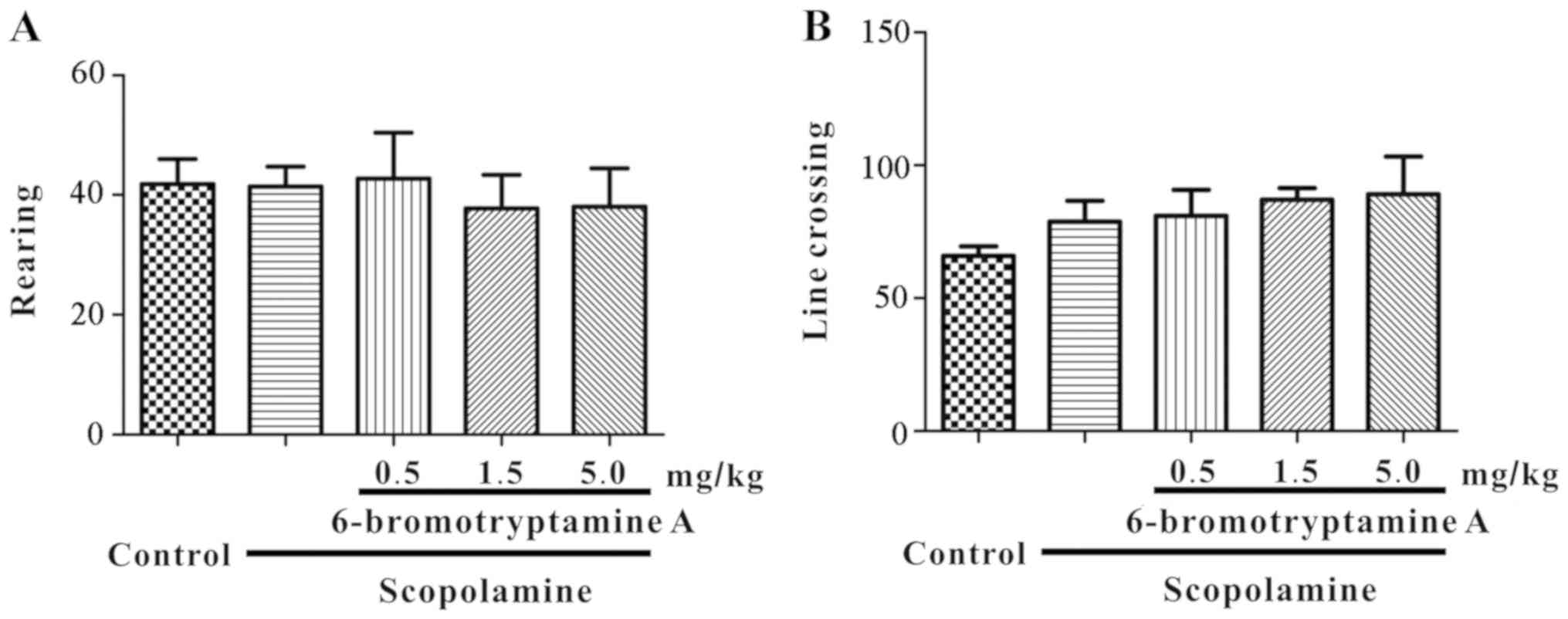
min prior to the daily testing. In the open-field test, the number
of line crossings and rearings were recorded for 5 min. As
demonstrated in Fig. 3, none of the
experimental group mice exhibited a significant change in the
number of line crossings or rearings following treatment [for line
crossing, one-way ANOVA, F(4, 35)=0.960, P=0.4418; for rearing,
F(4, 35)=0.167, P=0.9539; detailed data are provided in Table SI]. In this study, F-test was used
to determine whether group means are equal, and the F-value was
calculated as the variability between groups divided by the
variability within group. These results suggested that
6-bromotryptamine A treatment did not alter the motor functions of
mice.
6-bromotryptamine A prevents
scopolamine-induced short-term cognitive impairments
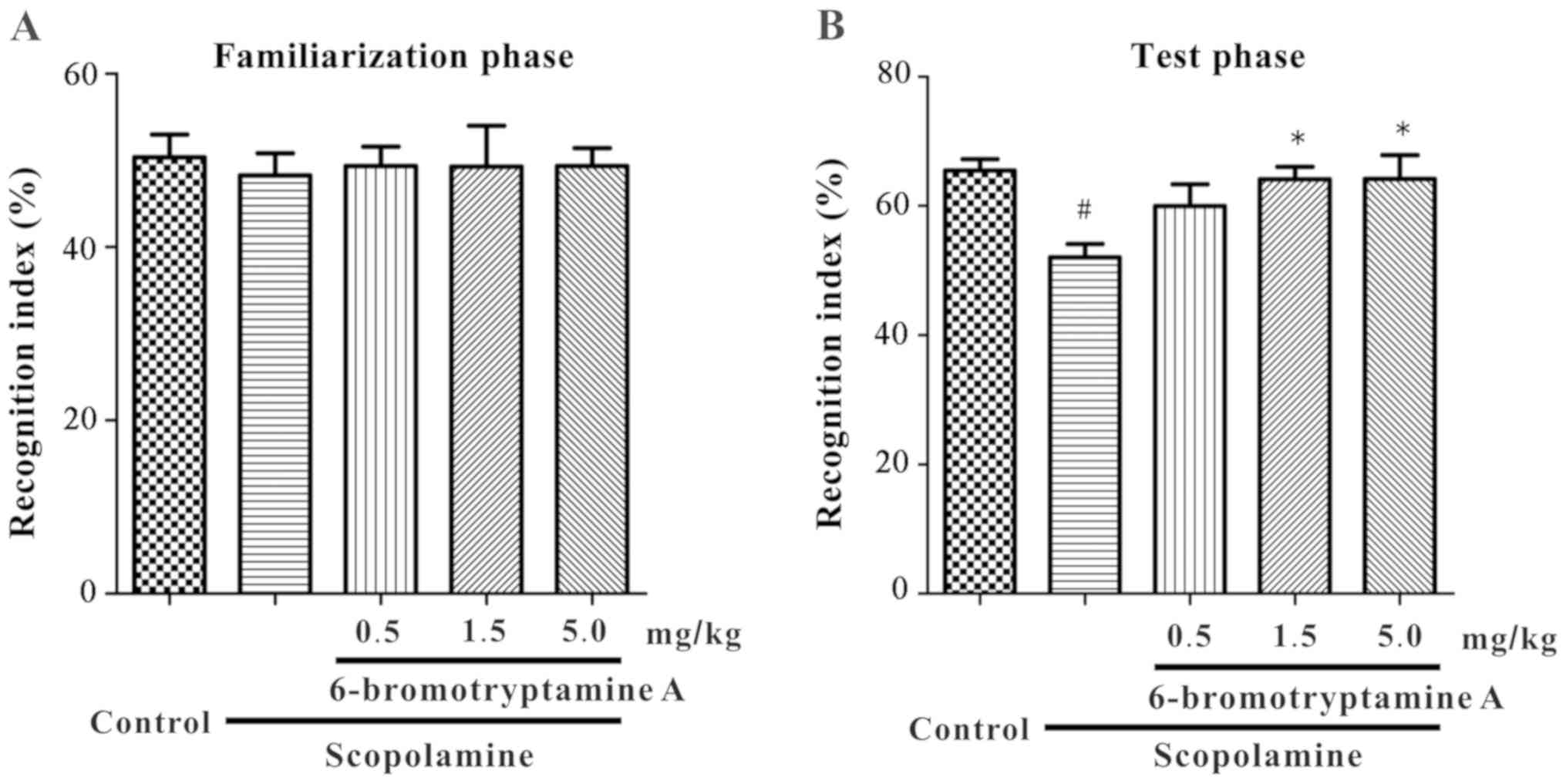
On day 1 of NOR test, mice were accustomed to the
experimental arena without any behaviorally-relevant stimulus.
Following this habituation phase, the familiarization phase of the
test was performed, and the exploration time of two identical
objects was recorded. As shown in Fig.
4A, in the familiarization phase, all groups exhibited similar
recognition indexes for identical objects [F(4, 35)=0.061,
P=0.9927; detailed data are provided in Table SII]. On the following day, the test
phase was conducted. As shown in Fig.
4B, the recognition index for the novel object was
significantly altered in the treated mice compared with the control
mice [one-way ANOVA, F(4, 35)=4.058, P=0.0083; detailed data are
provided in Table SII]. The
recognition index was significantly reduced in the
scopolamine-treated mice compared with that in the control mice
(Tukey's test, P<0.05), whereas 6-bromotryptamine A treatment
(1.5 and 5.0 mg/kg) significantly prevented the scopolamine-induced
decrease in this index (Tukey's test, P<0.05; Fig. 4B).
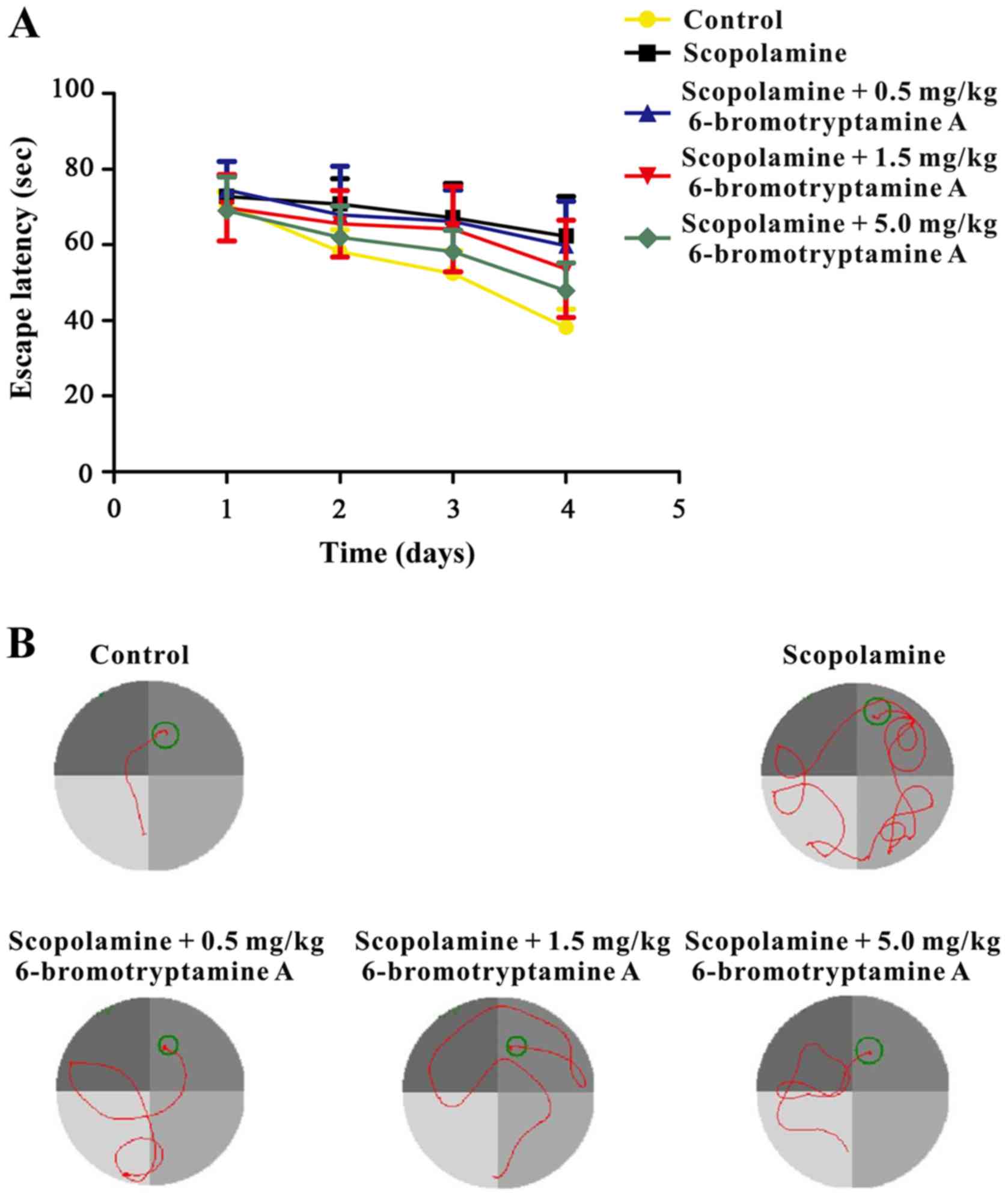
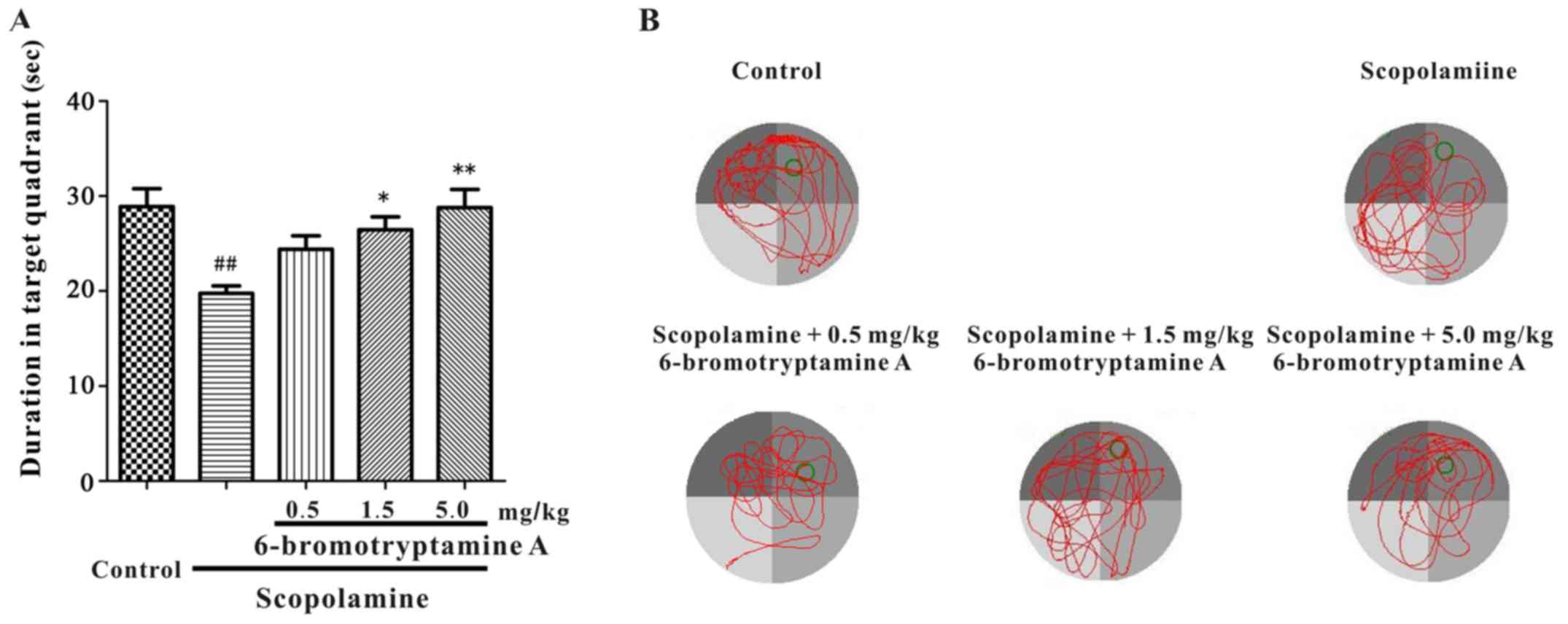
Furthermore, a Morris water maze test was conducted
to examine whether 6-bromotryptamine A was able to prevent
scopolamine-induced spatial cognitive impairments. The performance
of mice in all groups improved throughout the training session, as
indicated by the shortened escape latency (Fig. 5; detailed data are provided in
Table SIII). However, during the
probe trial, the time spent in the target quadrant was
significantly different among all groups, as shown in Fig. 6 [one-way ANOVA, F(4, 35)=6.102,
P=0.008; detailed data are provided in Table SIII]. Scopolamine treatment markedly
reduced the time mice spent in the target quadrant, as compared
with the control group. By contrast, treatment with
6-bromotryptamine A at a dose of 1.5 and 5 mg/kg significantly
increased the swimming time of mice in the target quadrant compared
with that in the scopolamine group (Tukey's test, P<0.05;
Fig. 6).
6-bromotryptamine A directly inhibits
AChE activity
AChE inhibitors have been reported to prevent
learning and memory impairments caused by scopolamine (25). Therefore, it was speculated that
6-bromotryptamine A may also act on AChE. In the current study, an
AChE activity assay was conducted to examine this. It was observed
that 6-bromotryptamine A dose-dependently inhibited the activity of
AChE in the test-tube assay, and 6-bromotryptamine A at a dose of
50 µM reduced the AChE activity to ~45% of the control group
without treatment of 6-bromotryptamine A (Table I). The IC50 of
6-bromotryptamine A on the inhibition of AChE was predicted to be
73.73 µM, as calculated using GraphPad Prism based on the results
shown in Table I.
 | Table I.Inhibitory effect of
6-bromotryptamine A on AChE activity. |
Table I.
Inhibitory effect of
6-bromotryptamine A on AChE activity.
| 6-bromotryptamine A
(µM) | Inhibition of AChE
(% of control) |
|---|
| 0.1 | 3.97±0.94 |
| 0.3 | 5.98±0.81 |
| 1 | 7.19±1.15 |
| 3 | 11.58±1.34 |
| 10 | 29.32±0.31 |
| 25 | 33.42±1.68 |
| 50 | 44.97±0.46 |
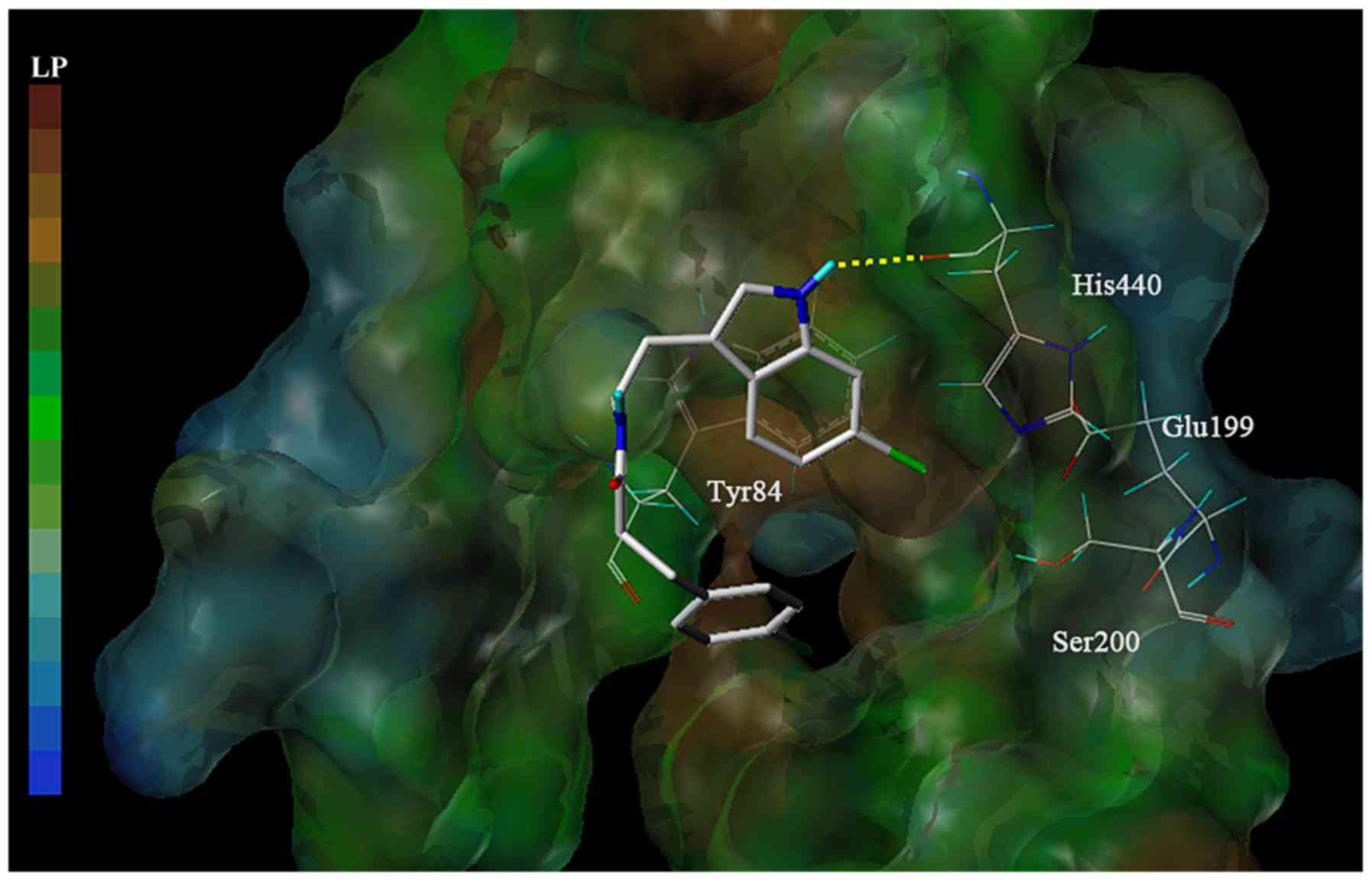
Interaction between 6-bromotryptamine
A and AChE
Molecular docking analysis was subsequently
performed to investigate the interaction between 6-bromotryptamine
A and AChE. The docking model suggested that 6-bromotryptamine A
may form a hydrogen bond with His440 residue at the catalytic
active site (CAS) within AChE (Fig.
7). Furthermore, the benzene ring of 6-bromotryptamine A may
extend to the peripheral anionic site (PAS) of AChE.
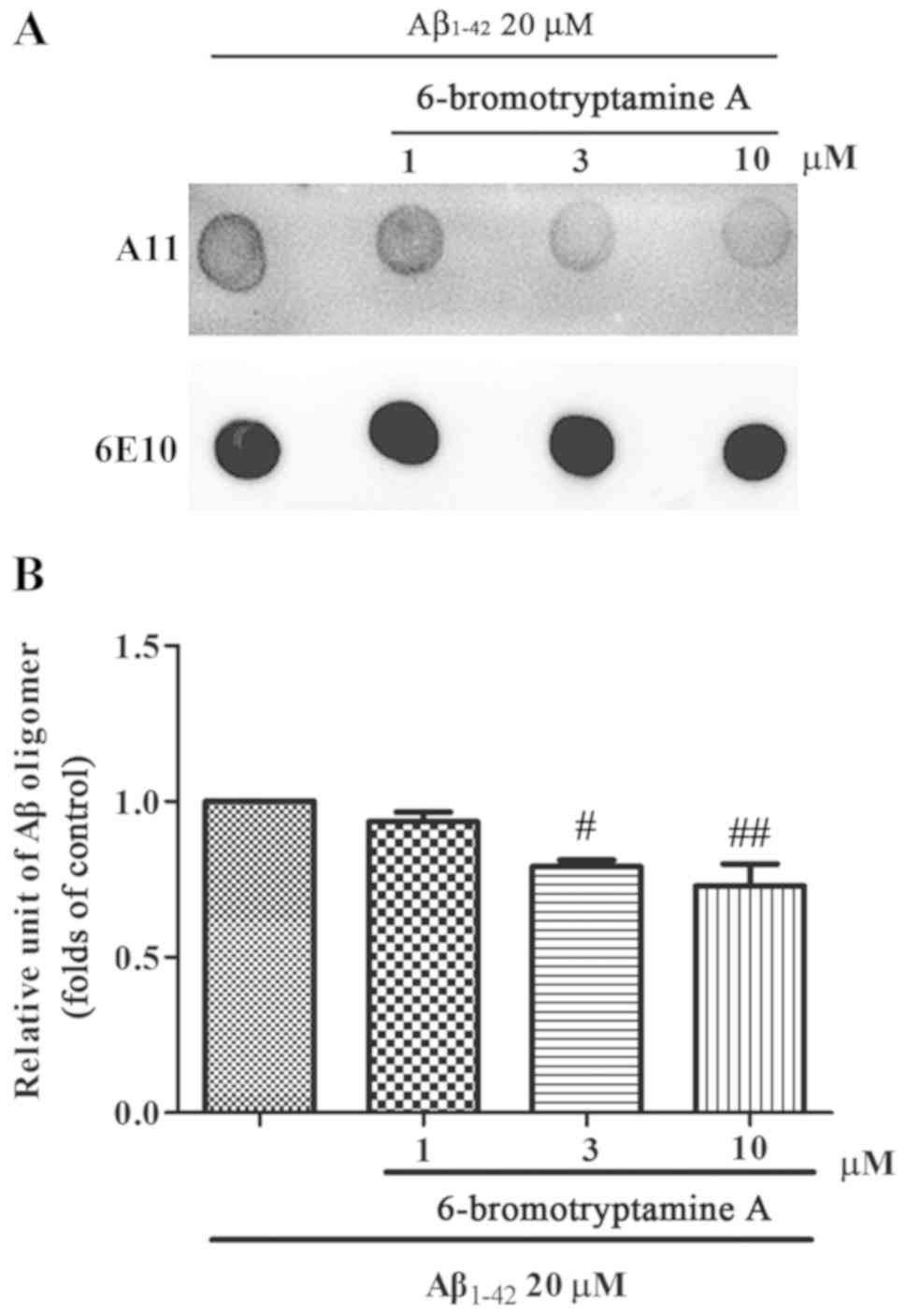
6-bromotryptamine A inhibits
Aβ1–42 oligomer formation
A dot blot assay was further performed to evaluate
the effects of 6-bromotryptamine A on the formation of Aβ oligomer.
In the control group, Aβ1–42 monomer formed
Aβ1–42 oligomer after 2 days of incubation under
stirring. Notably, co-incubation with 6-bromotryptamine A at doses
of 3 and 10 µM significantly reduced the amount of the
Aβ1–42 oligomer compared with the control condition
(Tukey's test, P<0.05; Fig.
8).
Discussion
In the present study, the data demonstrated that
6-bromotryptamine A treatment prevented scopolamine-induced
short-term learning and memory impairments in vivo,
suggesting that this compound may serve as an anti-AD agent
(26). In addition, it was observed
that 6-bromotryptamine A inhibited AChE, possibly by acting on both
the CAS and PAS of AChE. However, compared with the clinically used
AChE inhibitors, such as donepezil (IC50 on AChE
activity, 12.3 nM) and galantamine (IC50 on AChE
activity, 410 nM), the AChE inhibition activity of
6-bromotryptamine A was not as potent (27).
Several studies have reported that AChE is involved
not only in neurotransmission, but also in the regulation of cell
proliferation, differentiation and apoptosis (28), while overexpression of AChE was
observed in numerous types of cancer (29,30).
Notably, a number of AChE inhibitors are currently used or are
under investigation for their potential application in cancer
treatment. For instance, donepezil has been reported to induce
apoptosis of HL-60 human promyelocytic leukemia cells (31). Galantamine has also been proven to
produce anti-proliferation effects against 3T3 cells (28). Similarly, certain approved
chemotherapeutics have been demonstrated to inhibit AChE at their
efficient anti-cancer concentrations. Irinotecan, a
chemotherapeutic used for colon and ovarian cancer therapy,
inhibited AChE with an IC50 value of 0.97 µM (32). Furthermore, cyclophosphamide
monohydrate, an alkylating agent used for the treatment of lymphoma
and leukemia, inhibited AChE activity with an IC50 of
511 µM (33). Therefore, in the
present study, it is speculated that 6-bromotrypamine A, a novel
AChE inhibitor, may be used not only in the treatment of AD, but
also in the treatment of cancer.
The moderate AChE inhibitory activity may not fully
explain the cognitive-enhancing effects of 6-bromotryptamine A.
Cheng et al (34) have
demonstrated that (−)-meptazinol-indole amine hybrids, which have
structures similar to 6-bromotryptamine A, are potential anti-AD
candidates with dual inhibitory potency against AChE and Aβ
aggregation. Their results further indicated that the indole group
of chemicals may lead to the deposition of Aβ aggregates. In the
present study, it was speculated that 6-bromotryptamine A may also
act on Aβ aggregation due to its similar pharmacophore. Indeed, the
results of dot blot analysis further suggested that
6-bromotryptamine A was able to directly inhibit Aβ
oligomerization, indicating that the interactions between
6-bromotryptamine A and Aβ may decrease the interaction between Aβ
molecules and therefore prevent the formation of Aβ aggregates.
Previous studies have reported that several Aβ oligomer inhibitors,
such as epigallocatechin gallate and brazilin, interact with Aβ by
hydrogen bonds and π-π interactions (35,36). The
compound 6-bromotryptamine A has a benzene ring and indole group to
form π-π interactions, and active atoms to form hydrogen bonds.
Thus, it is speculated that 6-bromotryptamine A may also interact
with Aβ through hydrogen bonds and π-π interactions, and
consequently inhibit the intermolecular interactions among Aβ
oligomers.
To date, there are no effective drugs available for
AD treatment (37), and the current
therapeutic approach of ‘one molecule-one target’ strategy for AD
fails due to the complexity of AD (38). Furthermore, the pharmaceutical
combination of single-target drugs has certain challenges, such as
different degrees of bioavailability and metabolisms of various
drugs (39). Therefore, an
alternative one-molecule multi-target strategy may be suitable for
treating AD. The present study has provided evidence that
6-bromotryptamine A may be such a multi-target anti-AD
molecule.
Although the current study demonstrated that
6-bromotryptamine A was able to prevent learning and memory
impairments in mice, the possible applications of this compound in
humans cannot be clearly determined based on these preliminary
results. The main limitation of the present study is the lack of
examination of the toxicity, absorption, distribution, metabolism
and excretion of 6-bromotryptamine A, which would impact its
application in humans. Although i.p. injection of 5.0 mg/kg
6-bromotryptamine A + scopolamine did not significantly alter the
motor functions of mice, it cannot be concluded that
6-bromotryptamine A at this concentration is safe for animals.
Prior to the use of this compound in clinical trials, the acute and
chronic in vivo toxicity should be first evaluated by
administering 6-bromotryptamine A at high concentrations. In
addition, the pharmacokinetics and bioavailability of
6-bromotryptamine A should be investigated in various animal
models.
The quality of the finding reported in present study
may also be enhanced by performing in vivo experiments to
verify whether 6-bromotryptamine A reduces Aβ oligomer formation.
During the AD process, the formation of Aβ oligomer is relatively
slow and cannot be observed in the normal neurotoxin-induced AD
animal models, such as the model established by i.p. scopolamine
injection or intracerebroventricular Aβ injection of rodents.
Typically, in vivo formation of Aβ oligomer can only be
tested in AD transgenic mice, including APP/PS1 and APP/PS1/tau
mice. In such transgenic mice, drugs should be used for a long
period (for example, 3–6 months) and the quantity of Aβ oligomer
can only be measured in older animals (6-9 month of age). However,
such in vivo experiments were not conducted in the present
study due to the low supply of 6-bromotryptamine A available.
Besides the cholinergic system dysfunction and Aβ
neurotoxicity, numerous other factors contribute to the onset of
AD. For instance, tau aggregates in the cortex precede Aβ
deposition at the early stage of AD (40). Furthermore, there are several
mutations of APP, PSEN1 or PSEN2 in familial AD cases
(41). However, the present study
mainly evaluated the effects of 6-bromotryptamine A on AChE and Aβ
oligomer inhibition, which is a limitation of the study. Further
studies are required to investigate whether this compound acts on
other AD targets, such as tau, PSEN1 or PSEN2, to exert its anti-AD
effects.
In conclusion, the present study revealed that
6-bromotryptamine A, a novel tryptamine derivative, inhibited AChE
activity and Aβ oligomer formation, as well as prevented the
scopolamine-induced short-term impairments in learning and memory
in vivo. These results suggest that 6-bromotryptamine A, a
molecule with multiple targets, may be used to treat AD.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81673407, 31600402,
U1503223, 41776168, 41706167 and 31801165), the Applied Research
Project on Nonprofit Technology of Zhejiang Province (grant nos.
2016C37110 and 2015C33155), the Ningbo Natural Science Foundation
(grant nos. 2017A610216, 2015A610219 and 2018A610213), the Ningbo
Sci & Tech Project for Common Wealth (grant nos. 2017C50042 and
2017C10016), the Ningbo Municipal Innovation Team of Life Science
and Health (grant no. 2015C110026), the 111 Project (grant no.
D16013), the Li Dak Sum Yip Yio Chin Kenneth Li Marine
Biopharmaceutical Development Fund, and the K. C. Wong Magna Fund
in Ningbo University.
Availability of data and materials
All data generated or analyzed during this study are
included in this published study.
Authors' contributions
WC and SH conceived and designed the experiments.
XJ, MW, JS, CH, YB, HP, DZ, ZY, XX, HZ, LD, QW and XW performed the
experiments. XJ and MW analyzed the data. MW and WC wrote the
manuscript. All authors read and approved the final manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments followed the guidelines of
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals (publication no. 80-23, revised 1996), and were
approved by the Animal Ethics and Welfare Committee of Ningbo
University (Ningbo, China; approval no. SYXK-2008-0110).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
Glossary
Abbreviations
Abbreviations:
|
AD
|
Alzheimer's disease
|
|
AChE
|
acetylcholinesterase
|
|
Aβ
|
β-amyloid
|
|
CAS
|
catalytic active site
|
|
NIH
|
National Institutes of Health
|
|
i.p.
|
intraperitoneal
|
|
NOR
|
novel object recognition
|
|
PAS
|
peripheral anionic site
|
References
|
1
|
Luo W, Wang T, Hong C, Yang YC, Chen Y,
Cen J, Xie SQ and Wang CJ: Design, synthesis and evaluation of
4-dimethylamine flavonoid derivatives as potential multifunctional
anti-Alzheimer agents. Eur J Med Chem. 122:17–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bhounsule AS, Bhatt LK, Prabhavalkar KS
and Oza M: Cyclin dependent kinase 5: A novel avenue for
Alzheimer's disease. Brain Res Bull. 132:28–38. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao XJ, Gong DM, Jiang YR, Guo D, Zhu Y
and Deng YC: Multipotent AChE and BACE-1 inhibitors for the
treatment of Alzheimer's disease: Design, synthesis and
bio-analysis of 7-amino-1,4-dihydro-2H-isoquilin-3-one derivates.
Eur J Med Chem. 138:738–747. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cieslikiewicz-Bouet M, Chao S, Jean L, et
al: Toward an innovative treatment of Alzheimer's disease:
Synthesis and evaluation of multi-target directed ligands (MTDLs)
targeting acetylcholinesterase (AChE) and alpha7 nicotinic
acetylchloline receptors (alpha7 nAChRs). J Neurochem. 142:209–210.
2017.
|
|
5
|
Barnett R: Alzheimer's disease. Lancet.
393:15892019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saxena M and Dubey R: Target enzyme in
Alzheimer's disease: Acetylcholinesterase inhibitors. Curr Top Med
Chem. 19:264–275. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang L, Lin J, Xiang S, Zhao K, Yu J,
Zheng J, Xu D, Mak S, Hu S, Nirasha S, et al: Sunitinib, a
clinically used anticancer drug, is a potent AChE inhibitor and
attenuates cognitive impairments in mice. Acs Chem Neurosci.
7:1047–1056. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghumatkar PJ, Patil SP, Jain PD, Tambe RM
and Sathaye S: Nootropic, neuroprotective and neurotrophic effects
of phloretin in scopolamine induced amnesia in mice. Pharmacol
Biochem Behav. 135:182–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sang ZP, Qiang XM, Li Y, Xu R, Cao Z, Song
Q, Wang T, Zhang X, Liu H, Tan Z and Deng Y: Design, synthesis and
evaluation of scutellarein-O-acetamidoalkylbenzylamines as
potential multifunctional agents for the treatment of Alzheimer's
disease. Eur J Med Chem. 135:307–323. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ding L, He S, Wu W, Jin H, Zhu P, Zhang J,
Wang T, Yuan Y and Yan X: Discovery and structure-based
optimization of 6-bromotryptamine derivatives as potential 5-HT2A
receptor antagonists. Molecules. 20:17675–17683. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He S, Ding L and Yan X: New
6-bromotryptamine derivatives from marine bacterium
pseudoalteromonas rubra QD1-2 and the impact of side chain length
on their cytotoxicity. Planta Med. 79:8452013. View Article : Google Scholar
|
|
12
|
Chen HX, Xiang SY, Huang L, Lin J, Hu S,
Mak SH, Wang C, Wang Q, Cui W and Han Y: Tacrine(10)-hupyridone, a
dual-binding acetylcholinesterase inhibitor, potently attenuates
scopolamine-induced impairments of cognition in mice. Metab Brain
Dis. 33:1131–1139. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bae HJ, Sowndhararajan K, Park HB, Kim SY,
Kim S, Kim DH, Choi JW, Jang DS, Ryu JH and Park SJ: Danshensu
attenuates scopolamine and amyloid-β-induced cognitive impairments
through the activation of PKA-CREB signaling in mice. Neurochem
Int. 131:1045372019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kraeuter AK, Guest PC and Sarnyai Z: The
open field test for measuring locomotor activity and anxiety-like
behavior. Methods Mol Biol. 1916:99–103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Overstreet DH: The open field test for
two. J Psychopharmacol. 21:1402007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lueptow LM: Novel object recognition test
for the investigation of learning and memory in mice. J Vis Exp;
2017, View Article : Google Scholar
|
|
17
|
Chen L, Huang C, Shentu J, Wang M, Yan S,
Zhou F, Zhang Z, Wang C, Han Y, Wang Q and Cui W: Indirubin
derivative 7-bromoindirubin-3-oxime (7Bio) attenuates Aβ
oligomer-induced cognitive impairments in mice. Front Mol Neurosci.
10:3932017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
D'Hooge R and De Deyn PP: Applications of
the Morris water maze in the study of learning and memory. Brain
Res Brain Res Rev. 36:60–90. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen H, Wu X, Gu X, Zhou Y, Ye L, Zhang K,
Pan H, Wang J, Wei H, Zhu B, et al: Tacrine(10)-hupyridone prevents
post-operative cognitive dysfunction via the activation of BDNF
pathway and the inhibition of AChE in aged mice. Front Cell
Neurosci. 12:3962018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Santos WP, da Silva Carvalho AC, dos
Santos Estevam C, Santana AE and Marçal RM: In vitro and ex vivo
anticholinesterase activities of Erythrina velutina leaf extracts.
Pharm Biol. 50:919–924. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li FJ, Liu Y, Yuan Y, Yang B, Liu ZM and
Huang LQ: Molecular interaction studies of acetylcholinesterase
with potential acetylcholinesterase inhibitors from the root of
Rhodiola crenulata using molecular docking and isothermal titration
calorimetry methods. Int J Biol Macromol. 104:527–532. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jain AN: Surflex-Dock 2.1: Robust
performance from ligand energetic modeling, ring flexibility, and
knowledge-based search. J Comput Aided Mol Des. 21:281–306. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiang S, Liu F, Lin J, Chen H, Huang C,
Chen L, Zhou Y, Ye L, Zhang K, Jin J, et al: Fucoxanthin inhibits
β-amyloid assembly and attenuates β-amyloid oligomer-induced
cognitive impairments. J Agric Food Chem. 65:4092–4102. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chunhui H, Dilin X, Ke Z, Jieyi S, Sicheng
Y, Dapeng W, Qinwen W and Wei C: A11-positive β-amyloid oligomer
preparation and assessment using dot blotting analysis. J Vis Exp;
2018, View Article : Google Scholar
|
|
25
|
Sun K, Bai Y, Zhao R, Guo Z, Su X, Li P
and Yang P: Neuroprotective effects of matrine on
scopolamine-induced amnesia via inhibition of AChE/BuChE and
oxidative stress. Metab Brain Dis. 34:173–181. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakako T, Iwamura Y, Matsumoto A,
Matsumoto K, Ikejiri M and Ikeda K: Effects of donepezil on
scopolamine-induced cognitive impairment and Alzheimer's
disease-like change in quantitative EEG analysis in rhesus monkeys.
Eur Neuropsychopharm. 27:S736–S737. 2017. View Article : Google Scholar
|
|
27
|
Li WM, Kan KK, Carlier PR, Pang YP and Han
YF: East meets west in the search for Alzheimer's
therapeutics-novel dimeric inhibitors from tacrine and huperzine a.
Curr Alzheimer Res. 4:386–396. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lazarevic-Pasti T, Leskovac A, Momic T,
Petrovic S and Vasic V: Modulators of acetylcholinesterase
activity: From Alzheimer's disease to anti-cancer drugs. Curr Med
Chem. 24:3283–3309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xi HJ, Wu RP, Liu JJ, Zhang LJ and Li ZS:
Role of acetylcholinesterase in lung cancer. Thorac Cancer.
6:390–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ruiz-Espejo F, Cabezas-Herrera J, Illana
J, Campoy FJ, Muñoz-Delgado E and Vidal CJ: Breast cancer
metastasis alters acetylcholinesterase activity and the composition
of enzyme forms in axillary lymph nodes. Breast Cancer Res Tr.
80:105–114. 2003. View Article : Google Scholar
|
|
31
|
Ki YS, Park EY, Lee HW, Oh MS, Cho YW,
Kwon YK, Moon JH and Lee KT: Donepezil, a potent
acetylcholinesterase inhibitor, induces caspase-dependent apoptosis
in human promyelocytic leukemia HL-60 cells. Biol Pharm Bull.
33:1054–1059. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hyatt JL, Tsurkan L, Morton CL, Yoon KJ,
Harel M, Brumshtein B, Silman I, Sussman JL, Wadkins RM and Potter
PM: Inhibition of acetylcholinesterase by the anticancer prodrug
CPT-11. Chem-Biol Interact. 157-158:247–252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
al-Jafari AA, Duhaiman AS and Kamal MA:
Inhibition of human acetylcholinesterase by cyclophosphamide.
Toxicology. 96:1–6. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng S, Zheng W, Gong P, Zhou Q, Xie Q,
Yu L, Zhang P, Chen L, Li J, Chen J, et al:
(−)-Meptazinol-melatonin hybrids as novel dual inhibitors of
cholinesterases and amyloid-β aggregation with high antioxidant
potency for Alzheimer's therapy. Bioorg Med Chem. 23:3110–3118.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu FF, Dong XY, He LZ, Middelberg APJ and
Sun Y: Molecular insight into conformational transition of amyloid
β-peptide 42 inhibited by (−)-epigallocatechin-3-gallate probed by
molecular simulations. J Phys Chem B. 115:11879–11887. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Du WJ, Guo JJ, Gao MT, Hu SQ, Dong XY, Han
YF, Liu FF, Jiang S and Sun Y: Brazilin inhibits amyloid β-protein
fibrillogenesis, remodels amyloid fibrils and reduces amyloid
cytotoxicity. Sci Rep. 5:79922015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aykac A, Ozbeyli D, Uncu M, Ertaş B,
Kılınc O, Şen A, Orun O and Sener G: Evaluation of the protective
effect of Myrtus communis in scopolamine-induced Alzheimer model
through cholinergic receptors. Gene. 689:194–201. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bird TD: Genetic aspects of Alzheimer
disease. Genet Med. 10:231–239. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang T, Liu XH, Guan J, Ge S, Wu MB, Lin
JP and Yang LR: Advancement of multi-target drug discoveries and
promising applications in the field of Alzheimer's disease. Eur J
Med Chem. 169:200–223. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jagust W: Imaging the evolution and
pathophysiology of Alzheimer disease. Nat Rev Neurosci. 19:687–700.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Scheltens P, Blennow K, Breteler MM, de
Strooper B, Frisoni GB, Salloway S and Van der Flier WM:
Alzheimer's disease. Lancet. 388:505–517. 2016. View Article : Google Scholar : PubMed/NCBI
|