Introduction
Lung cancer is a major public health problem and is
the leading cause of cancer-associated mortality worldwide
(1–3). Cancer pathology often divides lung
cancer into non-small cell lung cancer (NSCLC) and small cell lung
cancer, which account for ~85 and ~15% of lung cancer cases,
respectively (4). Statistics have
estimated that there were ~1.8 million newly diagnosed lung cancer
cases and ~1.6 million lung cancer-associated mortalities in 2012
worldwide (5). Lung cancer is the
most frequently occurring human cancer and is the leading cause of
cancer-associated mortality among males, followed by prostate and
colorectal cancer for incidence, and liver and stomach cancer for
mortality (6). Currently, although
clinical therapeutic methods, including radiotherapy, chemotherapy,
Chinese medicinal herb treatment, immunotherapy, gene therapy and
targeted therapy, have been investigated and applied for the
treatment of patients with lung cancer (7–10), the
overall 5-year survival rate remains poor at <15% (11–13).
At present, lung tumor metastasis is the most
difficult treatment barrier in cancer therapy (14–16).
Therefore, obtaining an early diagnosis for human tumors is crucial
for the effective treatment of human lung cancer (17). Clinically, ultrasound, positron
emission tomography-computed tomography (PET/CT) and magnetic
resonance imaging have been applied for diagnosing human cancer
(18). Notably, PET/CT has become an
efficient protocol for tumor diagnosis in human lung cancer cases
(19–21). PET/CT also serves a vital role in the
differentiation of adrenal metastasis from a benign adrenal mass in
patients with lung cancer, with excellent diagnostic performance
(22). However, the diagnostic
efficacy in patients with early-stage lung cancer requires
improvement.
It has been reported that developing multimodal
contrast agent would enhance the diagnostic accuracy of PET/CT, as
well as increase the diagnostic accuracy sensitivity in patients
with lung cancer (23). A previous
study reported that contrast-enhanced ultrasound with a novel
nanoparticle contrast agent increases the diagnostic efficacy in
patients with NSCLC (24). In
addition, another study reported a composite nano-system composed
of gadolinium-doped mesoporous silica nanoparticles and gold
nanoparticles, which can be used as an efficient contrast agent for
in vivo cancer imaging (25).
In addition, previous studies have demonstrated that
Gd2O3-doped nanoparticles are promising
candidates of highly efficient contrast agents in diagnosing human
cancer (26–28).
Lenvatinib (Len) is a small-molecule tyrosine kinase
inhibitor that inhibits vascular endothelial growth factor
receptors, platelet-derived growth factor receptor α, fibroblast
growth factor receptors, stem cell factor receptor and rearranged
during transfection (29). In the
present study, Gd2O3-doped
carbon-11-choline-Len (GdCho-Len) nanoparticles contrast combined
with PET/CT (GdCho-Len-PET) was used to diagnose patients with lung
cancer. The present study characterized GdCho-Len-PET to visualize
the distribution of human lung tumor using PET/CT by performing
in vivo trails. The survival rate of patients with lung
cancer diagnosed by GdCho-Len-PET was identified during a 420-day
follow up.
Materials and methods
Subjects
A total of 172 patients with suspected lung cancer
were recruited from the Dongzhimen Hospital of Beijing University
of Traditional Chinese Medicine (Beijing, China) between May 2016
and September 2017. Lung cancer diagnosis was confirmed by biopsy
by three respiratory physicians who specialized in the
interpretation of clinical and radiological lung cancer data. All
patients with suspected lung cancer underwent GdCho-PET and
GdCho-Len-PET, which was further confirmed by a tissue biopsy
(n=172). The age range of patients was 36–60 years, and comprised
an equal number of men and women. The characteristics of the
patients are summarized in Table I.
The exclusion criteria were as follows: i) Patients with cancer
history; ii) patients with pulmonary infarction; iii) patients who
had been diagnosed with acute respiratory disease within 6 months;
iv) pregnant or lactating females; and v) patients with infection
suspected to cause coughs. The inclusion criteria were as follows:
i) age ≥25 years; and ii) individuals who were able to provide
informed consent for participation. The Ethics Committee of the
Dongzhimen Hospital of Beijing University of Traditional Chinese
Medicine (Beijing, China) approved the present study. All
participants provided written informed consent for inclusion.
 | Table I.Characteristics of patients with
suspected lung cancer. |
Table I.
Characteristics of patients with
suspected lung cancer.
|
Characteristics | n (%) | Mean ± standard
deviation |
|---|
| Sex |
|
|
|
Male | 86.0 (50.0) |
|
|
Female | 86.0 (50.0) |
|
| Age, years |
|
|
|
Mean | 47.6 |
|
|
Range | 36.0–60.0 |
|
| BMI |
| 26.2±5.6 |
| Heart rate,
beats/min |
| 88.0±8.0 |
| Smoking status |
|
|
|
Current/former | 160.0 (93.0) |
|
|
Never | 12.0 (7.0) |
|
Contrast agent
The GdCho and GdCho-Len contrast agents were
synthesized as described previously (30). Briefly, cetyltrimethylammonium
bromide (C16TAB; 0.2 g) was dissolved in distilled water
(50 ml). Subsequently, NH3.H2O (2 ml; 25%)
and tetraethoxysilane (4.49 mmol) were added and stirred at room
temperature for 10 min. Gd2O3 (0.5 mmol) was
then added to the solution and stirred at room temperature for 1 h,
and carbon-11 (0.1 mmol), choline (0.1 mmol) or carbon-11-choline
(0.1 mmol), and lenvatinib (0.2 mmol) were added to the solution
and stirred at room temperature for 1 h. All these compounds were
provided by Sigma-Aldrich; Merck KGaA. Samples were calcined at
37°C for 72 h, and the GdCho and GdCho-Len nanoparticles were
harvested. The synthesized GdCho-Len nanoparticles were imaged by
high-angle annular dark-field scanning electron microscopy
(magnification, ×100). The size distribution of the GdCho-Len
nanoparticles was measured using a DynaPro NanoStar Dynamic Light
Scattering Detector (Wyatt Technology Corporation). The
nanoparticles contrast agent was visualized by a PET/CT system. The
GdCho and GdCho-Len contrast agents were intravenously injected
prior to PET/CT.
PET/CT
Static PET/CT with a GEMINI TF Big Bore PET/CT
system (Philips Medical Systems, Inc.) was used to evaluate
patients with suspected lung cancer. PET/CT was performed at 3 h
following the administration of GdCho-Len (0.4–4.0 mg/kg; 0.4 mg
interval). A low dose CT of 30 sec (mAs, 80–175; kV, 120; slice
thickness, 5 mm) was performed and CT images were set at a 512
matrix. The emission time per bed position ranged between 1 and 2
min based on the body mass index of individuals.
Detection of GdCho-Len in plasma
concentration
The serum concentration levels of Len were analyzed
using an ELISA kit (cat. no. FAB357P; R&D Systems, Inc.),
according to the manufacturer's protocol. The results were analyzed
using an ELISA reader system (1775×Mark™; Bio-Rad Laboratories,
Inc.).
Hematoxylin and eosin staining
Biopsies of lung tissues were obtained from
individuals following diagnosis by GdCho-Len-PET or GdCho-PET.
Sections 4-µm-thick were prepared, fixed with 10% paraformaldehyde
for 15 min at room temperature and stained with hematoxylin and
eosin for 30 min at room temperature. Sections were washed with PBS
three times and then observed under a light microscope (Olympus
Corporation; magnification, ×100).
Stability assay
GdCho-Len nanoparticles were placed at 4, 15, 25 and
37°C for 7 days. Stability of GdCho-Len was analyzed by high
performance size exclusion chromatography performed using a TSKgel
G3000SWxl column (Tosoh Bioscience) and an Agilent HPLC 1200 system
(Agilent Technologies Gmbh).
Statistical analysis
Statistical analyses were analyzed using SPSS 18.0
software (SPSS, Inc., Chicago, IL, USA). Data are presented as the
mean ± standard error of the mean. All experiments were repeated at
least three times. A receiver operator characteristic curve was
generated to determine the cut-off point that optimized sensitivity
and specificity. A paired Student's t-test was used to compare two
independent groups of data. Survival curves were constructed using
the Kaplan-Meier method and were compared using a log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characterization of GdCho-Len
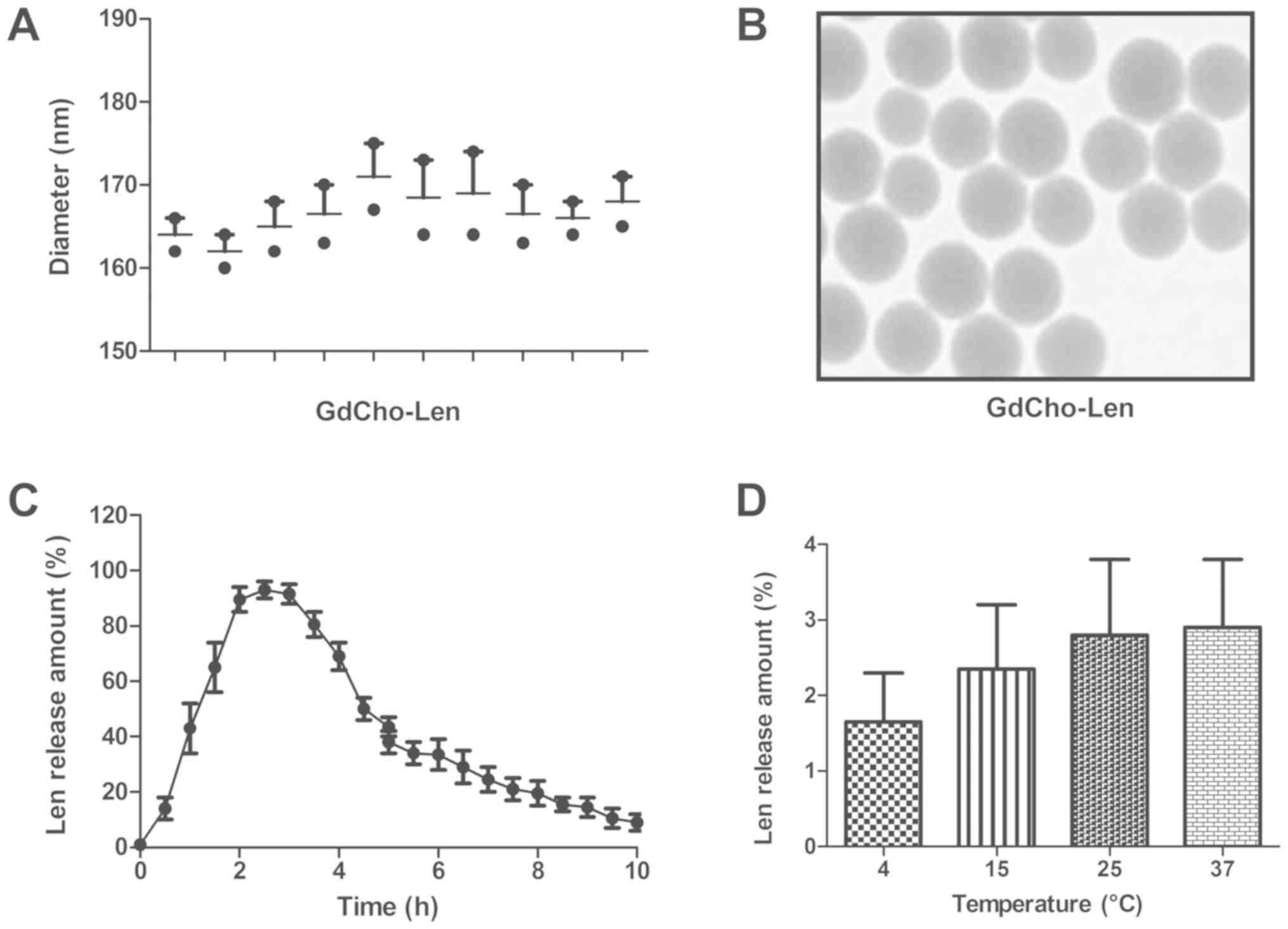
TEM revealed that the diameter of GdCho-Len was
168.2±6.8 nm (Fig. 1A). As presented
in Fig. 1B, GdCho-Len exhibited a
spherical and uniform shape. The in vitro release of Len
from the GdCho-Len was also investigated to determine its release
profile (Fig. 1C). The stability
assay demonstrated that GdCho-Len nanoparticles were stable
particles at 4, 15, 25 and 37°C for multiple laser irradiations
(Fig. 1D). These results indicate
the successful encapsulation of Len into the GdCho, and GdCho-Len
was demonstrated to be a stable nanoparticles contrast agent.
Diagnostic efficacy of GdCho-Len-PET
in patients with suspected lung cancer
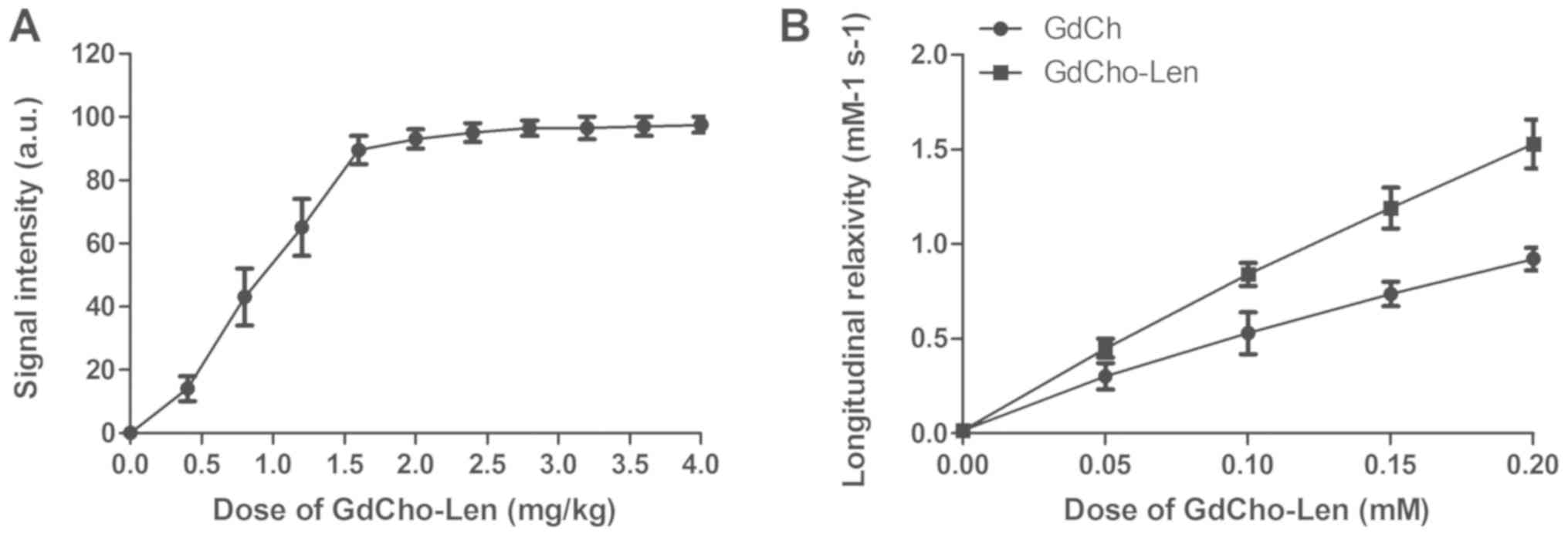
The diagnostic accuracy and sensitivity of
GdCho-Len-PET was investigated in patients with suspected lung
cancer. A clinical dose of GdCho-Len at 2.4 mg/kg was identified to
achieve the optimum signal intensity for PET/CT detection (Fig. 2A). GdCho-Len nanoparticles contrast
agent exhibited a markedly improved longitudinal relaxivity
compared with GdCho (Fig. 2B). The
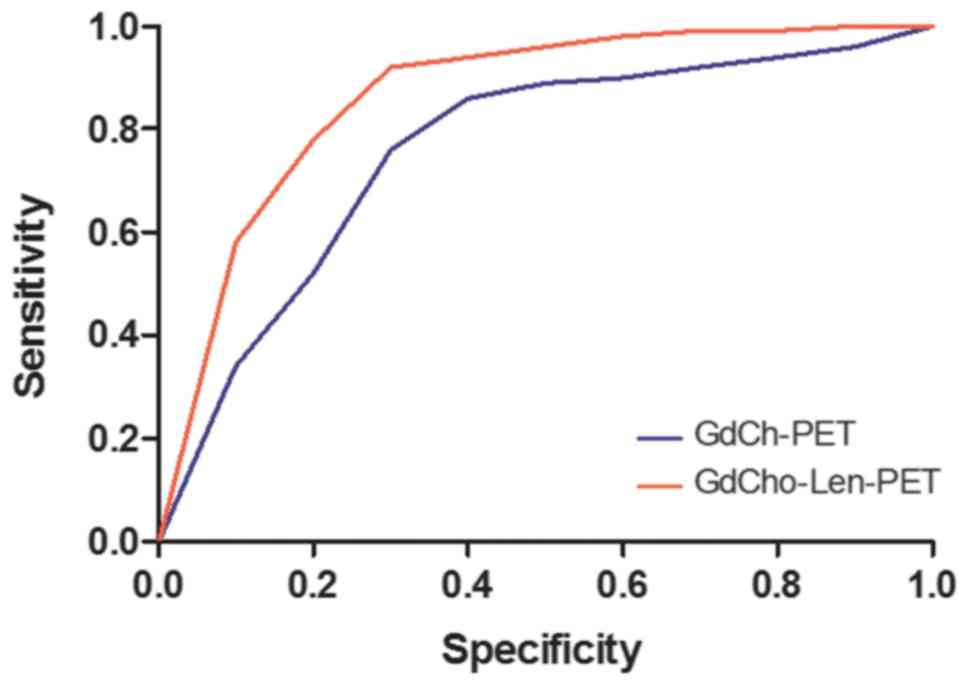
results indicated that GdCho-Len-PET diagnosed 152/172 patients
with lung cancer, while GdCho-PET diagnosed 130/172 patients with
lung cancer (Table II), and that
GdCho-Len-PET has higher accuracy and sensitivity compared with
GdCho-PET in diagnosing patients with lung cancer (Fig. 3).
 | Table II.Diagnostic outcomes of GdCho-Len-PET
and GdCho-PET. |
Table II.
Diagnostic outcomes of GdCho-Len-PET
and GdCho-PET.
| Presence of lung
cancer | GdCho-PET, n
(%) | GdCho-Len-PET, n
(%) | P-value |
|---|
| Lung cancer | 130 (75.6) | 152 (88.4) | 0.035 |
| No lung cancer | 42 (24.4) | 20 (11.6) | 0.023 |
Histopathological diagnoses of
patients with lung cancer
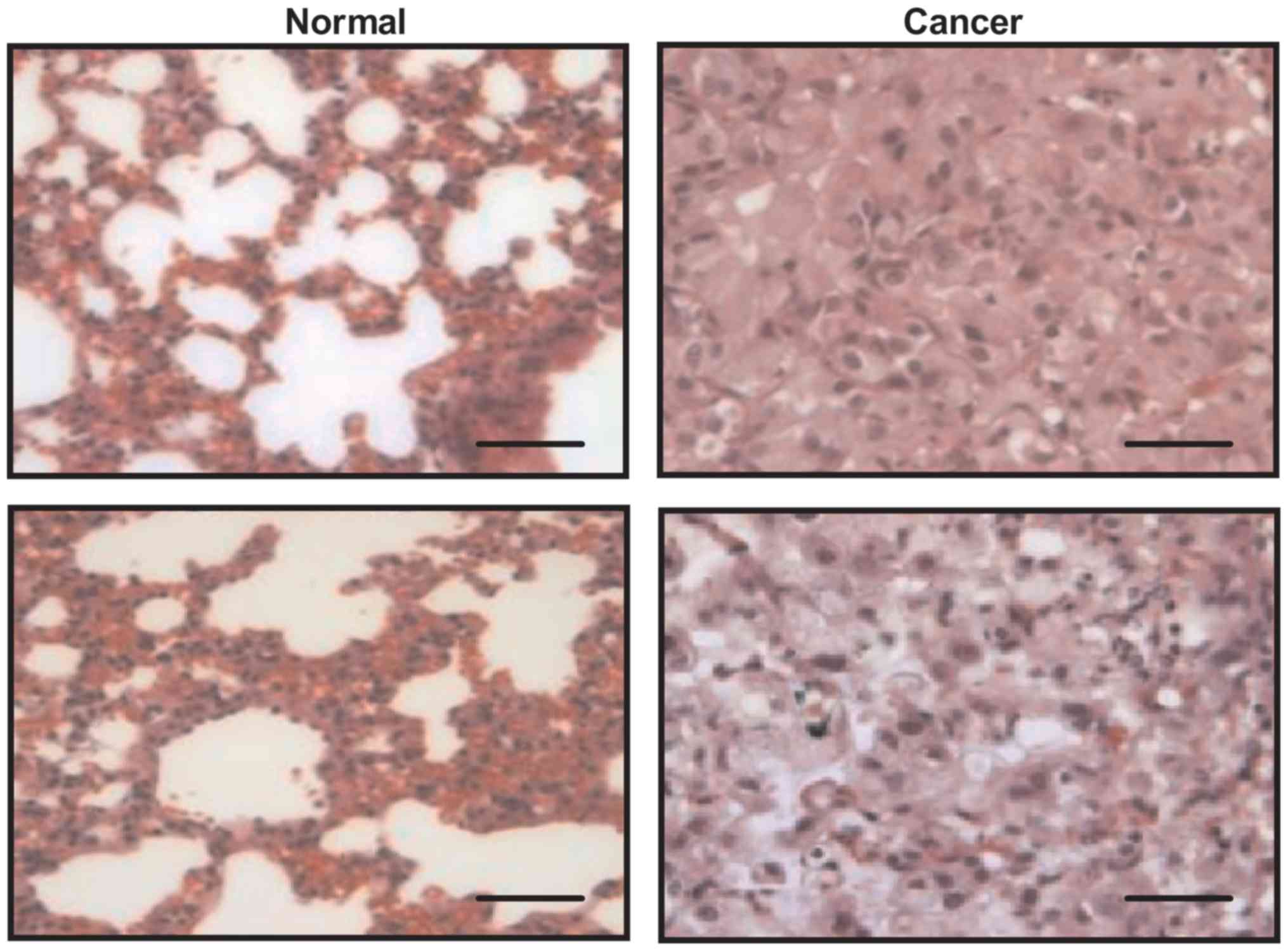
Immunohistochemistry was used to confirm the
diagnostic outcomes of GdCho-Len-PET. Fig. 4 presents representative cancer and
non-cancer tissues. Statistical analysis demonstrated that there
were 136 patients with lung cancer among 152 lung cancer patients
diagnosed by GdCho-Len-PET, and there were two lung cancer cases in
20 non-lung cancer cases diagnosed by GdCho-Len-PET (data not
shown).
Histopathological analyses revealed that there were
114 ‘true’ lung cancer cases in 130 lung cancer cases diagnosed by
GdCho-PET. This revealed that there were 102 patients with
confirmed lung cancer, as diagnosed by GdCho-PET. There were 21
patients with false positive cases as diagnosed by GdCho-Len-PET,
and 28 patients were false positive cases diagnosed by GdCho-PET.
In addition, there were five false negative cases diagnosed by
GdCho-Len-PET, while there were 34 false negative cases diagnosed
by GdCho-PET (Table III). These
outcomes indicate that GdCho-Len-PET exhibits higher accuracy
compared with GdCho-PET in diagnosing patients with lung
cancer.
 | Table III.Diagnostic efficacy of GdCho-Len-PET
for patients suspected of having lung cancer. |
Table III.
Diagnostic efficacy of GdCho-Len-PET
for patients suspected of having lung cancer.
| Result | GdCho-PET, n
(%) | GdCho-Len-PET, n
(%) | P-value |
|---|
| False positive | 28 (16.3) | 21 (12.2) | 0.030 |
| True positive | 102 (59.3) | 131 (76.2) | 0.017 |
| False negative | 34 (19.8) | 5 (2.9) | 0.001 |
| True negative | 8 (4.7) | 15 (8.7) | 0.0026 |
Plasma concentrations of GdCho-Len in
patients with lung cancer
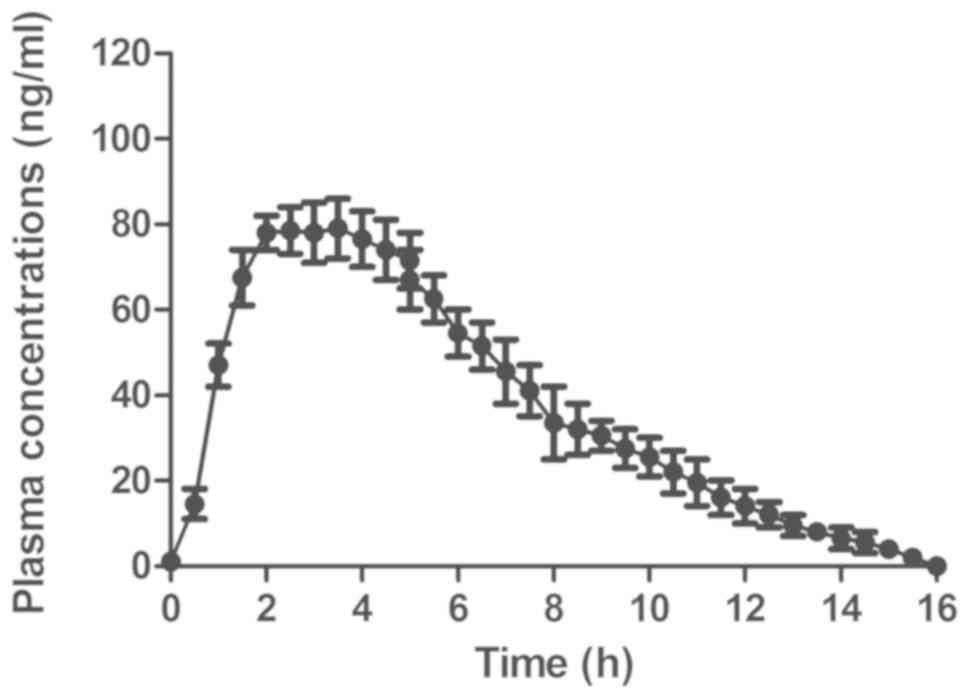
The pharmacodynamics of GdCho-Len was analyzed in
patients with lung cancer. The results revealed that GdCho-Len was
metabolized from the blood 16 h following injection (Fig. 5). The clinical data suggested that
GdCho-Len is a safe contrast agent when diagnosing patients with
lung cancer.
Outcomes for patients diagnosed by
GdCho-Len-PET
GdCho-Len-PET contributed to the anticancer
treatments in 56 out of 62 (90.3%) patients with lung cancer who
were candidates for radiation therapy, 52 out of 57 (91.2%)
patients undergoing adjuvant radiotherapy, and 13 out of 17 (76.5%)
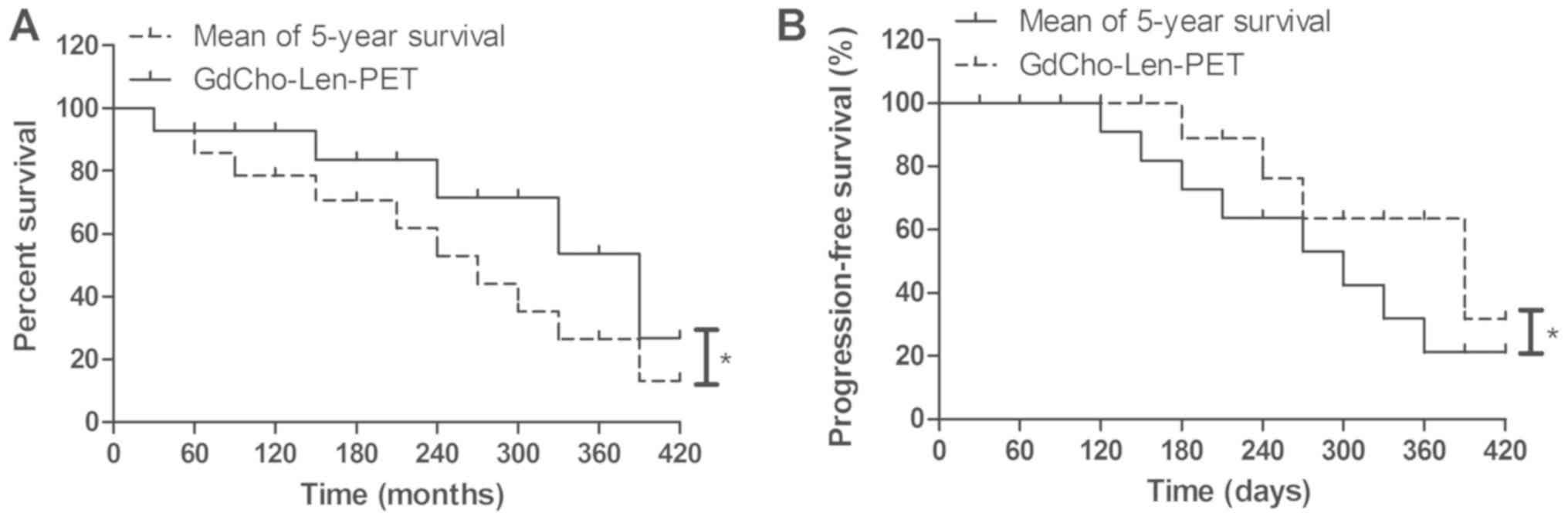
patients undergoing comprehensive therapy (Table IV). Patients diagnosed by
GdCho-Len-PET had a significantly improved mean overall survival
during the 420-day follow up (Fig.
6A). It was observed that GdCho-Len-PET-diagnosed patients
exhibited a significantly improved mean progression-free survival
compared with the mean 5-year survival (Fig. 6B). The results demonstrated that 82
patients were alive and tumor-free, 14 patients were still alive
with tumors, and 6 patients succumbed to the disease during the
420-day follow-up. These data suggested that patients with lung
cancer diagnosed by GdCho-Len-PET had longer median overall
survival times compared with the mean 5-year survival.
 | Table IV.Treatment of patients with lung
cancer diagnosed by GdCho-Len-PET. |
Table IV.
Treatment of patients with lung
cancer diagnosed by GdCho-Len-PET.
| Treatment | n (%) |
|---|
| Radiation
therapy | 56 (42.7) |
| Adjuvant
radiotherapy | 52 (39.7) |
| Comprehensive
therapy | 23 (17.6) |
Discussion
Lung cancer diagnosis is crucial for reducing
morbidity and increasing the quality of life of patients (24,31,32). An
early diagnosis of lung cancer may improve the administration of
timely anticancer treatments, including surgery, chemoradiotherapy
and immunotherapy, for patients with lung cancer, which can further
improve the overall survival and progression-free survival
(33–35). Clinically, PET/CT has been widely
used for diagnosing human lung cancer and evaluating metastatic
lesions (36). Previous studies have
indicated that contrast agent is useful in PET/CT scanning of human
lung cancer (37–39). In the present study, the nanoparticle
contrast agent GdCho-Len was administered and the diagnostic
efficacy of GdCho-Len-PET was investigated in a total of 172
patients with lung cancer. GdCho-Len-PET provided a 13.8% false
positive result in 152 cases. All cases excluded by GdCho-Len-PET
were patients without lung cancer. Taken together, the data
obtained in the present study indicates that GdCho-Len is a stable
and safe nanoparticle contrast agent for diagnosing patients with
lung cancer.
Contrast agent may increase the sensitivity and
accuracy of CT imaging for the diagnosis of early stage NSCLC
(23). A novel nano-sized
chistosan/Fe3O4-enclosed bispecific antibody
had been identified as an efficient contrast agent in lung cancer
diagnosis (40). However, a previous
study reported that a nonionic intravenous contrast agent did not
cause clinically significant improvement to 18F-FDG PET/CT in
patients with lung cancer (41).
Therefore, efficient nanoparticles contrast agent serves an
important role in diagnosing patients with lung cancer. In the
present study, successful encapsulation of Len into the GdCho was
achieved and GdCho-Len was produced, which was a stable
nanoparticle contrast agent. GdCho-Len exhibited an increased
accuracy and sensitivity when compared with GdCho-PET in diagnosing
patients suspected of having lung cancer. Indeed, the GdCho-Len
nanoparticles provided an improved resolution ratio for tumors than
GdCho due to the targeting of Len for tumor cells (42).
Apart from the intracellular environment of lung
tumor cells influencing the relaxivity of GdCho-Len, detection of
lung tumor cells was difficult to see on the imaging volume within
which these cells were embedded (43–45). The
present study indicated that the GdCho-Len allowed Len to
discriminate between lung cancer cells, which enhanced the
diagnostic sensitivity of PET/CT. Ideally, following detection of a
suspicious lesion on PET/CT, a plasma metabolic profile of contrast
agent could be used to evaluate the clinical safety of drugs
(46–48). The current study indicated that
GdCho-Len could be metabolized from blood 36 h post-injection. In
addition, GdCho-Len-PET contributed to the anticancer treatments
for patients with lung cancer, which further improved the median
overall survival and median progression-free survival compared with
the mean of 5-year survival. However, further studies that
investigate the effect GdCho-Len-PET on radiotherapy should be
performed with more patients with lung cancer in the future.
In conclusion, the present study is a clinical
report describing the characteristics of GdCho-Len and the
diagnostic efficacy of GdCho-Len-PET in patients with suspected
lung cancer. The results indicated that GdCho-Len-PET contributed
to the anticancer treatments and improved the survival of patients
with lung cancer. The results of the current study may aid the
diagnosis of lung cancer and the development of effective treatment
strategies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
TZ, DH and YL performed experiments. JZ, HYW and HGW
analyzed experimental data. ET and JY designed the current study
and wrote the manuscript.
Ethics approval and consent to
participate
The Ethical Committee of the Dongzhimen Hospital of
Beijing University of Traditional Chinese Medicine (Beijing, China)
approved the present study. Written informed consent was obtained
from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ridge CA and Boiselle PM: Optimizing the
lung cancer screening interval: The world is waiting. J Thorac Dis.
8:E1369–E1370. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xing P, Wang S, Hao X, Zhang T and Li J:
Clinical data from the real world: Efficacy of Crizotinib in
Chinese patients with advanced ALK-rearranged non-small cell lung
cancer and brain metastases. Oncotarget. 7:84666–84674. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gao L, Xie S, Liu H, Liu P, Xiong Y, Da J,
Que C, Dai H and Wang C: Lung cancer in patients with combined
pulmonary fibrosis and emphysema revisited with the 2015 World
Health Organization classification of lung tumors. Clin Respir J.
12:652–658. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhukovsky M, Varaksin A and Pakholkina O:
Statistical analysis of observational study of the influence of
radon and other risk factors on lung cancer incidence. Radiat Prot
Dosimetry. 160:108–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global Cancer Statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu B, Yuan M, Sun Y, Cheng Z, Zhang Z,
Hou S, Wang X and Liu J: Incidence and risk of hepatic toxicities
associated with anaplastic lymphoma kinase inhibitors in the
treatment of non-small-cell lung cancer: A systematic review and
meta-analysis. Oncotarget. 9:9480–9488. 2017.PubMed/NCBI
|
|
8
|
Leitinger M, Varosanec MV, Pikija S, Wass
RE, Bandke D, Weis S, Studnicka M, Grinzinger S, McCoy MR, Hauer L
and Sellner J: Fatal necrotizing encephalopathy after treatment
with nivolumab for squamous non-small cell lung cancer: Case report
and review of the literature. Front Immunol. 9:1082018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang JC, Mok T, Han B, Orlando M, Puri T
and Park K: A review of regimens combining pemetrexed with an
epidermal growth factor receptor tyrosine kinase inhibitor in the
treatment of advanced nonsquamous non-small-cell lung cancer. Clin
Lung Cancer. 19:27–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takamori S, Toyokawa G, Takada K, Shoji F,
Okamoto T and Maehara Y: Combination therapy of radiotherapy and
Anti-PD-1/PD-L1 treatment in non-small-cell lung cancer: A
Mini-review. Clin Lung Cancer. 19:12–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bamji-Stocke S, van Berkel V, Miller DM
and Frieboes HB: A review of metabolism-associated biomarkers in
lung cancer diagnosis and treatment. Metabolomics. 14:812018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vergnenègre A and Chouaïd C: Review of
economic analyses of treatment for non-small-cell lung cancer
(NSCLC). Expert Rev Pharmacoecon Outcomes Res. 18:519–528. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang X, Li M, Yang X, Zhao M, Huang Y, Dai
X, Jiang T, Feng M, Zhan C and Wang Q: Uniport versus multiport
video-assisted thoracoscopic surgery in the perioperative treatment
of patients with T1-3N0M0 non-small cell lung cancer: A systematic
review and meta-analysis. J Thorac Dis. 10:2186–2195. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suzuki H, Hyodo I and Hasegawa Y:
Prediction of decannulation, oral intake recovery, overall survival
and lung metastasis following oral malignant tumor resection and
reconstruction. Oncol Lett. 15:2686–2694. 2018.PubMed/NCBI
|
|
15
|
Deng Y, Yang Y, Yao B, Ma L, Wu Q, Yang Z,
Zhang L and Liu B: Paracrine signaling by VEGF-C promotes non-small
cell lung cancer cell metastasis via recruitment of
tumor-associated macrophages. Exp Cell Res. 364:208–216. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song W, Kuang J, Li CX, Zhang M, Zheng D,
Zeng X, Liu C and Zhang XZ: Enhanced immunotherapy based on
photodynamic therapy for both primary and lung metastasis tumor
eradication. ACS Nano. 12:1978–1989. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du Q, Yu R, Wang H, Yan D, Yuan Q, Ma Y,
Slamon D, Hou D, Wang H and Wang Q: Significance of
tumor-associated autoantibodies in the early diagnosis of lung
cancer. Clin Respir J. 12:2020–2028. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cormio A, Cormio G, Musicco C, Sardanelli
AM, Gasparre G and Gadaleta MN: Mitochondrial changes in
endometrial carcinoma: Possible role in tumor diagnosis and
prognosis (review). Oncol Rep. 33:1011–1018. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cherkashin M, Aniskhin M, Berezina N and
Puchkov D: CT and PET/CT fusion for lung cancer biopsy planning.
BMJ Case Rep. 2017(pii): bcr-2017-221972. 2017.PubMed/NCBI
|
|
20
|
Lim CG, Shin KM, Lim JS, Lim JK, Kim HJ,
Kim WH, Cho SH, Cha SI, Lee EB, Seock Y and Jeong SY: Predictors of
conversion to thoracotomy during video-assisted thoracoscopic
surgery lobectomy in lung cancer: Additional predictive value of
FDG-PET/CT in a tuberculosis endemic region. J Thorac Dis.
9:2427–2436. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gensheimer MF, Hong JC, Chang-Halpenny C,
Zhu H, Eclov NCW, To J, Murphy JD, Wakelee HA, Neal JW, Le QT, et
al: Mid-radiotherapy PET/CT for prognostication and detection of
early progression in patients with stage III non-small cell lung
cancer. Radiother Oncol. 125:338–343. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Q, Luo W, Zhao Y, Xu F and Zhou Q: The
utility of 18F-FDG PET/CT for the diagnosis of adrenal metastasis
in lung cancer: A PRISMA-compliant meta-analysis. Nucl Med Commun.
38:1117–1124. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan N, Zhang X, Cao Y, Jiang X, Zhao S,
Feng Y, Fan Y, Lu Z and Gao H: Contrast-enhanced computerized
tomography combined with a targeted nanoparticle contrast agent for
screening for early-phase non-small cell lung cancer. Exp Ther Med.
14:5063–5068. 2017.PubMed/NCBI
|
|
24
|
Li N, Han L and Jing H: Contrast-enhanced
ultrasound with a novel nanoparticle contrast agent for clinical
diagnosis in patients with non-small cell lung cancer. Exp Ther
Med. 14:3768–3773. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nicholls FJ, Rotz MW, Ghuman H, MacRenaris
KW, Meade TJ and Modo M: DNA-gadolinium-gold nanoparticles for in
vivo T1 MR imaging of transplanted human neural stem cells.
Biomaterials. 77:291–306. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiao L, Tian X, Harihar S, Li Q, Li L,
Welch DR and Zhou A: Gd2O3-doped silica @ Au
nanoparticles for in vitro imaging cancer biomarkers using
surface-enhanced Raman scattering. Spectrochim Acta A Mol Biomol
Spectrosc. 181:218–225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deng H, Chen F, Yang C, Chen M, Li L and
Chen D: Effect of Eu doping concentration on fluorescence and
magnetic resonance imaging properties of
Gd2O3:Eu3+ nanoparticles used as
dual-modal contrast agent. Nanotechnology. 29:4156012018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang H, Wang T, Zheng Y, Yan C, Gu W and
Ye L: Comparative toxicity and contrast enhancing assessments of
Gd2O3@BSA and MnO2@BSA
nanoparticles for MR imaging of brain glioma. Biochem Biophys Res
Commun. 499:488–492. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wirth LJ, Tahara M, Robinson B, Francis S,
Brose MS, Habra MA, Newbold K, Kiyota N, Dutcus CE, Mathias E, et
al: Treatment-emergent hypertension and efficacy in the phase 3
Study of (E7080) lenvatinib in differentiated cancer of the thyroid
(SELECT). Cancer. 124:2365–2372. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shao Y, Tian X, Hu W, Zhang Y, Liu H, He
H, Shen Y, Xie F and Li L: The properties of Gd2O3-assembled silica
nanocomposite targeted nanoprobes and their application in MRI.
Biomaterials. 33:6438–6446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wojcik E and Kulpa JK:
Pro-gastrin-releasing peptide (ProGRP) as a biomarker in small-cell
lung cancer diagnosis, monitoring and evaluation of treatment
response. Lung Cancer (Auckl). 8:231–240. 2017.PubMed/NCBI
|
|
32
|
Reck M and Rabe KF: Precision diagnosis
and treatment for advanced non-small-cell lung cancer. N Engl J
Med. 377:849–861. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou GH, Yang WH and Sun B: Clinical
impact of serum miR-661 in diagnosis and prognosis of non-small
cell lung cancer. Eur Rev Med Pharmacol Sci. 21:5696–5701.
2017.PubMed/NCBI
|
|
34
|
Labbé C, Anderson M, Simard S, Tremblay L,
Laberge F, Vaillancourt R and Lacasse Y: Wait times for diagnosis
and treatment of lung cancer: A single-centre experience. Curr
Oncol. 24:367–373. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tzouvelekis A, Spagnolo P, Bonella F,
Vancheri C, Tzilas V, Crestani B, Kreuter M and Bouros D: Patients
with IPF and lung cancer: Diagnosis and management. Lancet Respir
Med. 6:86–88. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Y, Jin G and Su D: Comparison of
Gadolinium-enhanced MRI and 18FDG PET/PET-CT for the diagnosis of
brain metastases in lung cancer patients: A meta-analysis of 5
prospective studies. Oncotarget. 8:35743–35749. 2017.PubMed/NCBI
|
|
37
|
Wang H, Machtaler S, Bettinger T, Lutz AM,
Luong R, Bussat P, Gambhir SS, Tranquart F, Tian L and Willmann JK:
Molecular imaging of inflammation in inflammatory bowel disease
with a clinically translatable dual-selectin-targeted US contrast
agent: Comparison with FDG PET/CT in a mouse model. Radiology.
267:818–829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Aschoff P, Plathow C, Beyer T, Lichy MP,
Erb G, Öksüz MÖ, Claussen CD and Pfannenberg C: Multiphase
contrast-enhanced CT with highly concentrated contrast agent can be
used for PET attenuation correction in integrated PET/CT imaging.
Eur J Nucl Med Mol Imaging. 39:316–325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hyafil F, Cornily JC, Rudd JH, Machac J,
Feldman LJ and Fayad ZA: Quantification of inflammation within
rabbit atherosclerotic plaques using the macrophage-specific CT
contrast agent N1177: A comparison with 18F-FDG PET/CT and
histology. J Nucl Med. 50:959–965. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gao J, Li L, Liu X, Guo R and Zhao B:
Contrast-enhanced magnetic resonance imaging with a novel nano-size
contrast agent for the clinical diagnosis of patients with lung
cancer. Exp Ther Med. 15:5415–5421. 2018.PubMed/NCBI
|
|
41
|
An YS, Sheen SS, Oh YJ, Hwang SC and Yoon
JK: Nonionic intravenous contrast agent does not cause clinically
significant artifacts to 18F-FDG PET/CT in patients with lung
cancer. Ann Nucl Med. 21:585–592. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nishio M, Horai T, Horiike A, Nokihara H,
Yamamoto N, Takahashi T, Murakami H, Yamamoto N, Koizumi F, Nishio
K, et al: Phase 1 study of lenvatinib combined with carboplatin and
paclitaxel in patients with non-small-cell lung cancer. Br J
Cancer. 109:538–544. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu Y, Zhang H, Xiang J, Mao Z, Shen G,
Yang F, Liu Y, Wang W, Du N, Zhang J and Tang Y: Ultrasensitive and
high specific detection of non-small-cell lung cancer cells in
human serum and clinical pleural effusion by aptamer-based
fluorescence spectroscopy. Talanta. 179:501–506. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cai NL, Lau ATY, Yu FY, Wu DD, Dai LJ, Mo
HY, Lin CM and Xu YM: Purification and characterization of a highly
specific polyclonal antibody against human extracellular
signal-regulated kinase 8 and its detection in lung cancer. PLoS
One. 12:e01847552017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sun Y, Liu S, Qiao Z, Shang Z, Xia Z, Niu
X, Qian L, Zhang Y, Fan L, Cao CX and Xiao H: Systematic comparison
of exosomal proteomes from human saliva and serum for the detection
of lung cancer. Anal Chim Acta. 982:84–95. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ohliger MA, von Morze C, Marco-Rius I,
Gordon J, Larson PEZ, Bok R, Chen HY, Kurhanewicz J and Vigneron D:
Combining hyperpolarized13 C MRI with a liver-specific
gadolinium contrast agent for selective assessment of hepatocyte
metabolism. Magn Reson Med. 77:2356–2363. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Uran S, Landmark K, Normann PT, Hals PA,
Toft KG and Skotland T: A respiration-metabolism chamber system and
a GC-MS method developed for studying exhalation of perfluorobutane
in rats after intravenous injection of the ultrasound contrast
agent Sonazoid. J Pharm Biomed Anal. 39:746–751. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Skotland T, Sontum PC and Oulie I: In
vitro stability analyses as a model for metabolism of ferromagnetic
particles (Clariscan), a contrast agent for magnetic resonance
imaging. J Pharm Biomed Anal. 28:323–329. 2002. View Article : Google Scholar : PubMed/NCBI
|