Introduction
Pancreatic cancer is the fourth most common cause of
cancer-associated mortality, and pancreatic ductal adenocarcinoma
(PDAC) accounts for 90% of total pancreatic cancer cases (1,2). PDAC
exhibits a 5-year survival rate of ~5% and a median survival time
of <6 months (3,4). Hypoxia is commonly observed in solid
tumors, particularly in pancreatic cancer (5). Previous studies have reported that
hypoxia enhances tumor invasion and metastasis, serving as an
activator of epithelial-mesenchymal transition (6,7).
However, the mechanisms underlying hypoxia-induced tumor
progression in pancreatic cancer are yet to be determined.
MicroRNAs (miRNAs or miRs) are implicated in the
regulation of a number of biological processes, under normal and
pathological conditions (8). They
regulate putative downstream targets by binding to their
3′-untranslated region (UTR) sequences (9). It has been demonstrated that the
aberrant expression of certain miRNAs influences the genesis and
progression of various types of cancer (10). Previous studies have revealed that
certain miRNAs, including miR-21, miR-217 and miR-135a, may serve a
diagnostic and prognostic role in patients with PDAC (11–13). To
the best of our knowledge, no previous study has reported the role
of miR-519 in pancreatic cancer.
The immune checkpoint, programmed death ligand 1
(PD-L1; also known as CD274 or B7-H1), has been demonstrated to be
activated via binding to its cognate receptor, programmed death 1
(PD-1), in numerous types of cancer, including oral squamous cell
carcinoma (14). Upon activation,
the PD-L1/PD-1 pathway facilitates escape from T cell-mediated
immune function (15). In cancer,
high PD-L1 levels are associated with tumor progression and a poor
prognosis (16). Recently, PD-L1 has
been revealed to influence tumor progression via the inhibition of
the T cell-mediated immune response (17,18).
Unfortunately, therapies targeting the PD-L1/PD-1 signaling pathway
have not yet exhibited a marked effect in pancreatic cancer
treatment (19). Therefore,
determination of the underlying mechanisms underpinning this
phenomenon require further study.
The present study aimed to investigate the molecular
mechanisms of hypoxia-induced tumorigenesis in pancreatic cancer.
The results revealed that miR-519 suppressed the invasion, and
induced the apoptosis, of pancreatic cancer cells via
downregulation of PD-L1. The conclusions of the present study may
advance the understanding of pancreatic cancer treatment.
Materials and methods
Cell culture and hypoxic
treatment
The human pancreatic cancer PANC-1 and SW1990 cell
lines were purchased from the American Type Culture Collection and
cultured at 37°C in RPMI1640 medium (HyClone; GE Healthcare Life
Sciences), supplemented with 1% penicillin/streptomycin and 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.). PANC-1 and SW1990 cells
were then plated separately either into cell culture dishes or
plates, and cultured under normoxic (94% N2, 5%
CO2 and 1% O2) or hypoxic conditions (95%
N2 and 5% CO2 with 100 µM
CoCl2).
Cell transfection
PD-L1 cDNA was subcloned and ligated into pCMV
vectors. Lipofectamine 2000® (Thermo Fisher Scientific,
Inc.) was subsequently used to incorporate 2 µg pCMV-PD-L1 into
5×105 PANC-1 and SW1990 cells. Next, ~48 h after
transfection, the cells were harvested to perform subsequent
experiments. Negative control (NC) mimic, NC inhibitor, miR-519
mimics or miR-519 inhibitors (Shanghai GenePharma Co., Ltd.) were
transfected into PANC-1 or SW1990 cells using
Lipofectamine® RNAiMAX reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). The sequences were as follows: NC mimic,
5′-GGUUCGUACGUACACUGUUCA-3′; miR-519 mimic,
5′-CUCUAGAGGGAAGCGCUUUCUG-3′; NC inhibitor,
5′-CCAUCAGUCCCAAAUCCA-3′; miR-519 inhibitor,
5′-CCAGAGGGAAGCGCCG-3′. NC mimic and NC inhibitor represented
non-targeting sequences. At 36 h after transfection, the cells were
subject to subsequent experiments.
Immunofluorescence
PANC-1 and SW1990 cells were fixed using 4%
paraformaldehyde, and 0.5% Triton X-100 was used to permeabilize
cells. Cells were then incubated with Annexin V-fluorescein
isothiocyanate antibodies (cat. no. 556547; 1:20; BD Pharmingen; BD
Biosciences) for 1 h at room temperature. Apoptosis rates were
subsequently determined by calculating the ratio of Annexin
V-positive cells to the total cell number under a fluorescent
microscope (Olympus Corporation) with a magnification of ×400.
Bioinformatics analysis
The downstream target of miR-519 was predicted using
TargetScan online program version 7.2 (http://www.targetscan.org/).
Dual-luciferase reporter assay
The wild-type and mutant 3′-UTR sequences of PD-L1
were subcloned and ligated into pGL3 vectors. The 3′-UTR sequence
containing the pGL3 vector (Promega Corporation) was co-transfected
with Lipofectamine 2000® (Thermo Fisher Scientific,
Inc.) into PANC-1 cells with miRNA (NCs, miR-519 mimics or miR-519
inhibitor). Luciferase activities were measured using a
Dual-Luciferase Reporter system (Promega Corporation) at 48 h after
transfection. The Firefly luciferase activities were normalized to
Renilla.
Transwell invasion assay
A total of ~1×105 cells were resuspended
in 200 µl DMEM (HyClone; GE Healthcare Life Sciences).
Subsequently, the medium was transferred to the top chamber with
8.0-µm pore membranes (EMD Millipore) pre-coated with Matrigel for
30 min at 37°C. DMEM (~350 µl) supplemented with 20% FBS (Gibco;
Thermo Fisher Scientific, Inc.) was plated in the lower chamber.
Invasive cells were stained with crystal violet at room temperature
for 2 h following incubation for 48 h. Cell images were obtained
using an inverted light microscope with magnification of ×400.
Statistical analysis was subsequently performed using GraphPad
prism software 6.0 (GraphPad Software, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted as previously described.
Takara- PrimeScriptTM RT reagent Kit (Takara Bio, Inc.) was used to
reverse transcribe RNA into cDNA according to the manufacturer's
protocol. The reverse transcription thermocycling program was 37°C
for 15 min followed by 85°C for 5 sec. SYBR®-Green dye
(Roche Diagnostics) was used to perform qPCR according to the
manufacturer's protocol. The thermocycling program was: Step 1,
95°C for 30 sec; step 2, 95°C for 3 sec; step 3, 60°C for 30 sec
(step 2–3, 40 cycles); and step 4, holding at 10°C. GAPDH and U6
were utilized as housekeeping genes for the detection of PD-L1 and
miR-519, respectively. Relative expression levels of genes were
calculated using the 2−ΔΔCt method (20). The primer sequences were as follows:
miR-519 forward, 5′-CATGCTGTGACCCTCCAAAG-3′ and reverse,
5′-GAGAAAACAAACAGAAAGCGCT-3′; PD-L1 forward,
5′-CTGAACGCCCCATACAACAA-3′ and reverse, 5′-CTTGGAATTGGTGGTGGTGG-3′;
GAPDH forward, 5′-GAGAAGTATGACAACAGCCTC-3′ and reverse,
5′-ATGGACTGTGGTCATGAGTC-3′; and U6 forward,
5′-CTCGCTTCGGCAGCACATATACTA-3′ and reverse,
5′-ACGAATTTGCGTGTCATCCTTGCG-3′.
Xenograft tumor experiment
Immunodeficient mice (n=8; 4 males and 4 females;
NOD-SCID; age, ~6 weeks; weight, 20–22 g) were utilized, and
purchased from Shanghai SLAC Laboratory Animal Co., Ltd. PANC-1
cells (1×106) suspended in RPMI1640 medium were injected
subcutaneously into right armpit of each mouse following anesthetic
treatment with 2% isoflurane (Baxter). The mice were housed in a
specific-pathogen-free room with enough distilled water and food,
under controlled conditions (25°C; 40–60% humidity; 10 h light/14 h
dark). Animal health and behavior were monitored daily. Body
temperature and weight, behavioral changes, pathological changes
(such as autonomous tumors, observed using micro-Computer
Tomography imaging technology) and blood oxygen saturation were the
criteria used to determine whether animals should be euthanized.
Mice were sacrificed by decapitation 4 weeks after injection.
Animal death was verified by cardiac and respiratory arrest, muscle
relaxation and lack of reflection. Murine tumors were subsequently
removed and weighed. No premature mortalities or significant
decreases in body weight were observed during the experiment. The
maximum percentage weight of the tumor compared with total body
weight was 2%. The protocol of the present study was approved by
the Animal Welfare Committee of The Third Affiliated Hospital of
Soochow University (Changzhou, China).
Statistical analysis
All experiments were performed in triplicate and
data are presented as the mean ± standard deviation. Statistical
analysis was performed using GraphPad prism software 6.0 (GraphPad
Software, Inc.). Comparisons between two groups were performed
using a two-tailed Student's t-test and comparisons among multiple
groups were performed using ANOVA (Tukey's post-hoc test).
*P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-519 attenuates the hypoxia-induced
tumorigenesis of pancreatic cancer cells
To investigate the influence of miR-519 on the
tumorigenesis and progression of pancreatic cancer cells, miR-519
expression in PANC-1 and SW1990 cells was determined under normoxic
and hypoxic conditions. The results of RT-qPCR revealed that
miR-519 expression was significantly reduced in both cell lines
when they were cultured in a hypoxic environment (Fig. 1A), indicating that miR-519 may serve
a suppressive role in hypoxia-induced phenotypes of pancreatic
cancer.
 | Figure 1.miR-519 attenuates the hypoxia-induced
tumorigenesis of pancreatic cancer cells. miR-519 levels in PANC-1
or SW1990 cells were determined via reverse
transcription-quantitative PCR under (A) normoxic or hypoxic
conditions; or (B) following transfection with NCs, miR-519 mimics
or miR-519 inhibitors. (C) Invasive abilities of PANC-1 or SW1990
cells transfected with NCs, miR-519 mimics or miR-519 inhibitors
were examined by performing a Transwell assay under normoxic or
hypoxic conditions. Magnification, ×200. (D) Statistical analysis
of invasive cell numbers. (E) E-cadherin and vimentin expression
were examined using western blotting in PANC-1 cells transfected
with either the NC inhibitor or miR-519 inhibitor under normoxic or
hypoxic conditions. GAPDH was used as the loading control. (F)
Apoptosis of NC-, miR-519 mimic- or miR-519 inhibitor-transfected
PANC-1 and SW1990 cells was determined using an immunofluorescence
assay following staining with Annexin V under normoxic or hypoxic
conditions, merge denotes that Annexin V was merged with DAPI
pictures. Magnification, ×200. (G) Statistical analysis of
apoptosis. Data are presented as the mean ± standard deviation of
three independent experiments. *P<0.05, **P<0.01 and
***P<0.001, as indicated. miR, microRNA; NC, negative
control. |
Invasion was subsequently assessed using a Transwell
assay in cells with indicated oligonucleotides. The results
revealed that hypoxia significantly increased the invasiveness of
both cell lines transfected with NC mimic (Fig. 1C and D). Furthermore, treatment with
miR-519 mimics reduced the number of invasive cells when compared
with the NC group, under hypoxic conditions. Furthermore, treatment
with miR-519 inhibitors promoted cell invasion (Fig. 1B-D). Additionally, it was revealed
that hypoxia and miR-519 inhibitor reduced E-cadherin expression,
whereas vimentin expression was elevated by hypoxia treatment and
miR-519 inhibitor (Fig. 1E). The
apoptosis of PANC-1 and SW1990 cells was examined via Annexin V
staining. As hypothesized, hypoxic treatment decreased Annexin V
signals produced in pancreatic cancer cells. In addition, miR-519
mimics elevated Annexin V positive cell numbers when compared with
NC treated cells under hypoxic conditions. The miR-519 inhibitor
reduced the apoptosis of pancreatic cancer cells (Fig. 1F and G). The results revealed that
miR-519 suppressed the hypoxia-induced tumorigenesis of pancreatic
cancer cells.
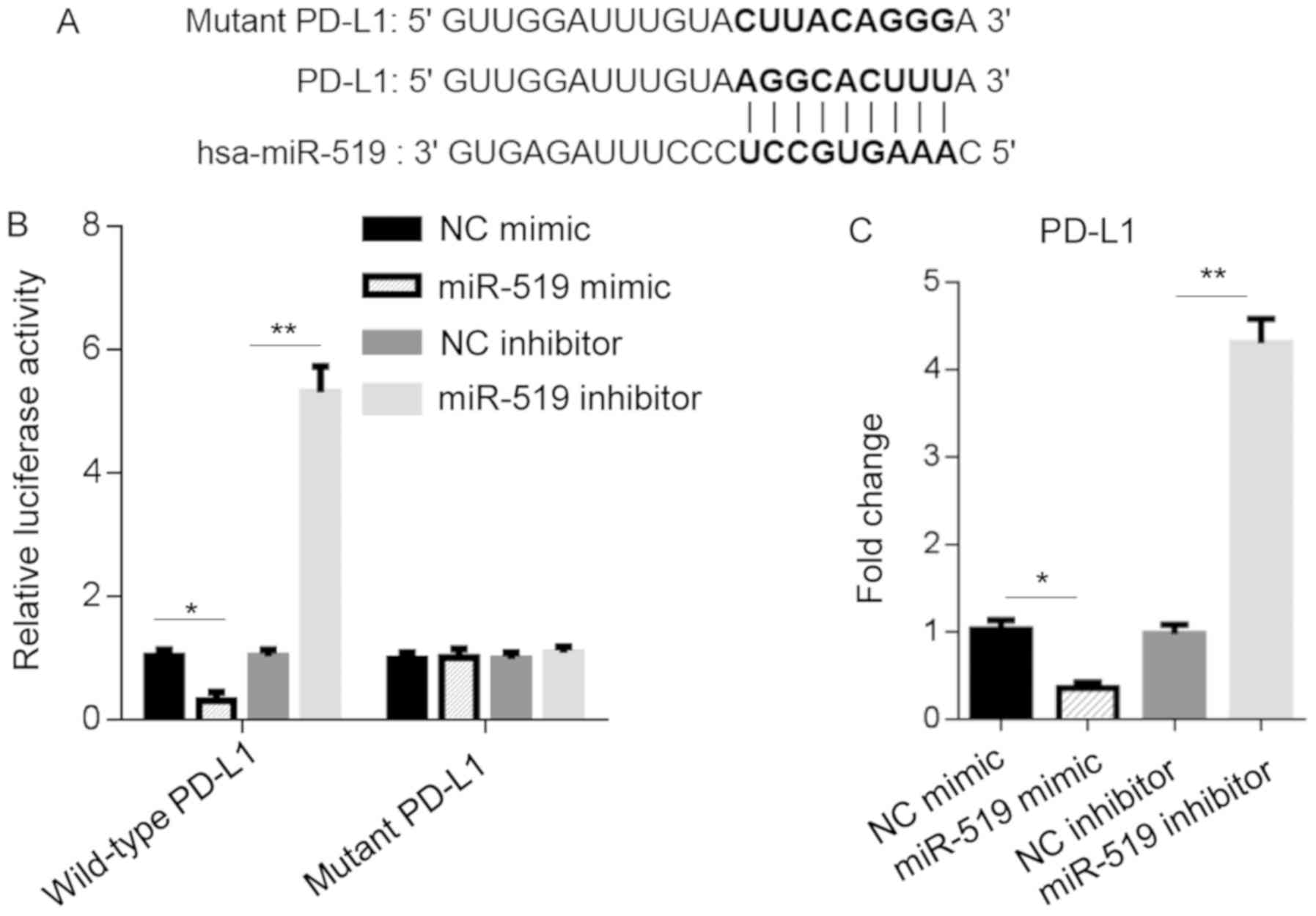
miR-519 binds to PD-L1 and regulates
its expression
To investigate the downstream targets of miR-519 in
pancreatic cancer, bioinformatics analysis was performed. The
results revealed that miR-519 was associated with PD-L1, as direct
binding to its 3′-UTR regions was predicted using the TargetScan
online program (Fig. 2A). To assess
whether miR-519 was directly associated with PD-L1 and regulated
its expression, a dual-luciferase reporter assay was performed. The
results demonstrated that the miR-519 mimic significantly reduced
luciferase activity in PANC-1 cells transfected with wild-type
3′-UTR-pGL3 vectors. Additionally, the miR-519 inhibitor promoted
luciferase activity (Fig. 2B). To
validate these results, RT-qPCR was performed and revealed that
miR-519 mimics decreased PD-L1 expression by 60–70%, whereas
transfection with the miR-519 inhibitor upregulated PD-L1 by
4.4-fold in PANC-1 cells (Fig. 2C).
Overall, the results indicated that miR-519 directly regulated
PD-L1 expression and activity via binding to its 3′-UTR region.
PD-L1 mediates the miR-519-attenuated
tumorigenesis of pancreatic cancer cells under hypoxic
conditions
After it was revealed that PD-L1 represents a
molecular downstream target of miR-519 in PANC-1 and SW1990 cells,
the present study aimed to confirm whether PD-L1 is responsible for
miR-519-associated tumorigenesis in pancreatic cancer cells. The
mRNA levels of PD-L1 in PANC-1 and SW1990 cells were detected under
normoxic and hypoxic conditions. RT-qPCR analysis revealed that
hypoxia significantly increased PD-L1 expression (2-3-fold;
Fig. 3A). These data indicated that
PD-L1 may promote hypoxia-induced phenotypes of pancreatic
cancer.
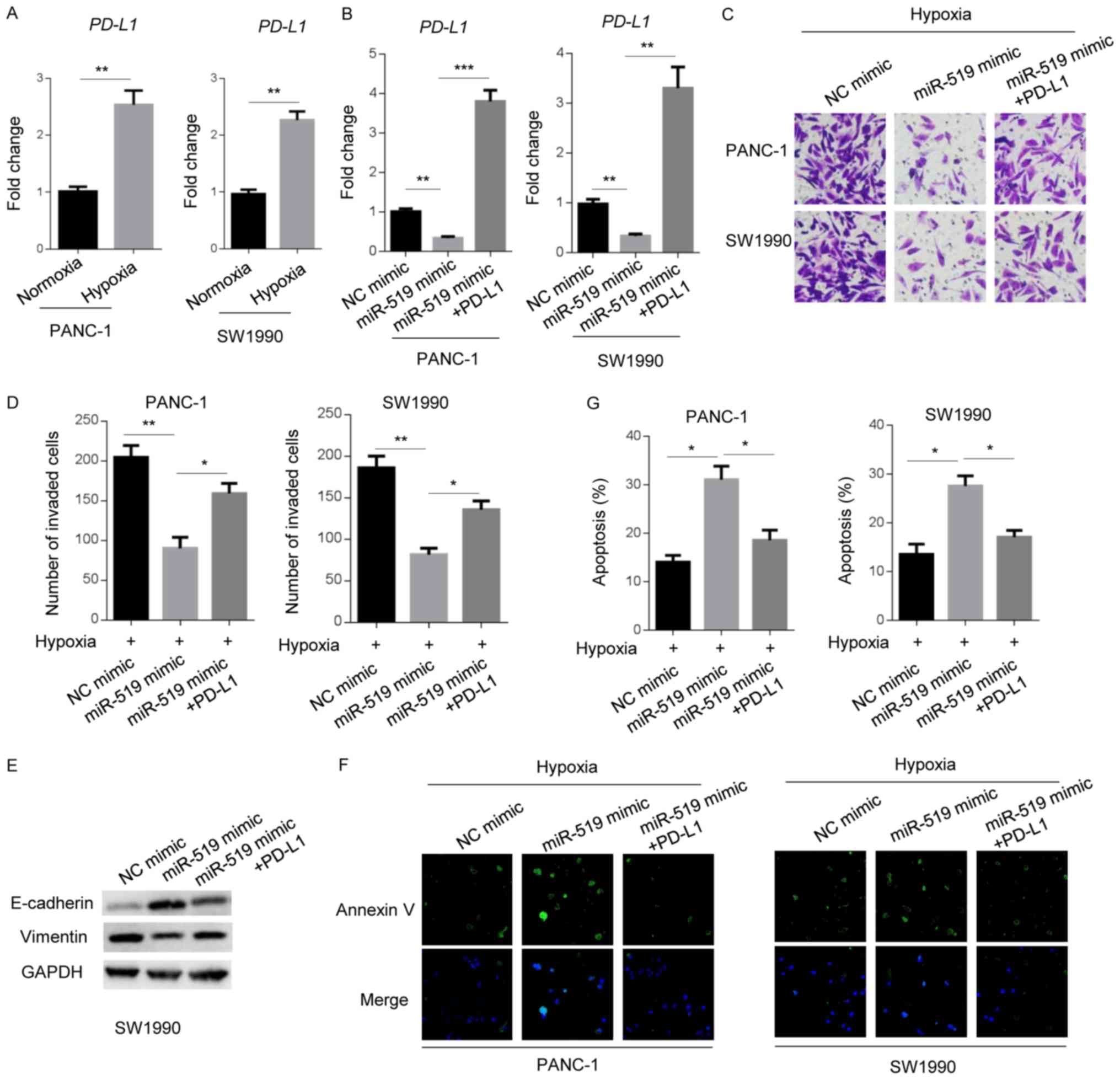 | Figure 3.PD-L1 mediates the miR-519-attenuated
tumorigenesis of pancreatic cancer cells under hypoxia. PD-L1 mRNA
levels were determined in PANC-1 or SW1990 cells via reverse
transcription-quantitative PCR (A) under normoxic or hypoxic
conditions, or (B) when transfected with NCs, miR-519 mimics, or
miR-519 mimics and PD-L1. (C) Invasive abilities of NC, miR-519
mimic, or miR-519 mimic and PD-L1-transfected PANC-1 or SW1990
cells were examined by performing a Transwell assay under hypoxic
conditions. Magnification, ×200. (D) Statistical analysis of
invasion assay. (E) E-cadherin and vimentin expression were
examined in SW1990 cells transfected with NC mimic, miR-519 mimic,
miR-519 mimic and PD-L1 by western blotting under hypoxic
conditions. GAPDH was used as the loading control. (F) Apoptosis of
PANC-1 or SW1990 cells transfected with NCs, miR-519 mimics, or
miR-519 mimics and PD-L1 were examined using an immunofluorescence
assay with Annexin V staining under hypoxic conditions.
Magnification, ×200. (G) Statistical analysis of apoptosis assay.
Data are presented as the mean ± standard deviation of three
independent experiments. *P<0.05, **P<0.01 and ***P<0.001,
as indicated. PD-L1, programmed death ligand 1; miR, microRNA; NC,
negative control. |
The miR-519 mimic decreased PD-L1 expression
compared with the NC mimic (Fig.
3B). In addition, PD-L1 level was higher when PD-L1 was
overexpressed in miR-519 mimic PANC-1 and SW1990 cells (Fig. 3B). Furthermore, the Transwell assay
demonstrated that PD-L1 significantly increased the number of
invasive miR-519 mimic cells under hypoxic conditions (Fig. 3C and D). miR-519 mimic increased the
E-cadherin level and decreased the vimentin level, which was
partially restored by PD-L1 overexpression. However, PD-L1
attenuated the number of pancreatic cancer cells that stained
positive for Annexin V, indicating that PD-L1 functioned as an
effector of miR-519 and served an apoptosis-inhibiting role in
pancreatic cancer cells, under hypoxic conditions (Fig. 3F and G). The present results
indicated that, when exposed to hypoxic conditions, PD-L1 served as
an effector of the miR-519-attenuated tumorigenesis of pancreatic
cancer.
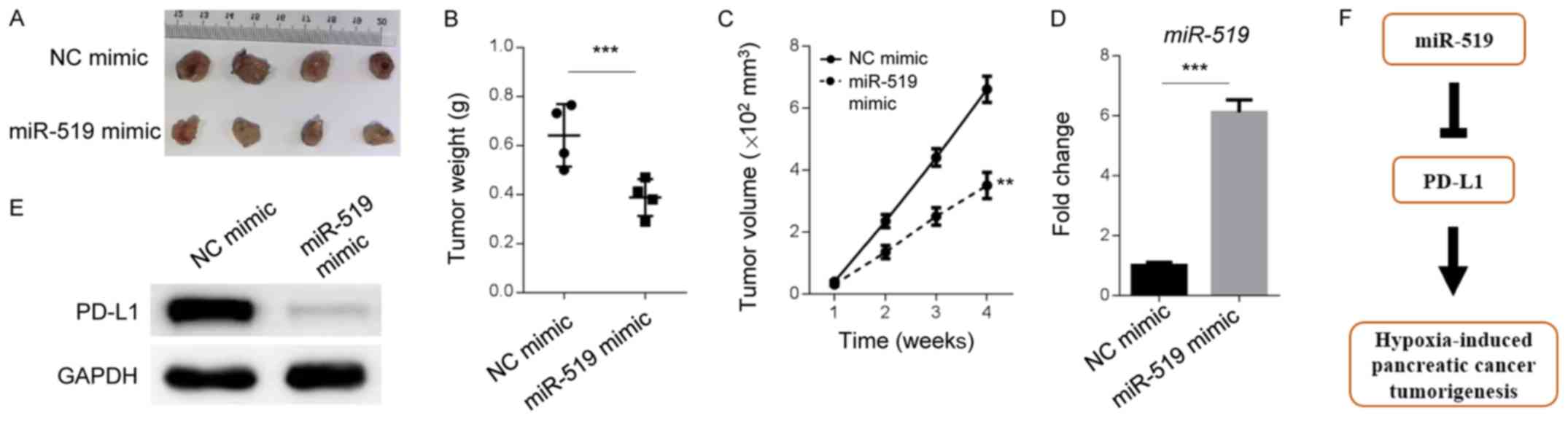
miR-519 and PD-L1 levels are
dysregulated in vivo
To determine whether miR-519 and PD-L1 were
dysregulated in an animal model, a xenograft tumor experiment was
performed to assess the role of miR-519 in the tumorigenesis of
PANC-1 cells. Cells treated with miR-519 mimics formed smaller
tumors compared with NC-treated cells (Fig. 4A-C). In addition, the results
demonstrated that miR-519 and PD-L1 levels were up- and
downregulated, respectively, in mouse tumors with overexpressed
miR-519 mimic (Fig. 4D and E). The
present results indicated that miR-519 and PD-L1 are aberrantly
expressed in mouse models, in vivo.
Discussion
The present study characterized the critical roles
of miR-519 and PD-L1 in hypoxia-induced pancreatic cancer cell
tumorigenesis (Fig. 4F). The results
also revealed that miR-519 interacted with PD-L1 and regulated its
expression. Additionally, miR-519 treatment inhibited invasiveness
and tumor growth in a mouse model, and induced pancreatic cancer
cell apoptosis by negatively regulating PD-L1. Clinically, it was
determined that miR-519 and PD-L1 were aberrantly expressed in
human pancreatic tumors compared with adjacent paracancerous
tissues.
Pancreatic cancer is a highly malignant form of
cancer, which represents the fourth most common cause of
cancer-associated mortality worldwide (21). As early diagnosis is challenging and
the prognosis is poor, surgery and chemotherapy remain the most
effective and common therapeutic strategies for pancreatic cancer
treatment (22,23). Recently, researchers and clinicians
have demonstrated that immune checkpoint inhibitors have an
efficacy of 50% in phase I clinical trials of patients with
pancreatic cancer (24). However,
objective responses were not observed. The present study indicated
that miR-519 inhibited the tumorigenesis of pancreatic cancer via
the PD-L1 signaling pathway. The results may catalyze the
development of novel approaches by targeting miR-519 and PD-L1.
MicroRNAs have been demonstrated to be aberrantly
expressed and implicated in hypoxia-induced tumor phenotypes
(25). Hypoxic conditions are also
associated with the upregulation of miR-21 expression in pancreatic
cancer cells (26). Similarly, the
present study revealed that miR-519 was modulated by hypoxia.
Certain research groups have demonstrated that various miRNAs,
including miR-212 and miR-224, promote pancreatic cancer
progression via hypoxia-inducible factor 1α (27,28). By
contrast, the present study revealed that miR-519 inhibited the
tumorigenesis of pancreatic cancer cells, in accordance with prior
conclusions (29,30). Additionally, the present study
investigated and confirmed the inhibitory role of miR-519 under
hypoxic conditions.
It has been demonstrated that immune checkpoint
inhibitors, such as the cytotoxic T-lymphocyte-associated protein 4
receptor antibody Ipilimumab, increase the overall survival rate of
patients with pancreatic cancer, when used in combination with GVAX
(31,32). Furthermore, anti-PD-L1 drugs alone
have exhibited less efficacy for the treatment of pancreatic cancer
(33). Therefore, the synergistic
therapeutic mechanisms of immune checkpoint inhibitors and
chemotherapy or radiotherapy may represent a promising area for the
development of therapeutics (34).
The present study determined that PD-L1 served as a mediator of
miR-519 in pancreatic cancer cells, indicating that miR-519 and
PD-L1 targeting may facilitate the development of improved
pancreatic cancer treatments.
In conclusion, the present study demonstrated a
novel interaction between miR-519 and PD-L1, which influenced
genesis and growth of pancreatic tumors. The in vitro and
in vivo experiments performed represent solid foundations
for explaining miR-519-mediated tumorigenesis. The current results
may aid the development of an effective therapeutic method for
patients with pancreatic cancer.
Acknowledgements
The authors would like to thank Dr Guo Jing (Tongji
University School of Medicine) for the critical reading of this
manuscript and for providing helpful suggestions.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KN, DZ and HC designed the study. KN, DZ, CC, YuY
and HC collected and analyzed the data. KN and HC drafted and wrote
the manuscript. YoY and SL were involved in the interpretation of
data and critically revised the manuscript. All authors had
intellectual input into the study and approved the final version of
the manuscript.
Ethics approval and consent to
participate
All animal protocols were approved by the Animal
Care and Use Committee at the The Third Affiliated Hospital of
Soochow University (Jiangsu, China). All procedures in the animal
studies were performed in accordance with the ethical standards of
the institution.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen S, Chen JZ, Zhang JQ, Chen HX, Qiu
FN, Yan ML, Tian YF, Peng CH, Shen BY, Chen YL and Wang YD:
Silencing of long noncoding RNA LINC00958 prevents tumor initiation
of pancreatic cancer by acting as a sponge of microRNA-330-5p to
down-regulate PAX8. Cancer Lett. 446:49–61. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin QJ, Yang F, Jin C and Fu DL: Current
status and progress of pancreatic cancer in China. World J
Gastroenterol. 21:7988–8003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang G, Pan J, Zhang L, Wei Y and Wang C:
Long non-coding RNA CRNDE sponges miR-384 to promote proliferation
and metastasis of pancreatic cancer cells through upregulating
IRS1. Cell Prolif. 50:2017. View Article : Google Scholar
|
|
5
|
Chang J and Erler J: Hypoxia-mediated
metastasis. Adv Exp Med Biol. 772:55–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lv WL, Liu Q, An JH and Song XY:
Scutellarin inhibits hypoxia-induced epithelial-mesenchymal
transition in bladder cancer cells. J Cell Physiol.
234:23169–23175. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Erkan M, Kurtoglu M and Kleeff J: The role
of hypoxia in pancreatic cancer: A potential therapeutic target?
Expert Rev Gastroenterol Hepatol. 10:301–316. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iorio MV and Croce CM: MicroRNAs in
cancer: Small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Zhao CY, Zhang SH, Yu DH, Chen Y,
Liu QH, Shi M, Ni CR and Zhu MH: Upregulation of miR-194
contributes to tumor growth and progression in pancreatic ductal
adenocarcinoma. Oncol Rep. 31:1157–1164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abue M, Yokoyama M, Shibuya R, Tamai K,
Yamaguchi K, Sato I, Tanaka N, Hamada S, Shimosegawa T, Sugamura K
and Satoh K: Circulating miR-483-3p and miR-21 is highly expressed
in plasma of pancreatic cancer. Int J Oncol. 46:539–547. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao WG, Yu SN, Lu ZH, Ma YH, Gu YM and
Chen J: The miR-217 microRNA functions as a potential tumor
suppressor in pancreatic ductal adenocarcinoma by targeting KRAS.
Carcinogenesis. 31:1726–1733. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dang Z, Xu WH, Lu P, Wu N, Liu J, Ruan B,
Zhou L, Song WJ and Dou KF: MicroRNA-135a inhibits cell
proliferation by targeting Bmi1 in pancreatic ductal
adenocarcinoma. Int J Biol Sci. 10:733–745. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin YM, Sung WW, Hsieh MJ, Tsai SC, Lai
HW, Yang SM, Shen KH, Chen MK, Lee H, Yeh KT and Chen CJ: High
PD-L1 expression correlates with metastasis and poor prognosis in
oral squamous cell carcinoma. PLoS One. 10:e01426562015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Francisco LM, Sage PT and Sharpe AH: The
PD-1 pathway in tolerance and autoimmunity. Immunol Rev.
236:219–242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang HB, Yao H, Li CS, Liang LX, Zhang Y,
Chen YX, Fang JY and Xu J: Rise of PD-L1 expression during
metastasis of colorectal cancer: Implications for immunotherapy. J
Dig Dis. 18:574–581. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Chen L, Xiong Y, Zheng X, Xie Q,
Zhou Q, Shi L, Wu C, Jiang J and Wang H: Knockdown of PD-L1 in
human gastric cancer cells inhibits tumor progression and improves
the cytotoxic sensitivity to CIK therapy. Cell Physiol Biochem.
41:907–920. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang XL, Xu LL and Wang F:
Hsa_circ_0020397 regulates colorectal cancer cell viability,
apoptosis and invasion by promoting the expression of the miR-138
targets TERT and PD-L1. Cell Biol Int. 41:1056–1064. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brahmer JR: PD-1-targeted immunotherapy:
Recent clinical findings. Clin Adv Hematol Oncol. 10:674–675.
2012.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Gao F, Zhou L, Wang H, Shi G and
Tan X: UCA1 regulates the growth and metastasis of pancreatic
cancer by sponging miR-135a. Oncol Res. 25:1529–1541. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fogel EL, Shahda S, Sandrasegaran K,
DeWitt J, Easler JJ, Agarwal DM, Eagleson M, Zyromski NJ, House MG,
Ellsworth S, et al: A multidisciplinary approach to pancreas cancer
in 2016: A review. Am J Gastroenterol. 112:537–554. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Landi S: Genetic predisposition and
environmental risk factors to pancreatic cancer: A review of the
literature. Mutat Res. 681:299–307. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kubo T, Ninomiya T, Hotta K, Kozuki T,
Toyooka S, Okada H, Fujiwara T, Udono H and Kiura K: Study
protocol: Phase-Ib trial of nivolumab combined with metformin for
refractory/recurrent solid tumors. Clin Lung Cancer. 19:e861–e864.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo G, Xia X, Wang X, Zhang K, Cao J,
Jiang T, Zhao Q and Qiu Z: miR-301a plays a pivotal role in
hypoxia-induced gemcitabine resistance in pancreatic cancer. Exp
Cell Res. 369:120–128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mace TA, Collins AL, Wojcik SE, Croce CM,
Lesinski GB and Bloomston M: Hypoxia induces the overexpression of
microRNA-21 in pancreatic cancer cells. J Surg Res. 184:855–860.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yue H, Liu L and Song Z: miR-212 regulated
by HIF-1α promotes the progression of pancreatic cancer. Exp Ther
Med. 17:2359–2365. 2019.PubMed/NCBI
|
|
28
|
Zhu G, Zhou L, Liu H, Shan Y and Zhang X:
MicroRNA-224 promotes pancreatic cancer cell proliferation and
migration by targeting the TXNIP-mediated HIF1α pathway. Cell
Physiol Biochem. 48:1735–1746. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abdelmohsen K, Kim MM, Srikantan S,
Mercken EM, Brennan SE, Wilson GM, Cabo Rd and Gorospe M: miR-519
suppresses tumor growth by reducing HuR levels. Cell Cycle.
9:1354–1359. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu G, Zhang T, Jing Y, Bao Q, Tang Q and
Zhang Y: miR-519 suppresses nasopharyngeal carcinoma cell
proliferation by targeting oncogene URG4/URGCP. Life Sci.
175:47–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Royal RE, Levy C, Turner K, Mathur A,
Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I and
Rosenberg SA: Phase 2 trial of single agent Ipilimumab
(anti-CTLA-4) for locally advanced or metastatic pancreatic
adenocarcinoma. J Immunother. 33:828–833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Le DT, Lutz E, Uram JN, Sugar EA, Onners
B, Solt S, Zheng L, Diaz LA Jr, Donehower RC, Jaffee EM and Laheru
DA: Evaluation of ipilimumab in combination with allogeneic
pancreatic tumor cells transfected with a GM-CSF gene in previously
treated pancreatic cancer. J Immunother. 36:382–389. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feng M, Xiong G, Cao Z, Yang G, Zheng S,
Song X, You L, Zheng L, Zhang T and Zhao Y: PD-1/PD-L1 and
immunotherapy for pancreatic cancer. Cancer Lett. 407:57–65. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Azad A, Yin Lim S, D'Costa Z, Jones K,
Diana A, Sansom OJ, Kruger P, Liu S, McKenna WG, Dushek O, et al:
PD-L1 blockade enhances response of pancreatic ductal
adenocarcinoma to radiotherapy. EMBO Mol Med. 9:167–180. 2017.
View Article : Google Scholar : PubMed/NCBI
|