Introduction
Human melanoma is a highly malignant tumor derived
from abnormal melanocytes. It is responsible for >75% of skin
cancer deaths (1). Melanomas
metastasize in the lymph nodes through blood vessels and the
prognosis is poor. Melanoma treatment by known drugs and therapies
is limited and outcome is poor. The incidence of a five-year
survival rate ranges between 5–15% (2). Although the effectiveness of
dacarbazine as a single-agent therapy is only 4.5%, it is commonly
used for the treatment of advanced melanoma as a chemotherapeutic
agent (3). Moreover the majority of
patients using a mono- or combination therapy comprising
single-agent BRAF and/or dual specificity mitogen-activated protein
kinase kinase (MEK) kinase inhibitors only exhibited a clinical
improvement for a limited time (4).
Therefore, the discovery of new treatments is important for the
short-term prognosis of patients with melanoma.
Natural products are a potential source for new
therapies. More than 75% of new anti-tumor drugs have originated
from natural products, for example, paclitaxel and colchicine
(5). The Food and Drug
Administration (FDA) has approved 25–48% of plant derived medicines
(6).
2′,4′-dihydroxy-6′-methoxychalcone (cardamonin) is a
natural chalcone compound that is extracted from members of the
Zingiberaceae family (7). It may
increase cell apoptosis in several tumors, including nasopharyngeal
carcinoma, prostate cancer and glioblastoma (8–10).
Cardamomin may be effective via multiple signaling pathways,
including nuclear factor (NF)-κB, signal transducer and activator
of transcription 3 and mammalian target of rapamycin pathways
(8,10,11).
However, the underlying effects and mechanisms of cardamonin on
melanoma cells remain unclear. In the present study, several assays
were used to determine the anticancer activity and effects of
cardamonin on human melanoma cells.
Materials and methods
Cell viability assay
An MTS assay was used to determine the effect of
cardamonin on melanoma cell viability. Cells were cultured in
RPMI-1640 Medium (Corning, cat. no. 10-040-CV) with 10% FBS
(Shanghai ExCell Biology, Inc.; cat. no. FND500) in an incubator
(37°C, 50% CO2) Human melanoma M14 cells, supplied by
the Chinese Academy of Medical Sciences, (2–5 ×103/100
µl/well) were seeded in a 96-well plate and incubated for 24 h
pre-cardamonin treatment at different concentrations (0, 30, 60 and
90 µM) for 24 h. Thereafter, 20 µl MTS solution (2 mg/ml) was added
to each well followed by a 2 h incubation period. Absorbance was
measured with a microplate reader (Infinite F50; Tecan
Schweiz AG) at a wavelength of 495 nm.
Flow cytometry
Cell apoptosis caused by cardamonin was measured
with a fluorescein isothiocyanate (FITC) Annexin V apoptosis
detection kit (BD Biosciences; cat. no. 556547). M14 cells were
seeded in a 6-well plate and treated with varying cardamonin
concentrations (0, 30, 60 and 90 µM) for 24 h. Cells were collected
and washed with ice-cold PBS and incubated with Annexin V-FITC and
propidium iodide (PI) in the dark for 20 min. The cells were then
resuspended with binding buffer and measured using a Navios flow
cytometer B47903 and Navios Platform System Software v2.0 (both
Beckman Coulter, Inc.).
Western blot analysis
M14 and A375 cell lines (Chinese Academy of
Medical Science) were seeded in 6-well plates (2×105
per well) and treated with varying cardamonin concentrations (0,
30, 60 and 90 µM) for 24 h. Cell proteins were collected with RIPA
buffer (Beijing Solarbio Science & Technology Co., Ltd.; cat.
no. R0020-100 ml) supplemented with PMSF. Protein concentrations
were measured using a bicinchoninic acid protein assay kit
(MultiSciences). The proteins (30 µg) were separated via
SDS-PAGE (15% gel) electrophoresis and transferred to PVDF
membranes. The membranes were then blocked in 5% non-fat milk in
TBST (0.05%Tween20) for 1 h at room temperature and incubated with
homologous primary antibodies (all 1:1,000) overnight at 4°C
[β-actin, cat. no. AC026; P65, cat. no. A2547; BCL2, cat. no.
A2212; BAX, cat. no. A7626 (all ABclonal Biotech Co., Ltd.);
Cleaved Caspase-8, cat. no. 9496; Cleaved Caspase-9, cat. no.
20750; Cleaved PARP, cat. no. 5625 (all Cell Signaling Technology,
Inc.)]. The membranes were incubated with horse radish peroxidase
conjugated secondary antibodies (Goat Anti-Rabbit IgG
horseradish-peroxidase conjugated; cat. no. ab205718; 1:10,000) at
room temperature for 2 h. After washing the membranes, enhanced
chemiluminescence reagents (Beijing Solarbio Science &
Technology Co., Ltd.; cat. no. PE0010) were applied to them before
being scanned by a ChemiDoc XRS+ system (Bio-Rad
Laboratories, Inc.).
Wound healing assay
A wound healing assay was used to test the effect of
cardamonin on cell migration. M14 cells were seeded into 6-well
plates (5×105 per well) (Guangzhou Jet Bio-Filtration
Co., Ltd.; cat. no. TCP010006) and cultured to 80% confluence. They
were then scratched through the cell monolayer using a 10 µl
pipette tip before being washed with serum-free RPMI-1640 medium
(Corning, Inc., cat. no. 15-040-CV) to remove floating cells.
Subsequently, cells were treated with varying cardamonin
concentrations (0, 30, 60 and 90 µM) for 24 h and images were
captured using the ChemiDoc™ XRS+ System light miscroscope with
Image Lab™ Software (Bio-Rad Laboratories, Inc., cat. no.
1708265).
Data analysis
The Gray value of three repeats was measured using
ImageJ (1.50i; National Institutes of Health). Data are
presented as the mean ± standard deviation. The
significance difference between distinct groups were determined
using one-way ANOVA (SPSS 21.0; IBM Corp.) with post-hoc test
(Fisher's Least Significant Difference). P<0.05 was considered
to indicate a statistically significant difference.
Results
Cardamonin inhibits cell
viability
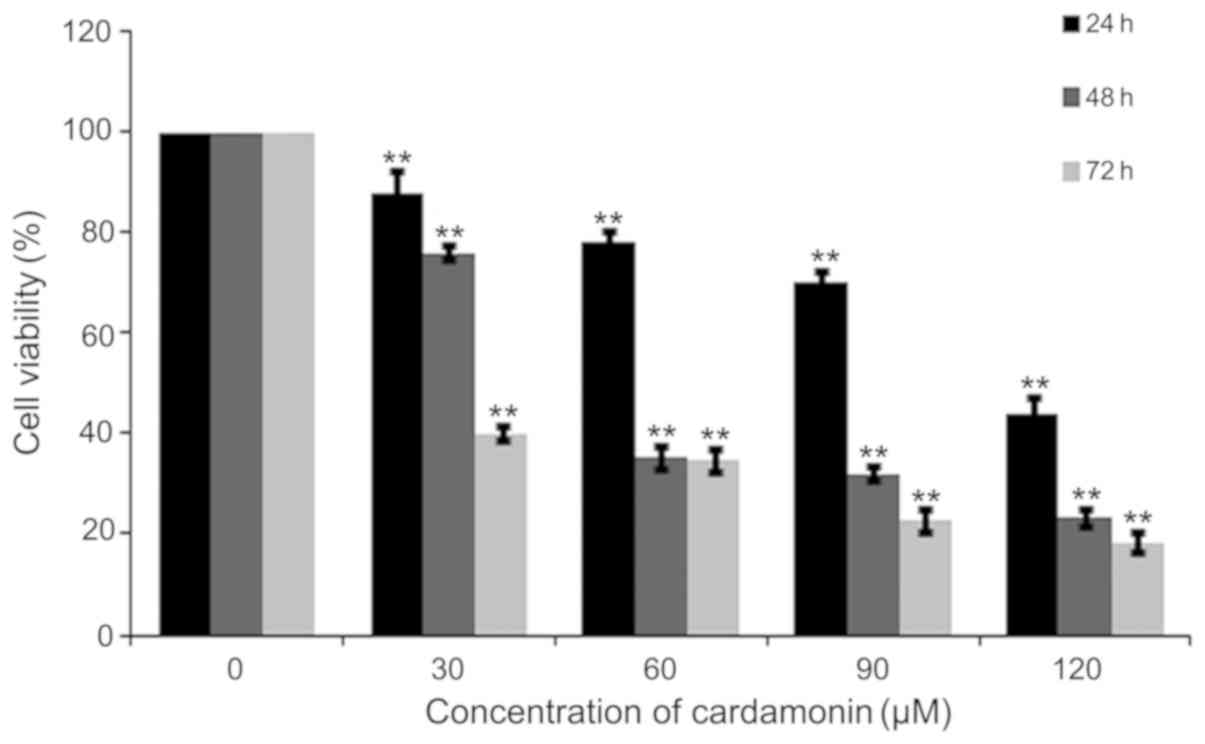
M14 cells were treated with varying cardamonin
concentrations (0, 30, 60, 90 and 120 µM; n=4) for 24, 48 and 72 h.
The MTS assay showed that cardamonin significantly decreased M14
cell viability (P<0.01; Fig. 1).
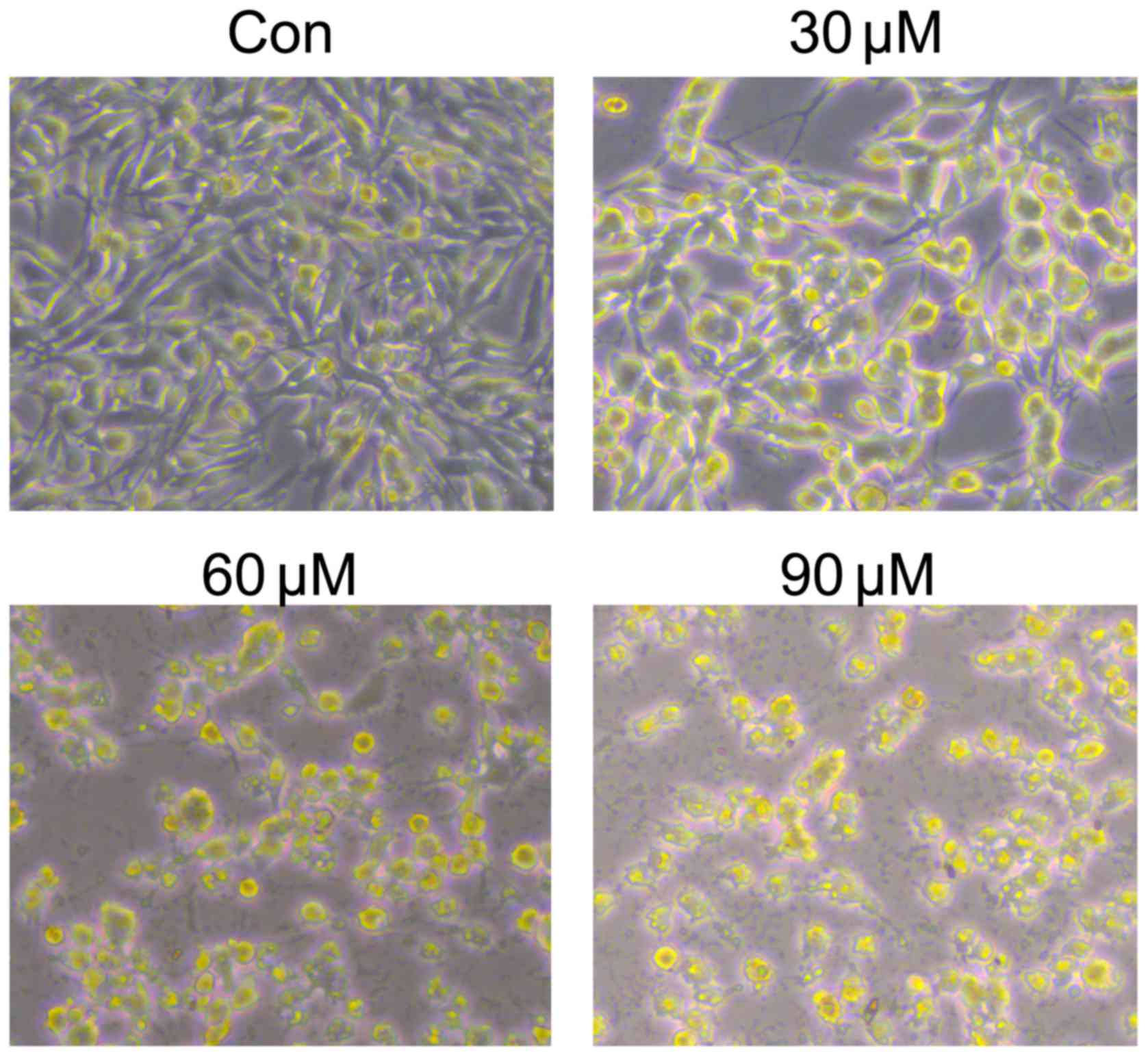
Cell density was also decreased after cardamonin treatment for 24 h
(Fig. 2). The results indicated that
cardamonin could inhibit M14 cell growth.
Cardamonin induces apoptosis in M14
cells
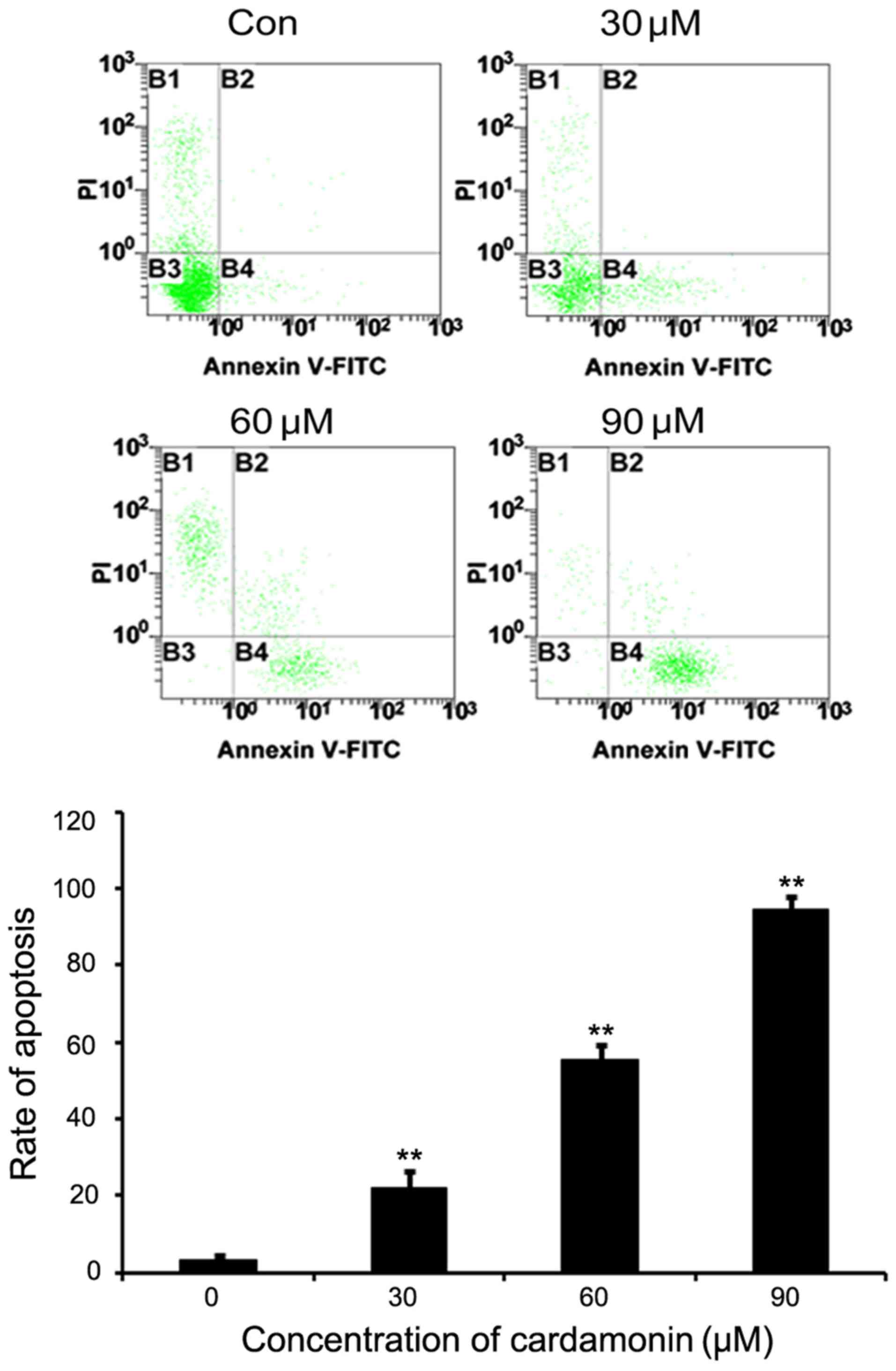
M14 cells were cultured in varying cardamonin
concentrations (0, 30, 60 and 90 µM, n=3) for 24 h and then stained
with Annexin V-FITC and PI for flow cytometry analysis. Results
indicated that the untreated M14 cells were not stained by Annexin
V and PI, whereas a significant dose-dependent increase was noted
in the Annexin V and PI positive cells following cardamonin
treatment (P<0.01; Fig. 3).
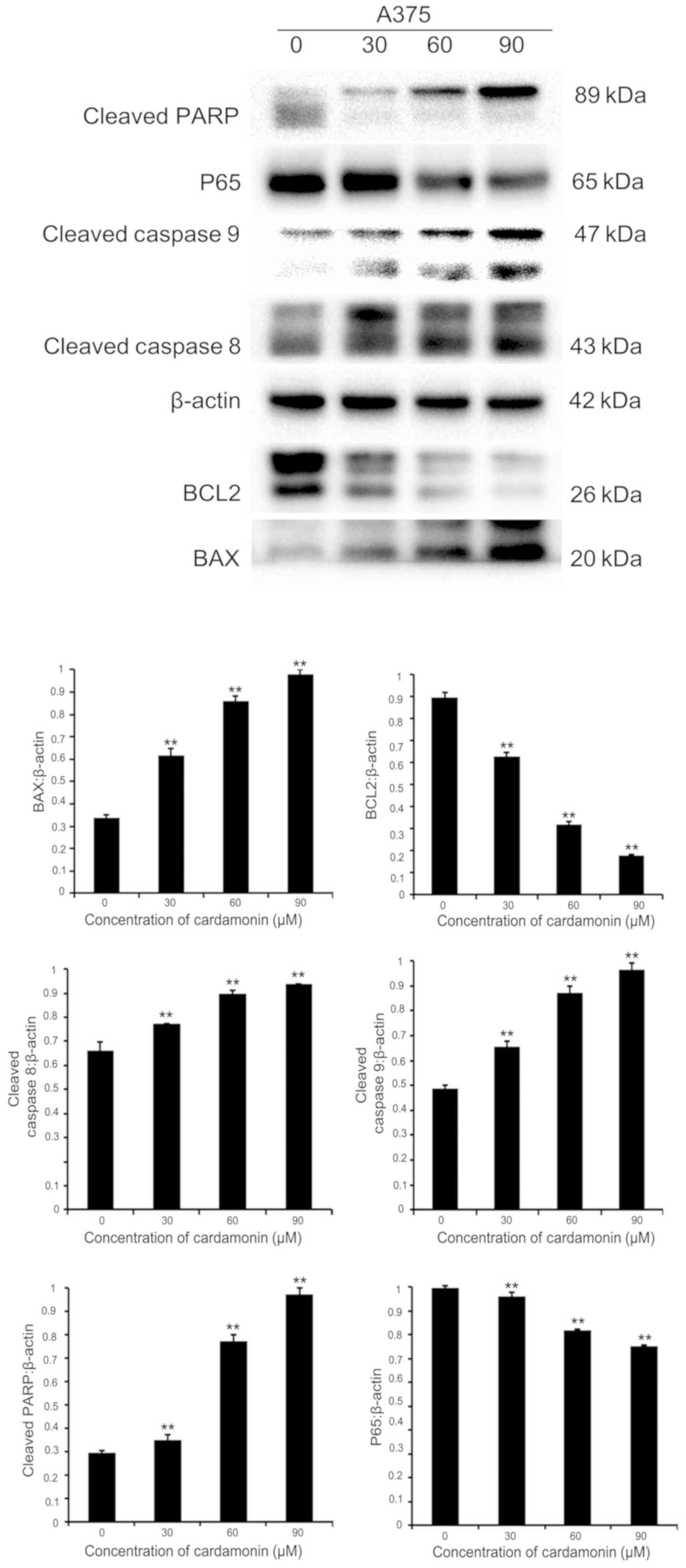
Cardamonin alters apoptosis-associated
proteins in M14 cells and A375 cells
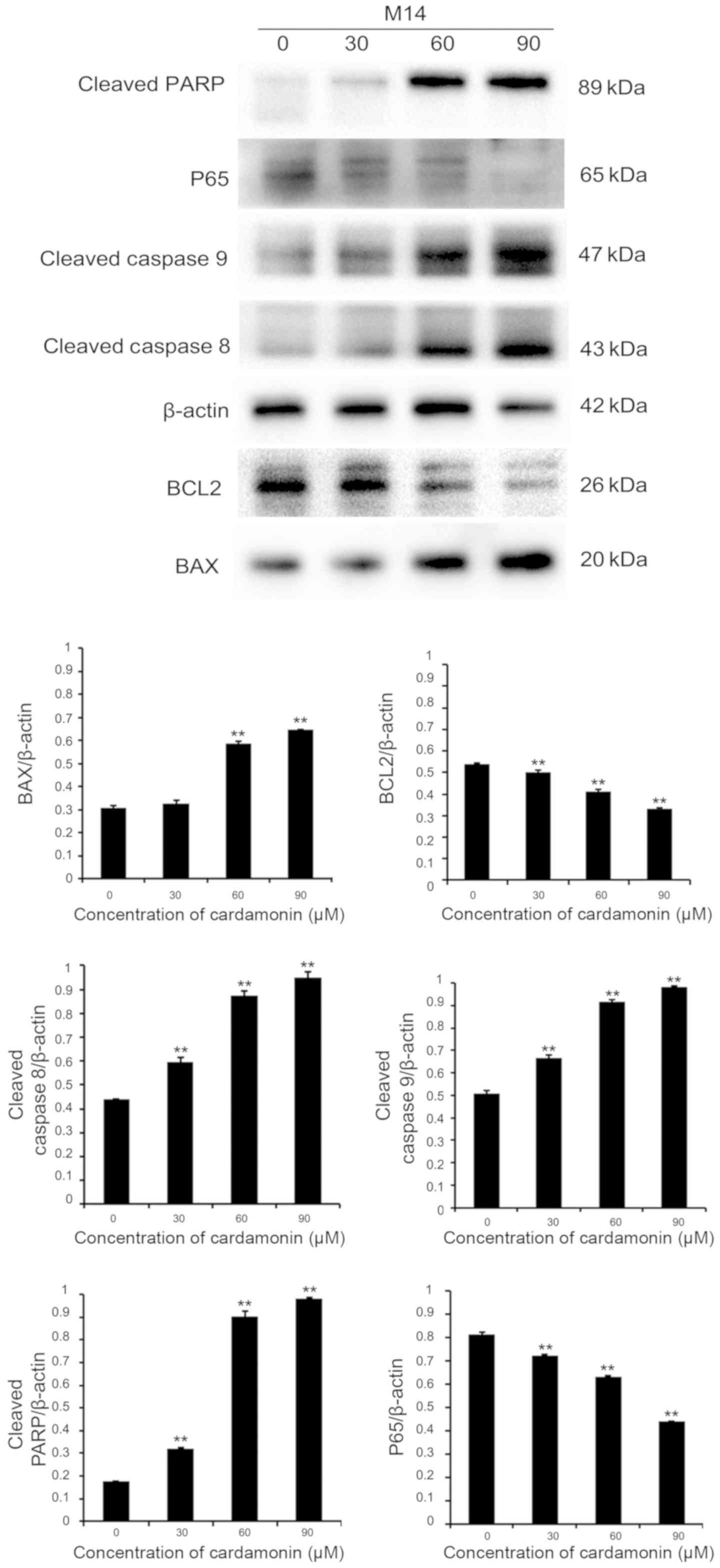
In order to investigate whether cardamonin induced
melanoma cell apoptosis via the effects of apoptosis-associated
proteins, immunoblotting was performed. Protein lysates were
collected from two different cell lines (M14 and A375). They were
cultured in varying cardamonin concentrations (0, 30, 60 and 90 µM;
n=3) for 24 h. It was found that the pro-apoptotic protein BAX was
upregulated, while the anti-apoptotic protein B-cell lymphoma-2
(BCL2) was downregulated in both cell lines. Intrinsic or extrinsic
pathways may be involved in apoptosis, therefore the expression of
cleaved caspase-8, −9 and cleaved PARP were investigated (12). These apoptotic signaling proteins
increased significantly after 24-h treatment with varying
cardamonin concentrations (P<0.01). P65 expression significantly
decreased, thus indicating that cardamonin is a major
anti-apoptotic regulator (P<0.01; Figs. 4 and 5). Results showed that cardamonin regulates
the expression of important pro-apoptotic and anti-apoptotic
proteins.
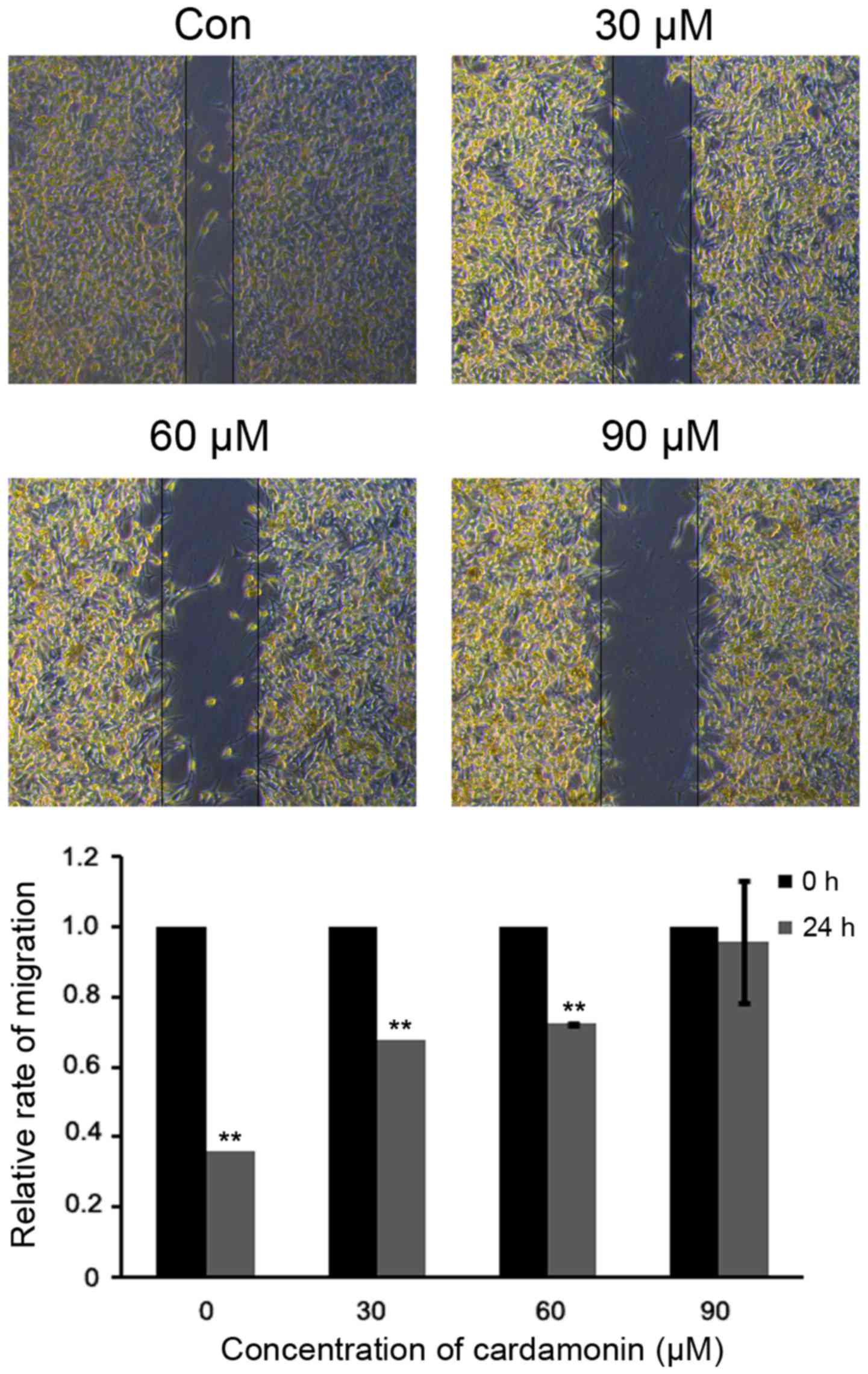
Cardamonin suppresses M14 cell
migration
To assess whether cardmonin affects cell migration,
a scratch-wounding assay was performed on M14 cells. Under varying
cardamonin concentrations (0, 30, 60 and 90 µM; n=3), the open
wound areas were significantly increased in comparison to the
untreated cells (P<0.01). These data indicated that cardamonin
suppressed M14 cell migration in a dose-dependent manner (Fig. 6).
Discussion
Cutaneous melanoma is responsible for 1.6% of new
cancer cases and 0.7% of cancer-related deaths worldwide (13). Traditional chemotherapy is
ineffective in melanoma treatment and the mortality rate remains
high. Dakabazine (DTIC) is the only FDA-approved anti-melanoma
chemotherapy drug in use to date. In comparison to supportive
therapies (5–11 months), the response rate to DTIC ranges from
between 5–25% and does not increase overall survival. The
development of targeted therapy has improved the response and
overall survival rate in melanoma patients. Vemurafenib as an
orally active BRAF inhibitor, exhibits partial and complete
responses in BRAF mutated melanoma patients. Melanoma cells may
easily develop drug resistance (14). Dabrafenib as another BRAF inhibitor
that is similar to vemurafenib in terms of progression-free
survival (PFS) and reaction rate (15). Approved immunotherapeutics include
ipilimumab and nivolumab, the former being a monoclonal antibody
that blocks the cytotoxic T-lymphocyte protein-4 receptor. Previous
studies have shown that the combination of nivolumab (a PD-1
inhibitor) may achieve better results than the single use of
ipilimumab (16–18). Nivolumab monotherapy has a higher
objective response rate, a longer PFS and overall survival rate
compared to chemotherapy treatments. Despite the positive effects
of immunotherapy drugs, a large number of patients continue to
succumb to melanoma metastasis. The ubiquitous resistance to
molecular targeted therapy remains a challenge for the treatment of
melanoma.
Therefore, the effective treatment of melanoma
depends on the development of novel medicines. Furthermore,
researchers are committed to finding drugs that are effective on
cancer cells while having minimal cytotoxic effects on normal
cells.
Alpinia katsumadai hayata is a traditional
Chinese herbal medicine. It induces a warming of the stomach and is
used for relieving gastric discomfort and a distended abdomen
(19). Cardamonin is extracted from
the seed of cardamom spices and it is an active ingredient of
Alpinia katsumadai hayata, which has antinociceptive effects
(15,20). Cardamonin has been tested as an
anticancer treatment in several tumors, except melanoma.
Cardamonin's proven safety and effects in tumor treatment make it a
promising anti-cancer reagent. An MTS assay was performed to verify
its anti-proliferative effect on M14 melanoma cells.
Apoptosis is an important physiological process
whereby cells commit suicide (21).
There are two major pathways of apoptosis, namely the intrinsic and
the extrinsic pathway. The intrinsic pathway is controlled by the
BCL2 family of proteins, which may be classified as pro-apoptotic
proteins like BAX and anti-apoptotic proteins such as BCL2. BAX as
a factor in the BCL2 family, plays an important role in cell
apoptosis and mitochondrial function (22). BCL2 may induce apoptosis by binding
to anti-apoptotic proteins, thereby replacing activators that may
activate BAX (23). BCL2 is a
fundamental anti-apoptotic gene that that is recognized in cancer
development (24). Overexpression of
BCL2 promotes tumorigenesis and tumor progression and is associated
with poor patient prognosis in numerous types of cancer, for
example, breast cancer, prostate cancer and melanoma (25). In order to achieve more reliable
results, both M14 and A375 cell were treated with cardamonin and
analyzed by western blotting. It was noted that treatment with
cardamonin resulted in the downregulation of BCL2 and upregulation
of BAX protein levels in M14 and A375 cells. Both proteins play a
pivotal role in apoptotic pathway regulation.
Caspases are also vital pro-apoptotic proteins.
Caspase-8 occurs in extrinsic apoptotic pathways while caspase-9 is
involved in intrinsic (mitochondrial) pathways. Caspase-8 can
process classical apoptotic caspases including caspase-9, which may
lead to apoptotic initiation (26).
Proenzymes synthesized by caspases, are activated by cleaving the
pro-domain at a specific aspartic acid cleaving site. Activation of
caspase-8 or −9 may eventually lead to the cleavage of poly
(ADP-ribose) polymerase (PARP) which results in DNA fragmentation
and apoptosis (27). In a previous
study, upregulation of cleavage of caspase-8, caspase-9 and PARP
was investigated in the treatment of prostate cancer cells with
cardamonin (9). Western blot
analysis results showed apoptotic induction by cardamonin via the
cleavage of caspase-8, −9 and PARP. These results reflected those
of a previous study that performed a Caspase-Glo 3/7, 8 and 9 assay
and western blot analysis in A549 and HK1 cells (28). Ma et al (29) discovered that Deoxyarbutin inhibited
Bcl-2, activated Bax and voltage-dependent anion-selective channel
protein, which in turn sequentially activated caspase-9, PARP,
caspase-3 and finally led to mitochondria associated apoptosis.
While in a study by Bush et al (30) it was revealed that another drug
curcumin induced melanoma cell death by activating caspases-3 and
caspases-8 but not caspase-9. Therefore, the role of caspase-9 in
melanoma cells needs to be further investigated.
NF-κB (P65) which combines with fixed nucleotide
sequences of various gene promoters, is an anti-apoptotic factor.
P65 has an important influence on cell proliferation and
differentiation, tumor formation, invasion and metastasis. P65
directly mediates vital tumor-promoting mechanisms. NF-κB is known
to activate cell proliferation, prevent apoptosis, promote tumor
angiogenesis, epithelial-to-mesenchymal transition, invasiveness
and metastasis (31). P65
phosphorylation is associated to the expression of the
pro-apoptotic BCL2 family, which includes Bcl-2 (32). A study by Wang and Liu (33) confirmed that ingenol-3-angelate
suppressed the growth of melanoma cells, in which P65 activity was
downregulated. Furthermore, the effects of cardamonin on cancer
cell apoptosis via the P65 signaling pathway have been proven. P65
expression was tested in M14 and A375 cell lines exposed to
cardamonin by means of western blot analysis in the present study.
Cardamonin did not inhibit P65 in these cell lines.
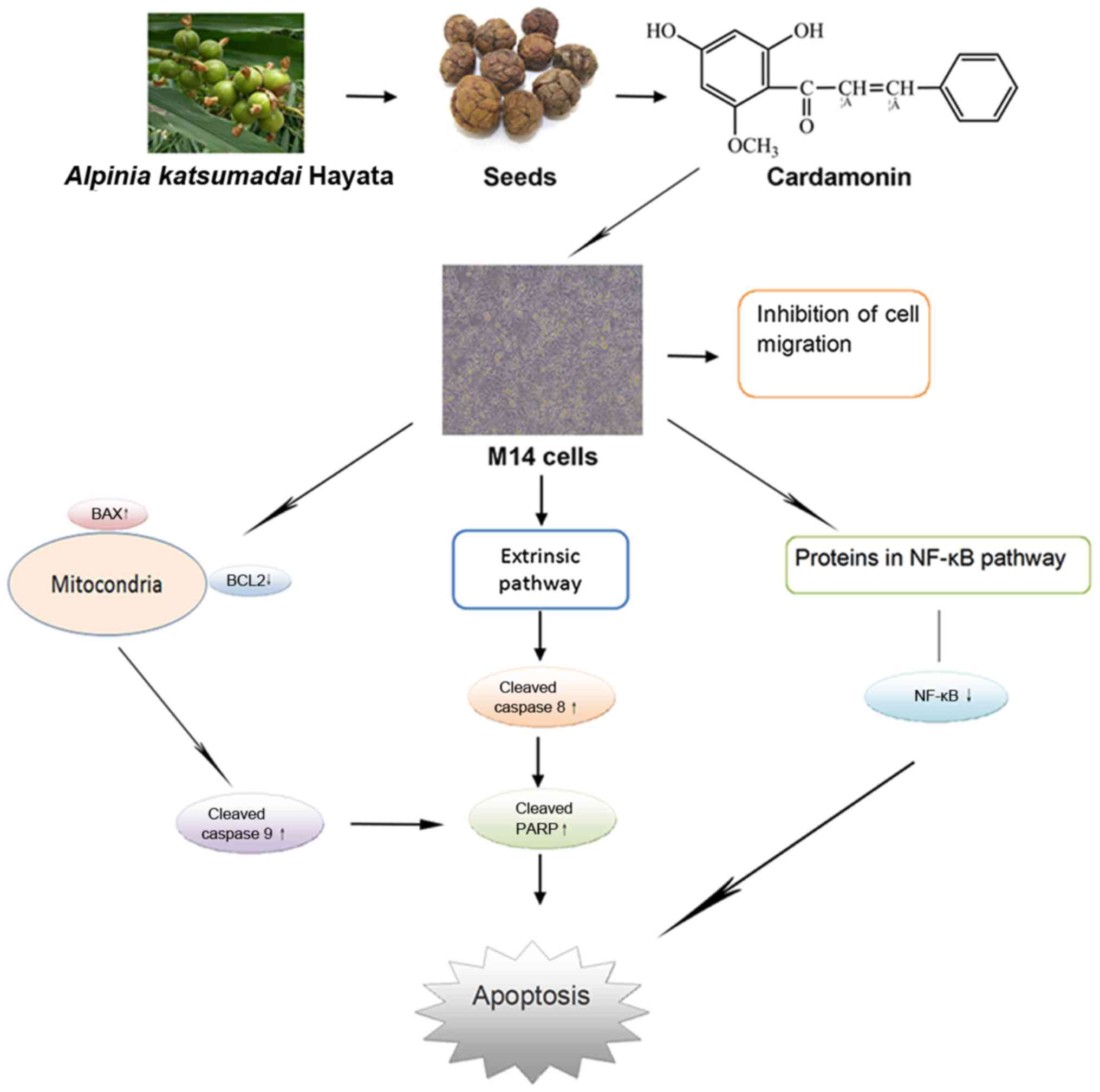
The present study demonstrated that cardamonin
promotes apoptosis of melanoma cells via the regulation of proteins
in the intrinsic, extrinsic and NF-κB pathways (Fig. 7). Cardamonin, as a natural compound,
may be a potentially valuable factor for human melanoma
treatment.
Acknowledgements
Not applicable.
Funding
Financial assistance was provided by the Hebei
Provincial Administration of Traditional Chinese Medicine (project
number: 2018055).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY, LL, YTL, PPL and YJL analyzed and collected the
data regarding the MTS and the western blotting analysis. HL
analyzed and collected the data regarding the flow cytometry
analysis. GZ and XD helped with experimental design, interpretation
of data and made major contributions to the writing of the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee C, Collichio F, Ollila D and Moschos
S: Historical review of melanoma treatment and outcomes. Clin
Dermatol. 31:141–147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chien AJ, Moore EC, Lonsdorf AS,
Kulikauskas RM, Rothberg BG, Berger AJ, Major MB, Hwang ST, Rimm DL
and Moon RT: Activated Wnt/beta-catenin signaling in melanoma is
associated with decreased proliferation in patient tumors and a
murine melanoma model. Proc Natl Acad Sci USA. 106:1193–1198. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cui C, Mao L, Chi Z, Si L, Sheng X, Kong
Y, Li S, Lian B, Gu K, Tao M, et al: A phase II, randomized,
double-blind, placebo-controlled multicenter trial of Endostar in
patients with metastatic melanoma. Mol Ther. 21:1456–1463. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sullivan R, LoRusso P, Boerner S and
Dummer R: Achievements and challenges of molecular targeted therapy
in melanoma. Am Soc Clin Oncol Educ Book. 2015.177–186. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Atanasov AG, Waltenberger B,
Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L,
Schwaiger S, Heiss EH, et al: Discovery and resupply of
pharmacologically active plant-derived natural products: A review.
Biotechnol Adv. 33:1582–1614. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harvey AL, Edrada-Ebel R and Quinn RJ: The
re-emergence of natural products for drug discovery in the genomics
era. Nat Rev Drug discov. 14:111–129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goncalves LM, Valente IM and Rodrigues JA:
An overview on cardamonin. J Med Food. 17:633–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Y, Qin Y, Yang C, Zhang H, Li Y, Wu B,
Huang J, Zhou X, Huang B, Yang K and Wu G: Cardamonin induces
ROS-mediated G2/M phase arrest and apoptosis through inhibition of
NF-κB pathway in nasopharyngeal carcinoma. Cell Death Dis.
8:e30242017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang J, Sikka S, Siveen KS, Lee JH, Um
JY, Kumar AP, Chinnathambi A, Alharbi SA, Basappa, Rangappa KS, et
al: Cardamonin represses proliferation, invasion, and causes
apoptosis through the modulation of signal transducer and activator
of transcription 3 pathway in prostate cancer. Apoptosis.
22:158–168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu N, Liu J, Zhao X, Yan Z, Jiang B, Wang
L, Cao S, Shi D and Lin X: Cardamonin induces apoptosis by
suppressing STAT3 signaling pathway in glioblastoma stem cells.
Tumour Biol. 36:9667–9676. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Niu P, Shi D, Zhang S, Zhu Y and Zhou J:
Cardamonin enhances the anti-proliferative effect of cisplatin on
ovarian cancer. Oncol Lett. 15:3991–3997. 2018.PubMed/NCBI
|
|
12
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rosen LS, Lorusso P, MA WW, Goldman JW,
Weise A, Colevas AD, Adjei A, Yazji S, Shen A, Johnston S, et al: A
first-in-human phase I study to evaluate the MEK1/2 inhibitor,
cobimetinib, administered daily in patients with advanced solid
tumors. Invest New Drugs. 34:604–613. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park MK, Lee HJ, Choi JK, Kim HJ, Kang JH,
Lee EJ, Kim YR, Kang JH, Yoo JK, Cho HY, et al: Novel
anti-nociceptive effects of cardamonin via blocking expression of
cyclooxygenase-2 and transglutaminase-2. Pharmacol Biochemistry
Behav. 118:10–15. 2014. View Article : Google Scholar
|
|
16
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M,
Rutkowski P, et al: Combined nivolumab and ipilimumab or
monotherapy in untreated melanoma. N Engl J Med. 373:23–34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Caroline R, Jacob S, Georgina VL, Ana A,
Jean JG, Laurent M, Adil D, Matteo SC, Catriona M, Michal L, et al:
Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med.
372:2521–2532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Caroline R, Georgina VL, Jacob S, Ana A,
Jean JG, Laurent M, Adil D, Matteo SC, Catriona M, Michal L, et al:
Long-term outcomes in patients (pts) with ipilimumab (ipi)-naïve
advanced melanoma in the phase 3 KEYNOTE-006 study who completed
pembrolizumab (pembro) treatment. J Clin Oncol. 15 (Suppl):S35.
2017.
|
|
19
|
Wang S, Zhai C, Zhang Y, Yu Y, Zhang Y, Ma
L, Li S and Qiao Y: Cardamonin, a novel antagonist of hTRPA1 cation
channel, reveals therapeutic mechanism of pathological pain.
Molecules. 21:E11452016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu L, Chen X and Hu Z: Separation and
determination of alpinetin and cardamonin in alpinia katsumadai
hayata by flow injection-micellar electrokinetic chromatography.
Talanta. 71:155–159. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fulda S, Gorman AM, Hori O and Samali A:
Cellular stress responses: Cell survival and cell death. Int J Cell
Biol. 2010:2140742010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chan SL and Yu VC: Proteins of the bcl-2
family in apoptosis signalling: From mechanistic insights to
therapeutic opportunities. Clin Exp Pharmacol Physiol. 31:119–128.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hata AN, Engelman JA and Faber AC: The
BCL2 family: Key mediators of the apoptotic response to targeted
anticancer therapeutics. Cancer Discov. 5:475–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu Y, Wen X and Zhao P: MicroRNA-365
inhibits cell growth and promotes apoptosis in melanoma by
targeting BCL2 and cyclin D1 (CCND1). Med Sci Monit. 24:3679–3692.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Radha G and Raghavan SC: BCL2: A promising
cancer therapeutic target. Biochim Biophys Acta Rev Cancer.
1868:309–314. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Monie TP and Bryant CE: Caspase-8
functions as a key mediator of inflammation and pro-IL-1β
processing via both canonical and non-canonical pathways. Immunol
Rev. 265:181–193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J and Yuan J: Caspases in apoptosis and
beyond. Oncogene. 27:6194–6206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Break MKB, Hossan MS, Khoo Y, Qazzaz ME,
Al-Hayali MZK, Chow SC, Wiart C, Bradshaw TD, Collins H and Khoo
TJ: Discovery of a highly active anticancer analogue of cardamonin
that acts as an inducer of caspase-dependent apoptosis and
modulator of the mTOR pathway. Fitoterapia. 125:161–173. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma L, Xu Y, Wei Z, Xin G, Xing Z, Niu H
and Huang W: Deoxyarbutin displays antitumour activity against
melanoma in vitro and in vivo through a p38-mediated mitochondria
associated apoptotic pathway. Sci Rep. 7:71972017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bush JA, Cheung KJ Jr and LI G: Curcumin
induces apoptosis in human melanoma cells through a Fas
receptor/caspase-8 pathway independent of p53. Exp Cell Res.
271:305–314. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaltschmidt B, Greiner JFW, Kadhim HM and
Kaltschmidt C: Subunit-specific role of NF-κB in cancer.
Biomedicines. 6:E442018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Turillazzi E, Neri M, Cerretani D,
Cantatore S, Frati P, Moltoni L, Busardò FP, Pomara C, Riezzo I and
Fineschi V: Lipid peroxidation and apoptotic response in rat brain
areas induced by long-term administration of nandrolone: The mutual
crosstalk between ROS and NF-kB. J Cell Mol Med. 20:601–612. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang D and Liu P: Ingenol-3-Angelate
suppresses growth of melanoma cells and skin tumor development by
downregulation of NF-κB-cox2 signaling. Med Sci Monit. 24:486–502.
2018. View Article : Google Scholar : PubMed/NCBI
|