Introduction
Glioblastoma (GBM) is one of the malignant tumors
with high recurrence rate and worst prognosis in humans (1,2). As the
mechanism of its development and invasion remains poorly
understood, its treatment regimens are still limited. The median
survival is <2 years, although the best optimal treatment has
been provided (1,2). Thus, a new treatment strategy should be
established. GBM is associated with a variety of genetic and
epigenetic changes, and these risk factors induce tumor development
and progression. Based on this, various therapeutic strategies such
as epigenetic drugs, micro-RNAs, and gene editing are considered
(3,4).
Recently, cancer has been thought to be
heterogeneous, comprising cancer stem cells (CSCs) with
self-renewal ability and multipotency. CSCs produce cancer cells
with differentiated potential (5,6). The
heterogeneity of cancer has been known to be associated with
resistance to chemotherapy and radiotherapy. The treatment
strategies for CSCs, which are involved in treatment resistance,
are attracting attention (7).
Previous studies showed that cells corresponding to CSCs also exist
in GBM (8–10). Therefore, to realize the long-term
survival and GBM treatment, the development of therapies targeting
glioma stem-like cells (GSCs) has been considered important
(11,12). Various strategies targeting GSCs have
been investigated (13–15), including those targeting the vascular
niche in GSCs (13,16); specific cell surface molecules in
GSCs, such as CD133 (9,17) and L1CAM (18); and specific signaling pathways in
GSCs, such as RTK-Akt (19–21) and Notch signaling (22). This study focused on strategies
targeting GSCs through the induction of differentiation, which is
different from those in a previous study. Bone morphogenetic
protein 4 (BMP4) is one of the most important factors in cell
dynamics. BMP is a member of the transforming growth factor beta
(TGF-β) family and plays a very important role in regulating cell
proliferation, differentiation, and development in various
biological systems (23–27). Interestingly, evidences that BMP4 can
promote differentiation of GBM cells have been increasing (28–31).
Moreover, dysfunction of the BMP pathway in GSCs has also been
reported (28–31). Furthermore, BMP4 has been reported to
inhibit the tumorigenic potential through GSC differentiation as
well as proliferation suppression (31).
CSCs balance self-renewal ability and multipotency
in order to adapt to various environments. CSCs divide into two
daughter cells using one of the two types of cell division:
asymmetric cell division (ACD) or symmetric cell division (SCD)
(32,33). With ACD, one CSC divides into one
differentiated cell and one self-renewing stem cell, whereas with
SCD, one CSC divides into two equal CSCs. SCD is advantageous for
CSC expansion in the tumor tissue. The concept termed ‘ACD therapy’
suppresses CSC symmetric cell division (self-renewal ability) and
inhibits CSC expansion. Thus, ‘ACD therapy’ may be established as a
new strategy for the treatment of cancer.
The present study aimed to examine GSCs' cell
division pattern under BMP4 treatment. Herein, we present the
evidence that BMP4 induces GSCs' ACD by inhibiting its self-renewal
ability.
Materials and methods
Cell culture
Patient-derived GSC MGG8 was obtained from
Massachusetts General Hospital as previously described (34). Cells were maintained in the stem cell
culture medium [Neurobasal medium (Gibco; Invitrogen), supplemented
with 3 mM of L-glutamine (Cellgro), B27 supplement (Gibco;
Invitrogen), N2 supplement (Gibco; Invitrogen), heparin (Sigma),
penicillin/streptomycin/amphotericin B (Cellgro), human recombinant
FGF-2 (Peprotech) for final 20 ng/ml, human recombinant EGF (R and
D systems) for final 20 ng/ml]. Furthermore, cells were cultured in
an atmosphere containing 5% CO2 at 37°C. Recombinant
human bone BMP-4 was purchased from R&D Systems.
Antibodies
Primary antibodies used were as follows: Anti-CD133
(W6B3C1) [1:10 for immunofluorescence (IF), 1:100 for
immunoblotting (WB), 130-092-395, Miltenyi Biotec], anti-Smad1
(1:1,000 for WB, cat. no. 9743; Cell Signaling Technology),
anti-phospho-Smad1/5/9 (1:1,000 for WB, cat. no. 13820; Cell
Signaling Technology), anti-Smad4 (1:1,000 for WB, 1:500 for IF,
cat. no. 46535; Cell Signaling Technology), anti-PCNA (1:1,000 for
WB, NB100-456; Novus Biologicals), anti-tubulin (1:1,000 for WB,
T5326; Sigma-Aldrich), anti-GAPDH (1:1,000 for WB, 60004-1-Ig;
Proteintech), and anti-MKLP-1 (1:100 for IF, sc-867; Santa Cruz
Biotechnology). Horseradish peroxidase-labeled secondary antibodies
were purchased from the General Electric (GE) Healthcare and used
at 1:10,000. Fluorescence-labeled Alexa secondary antibodies used
in this study were obtained from Molecular Probes and used at
1:500.
Flow cytometry
Cell surface antigen expressions were analyzed using
flow cytometry. CD133/1-PE (Miltenyi Biotec) and mouse IgG1-PE
isotype control antibody (Miltenyi Biotec) were used according to
manufacturer's instructions. MGG8 was incubated with these
antibodies for 10 min at 4°C and then washed and analyzed using a
MACSQuant Analyzer (Miltenyi Biotec).
RNA extraction and reverse
transcription-quantitative PCR (qPCR)
The total RNA was extracted using RNeasy Mini kit
from Qiagen according to the manufacturer's instructions. RNA was
reverse transcribed using the Omniscript Reverse Transcription kit
(Qiagen). The following primer sequences were used for PCR: CD133
(PROM1) sense 5′-agtggcatcgtgcaaacgata-3′ and antisense
5′-ctccgaatccattcgacgata-3′ and GAPDH (internal control) sense
5′-gcaccgtcaaggctgagaac-3′ and antisense 5′-tggtgaagacgccagtgga-3′.
The qPCR reactions were performed on the Step One Plus (Applied
Biosystems) using the Power Up SYBR Green Master Mix (Applied
Biosystems). Cycling conditions were initial denaturation at 95°C
for 10 min, then 40 cycles at 95°C for 15 sec and 60°C for 1
min.
Immunoblotting
Cells were lysed in the SDS/Nonidet P-40 lysis
buffer [1% SDS, 1% Nonidet P-40, 50 mM Tris (pH 8.0), 150 mM NaCl,
2 µg/ml leupeptin, 2 µg/ml aprotinin, 1 mM phenylmethylsulfonyl
fluoride (PMSF), 5 mM NaF, and 100 µM
Na3VO4]. Nucleus and cytosol protein
fractions were extracted using Nuclear/Cytosol Fractionation kit
(BioVision). The lysates were boiled for 5 min and then cleared by
centrifugation at 15,000 rpm and 4°C. A Bradford protein assay
reagent (BioRad) was used to determine the protein concentration of
supernatants. Lysates were further boiled for 5 min in the sample
buffer. Then, samples were resolved using the SDS-PAGE and
transferred onto Immobilon-P (Millipore Corp.) sheets. Blots were
first incubated in blocking buffer [5% (w/v) nonfat dry milk in
Tris-buffered saline (TBS) plus 0.05% Tween 20] for 30 min. Then,
they were incubated with a primary antibody for 16 h at 4°C,
followed by incubation with a horseradish peroxidase-conjugated
secondary antibody for 30 min at room temperature (RT). The
ECL-plus chemiluminescence (GE Healthcare) was used to visualize
antibody-antigen complex.
Indirect immunofluorescence and cell
imaging
Cells grown on coverslips were briefly washed with
phosphate-buffered saline (PBS) three times, and then fixed with 4%
paraformaldehyde for 15 min at RT or ice cold methanol for 20 min
at −20°C. A 1% NP-40 in PBS solution was used to treat the cells
for 10 min, which were incubated with blocking solution (15% bovine
serum albumin in PBS) for 1 h. The cells were then probed with
primary antibodies for 1 h at 37°C, and antibody-antigen complexes
were detected with either Alexa Fluor 594- or Alexa Fluor
488-conjugated donkey secondary antibody by incubation for 1 h at
RT. The samples were washed three times with TBS after each
incubation and then counterstained with
4′,6′-diamidino-2-phenylindole. Immunostained cells were examined
under a fluorescence microscope (Olympus IX73, Tokyo, Japan) using
a 100× or 60× objective lens. Fluorescence images were captured
using a CCD camera (Olympus, DP27) and processed with Adobe
Photoshop CC and ImageJ.
Mitotic analysis
TrypLE Express (Thermo Fisher) was used to separate
sphere-cultured cells. Mitotic cells were collected by settling
onto poly-L-lysine-coated coverslips at the bottom of 6-well plates
using a centrifugation at 1,200 rpm. for 5 min at RT. For
immunofluorescence analysis, cells were fixed, blocked, and stained
as described. Cells in the late telophase (cytokinesis) were
counted. ImageJ was used to measure the fluorescence intensity of a
given staining for the two dividing daughter cells. For these
values, the asymmetry cutoff was set with >25% difference
between the daughter cells (35).
Values from these three independent experiments were summarized
using frequencies and percentiles.
Sphere formation assay
Sphere-cultured MGG8 cells were suspended in the
stem cell culture medium at a density of 2.5×103
cells/ml, and 400 µl of the cell suspension were transferred to
each well in a non-coated 96-well plate. The spheres were counted
after 3 days. Then, a hemocytometer was used to determine the cell
number, and the dye exclusion method (0.1% trypan blue) was used
determine the viability. MGG8 spheres of >50 µm were counted.
Values from three independent experiments were summarized.
Statistical analysis
All statistical analyzes were performed using the
JMP Pro. The unpaired Student's t test was used to compare the
experimental groups, owing to the binary nature of data sets.
P<0.05 was considered to indicate a statistically significant
difference. Data are presented as the mean ± SEM.
Results
MGG8 cells responses in the BMP4-Smads
pathway
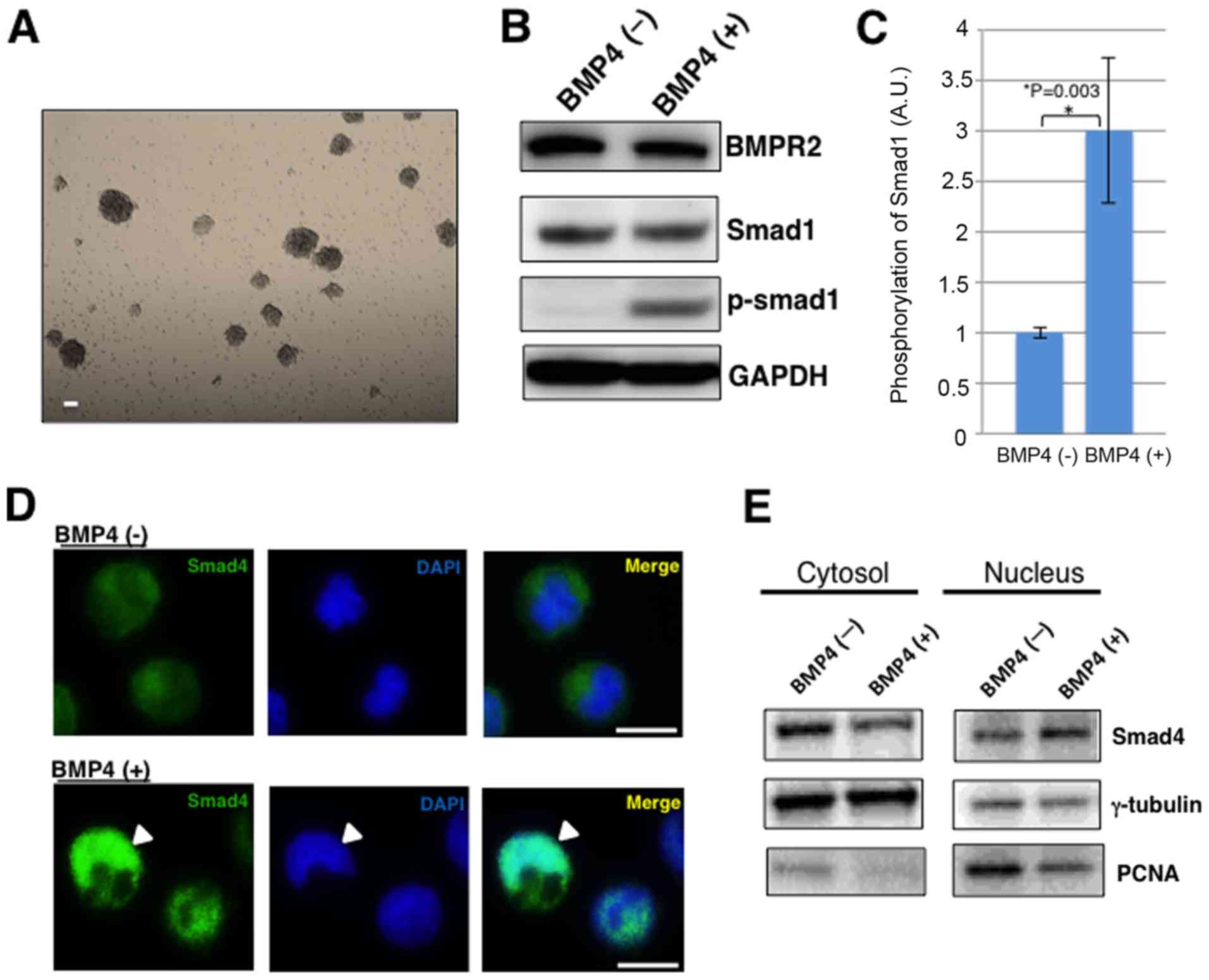
First, MGG8 cells were confirmed to stably perform
the sphere-forming activity under GBM cancer stem-like
cell-specific tissue culture conditions (Fig. 1A). MGG8 cells successfully continued
showing cell proliferation by forming tumor spheres. When BMP4
binds to the BMP receptor, Smad1/5/8 are phosphorylated.
Furthermore, the phosphorylated Smad1/5/8 forms a complex with
Smad4 and then transmits a signal into the nucleus. The activated
Smads regulate various biological effects, such as tissue
homeostasis in cooperation with transcription factors, and perform
transcriptional control for a specific cell state (23). Western blotting showed Smad1
phosphorylation under BMP4 treatment in MGG8 cells (Fig. 1B and C). Then, to confirm that Smads
complex were transmitting signals into the nucleus, an
immunofluorescence stained experiment for Smad4 was performed. As
expected, Smad4 was localized into the nucleus under the BMP4
treatment (Fig. 1D). Futhermore, the
western blotting of Smad4 in nucleus and cytosol fractions showed
that Smad4 in the nuclear fraction increases under BMP4 treatment
in MGG8 cells (Fig. 1E). These
findings indicate that MGG8 cells may respond to BMP4-Smads
pathway, the main pathway of BMP signaling.
BMP4 causes downregulation of CD133
expression in MGG8 cells
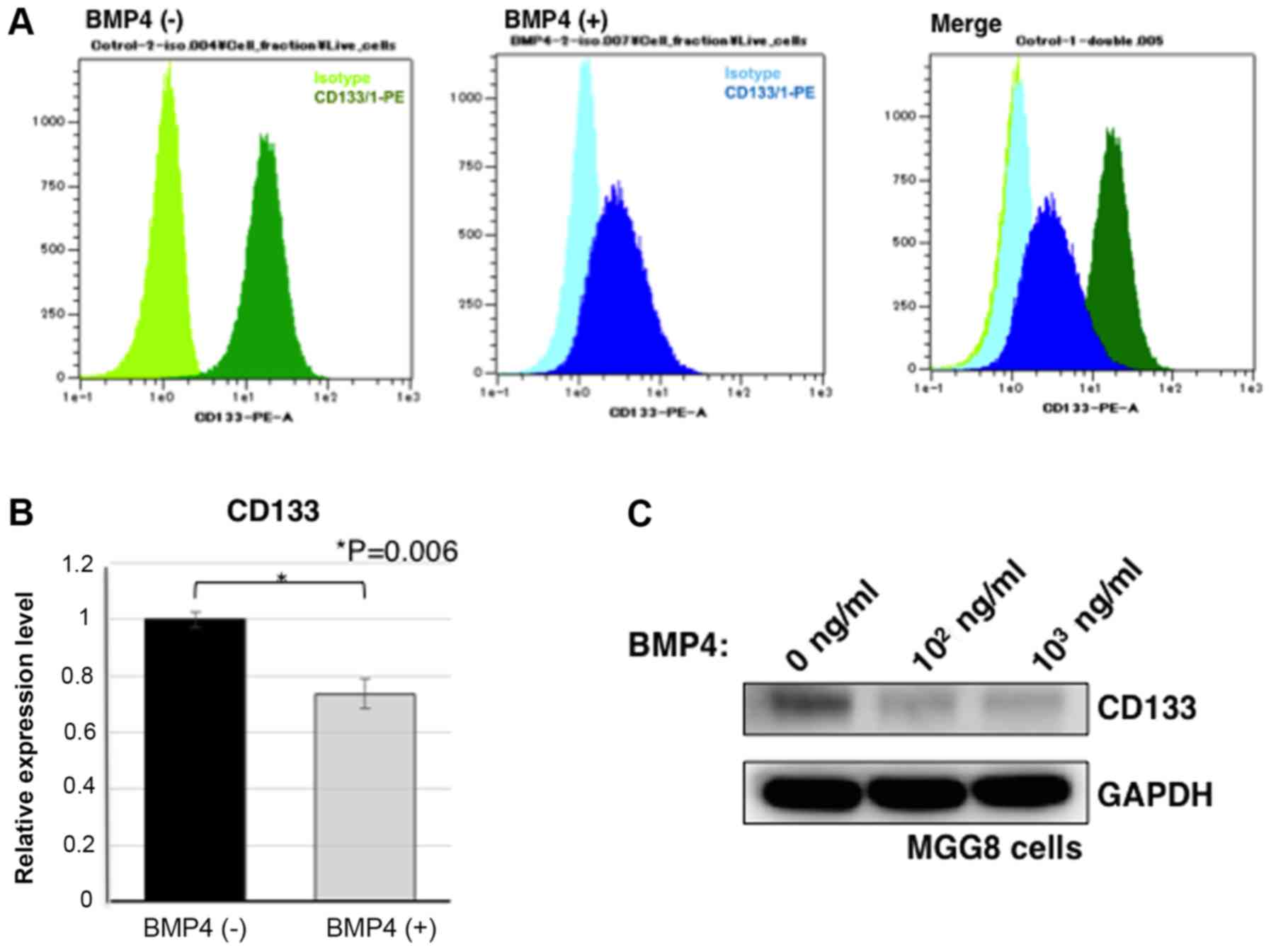
Sphere-cultured MGG8 cells were treated with BMP4
for 72 h. Then, the CD133 expression was considered as stemness
indicator (36,37). Flow cytometry analysis showed that
MGG8 cells expressed CD133 antigens and that BMP4 treatment
resulted in a cell population with low expression levels of CD133
antigen (Fig. 2A). Real-time PCR was
performed as quantitative gene expression analysis. As a result,
the gene expression of CD133 was reduced in MGG8 cells under BMP4
treatment (P=0.006; Fig. 2B).
Furthermore, western blotting was used to examine the expression
levels of CD133 protein. BMP4 treatment caused CD133 protein
downregulation in MGG8 cells. Therefore, upon BMP4 exposure, CD133
expression in MGG8 cells was reduced (Fig. 2C). These findings indicate that BMP4
exposure causes downregulation of CD133 expression in MGG8
cells.
Mitotic image analysis
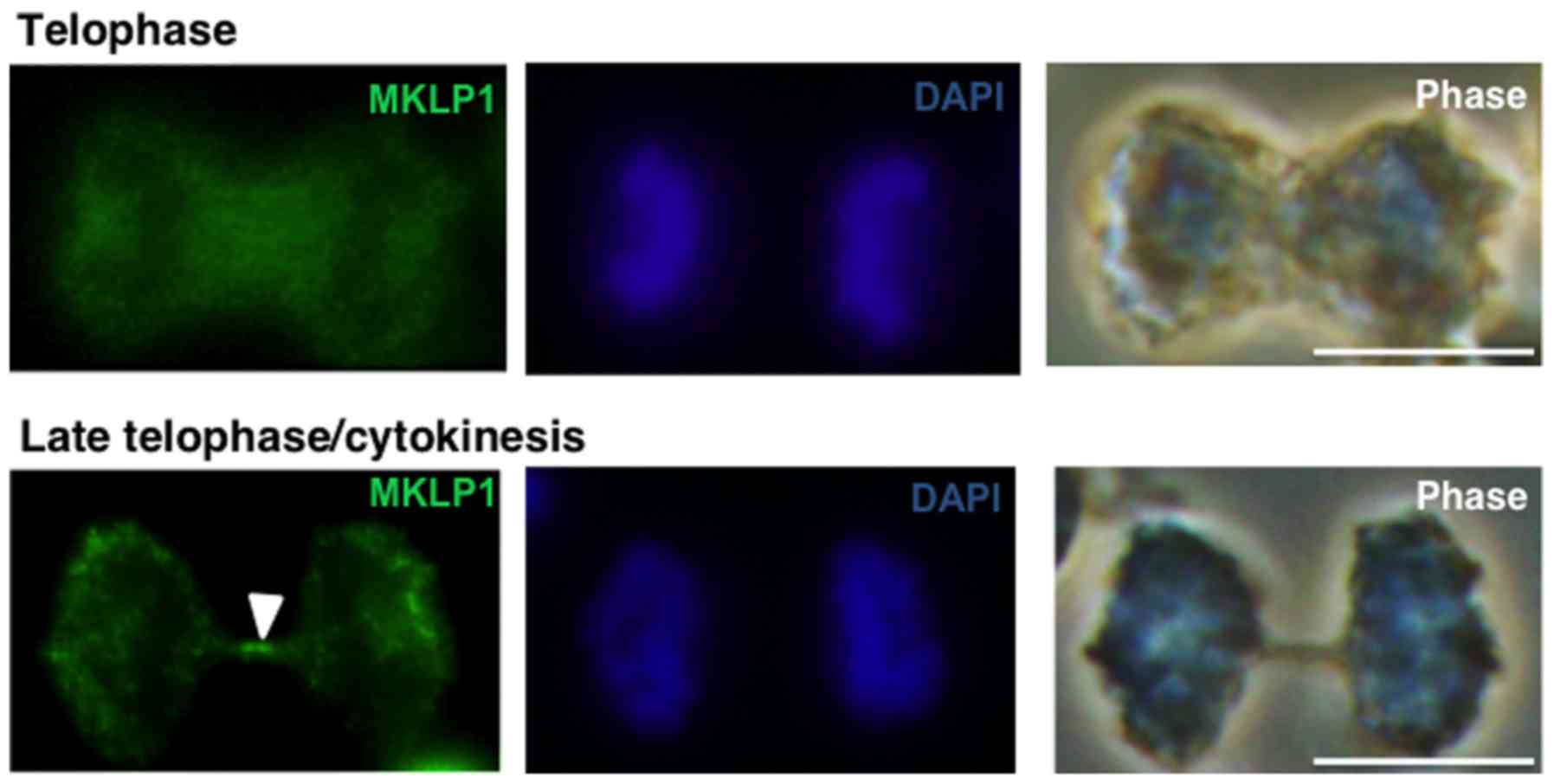
Mitotic kinesin-like protein 1 (MKLP1) is
specifically accumulated at the central part of microtubules
(Fleming body) in the late telophase/cytokinesis (38). Therefore, an immunostaining
experiment was performed using an anti-MKLP1 antibody (green
fluorescence) in MGG8 cells. In the early telophase, no specific
MKLP1 accumulation was observed. However, in its later stage, MKLP1
was specifically accumulated at the midbody, known as a Fleming
body (Fig. 3). Therefore, MKLP1
immunostaining was used as a cytokinesis marker for further
evaluation.
CD133-based ACDs induced by BMP4 in
MGG8 cells
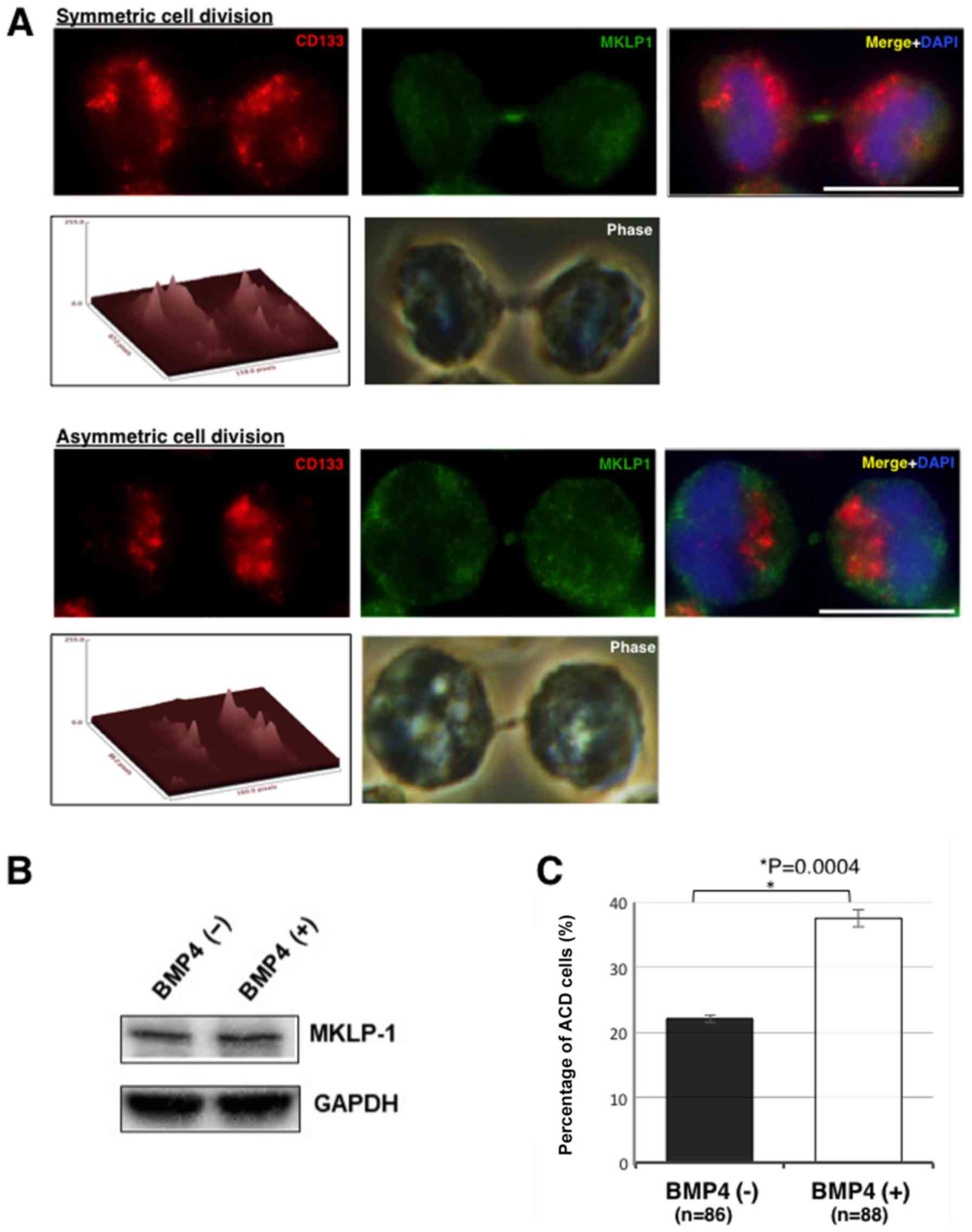
Next, whether BMP4 treatment induces ACD in MGG8
cells was examined. Representative immunostaining images in ACD and
symmetric cell division in MGG8 cells are shown in Fig. 4A. The asymmetry cutoff ratio was set
as >25% difference between the daughter cells (35). While the ACD ratio was found to be
23% (ACD/total cell divisions: 20/86) in the control cells, it was
38% (33/88) in cells treated with BMP4 (P=0.004). Thus, BMP4
induced ACD in MGG8 cells (Fig. 4B).
MKLP1 level with or without BMP4 treatment were compared using
western blotting. In this experiment, the expression of MKLP1 under
BMP4 treatment was not affected (Fig.
4C).
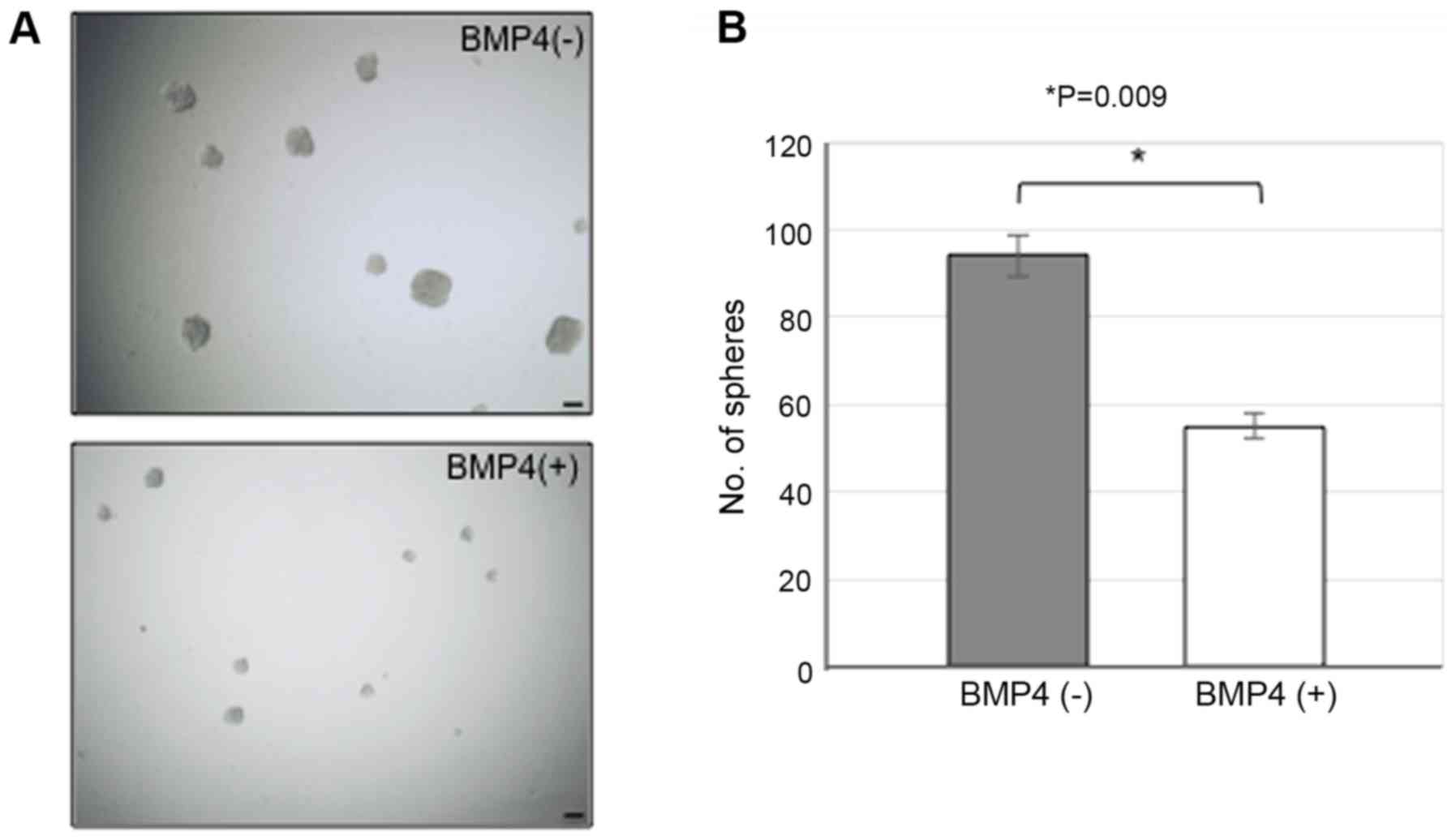
BMP4 signal suppressing sphere-forming
ability in MGG8 cells
Finally, whether BMP4 treatment affects self-renewal
division in MGG8 cells was evaluated using the sphere assay. After
a 3-day of BMP4 treatment, the large spheres of >50 µm in
diameter were counted (Fig. 5A and
B). Interestingly, those treated with BMP4 showed significantly
low number of spheres as compared with control (Fig. 5A and B). This finding strongly
suggests that BMP4 can suppress the self-renewal capability
(symmetric cell division) of MGG8 cells.
Discussion
BMP4 has been shown to suppress CD133 expression and
reduce the number of CD133-positive cells in GSCs (MGG8 cells).
Furthermore, the mechanism underlying the CD133 inhibitory effects
of BMP4 in GSCs mainly focused on cell division in this study.
Cells at late telophase/cytokinesis showed the proportion of
asymmetric division, and those with asymmetrically distributed
CD133 increased with BMP4 treatment. These results indicate that
the number of CD133-positive cells is suppressed with BMP4
treatment in GSCs.
BMP4 can inhibit cell proliferation and induce the
expression of neural differentiation markers without affecting cell
viability (29,31). BMP4 also promotes cell
differentiation in GSCs both in vitro and in vivo (29,31).
Therefore, BMP4 and its signaling pathway, Smad pathway, can
strongly be promising targets for differentiation therapy (31). Moreover, in our in vitro studies,
BMP4 reduced the CD133-positive cell ratio in GSCs and its
sphere-forming ability. Therefore, BMP4 treatment may improve the
chemotherapeutic effects. BMP4 expression is downregulated in GBM
as compared to normal brain in human samples (28), suggesting that this downregulation
may cause drug resistance. BMP4 reverses drug resistance, such as
temozolomide, by regulating the B-cell lymphoma 2(BCL-2) and glial
cell-derived neurotrophic factor (39). Thus, BMP4 may act as an important
inhibitory regulator of glioma tumorigenesis to improve the
therapeutic efficacy combined with conventional chemotherapy and
radiation therapy (31,39,40).
Several studies have reported that CD133-positive
GBM cells can effectively form tumors (36,37,41).
Studies have also been conducted to characterize the isolated cell
population, using an anti-CD133 antibody as a specific cell surface
marker to isolate CSCs (42).
However, the antibodies routinely used as cell surface markers for
CD133 target poorly specific glycoprotein epitopes, and skeptical
arguments exist in using CD133 as a cell surface marker for CSCs
(26). Recently, studies have been
focusing on the functional analysis of CD133 itself. The
phosphorylation of tyrosine-828 residue in CD133 C-terminal
cytoplasmic domain results in the activation of PI3K/Akt pathway,
which promotes GSC self-renewal and tumorigenesis (43). Furthermore, the role of CD133 in
maintaining the undifferentiated state of cancer cells has recently
been reported by inhibiting autophagy. That is, when CD133 is not
phosphorylated by Src, CD133 is preferentially transported to the
centrosome through the intracellular transport, and trap GABARAP,
resulting in autophagy inhibition (44). These findings not only facilitate
understanding the functional role of CD133 itself but also suggest
the CD133 and its regulatory mechanisms as attractive therapeutic
targets.
CSCs balance self-renewal and multipotency using the
ACD/SCD proportion and adapt to various environments. ACD/SCD
proportion is deeply involved in the maintenance of stemness of
tumor tissue and proliferation of cancer cells (32,33,45).
Researchers believe that as CSCs become more malignant, they become
more likely to divide symmetrically. Since SCD produces both two
daughter CSCs, SCD leads to effectively the expansion of the CSC
population (33,45–48). The
concept of ACD therapy, which suppresses CSCs symmetrical division
and inhibits CSCs expansion, may establish a new strategy for
cancer treatment.
Loss of function of the tumor suppressor gene
TP53 has been shown to promote stemness maintenance
(49). A recent study using a
mammary cancer model that used PKH fluorescent dye labeling for
stem cell mitotic analysis, showed that loss of p53 activity can
induce a shift from ACD to SCD, thereby contributing to tumor
growth (46). This study assumes
that PKH-high cells have the greater stemness and the higher
tumorigenic potential. In GBM, TRIM3 expression also attenuates the
stemness of GSCs. In fact, TRIM3 expression suppresses both sphere
formation and expression of stem cell markers such as CD133,
Nestin, and Nanog. TRIM3 expression leads to a larger proportion of
ACD rather than SCD (47). These
studies assume that PKH-high cell have the greater stemness and the
higher tumorigenic potential (46,47).
However, mitotic analysis using the PKH staining is not accompanied
with analysis of cancer stem cell markers. On the other hand, we
examined the mode of cell division using CD133, one of the most
common markers of GSCs, and provided more direct evidence that BMP4
induces to ACD and suppresses self-renewal ability.
Although our study have been limited to in vitro
experiments and have not clarified the effects of BMP4 in
vivo, recent study shows that BMP4 reduces tumorigenic
potential through the suppression of proliferation and the
differentiation of GSCs (31).
Therefore, our research approach may be also useful for further in
vivo study. In conclusion, BMP4 induces ACD and suppresses
self-renewal ability. This finding may provide a new perspective on
how BMP4 reduces the tumorigenicity of GSCs.
Acknowledgements
This paper was presented at The 24th Annual
Scientific Meeting and Education Day of The Society for
Neuro-Oncology November 22–24, 2019, Phoenix, Arizona. The authors
would like to thank Dr Hiroaki Wakimoto (Massahcusetts General
Hospital) for the gift of glioma cells. The authors would also like
to thank Mrs. Yumiko Oishi, Mrs. Chieko Mizukawa and Mrs. Akiko
Soejima (Department of Neurosurgery, Faculty of Medicine, Saga
University) for their secretarial assistance.
Funding
The present study was supported by JSPS KAKENHI
(grant no. JP18K16589).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
MK and HIz designed experiments. MK and HIz
performed experiments. MK, YN, HIt, TW, FY, AO, KI, JM, HIz and TA
analyzed the results. MK and HIz wrote the manuscript. MK, NY, HIz
and TA conceived and supervised the project.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rasras S, Zibara K, Vosughi T and Zayeri
ZD: Genetics and epigenetics of glioblastoma: Therapeutic
challenges. Clin Cancer Invest J. 7:43–49. 2018. View Article : Google Scholar
|
|
4
|
Deris Zayeri Z, Tahmasebi Birgani M,
Mohammadi Asl J, Kashipazha D and Hajjari M: A novel infram
deletion in MSH6 gene in glioma: Conversation on MSH6 mutations in
brain tumors. J Cell Physiol. 234:11092–11102. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Antoniou A, Hébrant A, Dom G, Dumont JE
and Maenhaut C: Cancer stem cells, a fuzzy evolving concept: A cell
population or a cell property? Cell Cycle. 12:3743–3748. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: Models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang T, Shigdar S, Gantier MP, Hou Y, Wang
L, Li Y, Shamaileh HA, Yin W, Zhou SF, Zhao X and Duan W: Cancer
stem cell targeted therapy: Progress amid controversies.
Oncotarget. 6:44191–44206. 2015.PubMed/NCBI
|
|
8
|
Soeda A, Hara A, Kunisada T, Yoshimura S,
Iwama T and Park DM: The evidence of glioblastoma heterogeneity.
Sci Rep. 5:79792015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sampetrean O and Saya H: Characteristics
of glioma stem cells. Brain Tumor Pathol. 30:209–214. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bleau AM, Hambardzumyan D, Ozawa T,
Fomchenko EI, Huse JT, Brennan CW and Holland EC: PTEN/PI3K/Akt
pathway regulates the side population phenotype and ABCG2 activity
in glioma tumor stem-like cells. Cell Stem Cell. 4:226–235. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng L, Bao S and Rich JN: Potential
therapeutic implications of cancer stem cells in glioblastoma.
Biochem Pharmacol. 80:654–665. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sunayama J, Sato A, Matsuda K, Tachibana
K, Watanabe E, Seino S, Suzuki K, Narita Y, Shibui S, Sakurada K,
et al: FoxO3a functions as a key integrator of cellular signals
that control glioblastoma stem-like cell differentiation and
tumorigenicity. Stem Cells. 29:1327–1337. 2011.PubMed/NCBI
|
|
15
|
Sato A, Sunayama J, Okada M, Watanabe E,
Seino S, Shibuya K, Suzuki K, Narita Y, Shibui S, Kayama T and
Kitanaka C: Glioma-initiating cell elimination by metformin
activation of FOXO3 via AMPK. Stem Cells Transl Med. 1:811–824.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bidlingmaier S, Zhu X and Liu B: The
utility and limitations of glycosylated human CD133 epitopes in
defining cancer stem cells. J Mol Med (Berl). 86:1025–1032. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
18
|
Bao S, Wu Q, Li Z, Sathornsumetee S, Wang
H, McLendon RE, Hjelmeland AB and Rich JN: Targeting cancer stem
cells through L1CAM suppresses glioma growth. Cancer Res.
68:6043–6048. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dreesen O and Brivanlou AH: Signaling
pathways in cancer and embryonic stem cells. Stem Cell Rev. 3:7–17.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eyler CE, Foo WC, LaFiura KM, McLendon RE,
Hjelmeland AB and Rich JN: Brain cancer stem cells display
preferential sensitivity to Akt inhibition. Stem Cells.
26:3027–3036. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gallia GL, Tyler BM, Hann CL, Siu IM,
Giranda VL, Vescovi AL, Brem H and Riggins GJ: Inhibition of Akt
inhibits growth of glioblastoma and glioblastoma stem-like cells.
Mol Cancer Ther. 8:386–393. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan X, Khaki L, Zhu TS, Soules ME, Talsma
CE, Gul N, Koh C, Zhang J, Li YM, Maciaczyk J, et al: NOTCH pathway
blockade depletes CD133-positive glioblastoma cells and inhibits
growth of tumor neurospheres and xenografts. Stem Cells. 28:5–16.
2010.PubMed/NCBI
|
|
23
|
Xiao YT, Xiang LX and Shao JZ: Bone
morphogenetic protein. Biochem Biophys Res Commun. 362:550–553.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao L, Michalski N, Kronander E, Gjoni E,
Genoud C, Knott G and Schneggenburger R: BMP signaling specifies
the development of a large and fast CNS synapse. Nat Neurosci.
16:856–864. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lim DA, Tramontin AD, Trevejo JM, Herrera
DG, García-Verdugo JM and Alvarez-Buylla A: Noggin antagonizes BMP
signaling to create a niche for adult neurogenesis. Neuron.
28:713–726. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Johnston MA and Lim DA: Keeping them
quiet: BMPs maintain adult neural stem cell quiescence. Cell Stem
Cell. 7:9–10. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qi X, Li TG, Hao J, Hu J, Wang J, Simmons
H, Miura S, Mishina Y and Zhao GQ: BMP4 supports self-renewal of
embryonic stem cells by inhibiting mitogen-activated protein kinase
pathways. Proc Natl Acad Sci USA. 101:6027–6032. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xi G, Best B, Mania-Farnell B, James CD
and Tomita T: Therapeutic potential for bone morphogenetic protein
4 in human malignant glioma. Neoplasia. 19:261–270. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee J, Son MJ, Woolard K, Donin NM, Li A,
Cheng CH, Kotliarova S, Kotliarov Y, Walling J, Ahn S, et al:
Epigenetic-mediated dysfunction of the bone morphogenetic protein
pathway inhibits differentiation of glioblastoma-initiating cells.
Cancer Cell. 13:69–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bonaguidi MA: LIF and BMP signaling
generate separate and discrete types of GFAP-expressing cells.
Development. 132:5503–5514. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Piccirillo SG, Reynolds BA, Zanetti N,
Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F and Vescovi
AL: Bone morphogenetic proteins inhibit the tumorigenic potential
of human brain tumour-initiating cells. Nature. 444:761–765. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morrison SJ and Kimble J: Asymmetric and
symmetric stem-cell divisions in development and cancer. Nature.
441:1068–1074. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bajaj J, Zimdahl B and Reya T: Fearful
symmetry: Subversion of asymmetric division in cancer development
and progression. Cancer Res. 75:792–797. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wakimoto H, Mohapatra G, Kanai R, Curry WT
Jr, Yip S, Nitta M, Patel AP, Barnard ZR, Stemmer-Rachamimov AO,
Louis DN, et al: Maintenance of primary tumor phenotype and
genotype in glioblastoma stem cells. Neuro Oncol. 14:132–144. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lathia JD, Hitomi M, Gallagher J, Gadani
SP, Adkins J, Vasanji A, Liu L, Eyler CE, Heddleston JM, Wu Q, et
al: Distribution of CD133 reveals glioma stem cells self-renew
through symmetric and asymmetric cell divisions. Cell Death Dis.
2:e2002011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Beier D, Hau P, Proescholdt M, Lohmeier A,
Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U and Beier
CP: CD133(+) and CD133(−) glioblastoma-derived cancer stem cells
show differential growth characteristics and molecular profiles.
Cancer Res. 67:4010–4015. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Joo KM, Kim SY, Jin X, Song SY, Kong DS,
Lee JI, Jeon JW, Kim MH, Kang BG, Jung Y, et al: Clinical and
biological implications of CD133-positive and CD133-negative cells
in glioblastomas. Lab Invest. 88:808–815. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Makyio H, Ohgi M, Takei T, Takahashi S,
Takatsu H, Katoh Y, Hanai A, Ueda T, Kanaho Y, Xie Y, et al:
Structural basis for Arf6-MKLP1 complex formation on the Flemming
body responsible for cytokinesis. EMBO J. 31:2590–2603. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu B, Chen Q, Tian D, Wu L, Dong H, Wang
J, Ji B, Zhu X, Cai Q, Wang L and Zhang S: BMP4 reverses multidrug
resistance through modulation of BCL-2 and GDNF in glioblastoma.
Brain Res. 1507:115–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rahman M, Azari H, Deleyrolle L, Millette
S, Zeng H and Reynolds BA: Controlling tumor invasion: Bevacizumab
and BMP4 for glioblastoma. Future Oncol. 9:1389–1396. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang J, Sakariassen PØ, Tsinkalovsky O,
Immervoll H, Bøe SO, Svendsen A, Prestegarden L, Røsland G, Thorsen
F, Stuhr L, et al: CD133 negative glioma cells form tumors in nude
rats and give rise to CD133 positive cells. Int J Cancer.
122:761–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wei Y, Jiang Y, Zou F, Liu Y, Wang S, Xu
N, Xu W, Cui C, Xing Y, Liu Y, et al: Activation of PI3K/Akt
pathway by CD133-p85 interaction promotes tumorigenic capacity of
glioma stem cells. Proc Natl Acad Sci USA. 110:6829–6834. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li Z: CD133: A stem cell biomarker and
beyond. Exp Hematol Oncol. 2:172013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Izumi H, Li Y, Shibaki M, Mori D, Yasunami
M, Sato S, Matsunaga H, Mae T, Kodama K, Kamijo T, et al: Recycling
endosomal CD133 functions as an inhibitor of autophagy at the
pericentrosomal region. Sci Rep. 9:22362019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Neumüller RA and Knoblich JA: Dividing
cellular asymmetry: Asymmetric cell division and its implications
for stem cells and cancer. Genes Dev. 23:2675–2699. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cicalese A, Bonizzi G, Pasi CE, Faretta M,
Ronzoni S, Giulini B, Brisken C, Minucci S, Di Fiore PP and Pelicci
PG: The tumor suppressor p53 regulates polarity of self-renewing
divisions in mammary stem cells. Cell. 138:1083–1095. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen G, Kong J, Tucker-Burden C, Anand M,
Rong Y, Rahman F, Moreno CS, Van Meir EG, Hadjipanayis CG and Brat
DJ: Human Brat ortholog TRIM3 is a tumor suppressor that regulates
asymmetric cell division in glioblastoma. Cancer Res. 74:4536–4548.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tominaga K, Minato H, Murayama T, Sasahara
A, Nishimura T, Kiyokawa E, Kanauchi H, Shimizu S, Sato A, Nishioka
K, et al: Semaphorin signaling via MICAL3 induces symmetric cell
division to expand breast cancer stem-like cells. Proc Natl Acad
Sci USA. 116:625–630. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Spike BT and Wahl GM: p53, Stem cells, and
reprogramming: Tumor suppression beyond guarding the genome. Genes
Cancer. 2:404–419. 2011. View Article : Google Scholar : PubMed/NCBI
|