Introduction
Uveal melanoma (UM) is the most common intraocular
malignant tumor in adults worldwide (1). Primary UM can be treated by surgery
with a low local recurrence rate. However, certain patients develop
distant metastasis, predominantly in the liver, following primary
tumor treatment (2). The development
of distant metastasis is associated with a high mortality rate in
up to half of the patients (3,4). To
date, the treatment of metastatic UM remains controversial
(5). Thus, in-depth studies on the
pathogenesis of UM are required for effective diagnosis and
treatment, and improved prognosis.
Long non-coding RNA (lncRNA) is defined as a
transcript>200 nucleotides long that lacks protein-coding
potential (6), which is involved in
various biological processes (such as chromatin remodeling, mRNA
splicing, mRNA editing and translation) (7) and can be regulated through a number of
different molecular mechanisms (8).
A number of studies have demonstrated that lncRNAs play a key role
in the occurrence and development of different types of tumor
(9–11). Studies have demonstrated that lncRNAs
regulate several cancer characteristics, including cell
proliferation, apoptosis and invasion (12–14).
lncRNAs are highly valuable for determining the pathological
characteristics of different types of tumor, analyzing the
prognosis and providing appropriate treatment (15). A number of lncRNAs, with either tumor
suppressive or carcinogenic function have been identified in the
past decades (16).
With regards to UM, lncRNA is considered to play a
role in regulating cell proliferation, migration and invasion, and
thus, is deemed essential to the occurrence and development of UM
(17–19). Small nucleolar RNA host gene 7
(SNHG7) is a recognized lncRNA, located on chromosome 9 q34.3
(20), with a total length of 2,176
base pairs (21). Previous studies
have demonstrated that SNHG7 acts as a carcinogenic non-coding RNA
in several types of cancer, including pancreatic (22), colorectal (20), bladder (23), gastric (24) and breast cancer (25), whereby it facilitates the
proliferation, migration and invasion of tumor cells. In addition,
enhancer of zeste homolog 2 (EZH2) is the catalytic subunit of
polycomb repressive complex 2 and is associated with several types
of tumor, including UM (26,27). Previous studies have demonstrated
that lncRNAs play significant roles in different types of tumor via
EZH2 (28,29). However, the role of SNHG7 in the
development of UM and the association between SNHG7 and EZH2 have
not yet been investigated.
The present study detected the expression of SNHG7
in UM and hypothesized that SNHG7 may be associated with UM
prognosis via EZH2. Further functional experiments were performed
by upregulating SNHG7. The expression of EZH2 was detected in
MEL270 and OMM2.5 cell lines overexpressing SNHG7. The results of
the present study suggest that SNHG7 may play a significant role in
UM development.
Materials and methods
The cancer genome atlas (TCGA) dataset
analysis
Detailed SNHG7 mRNA expression data and clinical
information of 80 patients with UM were obtained from TCGA database
(https://portal.gdc.cancer.gov/).
Patients were grouped according to median SNHG7 expression level
(cutoff value=73.695 FPKM). The overall survival (OS) analysis and
correlation analysis of SNHG7 and EZH2 were obtained from the Gene
Expression Profiling Interactive Analysis (GEPIA) database
(http://gepia.cancer-pku.cn) (30). The association between SNHG7 and
clinical staging, as well as between EZH2 and clinical staging and
histology type were obtained from the UALCAN data-mining platform
(http://ualcan.path.uab.edu) (31).
Cell lines and patient tissues
A total of six human UM cell lines (92.1, MEL202,
MEL270, MEL290, OMM2.3 and OMM2.5) were provided by WuXi AppTec
(https://www.wuxiapptec.com/zh-cn). The
92.1, MEL202, MEL270 and MEL290 cell lines derived from
non-metastatic tissues, while the OMM2.3 and OMM2.5 cell lines
derived from metastatic tissues. UM cell lines were cultured in
RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc.),
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin (Beyotime
Institute of Biotechnology), at 37°C with 5% CO2. A
total of seven UM tissue samples were acquired from the tissue bank
of Eye & ENT Hospital of Fudan University (Shanghai, China).
The seven participants included 2 men and 5 women, with a mean age
of 52 years (range, 24–71). Of these, three patients did not
develop tumor metastasis, while four patients did develop
metastasis. The present study was approved by the Ethics Committee
of The Eye & ENT Hospital of Fudan University, and all
procedures agreed with The Declaration of Helsinki. All patients
provided written informed consent prior to treatment.
Transfection
A lentiviral vector containing human lncRNA SNHG7
and an empty lentiviral vector were purchased from Genomeditech
Co., Ltd. MEL270 and OMM2.5 cells were transfected with lentiviral
vectors as follows: Cells were plated in 24-well plates and
incubated overnight at 37°C. Virus solution (4×105
TU/well; Genomeditech Co., Ltd.) and polybrene (5 µg/ml;
Genomeditech Co., Ltd.) were added to the cells after 24 h.
Following 16 h of transfection, the lentiviral-containing medium
was replaced with RPMI 1640 medium supplemented with 10% FBS. After
72 h, puromycin (2 µg/ml; Genomeditech Co., Ltd.) was added to the
culture medium and changes in gene expression were evaluated via
reverse transcription-quantitative (RT-q)PCR.
RT-qPCR analysis
Total RNA of six human UM cell lines (92.1, MEL202,
MEL270, MEL290, OMM2.3 and OMM2.5) and seven UM tissue samples was
extracted using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) and reverse transcribed into cDNA using
the PrimeScript™ RT Reagent kit (Takara Biotechnology Co., Ltd.).
qPCR was subsequently performed using the SYBR® Green
qPCR kit (Takara Biotechnology Co., Ltd.), on the ViiA™
7 Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The following thermocycling
conditions were used for PCR: Initial denaturation at 95°C for 30
sec; 40 cycles of 95°C for 5 sec and 60°C (annealing and extension)
for 34 sec, according to the manufacturer's protocol
(SYBR® Green qPCR kit; Takara Biotechnology Co.,Ltd.).
The following primer sequences were used for PCR: SNHG7: Forward,
5′-TTGCTGGCGTCTCGGTTAAT-3′ and reverse, 5′-GGAAGTCCATCACAGGCGAA-3′;
GAPDH: Forward, 5′-TGTTGCCATCAATGACCCCTT-3′ and reverse,
5′-CTCAGCCTTGACGGTGCCAT-3′; β-actin: Forward,
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse,
5′-CTCCTTAATGTCACGCACGAT-3′; U1: Forward,
5′-GACGGGAAAAGATTGAGCGG-3′ and reverse,
5′-GCCACGAAGAGAGTCTTGAAGG-3′. The relative mRNA levels were
calculated using the 2−ΔΔCq method (32) and normalized to the internal
reference gene GAPDH.
Cell counting kit-8 (CCK-8) assay
The Cell Counting Kit-8 assay was performed,
according to the manufacturer's protocol (CCK-8; Dojindo Molecular
Technologies, Inc.). UM cells were cultured in 96-well plates at a
density of 2,000 cells/well for 4 days, at 37°C with 5%
CO2. CCK-8 solution (10 µl/well) was added prior to
incubation at 37°C for 2 h. Cell viability was subsequently
analyzed at a wavelength of 450 nm, using a microplate reader
(Tecan Group, Ltd.).
Flow cytometric analysis
The apoptosis assay was performed using the
Annexin-V-FITC apoptosis kit (BD Biosciences). Cells were
trypsinised, collected and washed twice with pre-cooled PBS. A cell
suspension (1×106 cells/ml) was prepared with 1 ×
Binding buffer (BD Biosciences), and 100 µl of the solution was
added to each tube. Annexin V (5 µl) and Propidium Iodide (5 µl)
(BD Biosciences) were added and mixed manually. The sample was
placed in the dark for 15 min at 25°C and 1 × Binding Buffer (400
µl; BD Biosciences) was added to each sample. Apoptotic cells were
subsequently analyzed via flow cytometry, using MoFlo XDP (Beckman
Coulter, Inc.).
For the cell cycle analysis, the cells were
harvested in a tube and washed twice with 4 ml PBS. Pre-cooled 75%
ethanol was added and incubated overnight at 4°C. The cells were
washed twice to remove all ethanol. Cell staining was performed
using 1×106 cells for each tube sample. For PI/RNase
staining, the cells were resuspended in 0.5 ml PI/RNase staining
solution (BD Biosciences) and incubated for 15 min at 25°C in the
dark. The sample was stored at 4°C in the dark before analysis.
Flow cytometric analysis was performed within 1 h.
Western blotting
Total protein of MEL270 and OMM2.5 was extracted
using RIPA buffer with proteinase inhibitor (Beyotime Institute of
Biotechnology). Total protein was measured using a BCA assay. Equal
amounts of protein (20 µg/lane) were separated via 12% SDS-PAGE and
subsequently transferred onto a polyvinylidene difluoride membrane.
The membranes were blocked using 5% BSA, at 25°C for 2 h. and
subsequently incubated with the following primary antibodies,
overnight at 4°C: Specific monoclonal EZH2 (1:1,000; cat. no. 5246)
and GAPDH (1:1,000; cat. no. 2118), both from Cell Signaling
Technology, Inc. Membranes were washed three times with
Tris-buffered saline Tween 20 buffer (TBST; 10 mM Tris, 150 mM
NaCl, 0.05% Tween-20; Beijing Solarbio Science & Technology
Co., Ltd). Following the primary incubation, membranes were
incubated with the horseradish peroxidase-conjugated (HRP)
anti-rabbit secondary antibody (1:5,000; cat. no. 7074; Cell
Signaling Technology, Inc.) at 25°C for 2 h. Membranes were
re-washed three times with TBST buffer. Electrochemiluminescence
(cat. no. 6883; Cell Signaling Technology, Inc.) was used for
visualization of the protein bands. GAPDH was used as the loading
control and protein expression was quantified using ImageJ Software
version 1.47 (National Institutes of Health) (33).
Subcellular fractionation
The MEL270 and OMM2.5 cell lines were divided into
nuclear and cytoplasmic fractions to extract RNA prior to RT-qPCR,
in order to determine the cell localization of SNHG7 by using the
PARIS kit (Thermo Fisher Scientific, Inc.). U1 (nucleus control)
and β-actin (cytoplasm control) were used for normalization.
Subcellular localizations of SNHG7 in 15 cell lines were obtained
from LncATLAS (http://lncatlas.crg.eu).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 5.0 (GraphPad Software, Inc.). The data are presented
as the mean ± standard deviation. The mean of two independent
samples was compared using Student's t-test. The associations
between SNHG7 mRNA level and clinical pathological parameters of
the UM specimens were assessed using χ2 test.
Correlation analysis and survival curve analyses were performed
using Pearson's correlation analysis and the Kaplan-Meier method
respectively, through the GEPIA database. P<0.05 was considered
to indicate a statistically significant difference.
Results
Low expression of SNHG7 in UM is
associated with poor prognosis
The public database, TCGA was used for in-depth
analysis of UM. Clinical information and SNHG7 mRNA expression data
were downloaded from 80 UM cases in TCGA (Table I). Patients were divided into two
groups (low- and-high expression groups), according to the median
expression level of SNHG7 (cutoff value=73.695 FPKM). The results
of the present study suggest that low expression of SNHG7 was
associated with a higher proportion of epithelioid cells (P=0.002;
Table I), fewer cases of tumor-free
survival (P=0.009; Table I) and a
higher death rate (P=0.034; Table I)
The histological types of UM included epithelioid and spindle,
which accounted for 42.5 and 57.5% of total cases, respectively.
There were 61 tumor-free cases (76.25%) and 18 cases (22.5%) with
tumor. A total of 67 cases (83.75%) were alive whereas 13 cases
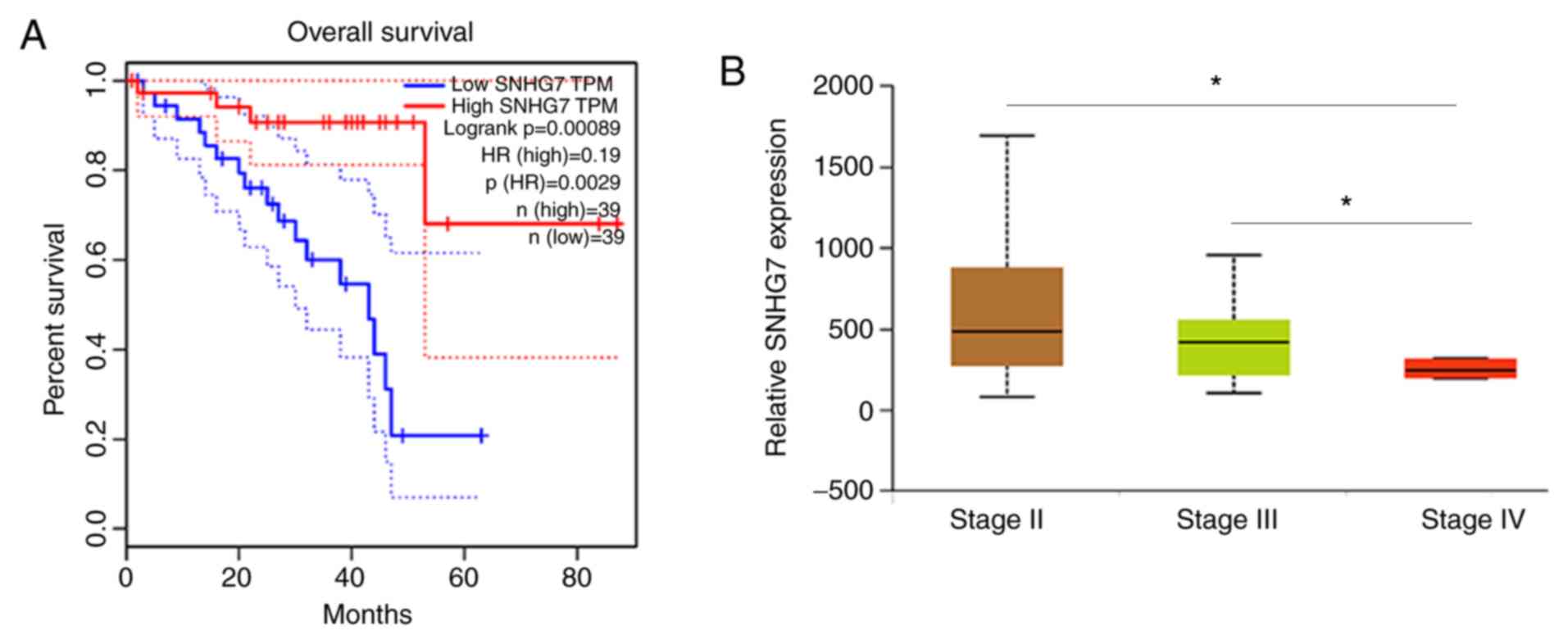
(16.25%) were dead. Furthermore, the OS analysis suggested that
lower SNHG7 expression is significantly associated with poor OS
compared with higher SNHG7 expression in patients with UM
(P<0.05; Fig. 1A). The present
study analyzed the results of TCGA database using the UALCAN
platform and confirmed that SNHG7 is associated with clinical
staging (Stage II vs. Stage IV, P<0.05; Stage III vs. Stage IV,
P<0.05; Fig. 1B). The results of
the present study demonstrated that downregulated expression of
SNHG7 was associated with poor prognosis in patients with UM.
 | Table I.SNHG7 expression and
clinicopathological features of 80 patients with uveal melanoma
from The Cancer Genome Atlas database. |
Table I.
SNHG7 expression and
clinicopathological features of 80 patients with uveal melanoma
from The Cancer Genome Atlas database.
|
|
| SNHG7
expression |
|
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | Number of cases,
n | High | Low | χ2 | P-value |
|---|
| Sex |
|
|
| 0.051 | 0.822 |
|
Male | 45 | 22 | 23 |
|
|
|
Female | 35 | 18 | 17 |
|
|
| Tumor basal
diameter, mm |
|
|
| 1.602 | 0.206 |
|
≤16 | 34 | 20 | 14 |
|
|
|
>16 | 45 | 20 | 25 |
|
|
| Tumor thickness,
mm |
|
|
| 1.257 | 0.262 |
|
≤10 | 37 | 21 | 16 |
|
|
|
>10 | 43 | 19 | 24 |
|
|
| Extrascleral
extension |
|
|
| 2.891 | 0.089 |
| No | 68 | 38 | 30 |
|
|
|
Yes | 7 | 1 | 6 |
|
|
| Histological
type |
|
|
| 10.026 | 0.002 |
|
Epithelioid cell | 34 | 10 | 24 |
|
|
| Spindle
Cell | 46 | 30 | 16 |
|
|
| Tumor status |
|
|
| 6.872 | 0.009 |
|
Tumor-free | 61 | 35 | 26 |
|
|
|
Tumor | 18 | 4 | 14 |
|
|
| Pathological T |
|
|
| 1.385 | 0.239 |
| II | 14 | 9 | 5 |
|
|
|
III&IV | 66 | 31 | 35 |
|
|
| Pathological N |
|
|
| N.A. | N.A. |
| N0 | 52 | 27 | 25 |
|
|
| NX
& null | 28 | 13 | 15 |
|
|
| Pathological M |
|
|
| 2.311 | 0.128 |
| M0 | 51 | 27 | 24 |
|
|
|
M1+ | 4 | 0 | 4 |
|
|
| Vital status |
|
|
| 4.501 | 0.034 |
|
Alive | 67 | 37 | 30 |
|
|
|
Dead | 13 | 3 | 10 |
|
|
SNHG7 expression in UM tissues and six
UM cell lines
In order to further determine the biological
functions of SNHG7 in the development of UM, the present study
investigated the differential expression of SNHG7 in UM tissues of
patients both with and without metastasis, using RT-qPCR. SNHG7
expression in UM was significantly downregulated in the metastatic
group compared with the non-metastatic group (P<0.001; Fig. 2A). Furthermore, SNHG7 expression
levels in the six UM cell lines were analyzed using RT-qPCR, and
the cell lines with low expression of SNHG7 were selected for the
SNHG7 overexpression experiment (Fig.
2B). The results of the present study demonstrated that the
expression levels of SNHG7 in the cell lines derived from
metastasis (OMM2.5) were relatively low. Among the cell lines
derived from non-metastasis (92.1, MEL202, MEL270 and MEL290),
MEL270 exhibited the lowest SNHG7 expression levels. Thus, the
present study selected the OMM2.5 cell line derived from
metastasis, and the MEL270 cell line derived from non-metastasis
for further investigation. Subcellular localizations of SNHG7 in 15
cell lines were obtained from lncATLAS (34), in order to determine the association
between cell localization and lncRNA function. SNHG7 was
predominantly expressed in the cytoplasm in HepG2 (liver cancer
cell line) and A549 (lung cancer cell line); however, SNHG7 was
predominantly expressed in the nucleus in K562 (human leukemia cell
line) (Fig. 2C). The present study
performed subcellular fractionation in order to detect the
localization of SNHG7 in UM cell lines. MEL270 and OMM2.5 cell
lines were divided into nuclear and cytoplasmic fractions.
Subsequently, RT-qPCR was performed in order to identify the
subcellular localization of SNHG7, which confirmed that SNHG7 was
preferentially located in the nucleus in both MEL270 and OMM2.5
cell lines (Fig. 2D and E).
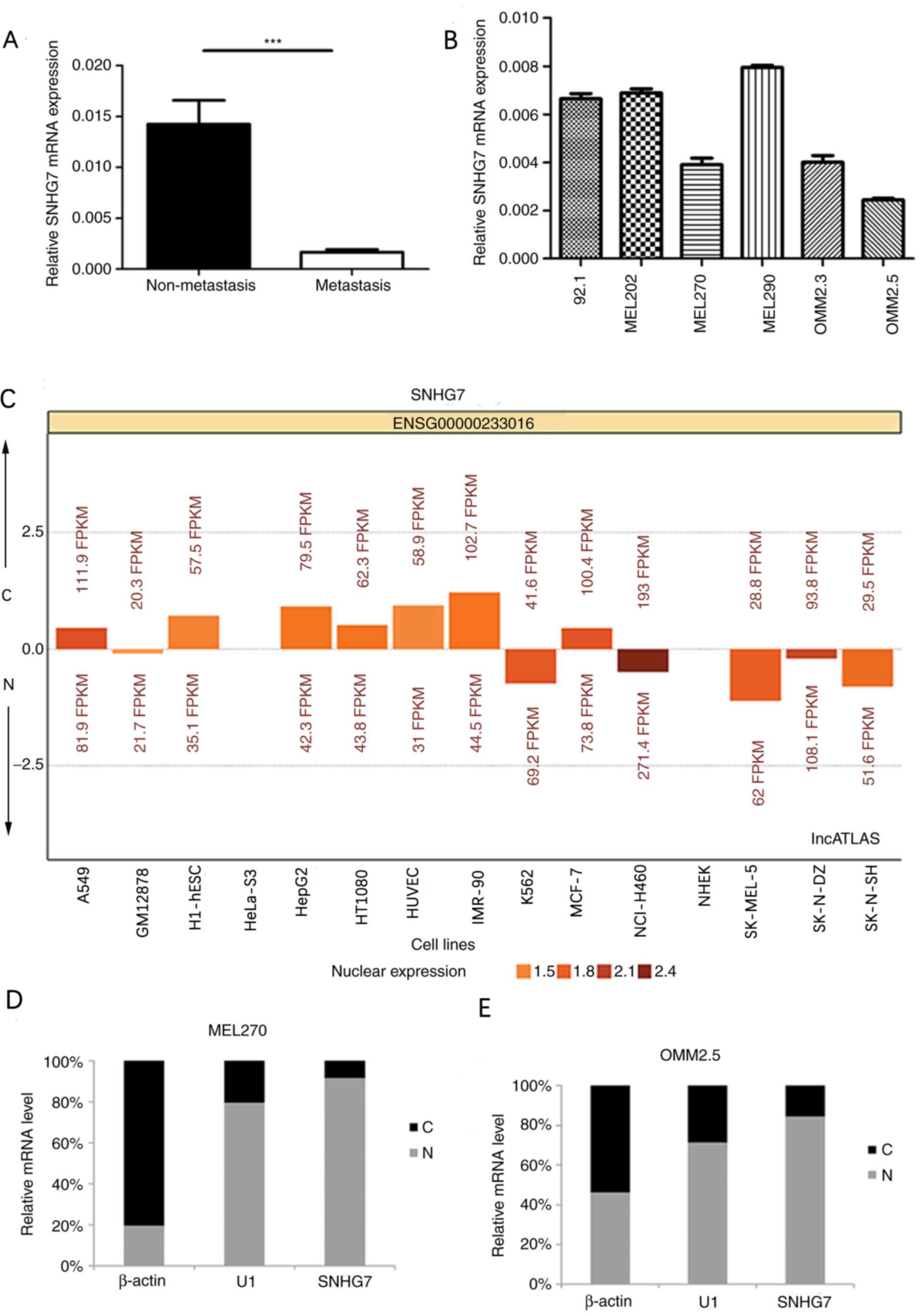 | Figure 2.Reverse transcription-quantitative
PCR analysis of SNHG7 expression levels in UM tissues and cell
lines. (A) Relative SNHG7 mRNA expression levels in tissues of
patients with UM. SNHG7 levels were significantly downregulated in
patients with distant metastasis compared with patients without
distant metastasis. (B) SNHG7 expression levels were measured in
six UM cell lines (92.1, MEL202, MEL270, MEL290, OMM2.3 and
OMM2.5). The MEL270 and OMM2.5 cell lines were selected for further
analyses. (C) Subcellular localization of SNHG7 in 15 cell lines
(A549, GM12878, H1-hESC, HeLa-S3, HepG2, HT1080, HUVEC, IMR-90,
K562, MCF-7, NCI-H460, NHEK, SK-MEL-5, SK-N-DZ and SK-N-SH). (D)
Localization of SNHG7 in MEL270 and (E) OMM2.5 cell lines. SNHG7
was preferentially located in the nucleus in both MEL270 and OMM2.5
cell lines. β-actin and U1 were used as cytoplasmic and nuclear
site markers, respectively. ***P<0.001 vs. non-metastasis group.
SNHG7, small nucleolar RNA host gene 7; UM, uveal melanoma; C,
cytoplasm; N, nucleus. |
Upregulation of SNHG7 inhibits cell
proliferation in UM cell lines
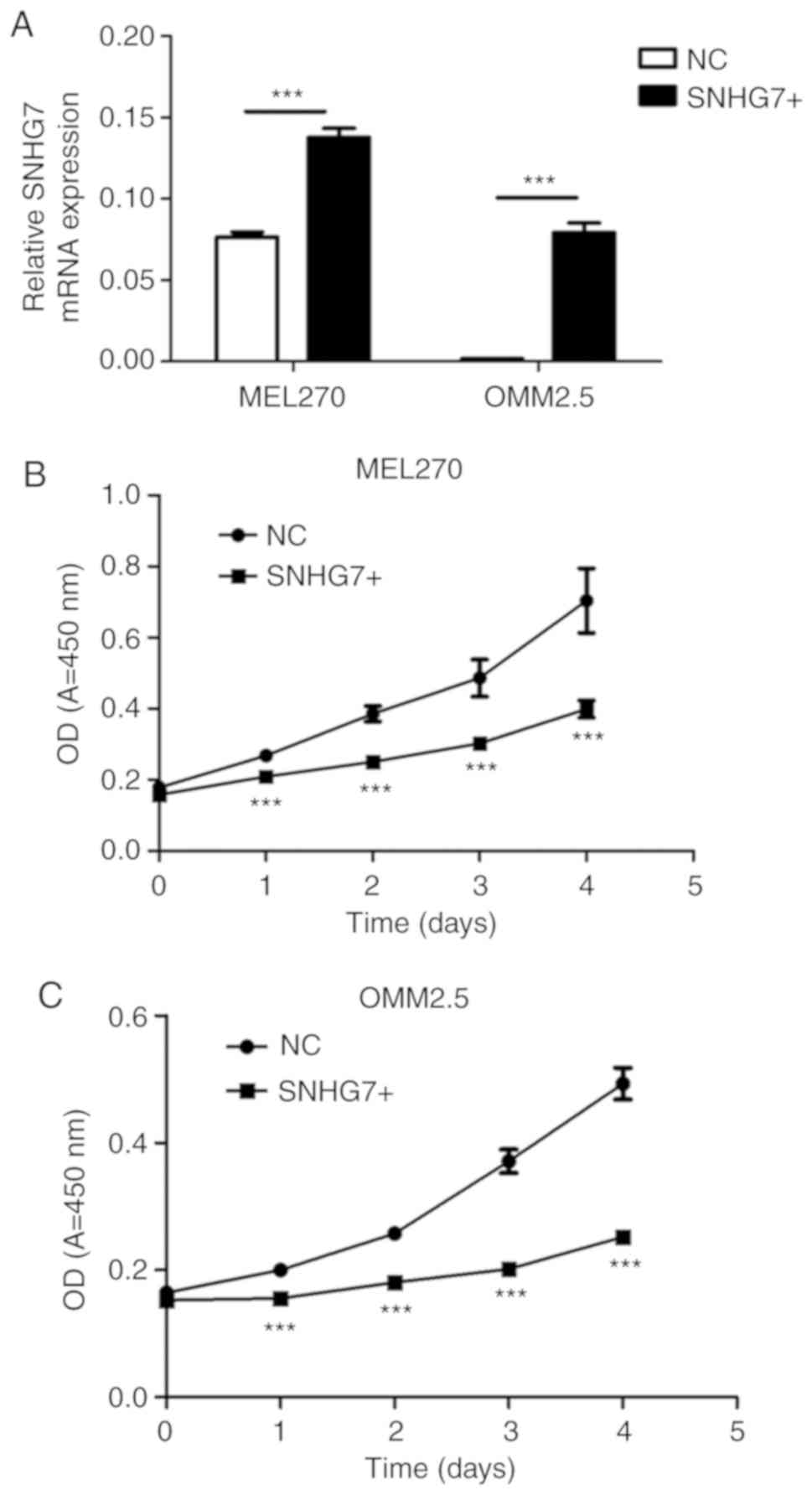
SNHG7 expression levels were significantly
upregulated by lentivirus infection in both the MEL270 and OMM2.5
cell lines, and the overexpression efficiency was detected via
RT-qPCR (P<0.001; Fig. 3A). As
presented in Fig. 3B and C,
overexpression of SNHG7 in the MEL270 and OMM2.5 cell lines
significantly inhibited cell proliferation. The cells
overexpressing SNHG7 in both MEL270 and OMM2.5 cell lines
demonstrated a significant decrease in cell proliferation following
incubation for 4 days (P<0.001; Fig.
3B and C). The results of the present study demonstrated that
SNHG7 significantly inhibited the proliferation of UM cells.
Overexpression of SNHG7 in UM cell
lines induces cell cycle arrest and promotes apoptosis in
vitro
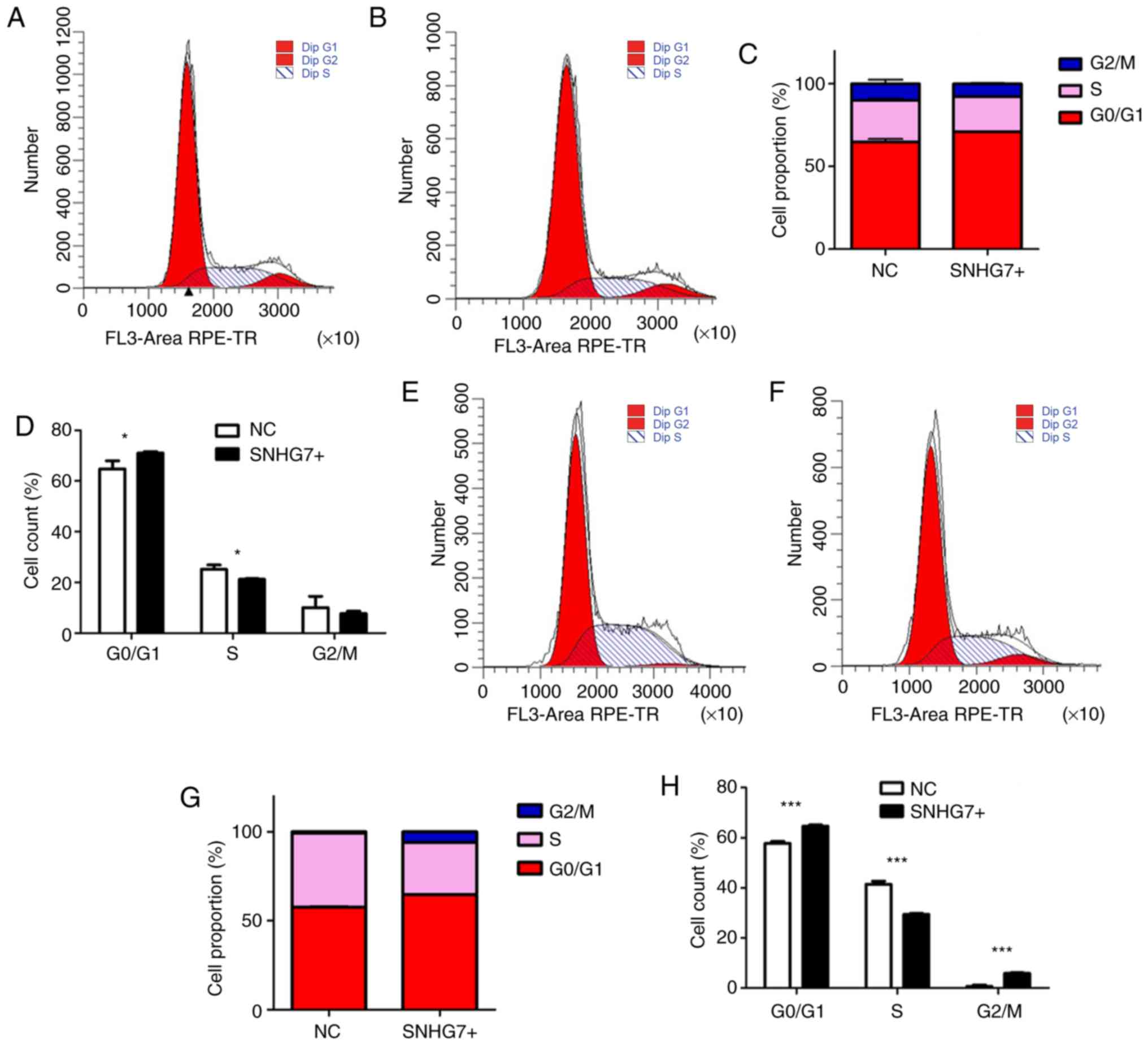
In order to investigate the effect of SNHG7
overexpression on cell cycle, the present study performed a cell
cycle analysis to determine whether SNHG7 also inhibited cell cycle
progression. The results of the present study suggest that
overexpression of SNHG7 could affect the cell cycle of both MEL270
and OMM2.5 cell lines. The proportion of overexpressed SNHG7 cells
in G0/G1 phase significantly increased, with
a decline in S phase in both MEL270 (P<0.05; Fig. 4A-D) and OMM2.5 (P<0.001; Fig. 4E-H) cell lines. The results of the
present study indicate that SNHG7 imposes a strong blocking effect
on UM cell cycle.
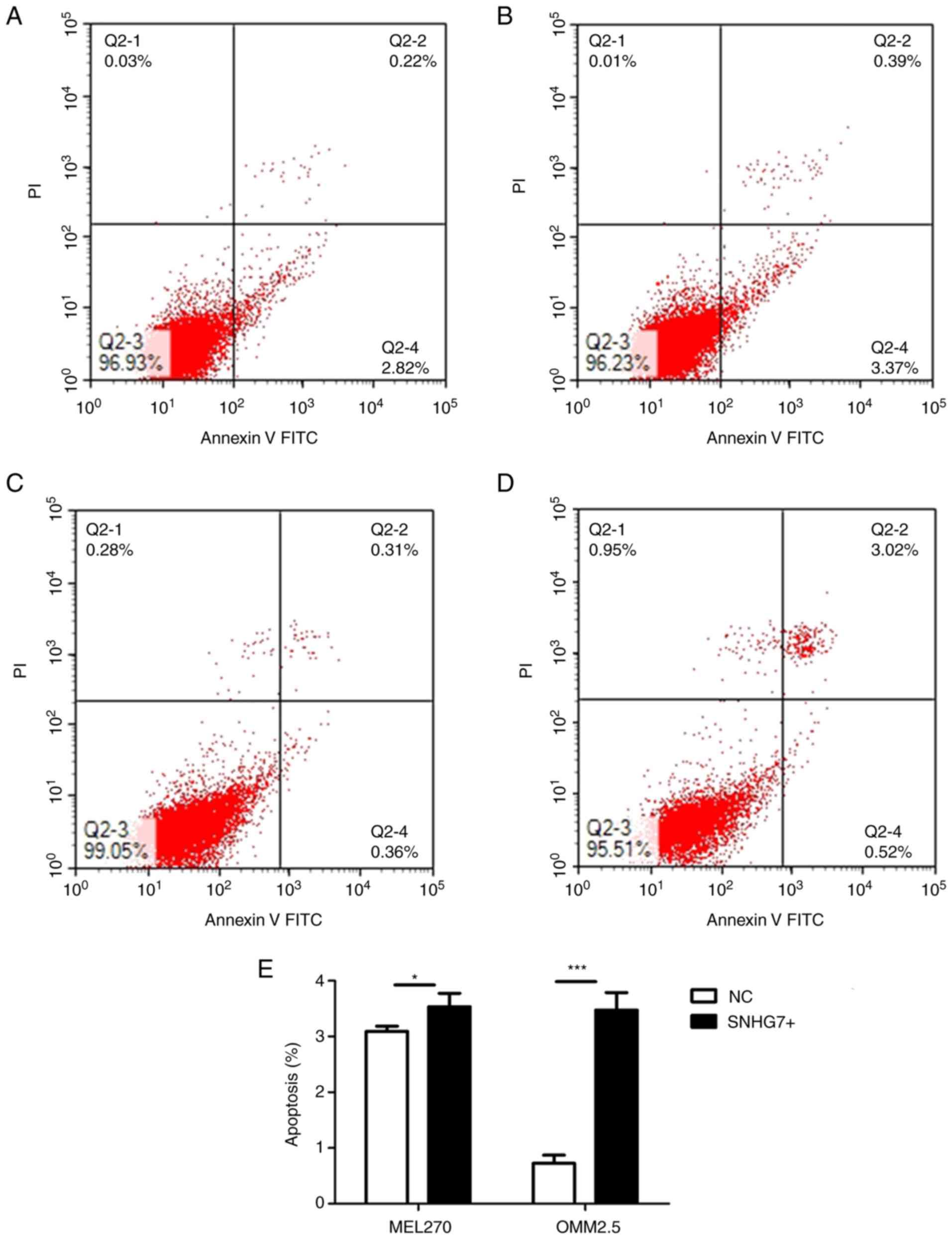
The apoptotic rate (early + late stage apoptosis) in
the SNHG7+ and the NC groups, in both MEL270 and OMM2.5 cell lines
was measured using the Annexin-V-FITC apoptosis kit, in order to
detect whether SNHG7 exerted anticancer effects on UM cells. The
results of the present study demonstrated that the apoptotic rate
in the MEL270 SNHG7+ group was 3.530±0.241% compared with the
MEL270 NC group (3.090±0.092%, P<0.05; Fig. 5A, B and E), the OMM2.5 SNHG7+ group
(3.47±0.315%) and the OMM2.5 NC group (0.72±0.147%, P<0.001;
Fig. 5C-E), and the differences were
statistically significant (Fig. 5E).
The results of the present study indicate that overexpression of
SNHG7 promotes apoptosis of UM cells.
SNHG7 suppresses UM cell line
proliferation, induces cell cycle arrest and promotes apoptosis by
inhibiting EZH2
It is well known that lncRNA can bind specific
proteins in order to exert its molecular function (35). Correlation analysis in the present
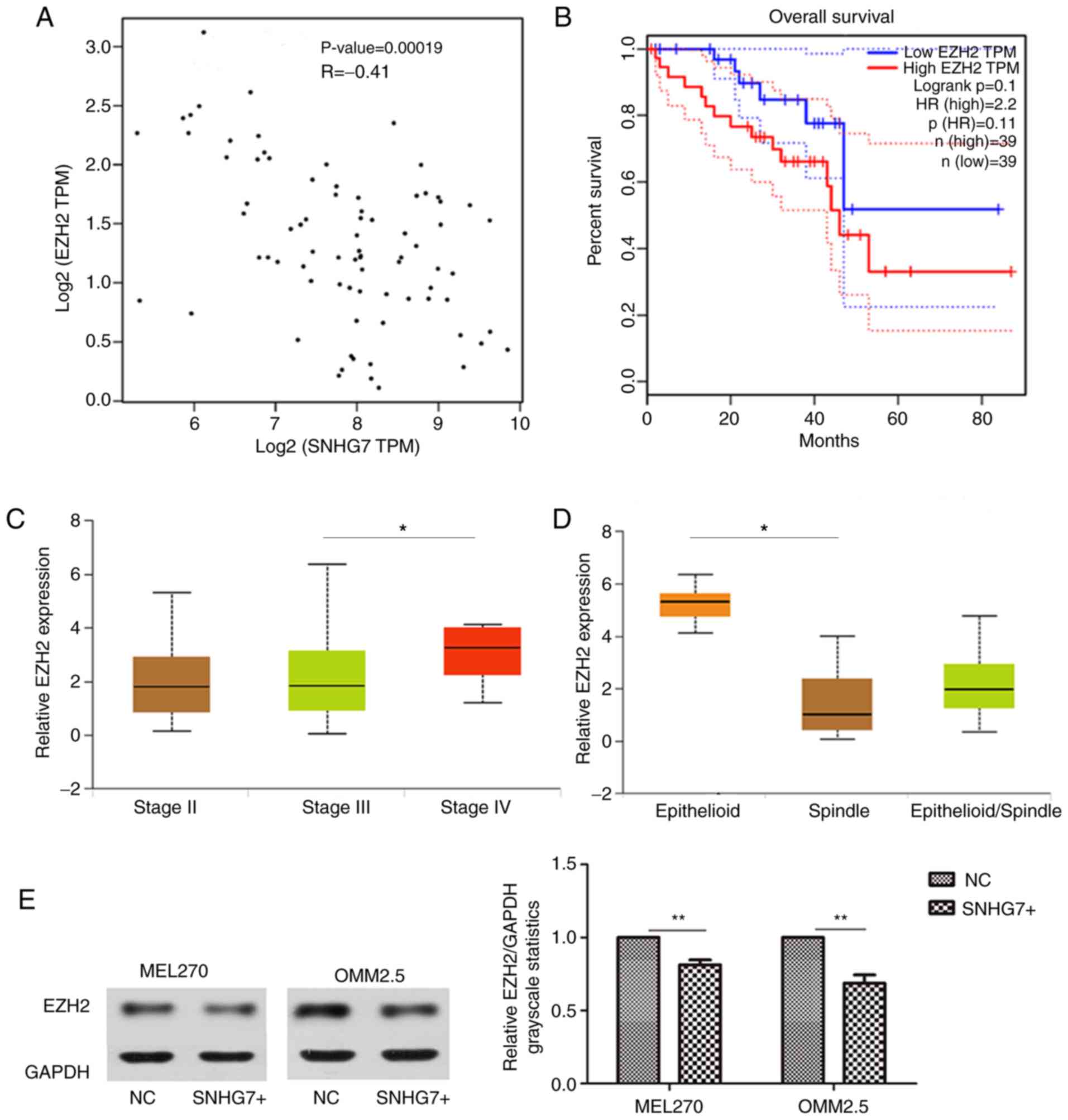
study indicated that SNHG7 and EZH2 were correlated (R=−0.41,
P=0.00019; Fig. 6A). The OS analysis
demonstrated that a higher EZH2 expression level was associated
with a poor OS; however, the difference was not significant between
the high and low EZH2 expression levels (P>0.05; Fig. 6B). Conversely, EZH2 was demonstrated
to be associated with clinical staging (stage III vs. stage IV,
P<0.05; Fig. 6C) and histological
type (P<0.05; Fig. 6D).
The present study detected EZH2 expression at the
protein level via western blot analysis. Overexpression of SNHG7
was demonstrated to have an inhibitory effect on EZH2. The SNHG7
overexpression group of MEL270 and OMM2.5 cell lines had a lower
expression of EZH2 compared with the NC groups (P<0.01; Fig. 6E). The results of the present study
indicate that overexpression of SNHG7 inhibits EZH2 expression,
suggesting that EZH2 may be a downstream target of SNHG7.
Discussion
UM is the most common intraocular tumor in adults
worldwide (36). UM has been widely
studied; however, the molecular mechanism underlying its occurrence
and development remains unknown. Thus, investigating the
pathogenesis of UM may aid in the development of novel therapeutic
targets. A number of lncRNAs are involved in the development and
progression of UM, as oncogenes or tumor suppressors (37). For example, FTH1P3 promotes the
proliferation and migration of UM cells by inhibiting the
expression of miR-224-5p (17).
Furthermore, the lncRNA PAUPAR inhibits the occurrence of UM by
preventing H3K4 demethylation, which exerts a tumor suppressing
effect (38). Although a large
number of lncRNAs have been demonstrated to be associated with UM,
numerous lncRNAs have not yet been discovered. In the present
study, 80 clinical UM specimens from TCGA database were analyzed.
The OS analysis indicated that low expression of SNHG7 resulted in
a significantly lower OS rate. Furthermore, the expression of SNHG7
was significantly associated with histological types, tumor-free
survival and vital status. The present study set out to investigate
the function and underlying molecular mechanism of SNHG7 in UM, and
demonstrated that overexpression of SNHG7 significantly suppressed
the proliferation and cell cycle, while promoting apoptosis in both
MEL270 and OMM2.5 cell lines.
SNHG7 has previously been studied as an oncogene
(20,22,23).
However, a number of lncRNAs play a dual role, namely, oncogenes or
tumor suppressors in different types of cancer. For example, ZFAS1
plays a tumor-suppressor role in breast cancer; however, it acts as
an oncogene in non-small cell lung, colorectal, gastric, liver,
ovarian and bladder cancer (39).
Furthermore, the lncRNA, H19 has also been reported to have either
anti-cancer or carcinogenic effects in different types of tumor
(40,41). As research progresses, the role of
SNHG7 in different types of tumor may gradually be confirmed. The
present study demonstrated that SNHG7 played a role in inhibiting
malignant transformation in UM. SNHG7 inhibited cell proliferation,
induced cell cycle arrest and promoted apoptosis. Contradictory to
a previous study (42), SNHG7 was
preferentially located in the nucleus in both MEL270 and OMM2.5
cell lines in the present study. UM is significantly different from
cutaneous melanoma and has unique tumorigenic processes and tumor
biology (43). Thus, the present
study suggests that the effect of SNHG7 in UM may be attributed to
the specificity of the intraocular tumor.
EZH2 serves a significant role in human carcinomas
(44–46) and regulates the processes of cell
proliferation, cell cycle and apoptosis (47–49).
Notably, EZH2 is involved in the malignant transformation of
different types of tumor in both cutaneous melanoma and UM
(27,50). In the present study, EZH2 expression
was demonstrated to be associated with SNHG7. Higher EZH2
expression levels were associated with a higher
Tumor-Node-Metastasis (TNM) stage (51) and a poor histological type. In
addition, the present study demonstrated that SNHG7 inhibited EZH2
expression. This suggests that SNHG7 could inhibit the
proliferation, mediate cell cycle and induce apoptosis in UM cells,
and its molecular mechanism may be associated with the inhibition
of EZH2.
Overall, the present study demonstrated that SNHG7
played a vital role in UM via EZH2. Although numerous studies have
been performed on UM, there are currently no effective treatments
for UM, particularly for high-risk patients. The development of
appropriate treatment is of great importance in order to improve
the survival rate of patients with UM. The present study set out to
investigate the molecular mechanism underlying UM development. The
results of the present study suggest that SNHG7 may be a potential
novel target for the diagnosis and treatment of UM. However,
further experiments in vivo and associated clinical trials
are required for verification.
Acknowledgements
The authors would like to thank Dr Yun Cheng
(Department of Ophthalmology, Eye & ENT Hospital of Fudan
University, Shanghai, China) for her help in performing the
experiments, and Dr Qian Wu (Department of Pathology, West China
Hospital, Sichuan University, Sichuan, China) for providing
suggestions for the revision of the manuscript.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant nos. 81970835 and 81800867).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
RZ designed and supervised the present study. XW
performed the majority of the experiments and drafted the initial
manuscript. YY helped design the study. RM helped perform the
experiments and revised the language of the manuscript. BX
interpreted the data. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of the Eye & ENT Hospital of Fudan University
(Shanghai, China). All patients provided written informed consent
prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
lncRNA
|
long non-coding RNA
|
|
SNHG7
|
small nucleolar RNA host gene 7
|
|
UM
|
uveal melanoma
|
|
TCGA
|
The Cancer Genome Atlas
|
|
GEPIA
|
Gene Expression Profiling Interactive
Analysis
|
|
OS
|
overall survival
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
PBS
|
phosphate- buffered saline
|
|
RIPA
|
radioimmunoprecipitation assay
buffer
|
|
EZH2
|
enhancer of zeste homolog 2
|
|
SNHG7+
|
SNHG7 overexpression group
|
|
NC
|
empty vector group
|
|
CCK-8
|
Cell Counting Kit-8
|
References
|
1
|
Chandran SS, Somerville RPT, Yang JC,
Sherry RM, Klebanoff CA, Goff SL, Wunderlich JR, Danforth DN, Zlott
D, Paria BC, et al: Treatment of metastatic uveal melanoma with
adoptive transfer of tumour-infiltrating lymphocytes: A
single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol.
18:792–802. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Robertson AG, Shih J, Yau C, Gibb EA, Oba
J, Mungall KL, Hess JM, Uzunangelov V, Walter V, Danilova L, et al:
Integrative analysis identifies four molecular and clinical subsets
in uveal melanoma. Cancer Cell. 32:204–220.e15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Field MG, Durante MA, Anbunathan H, Cai
LZ, Decatur CL, Bowcock AM, Kurtenbach S and Harbour JW: Punctuated
evolution of canonical genomic aberrations in uveal melanoma. Nat
Commun. 9:1162018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lane AM, Kim IK and Gragoudas ES: Survival
rates in patients after treatment for metastasis from uveal
melanoma. JAMA Ophthalmol. 136:981–986. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoo JH, Shi DS, Grossmann AH, Sorensen LK,
Tong Z, Mleynek TM, Rogers A, Zhu W, Richards JR, Winter JM, et al:
ARF6 is an actionable node that orchestrates oncogenic GNAQ
signaling in uveal melanoma. Cancer Cell. 29:889–904. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Batista PJ and Chang HY: Long noncoding
RNAs: Cellular address codes in development and disease. Cell.
152:1298–1307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Zhang R, Qiu F, Li K, Zhou Y, Shang
D and Xu Y: Construction of a lncRNA-PCG bipartite network and
identification of cancer-related lncRNAs: A case study in prostate
cancer. Mol Biosyst. 11:384–393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ye B, Liu B, Yang L, Zhu X, Zhang D, Wu W,
Zhu P, Wang Y, Wang S, Xia P, et al: LncKdm2b controls self-renewal
of embryonic stem cells via activating expression of transcription
factor Zbtb3. EMBO J. 37:e971742018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L, Meng X, Zhu XW, Yang DC, Chen R,
Jiang Y and Xu T: Long non-coding RNAs in Oral squamous cell
carcinoma: Biologic function, mechanisms and clinical implications.
Mol Cancer. 18:1022019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martens-Uzunova ES, Bottcher R, Croce CM,
Jenster G, Visakorpi T and Calin GA: Long noncoding RNA in
prostate, bladder, and kidney cancer. Eur Urol. 65:1140–1151. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Parasramka MA, Maji S, Matsuda A, Yan IK
and Patel T: Long non-coding RNAs as novel targets for therapy in
hepatocellular carcinoma. Pharmacol Ther. 161:67–78. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang G, Zhang ZJ, Jian WG, Liu PH, Xue W,
Wang TD, Meng YY, Yuan C, Li HM, Yu YP, et al: Novel long noncoding
RNA OTUD6B-AS1 indicates poor prognosis and inhibits clear cell
renal cell carcinoma proliferation via the Wnt/β-catenin signaling
pathway. Mol Cancer. 18:152019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Murugan AK, Munirajan AK and Alzahrani AS:
Long noncoding RNAs: Emerging players in thyroid cancer
pathogenesis. Endocr Relat Cancer. 25:R59–R82. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang JL, Zheng L, Hu YW and Wang Q:
Characteristics of long non-coding RNA and its relation to
hepatocellular carcinoma. Carcinogenesis. 35:507–514. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang FW, Cao CH, Han K, Zhao YX, Cai MY,
Xiang ZC, Zhang JX, Chen JW, Zhong LP, Huang Y, et al:
APC-activated long noncoding RNA inhibits colorectal carcinoma
pathogenesis through reduction of exosome production. J Clin
Invest. 129:727–743. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noh JH and Gorospe M: AKTions by
cytoplasmic lncRNA CASC9 promote hepatocellular carcinoma survival.
Hepatology. 68:1675–1677. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng X, Tang H, Zhao X, Sun Y, Jiang Y
and Liu Y: Long non-coding RNA FTH1P3 facilitates uveal melanoma
cell growth and invasion through miR-224-5p. PLoS One.
12:e01847462017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu Q, Zhao N, Zha G, Wang H, Tong Q and
Xin S: LncRNA HOXA11-AS exerts oncogenic functions by repressing
p21 and miR-124 in uveal melanoma. DNA Cell Biol. 36:837–844. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu L, Yu X, Zhang L, Ding X, Pan H, Wen X,
Xu S, Xing Y, Fan J, Ge S, et al: The long non-coding RNA RHPN1-AS1
promotes uveal melanoma progression. Int J Mol Sci. 18(pii):
E2262017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Zeng C, Hu J, Pan Y, Shan Y, Liu B
and Jia L: Long non-coding RNA-SNHG7 acts as a target of miR-34a to
increase GALNT7 level and regulate PI3K/Akt/mTOR pathway in
colorectal cancer progression. J Hematol Oncol. 11:892018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ota T, Suzuki Y, Nishikawa T, Otsuki T,
Sugiyama T, Irie R, Wakamatsu A, Hayashi K, Sato H, Nagai K, et al:
Complete sequencing and characterization of 21,243 full-length
human cDNAs. Nat Genet. 36:40–45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng D, Fan J, Ma Y, Zhou Y, Qin K, Shi M
and Yang J: LncRNA SNHG7 promotes pancreatic cancer proliferation
through ID4 by sponging miR-342-3p. Cell Biosci. 9:282019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhong X, Long Z, Wu S, Xiao M and Hu W:
LncRNA-SNHG7 regulates proliferation, apoptosis and invasion of
bladder cancer cells assurance guidelines. J BUON. 23:776–781.
2018.PubMed/NCBI
|
|
24
|
Wang MW, Liu J, Liu Q, Xu QH, Li TF, Jin S
and Xia TS: LncRNA SNHG7 promotes the proliferation and inhibits
apoptosis of gastric cancer cells by repressing the P15 and P16
expression. Eur Rev Med Pharmacol Sci. 21:4613–4622.
2017.PubMed/NCBI
|
|
25
|
Luo X, Song Y, Tang L, Sun DH and Ji DG:
LncRNA SNHG7 promotes development of breast cancer by regulating
microRNA-186. Eur Rev Med Pharmacol Sci. 22:7788–7797.
2018.PubMed/NCBI
|
|
26
|
Villanueva MT: Anticancer drugs: All roads
lead to EZH2 inhibition. Nat Rev Drug Discov. 16:2392017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang XM, Shi SS, Jian TM, Tang DR, Wu T
and Sun FY: LncRNA PVT1 knockdown affects proliferation and
apoptosis of uveal melanoma cells by inhibiting EZH2. Eur Rev Med
Pharmacol Sci. 23:2880–2887. 2019.PubMed/NCBI
|
|
28
|
Li Z, Hou P, Fan D, Dong M, Ma M, Li H,
Yao R, Li Y, Wang G, Geng P, et al: The degradation of EZH2
mediated by lncRNA ANCR attenuated the invasion and metastasis of
breast cancer. Cell Death Differ. 24:59–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jia B, Xie T, Qiu X, Sun X, Chen J, Huang
Z, Zheng X, Wang Z and Zhao J: Long noncoding RNA FALEC inhibits
proliferation and metastasis of tongue squamous cell carcinoma by
epigenetically silencing ECM1 through EZH2. Aging (Albany NY).
11:4990–5007. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45((W1)):
W98–W102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mas-Ponte D, Carlevaro-Fita J, Palumbo E,
Hermoso Pulido T, Guigo R and Johnson R: LncATLAS database for
subcellular localization of long noncoding RNAs. RNA. 23:1080–1087.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chaudhary R and Lal A: Long noncoding RNAs
in the p53 network. Wiley Interdiscip Rev RNA. 8:2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Van Raamsdonk CD, Griewank KG, Crosby MB,
Garrido MC, Vemula S, Wiesner T, Obenauf AC, Wackernagel W, Green
G, Bouvier N, et al: Mutations in GNA11 in uveal melanoma. N Engl J
Med. 363:2191–2199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun J, Pan LM, Chen LB and Wang Y: LncRNA
XIST promotes human lung adenocarcinoma cells to cisplatin
resistance via let-7i/BAG-1 axis. Cell Cycle. 16:2100–2107. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ding X, Wang X, Lin M, Xing Y, Ge S, Jia
R, Zhang H, Fan X and Li J: PAUPAR lncRNA suppresses tumourigenesis
by H3K4 demethylation in uveal melanoma. FEBS Lett. 590:1729–1738.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang X, Yang Z and Li Z: Zinc finger
antisense 1: A long noncoding RNA with complex roles in human
cancers. Gene. 688:26–33. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lan X, Sun W, Dong W, Wang Z, Zhang T, He
L and Zhang H: Downregulation of long noncoding RNA H19 contributes
to the proliferation and migration of papillary thyroid carcinoma.
Gene. 646:98–105. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liang WQ, Zeng, Chen CF, Sun SM, Lu XF,
Peng CY and Lin HY: Long noncoding RNA H19 is a critical oncogenic
driver and contributes to epithelial-mesenchymal transition in
papillary thyroid carcinoma. Cancer Manag Res. 11:2059–2072. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shan Y, Ma J, Pan Y, Hu J, Liu B and Jia
L: LncRNA SNHG7 sponges miR-216b to promote proliferation and liver
metastasis of colorectal cancer through upregulating GALNT1. Cell
Death Dis. 9:7222018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pandiani C, Beranger GE, Leclerc J,
Ballotti R and Bertolotto C: Focus on cutaneous and uveal melanoma
specificities. Genes Dev. 31:724–743. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim KH and Roberts CW: Targeting EZH2 in
cancer. Nat Med. 22:128–134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
McCabe MT, Ott HM, Ganji G, Korenchuk S,
Thompson C, Van Aller GS, Liu Y, Graves AP, Della Pietra A III,
Diaz E, et al: EZH2 inhibition as a therapeutic strategy for
lymphoma with EZH2-activating mutations. Nature. 492:108–112. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Varambally S, Dhanasekaran SM, Zhou M,
Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt
RG, Otte AP, et al: The polycomb group protein EZH2 is involved in
progression of prostate cancer. Nature. 419:624–629. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zingg D, Debbache J, Schaefer SM, Tuncer
E, Frommel SC, Cheng P, Arenas-Ramirez N, Haeusel J, Zhang Y,
Bonalli M, et al: The epigenetic modifier EZH2 controls melanoma
growth and metastasis through silencing of distinct tumour
suppressors. Nat Commun. 6:60512015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen S, Pu J, Bai J, Yin Y, Wu K, Wang J,
Shuai X, Gao J, Tao K, Wang G and Li H: EZH2 promotes
hepatocellular carcinoma progression through modulating
miR-22/galectin-9 axis. J Exp Clin Cancer Res. 37:32018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hubaux R, Thu KL, Coe BP, MacAulay C, Lam
S and Lam WL: EZH2 promotes E2F-driven SCLC tumorigenesis through
modulation of apoptosis and cell-cycle regulation. J Thorac Oncol.
8:1102–1106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bachmann IM, Halvorsen OJ, Collett K,
Stefansson IM, Straume O, Haukaas SA, Salvesen HB, Otte AP and
Akslen LA: EZH2 expression is associated with high proliferation
rate and aggressive tumor subgroups in cutaneous melanoma and
cancers of the endometrium, prostate, and breast. J Clin Oncol.
24:268–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kivelä T and Kujala E: Prognostication in
eye cancer: The latest tumor, node, metastasis classification and
beyond. Eye (Lond). 27:243–252. 2013. View Article : Google Scholar : PubMed/NCBI
|