Introduction
In China, esophageal cancer (EC) is ranked sixth and
second for the number of reported cases and mortality, respectively
(1). Additionally, the most common
pathological type of EC is esophageal squamous cell carcinoma
(ESCC) (2). Of patients with EC, ~70%
are diagnosed with advanced and inoperable EC (3). Furthermore, even following treatment
with chemotherapy and definitive or neoadjuvant chemoradiotherapy,
the outcome remains poor, with a 5-year overall survival rate of
<15% (4). It is therefore
important to identify and develop novel therapies and treatments
for EC.
Recent research has focused on the role of the
epidermal growth factor receptor (EGFR) signaling pathways in the
progression of EC (5,6). EGFR is a prototypic cell-surface
receptor that belongs to the ErbB/HER oncogene family (7). Additionally, EGFR overexpression or
mutations have been reported to serve an important role in
tumorigenesis in various EC types (8), and also participate in the development
of resistance to chemotherapy and radiation (9,10). It has
been demonstrated that EGFR inhibitors alone may be used to treat a
number of tumor types, such treatments cause fewer side effects
compared with traditional chemotherapy (11,12), and
notably improve the local control rate when combined with
radiotherapy (13,14).
EGFR inhibitors, including small-molecule EGFR
tyrosine kinase inhibitors (TKIs) and monoclonal antibodies, are
utilized for clinical treatment of EC. TKIs, including gefitinib
and erlotinib, repress EGFR phosphorylation and inhibit downstream
signals of EGFR (15). Monoclonal
antibodies, including cetuximab and nimotuzumab, have the ability
to bind to the extracellular domain of EGFR to prevent EGFR
receptor dimerization and the activation of its intracellular
tyrosine kinase (16). These drugs
have been demonstrated to have a notable radiosensitizing effect
and are frequently administered in combination with radiotherapy,
whether during a short time period or simultaneously (17). However, the optimal delivery time for
EGFR inhibitors has not been determined and administration of these
inhibitors at the wrong time may reduce the effects of combined
therapy (18). In the present study,
the aim was to determine the optimal time for administration of
nimotuzumab to enhance its radiosensitizing effect in EC.
Materials and methods
Cell culture
Human Eca109 cells were obtained from the Shanghai
Institute of Biochemistry and Cell Biology (Shanghai, China) and
maintained at the Fujian Provincial Key Laboratory of Translational
Cancer Medicine (Fuzhou, China). Cells were cultured in RPMI-1640
containing 10% fetal calf serum (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), 100 U/ml penicillin and 100 µg/ml
streptomycin at 37°C in a humidified air-incubator with an
atmosphere containing 5% CO2. Notably, the Eca109 cell
line has been reported to be contaminated with cervical carcinoma
HeLa cells, as reported by Ye et al (19).
Small interfering RNAs (siRNAs)
siRNAs were transfected into cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
Briefly, cells were seeded in a 6-well plate at a density of
5×104 cells/well 24 h prior to transfection. siRNA
complexes were added to cells when cultures reached 50% confluence
at a final concentration of 50 nM in the absence of serum.
Following incubation at 37°C for 4 h, the culture medium (Opti-MEMI
low serum medium; cat. no. 31985-062) was replaced with 2 ml fresh
Opti-MEMI medium supplemented with 10% fetal bovine serum (both
Thermo Fisher Scientific, Inc.). Cells were cultured under standard
conditions (37°C) for a further 72 h before being examined by
western blot analysis.
A total of three different sequences of siRNA used
in the experiment, including EGFR siRNA1, EGFR
siRNA2 and EGFR siRNA3, which were designed
by Invitrogen; Thermo Fisher Scientific, Inc., to determine the
most effective RNA interference sequence. For the negative control
(NC) a random sequence siRNA(−) was used. NC siRNA(−) forward,
5′-CGUGAUUGCGAGACUCUGAdTdT-3′ and reverse,
3′-dTdTGCACUAACGCUCUGAGACU-5′, which were also obtained from Thermo
Fisher Scientific, Inc. (Invitrogen; Thermo Fisher Sientific,
Inc.). The siRNAs used were as follows: EGFR siRNA1
forward, 5′-UGAUCUGUCACCACAUAAUUACGGG-3′ and reverse,
3′-CCCGUAAUUAUGUGGUGACAGAUCA-5′; EGFR siRNA2 forward,
5′-UUAGAUAAGACUGCUAAGGCAUAGG-3′ and reverse,
3′-CCUAUGCCUUAGCAGUCUUAUCUAA-5′; and EGFR siRNA3
forward, 5′-UUUAAAUUCACCAAUACCUAUUCCG-3′ and reverse,
3′-CGGAAUAGGUAUUGGUGAAUUUAAA-5′.
Western blot analysis
Cells were seeded at a density of 1×103
cells/well in 3-well plates for 48 h and washed for 5 min three
times in ice-cold PBS. Protein was extracted using
radioimmunoprecipitation assay lysis buffer (Wuhan Boster
Biological Technology Co., Ltd., Wuhan, China). Total protein (20
µg/lane) was separated by 10% SDS-PAGE and transferred to a
polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA), followed by incubation with 10 ml 5% skim milk at room
temperature for 1 h. A primary antibody against EGFR (cat. no.
ab40815; 1:500; Abcam, Cambridge, UK) and β-tubulin (cat. no. 2128;
Cell Signaling Technology Inc., Danvers, MA, USA) was used as the
loading control at 4°C overnight. A horseradish
peroxidase-conjugated goat anti-rabbit IgG (cat. no. A0277;
1:2,500; Beyotime Institute of Biotechnology, Shanghai, China) was
used as the secondary antibody at room temperature for 2 h.
Subsequently, the coloration was completed by DAB (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). Images were captured with a
Bio-Rad Gel Doc XR and Quantity One v4.6.8 (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
MTT assay
Cells in the logarithmic-growth phase were cultured
in 96-well plates at a density of 1×105 cells/well in
triplicate. Following incubation for 24 h, nimotuzumab (Trinity
Biotech Plc, Beijing, China) was added at concentrations of 2,000,
1,000, 500, 250, 125 or 62.5 µg/ml. MTT (50 µl; Amresco, LLC,
Solon, OH, USA) was added following incubation at 37°C for 24, 48
or 72 h, followed by the addition of 150 µl dimethyl sulfoxide
(Sigma-Aldrich; Merck KGaA) into each well. A microplate reader was
used to determine the absorbance of the formed product at 570 nm
[optical density (OD)570]. Cell viability (%) was
calculated as follows:
(ODsample-ODblank)/(ODcontrol-ODblank)
×100. The IC50 was also calculated.
Radiation and colony formation
assay
Cells were seeded at a density of 6×105
cells/well in 3-well plates with a 60-mm diameter. Cultured cells
were divided into five groups: Control without any treatment (O
group); irradiation without nimotuzumab treatment (R group);
treatment with nimotuzumab 24 h prior to irradiation (24NR group);
nimotuzumab 24 h after irradiation (24RN group); and nimotuzumab
administered with irradiation simultaneously (NR group) Nimotuzumab
was administered at different doses, including 2,000, 1,000, 500,
250, 125 and 62.5 µg/ml. Cells were irradiated using a Synergy
linear accelerator (Siemens AG, Munich, Germany) at 6 MV exposure
with a source-skin distance of 100 cm at a rate of 3 Gy/min and a
field of 20×20 cm. Cells were irradiated with doses of 0, 1, 2, 4,
6 or 8 Gy, with three complex holes for each dose. Following
irradiation, cells were cultured at 37°C for 14 days. The number of
colonies with >50 cells was recorded by eye. The plating
efficiency (%) was determined as follows: (Number of
clones/inoculated cells) ×100. The surviving fraction (SF; %) was
determined as follows: (Colony formation rate of irradiated
cells/colony formation rate of control cells) ×100. Origin 7.5
software (OriginLab, Northampton, MA, USA) was used to calculate
the mean lethal dose (D0), quasi-threshold dose (Dq), SF and
sensitization enhancement ratio (SER). The SER of different groups
were analyzed at a dosage of 0.2 IC50 and 0.3
IC50.
Apoptosis and cell cycle distribution
analysis
Trypsin-digested (37°C for 3 min) Eca109 cells were
filtered to prepare a cell suspension. Annexin-V-fluorescein
isothiocyanate from Dead Cell Apoptosis kit with Annexin V FITC and
PI (eBioscience; Thermo Fisher Scientific, Inc.) was added to the
cell suspension for 30 min at 4°C for labeling. Following washing
with PBS for 5 min twice, propidium iodide or 7-aminoactinomycin D
staining solution (eBioscience; Thermo Fisher Scientific, Inc.) was
added at 4°C for 30 min, followed by immediate detection of
apoptosis in Eca109 cells using a flow cytometer (FACSCalibur™; BD
Biosciences, San Jose, CA, USA). The cell cycle was analyzed with
the DNA was labeled with nucleic acid dyes.
Statistical analysis
The data were presented mean ± standard error of the
mean. One-way analysis of variance followed by a least-significant
difference test was performed using SPSS version 17.0 software
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
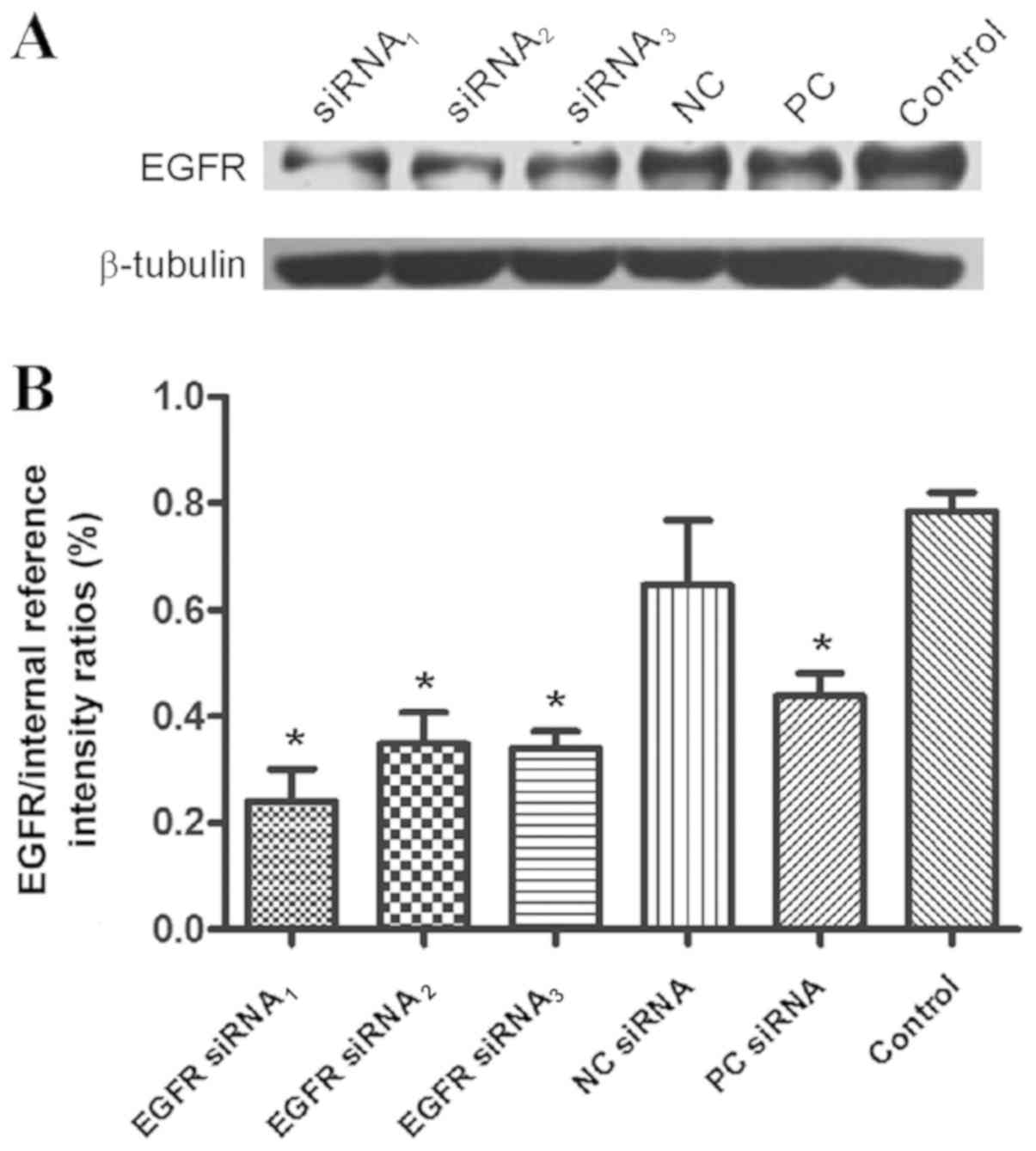
EGFR protein expression
A total of three different sequences of siRNA,
including EGFR siRNA1, EGFR siRNA2 and EGFR
siRNA3, were used to determine the most effective RNA
interference sequence. Additionally, NC was included. Subsequently,
the gray level of EGFR:NC was 0.7857±0.03581, compared with NC
(Fig. 1). The local expression of
EGFR was increased in Eca109 cells with siRNAs.
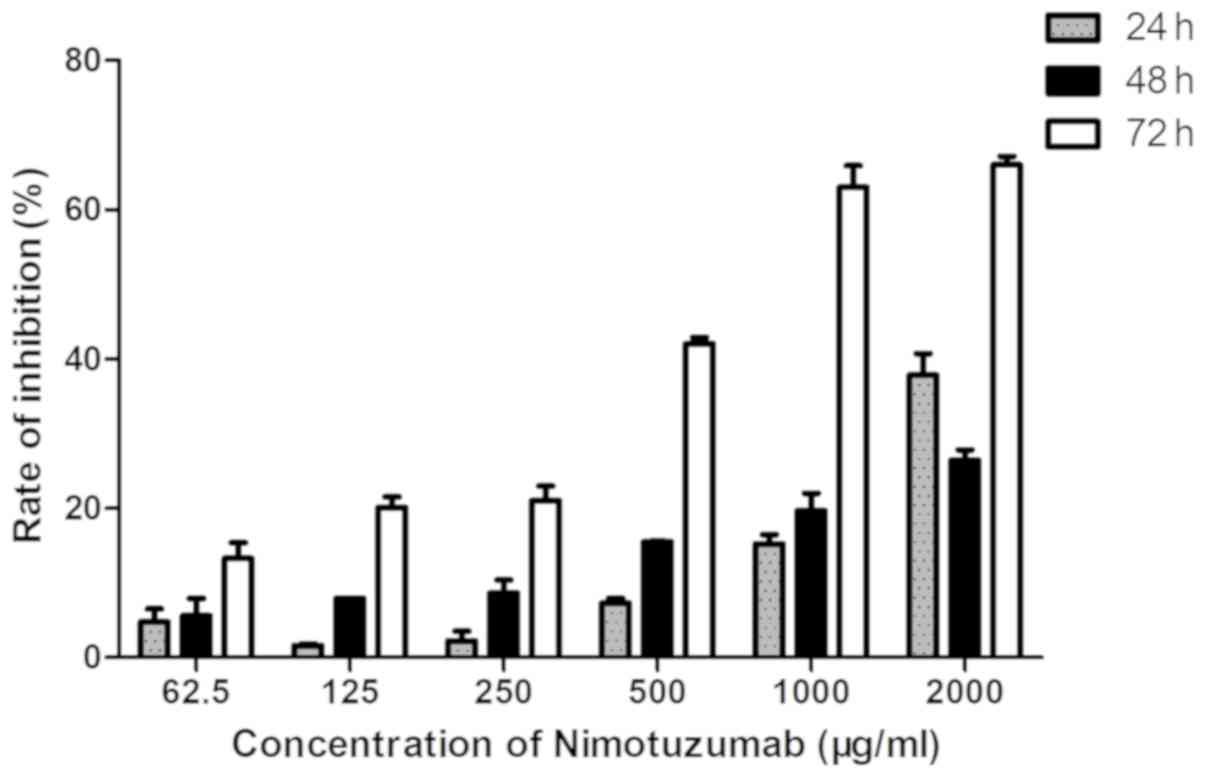
Changes in cell proliferation
Growth inhibition was observed in the cells
following exposure to nimotuzumab for 24 and 48 h; additionally,
there was growth inhibition following exposure to nimotuzumab for
72 h. The rate of inhibition was increased with the concentration
of nimotuzumab, which was notably increased when the concentration
was >250 µg/ml. The IC50 of nimotuzumab was
calculated as 768 µg/ml (Fig. 2).
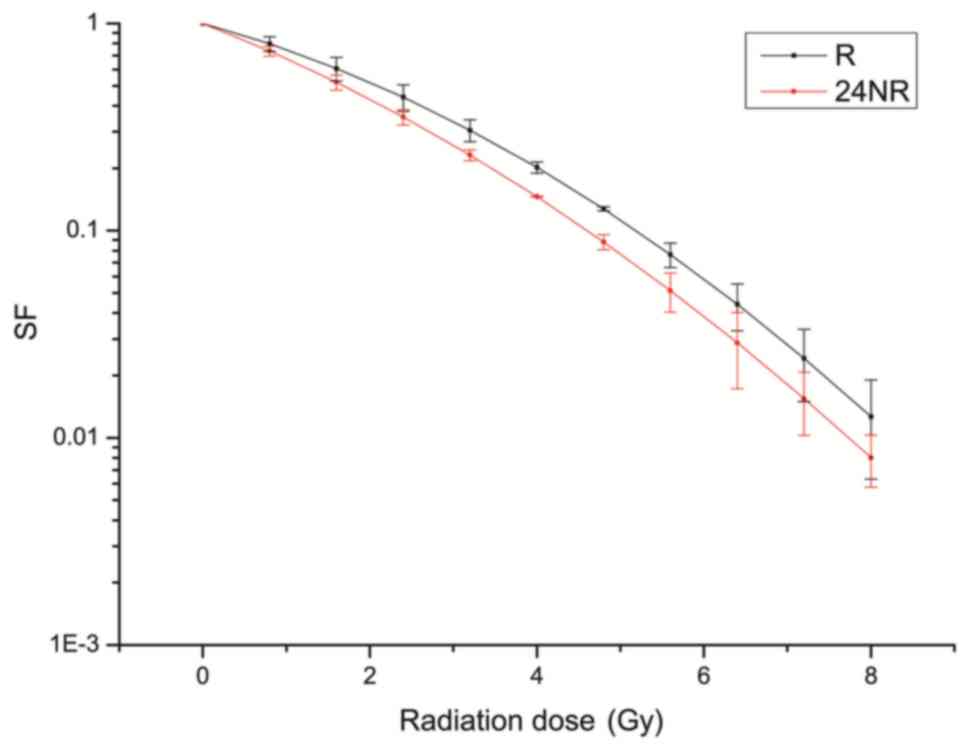
Colony formation assay
Radiation biology parameters are presented in
Table I. The SER of the 24NR, NR and
24RN groups were 1.09, 0.99 and 0.88, respectively, at a dosage of
0.2 IC50 (150 µg/ml). The SER of the 24NR, NR and 24RN
groups were 1.22, 1.04 and 0.98, respectively, at a dosage of 0.3
IC50 (200 µg/ml; Fig. 2).
At these concentrations, the 24NR group demonstrated the greatest
increase in radio sensitivity, compared with the other groups.
Additionally, treatment with increased doses of nimotuzumab
proportionally raised the radio sensitivity of cells. The survival
curve of the 24NR group at 0.3 IC50 demonstrated a
notable decrease in SF compared with the R group (Fig. 3). This colony formation assay
demonstrated that the 24NR group had reduced D0, compared with the
NR group.
 | Table I.Radiation biology parameters of
Eca109 cells fitted using a multi-target model. |
Table I.
Radiation biology parameters of
Eca109 cells fitted using a multi-target model.
| Drug doses,
µg/ml | Group | D0 | Dq | NR | SER |
|---|
|
| R | 1.65 | 3.75 | 2.27 | – |
| 150 | 24NR | 1.52 | 6.64 | 4.36 | 1.09 |
|
| NR | 1.66 | 2.93 | 1.76 | 0.99 |
|
| 24RN | 1.88 | 2.22 | 1.18 | 0.88 |
| 200 | 24NR | 1.35 | 3.24 | 2.39 | 1.22 |
|
| NR | 1.58 | 2.88 | 1.81 | 1.04 |
|
| 24RN | 1.68 | 3.36 | 2.0 | 0.98 |
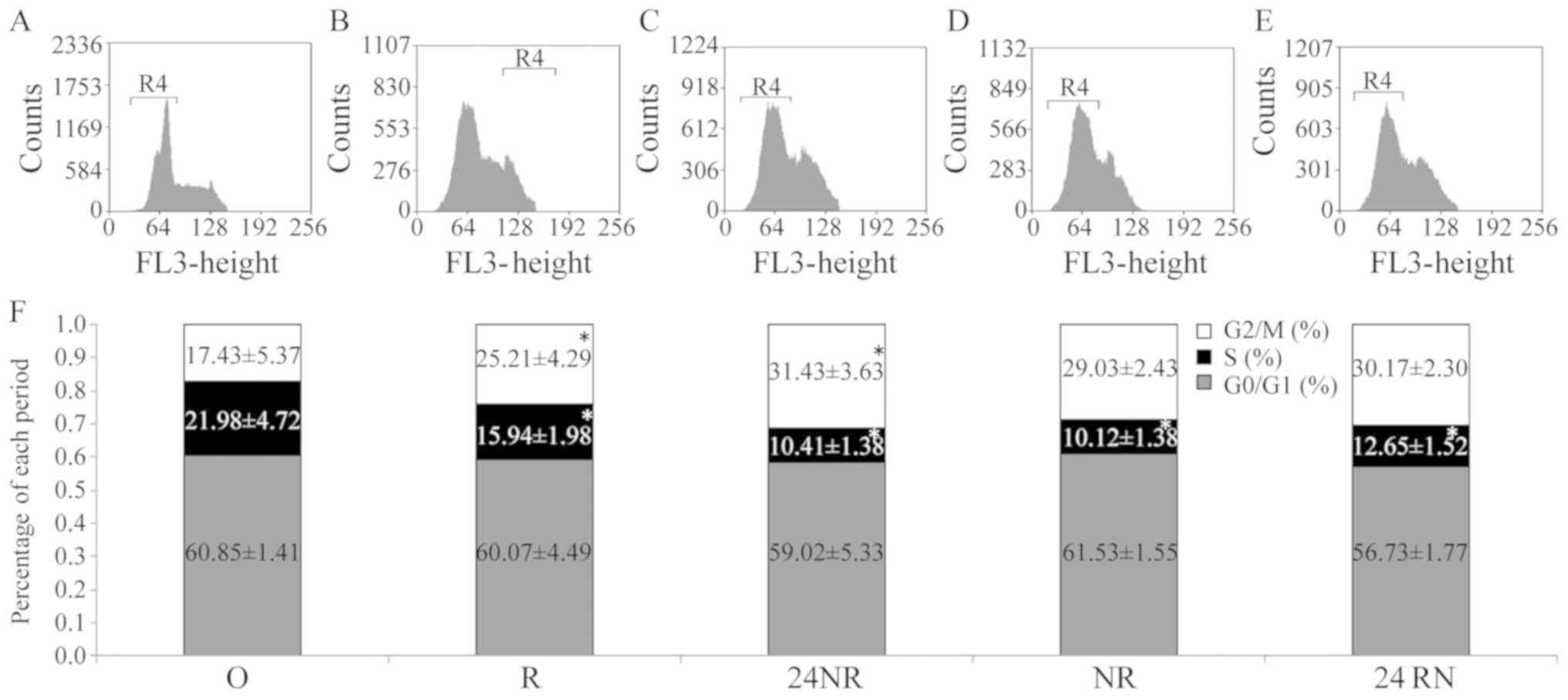
Cell cycle distribution
Cell cycle distribution for each group is depicted
in Fig. 4. The percentage of cells in
the G2/M phase in the R group was significantly
increased compared with that of the O control group, whereas the
percentage of S phase cells was significantly reduced (both
P<0.05). In the 24NR, NR and 24RN groups, the proportion of the
S-phase cells decreased significantly, while the proportion of
G2/M-phase cells increased compared with the O control
group (all P<0.05), indicating that nimotuzumab and radiation
exhibit a synergistic effect. Furthermore, the 24NR and NR groups
demonstrated significant differences in the proportion of S-phase
cells (P=0.041). Although an overall increase in
G2/M-phase cells was evident, no significant differences
were reported between the three groups (P=0.62). Additionally, the
24NR group had increased proportions of S and G2/M-phase
cells, compared with the 24RN or NR groups (P=0.53).
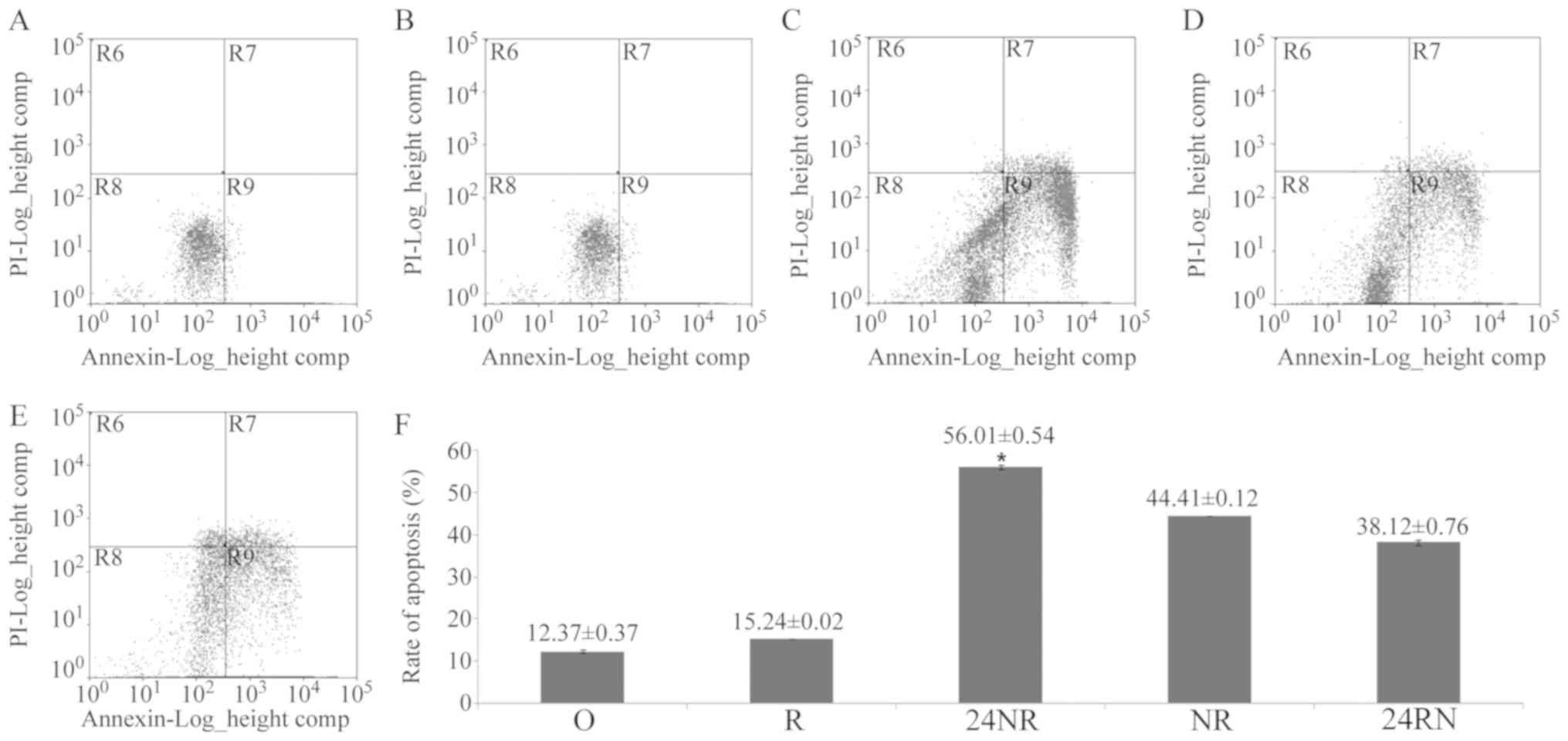
Cell apoptosis
Apoptosis rates for each group are depicted in
Fig. 5. Nimotuzumab enhanced
radiation-induced apoptosis in Eca109 cells at different delivery
times. A significantly increased apoptosis ratio was observed in
the 24NR (56.01±0.54; P=0.032), NR (44.41±0.12; P=0.025) and 24RN
(38.12±0.76; P=0.036) groups, compared with the R group
(15.24±0.02). The apoptosis ratio was significantly increased in
the 24NR group compared with NR (P=0.045) and 24RN (P=0.047)
groups.
Discussion
In the present study, it was determined that the
combination of EGFR inhibition and radiotherapy was more effective
compared with individual treatment, which is consistent with
another study (20). EGFR is an
epidermal receptor with tyrosine kinase activity, which is usually
over expressed in epithelial tumor types (21), including epithelial ovarian cancer
(22), and breast tumor cells
(23). Over expression of EGFR is
associated with poor overall survival and increased rates of tumor
recurrence, therefore it is an important target for treatment
(24). Previous research has
demonstrated that EGFR overexpression may regulate cell
proliferation and assist tumor cells in avoiding apoptosis
(25,26). Additionally, monoclonal antibodies
prevent the activation of this intracellular tyrosine kinase, thus
inhibiting cell proliferation (27).
Nimotuzumab is a humanized monoclonal antibody. It
blocks EGFR, and its downstream signals induce antibody-dependent
cell-mediated cytotoxicity, tumor cytotoxicity and effectively
stimulate EGFR internalization. Its anticancer properties have been
demonstrated in vitro, and clinical trials have demonstrated
that it is effective for treating tumor types of the head, neck and
brain (28–30). Thus far, nimotuzumab has been used to
treat >4,000 patients with milder side effects compared with
those of cetuximab (31), although
cetuximab has also been demonstrated to be more effective against
advanced non-small cell lung cancer (NSCLC) (32). Numerous clinical studies have
confirmed that treatment with EGFR inhibitors in combination with
other therapies is effective and improves the prognosis of patients
(33,34). For example, treatment with nimotuzumab
in combination with radiation and chemotherapy in head and neck
cancer demonstrated positive results (35). Ramos-Suzarte et al (34) performed a phase II clinical trial
where patients received radiotherapy, alone or combined with
nimotuzumab, and determined that the objective response (15.4% vs.
47.8%, respectively) and disease control (26.9% vs. 60.9%,
respectively) rates were increased in the combination treatment
group, compared with the control group.
The mechanisms of EGFR inhibitors include modifying
signal transduction to enhance cellular radiosensitivity, killing
cancer stem cells directly, inhibiting repair of DNA damage,
reducing repopulation and improving reoxygenation during
fractionated radiotherapy (35).
Although EGFR inhibitors have been studied extensively, the optimal
delivery time for EGFR inhibitors combined with radiation has not
been determined, particularly for EC.
In the present study, the effect of delivery time on
the effectiveness of EGFR inhibitors was investigated. A colony
formation assay demonstrated that the 24NR group reduced D0,
compared with the NR group. These results suggest that nimotuzumab
enhances the radiosensitivity of Eca109 cells when combined with
radiotherapy. The present study also demonstrated that the ratio of
S-phase cells in the 24NR group and NR group was significantly
reduced, compared with group O (P<0.05), and the proportion of
G2/M-phase cells was increased in the 24NR, NR and 24RN
groups, particularly the 24NR group. These results demonstrated
that nimotuzumab has a weak effect on cell cycle distribution, but
treatment with nimotuzumab 24 h prior to irradiation is most
effective. Cells exposed to nimotuzumab in combination with
radiation had an increased apoptosis ratio, compared with cells
treated with radiation only. Generally, the cell cycle stagnates at
the same phase to repair damage and prevent apoptosis (36). Radiation therapy arrested the cell
cycle at the G2/M phase, where multiple growth factors
would be required to repair it (37).
When the EGFR signal pathway is blocked by nimotuzumab, cells
undergo apoptosis as they lack the necessary growth factors
(38).
Western blotting demonstrated that EGFR is expressed
in Eca109 cells. A number of studies have determined that no
significant association between EGFR expression levels and the
antitumor effects of EGFR inhibitors (39–41).
Garrido et al (35) observed
that nimotuzumab selectively binds to cells with moderate to high
EGFR expression levels, and its antitumor capabilities decreased
proportionally with EGFR expression levels. In contrast, the
efficacy of cetuximab does not appear to depend on EGFR expression
levels. Zhao et al (40)
demonstrated that nimotuzumab enhances the radiation response and
increases the rate of radiation-mediated apoptosis in KYSE30 cells
that exhibit high EGFR activity; however, these effects were not
observed in TE-1 cells that exhibit low EGFR activity. Akashi et
al (41) examined the effects of
nimotuzumab combined with radiation therapy on human NSCLC cell
lines with different EGFR expression levels, and determined that
nimotuzumab enhances the effectiveness of radiation therapy in
human NSCLC cell lines with high levels of EGFR expression, in
vitro and in vivo. These trials demonstrated that the
radiosensitivity of nimotuzumab depends on the expression levels of
EGFR. Nimotuzumab and cetuximab are antibodies that inhibit ligand
binding upon interaction with EGFR, thereby indirectly inactivating
the EGFR kinase. Nimotuzumab has a reduced binding affinity for
EGFR, compared with cetuximab. In EGFR-overexpressing cells,
nimotuzumab inhibits EGFR-stimulated signaling and
ligand-independent basal signaling (42). Additionally, cetuximab is effective at
reduced concentrations (43).
Compared with the study by Yang et al (44), where different cell lines were used,
the present study used one cell line. The present study focused on
different delivery times in the mixed cancer Eca109 cell line,
which is comparative to the study by Yang et al (44). Additionally, Yang et al
(44) focused on the combined use of
h-R3 with cisplatin and fluorouracil and determined that the
sensitization effect of h-R3 on chemotherapy drugs is associated
with the expression level of EGFR in EC1 or EC9706 cells.
Furthermore, in the present study it was demonstrated that the
concentration of Nimotuzumab administered was directly proportional
to the increase in radiosensitivity of the cells (44). The present study demonstrated that
treatment with nimotuzumab in combination with radiation affected
cell cycle distribution, and enhanced radiation-induced apoptosis
and radiosensitivity in human Eca109 cells. The absence of RT-qPCR
data is one of the limitations of the present study. Additionally,
due to only one cell line being used in the present study, the
significance of these data is limited.
In conclusion, the present study demonstrated that
the most optimal radiosensitizing effect was observed in the ESCC
Eca109 cell line when nimotuzumab was delivered 24 h prior to
radiation. The concentration of nimotuzumab administered was
directly proportional to the increase in radiosensitivity.
Furthermore, due to the effects of nimotuzumab-induced
radiosensitization in vivo being more complicated than in
vitro, future studies should focus on in vivo
experiments to confirm the effects of nimotuzumab.
Acknowledgements
The authors would like to thank Professor Fuzhou
Chen (Fujian Provincial Tumor Hospital, Fuzhou, China) for critical
reading.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JL and LW conceived and designed the experiments.
LW, YS and JL performed the experiments. ZQ and JL analyzed the
data. ZQ and YS contributed reagents, materials and analysis
tools.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the Human
Development Index (2008–2030): A population-based study. Lancet
Oncol. 13:790–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baba Y, Saeki H, Nakashima Y, Oki E,
Shigaki H, Yoshida N, Watanabe M, Maehara Y and Baba H: Review of
chemotherapeutic approaches for operable and inoperable esophageal
squamous cell carcinoma. Dis Esophagus. 30:1–7. 2017.
|
|
3
|
Tamaki Y, Hieda Y, Nakajima M, Kitajima K,
Yoshida R, Yoshizako T, Ue A, Tokudo M, Hirahara N, Moriyama I, et
al: Concurrent chemoradiotherapy with docetaxel, cisplatin, and
5-fluorouracil improves survival of patients with advanced
esophageal cancer compared with conventional concurrent
chemoradiotherapy with cisplatin and 5-fluorouracil. J Cancer.
9:2765–2772. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fokas E, Weiss C and Rodel C: The role of
radiotherapy in the multimodal management of esophageal cancer. Diq
Dis. 31:30–37. 2013. View Article : Google Scholar
|
|
5
|
Wang Q, Zhu H, Xiao Z, Zhang W, Liu X,
Zhang X, He J, Sun K, Wang L and Xu N: Expression of epidermal
growth factor receptor is an independent prognostic factor for
esophageal squamous cell carcinoma. World J Surg Oncol. 11:2782013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abdo J, Agrawal DK and Mittal SK: Basis
for molecular diagnostics and immunotherapy for esophageal cancer.
Expert Rev Anticancer Ther. 17:33–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hong L, Han Y and Brain L: Epidermal
growth factor receptor: An important target in esophageal cancer.
Expert Opin Ther Targets. 17:1179–1185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sasada T, Azuma K, Ohtake J and Fujimoto
Y: Immune responses to epidermal growth factor receptor (EGFR) and
their application for cancer treatment. Front Pharmacol. 7:4052016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cohen RB: Current challenges and clinical
investigations of epidermal growth factor receptor (EGFR)- and ErbB
family-targeted agents in the treatment of head and neck squamous
cell carcinoma (HNSCC). Cancer Treat Rev. 40:567–577. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chi A, Remick S and Tse W: EGFR inhibition
in non-small cell lung cancer: Current evidence and future
directions. Biomark Res. 1:22013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cohen EE, Rosen F, Stadler WM, Recant W,
Stenson K, Huo D and Vokes EE: Phase II trial of ZD1839 in
recurrent or metastatic squamous cell carcimoma of the head and
neck. J Clin Oncol. 21:1980–1987. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan CM, Ma BB, Wong SC and Chan AT:
Celecoxib induces dose dependent growth inhibition in nasopharygeal
carcinoma cell lines independent of cyclooxygenase-2 expression.
Biomed Phamacother. 59:S268–S271. 2005. View Article : Google Scholar
|
|
13
|
Provencio M and Sánchez A: Therapeutic
integration of new molecule-targeted therapies with radiotherapy in
lung cancer. Transl Lung Cancer Res. 3:89–94. 2014.PubMed/NCBI
|
|
14
|
Chinnaiyan P, Huang S, Vallabhaneni G,
Armstrong E, Varambally S, Tomlins SA, Chinnaiyan AM and Harari PM:
Mechanisms of enhanced radiation response following epidermal
growth factor receptor signaling inhibition by erlotinib (Tarceva).
Cancer Res. 65:3328–3335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ku GY and Ilson DH: Emerging tyrosine
kinase inhibitors for esophageal cancer. Expert Opin Emerg Drugs.
18:219–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu M, Wang X, Shen L, Jia J, Gong J, Li J,
Li J, Li Y, Zhang X, Lu Z, et al: Nimotuzumab plus paclitaxel and
cisplatin as the first line treatment for advanced esophageal
squamous cell cancer: A single centre prospective phase II trial.
Cancer Sci. 107:486–490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Diaz-Miqueli A and Martinez GS:
Nimotuzumab as a radiosensitizing agent in the treatment of high
grade glioma: Challenges and opportunities. Onco Targets Ther.
6:931–942. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang T, Min W, Li Y, Yue Z, Wu C and Zhou
C: Radiotherapy plus EGFR TKIs in non-small cell lung cancer
patients with brain metastases: An update meta-analysis. Cancer
Med. 5:1055–1065. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye F, Chen C, Qin J, Liu J and Zheng C:
Genetic profiling reveals an alarming rate of cross-contamination
among human cell lines used in China. FASEB J. 29:4268–4272. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Crombet T, Osorio M, Cruz T, Roca C, del
Castillo R, Mon R, Iznaga-Escobar N, Figueredo R, Koropatnick J,
Renginfo E, et al: Use of the humanized anti-epidermal growth
factor receptor monoclonal antibody h-R3 in combination with
radiotherapy in the treatment of locally advanced head and neck
cancer patients. J Clin Oncol. 22:1646–1654. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mukhopadhyay C, Zhao X, Maroni D, Band V
and Naramura M: Distinct effects of EGFR ligands on human mammary
epithelial cell differentiation. PLoS One. 8:e759072013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou X, Hu Y, Dai L, Wang Y, Zhou J, Wang
W, Di W and Qiu L: MicroRNA-7 inhibits tumor metastasis and
reverses epithelial-mesenchymal transition through AKT/ERK1/2
inactivation by targeting EGFR in epithelial ovarian cancer. PLoS
One. 9:e967182014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Andrade SS, Sumikawa JT, Castro ED,
Batista FP, Paredes-Gamero E, Oliveira LC, Guerra IM, Peres GB,
Cavalheiro RP, Juliano L, et al: Interface between breast cancer
cells and the tumor microenvironment using platelet-rich plasma to
promote tumor angiogenesis-influence of platelets and fibrin
bundles on the behavior of breast tumor cells. Oncotarget.
8:16851–16874. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Navarini D, Gurski RR, Madalosso CA, Aita
L, Meurer L and Fornari F: Epidermal growth factor receptor
expression in esophageal adenocarcinoma: Relationship with tumor
stage and survival after esophagectomy. Gastroenterol Res Pract.
2012:9419542012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Szabó B, Nelhubel GA, Kárpáti A, Kenessey
I, Jóri B, Székely C, Peták I, Lotz G, Hegedus Z, Hegedus B, et al:
Clinical significance of genetic alterations and expression of
epidermal growth factor receptor (EGFR) in head and neck squamous
cell carcinomas. Oral Oncol. 47:487–496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park HS, Jang MH, Kim EJ, Kim HJ, Lee HJ,
Kim YJ, Kim JH, Kang E, Kim SW, Kim IA and Park SY: High EGFR gene
copy number predicts poor outcome in triple-negative breast cancer.
Mod Pathol. 27:1212–1222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ayyappan S, Prabhakar D and Sharma N:
Epidermal growth factor receptor (EGFR)-targeted therapies in
esophagogastric cancer. Anticancer Res. 33:4139–4155.
2013.PubMed/NCBI
|
|
28
|
Wang Y, Pan L, Sheng XF, Chen S and Dai
JZ: Nimotuzumab, a humanized monoclonal antibody specific for the
EGFR, in combination with temozolomide and radiation therapy for
newly diagnosed glioblastoma multiforme: First results in Chinese
patients. Asia Pac J Clin Oncol. 12:e23–e29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nitta Y, Shimizu S, Shishido-Hara Y,
Suzuki K, Shiokawa Y and Nagane M: Nimotuzumab enhances
temozolomide-induced growth suppression of glioma cells expressing
mutant EGFR in vivo. Cancer Med. 5:486–499. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chong DQ, Toh XY, Ho IA, Sia KC, Newman
JP, Yulyana Y, Ng WH, Lai SH, Ho MM, Dinesh N, et al: Combined
treatment of Nimotuzumab and rapamycin is effective against
temozolomide-resistant human gliomas regardless of the EGFR
mutation status. BMC Cancer. 15:2552015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boland WK and Bebb G: Nimotuzumab: A novel
anti-EGFR monoclonal antibody that retains anti-EGFR activity while
minimizing skin toxicity. Expert Opin Biol Ther. 9:1199–1206. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gridelli C, Maione P, Ferrara ML and Rossi
A: Cetuximab and other anti-epidermal growth factor receptor
monoclonal antibodies in the treatment of non-small cell lung
cancer. Oncologist. 14:601–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Basavaraj C, Sierra P, Shivu J, Melarkode
R, Montero E and Nair P: Nimotuzumab with chemoradiation confers a
survival advantage in treatment-naïve head and neck tumors over
expressing EGFR. Cancer Biol Ther. 10:673–681. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ramos-Suzarte M, Lorenzo-Luaces P, Lazo
NG, Perez ML, Soriano JL, Gonzalez CE, Hernadez IM, Albuerne YÁ,
Moreno BP, Alvarez ES, et al: Treatment of malignant,
non-resectable, epithelial origin esophageal tumours with the
humanized anti-epidermal growth factor antibody nimotuzumab
combined with radiation therapy and chemotherapy. Cancer Biol Ther.
13:600–605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baumann M, Krause M, Dikomey E, Dittmann
K, Dörr W, Kasten-Pisula U and Rodemann HP: EGFR-targeted
anti-cancer drugs in radiotherapy: Preclinical evaluation of
mechanisms. Radiother Oncol. 83:238–248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shaltiel IA, Krenning L, Bruinsma W and
Medema RH: The same, only different -DNA damage checkpoints and
their reversal throughout the cell cycle. J Cell Sci. 128:607–620.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fan QW and Weiss WA: RNA interference
against a glioma-derived allele of EGFR induces blockade at G2M.
Oncogene. 24:829–837. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin S, Yan Y, Liu Y, Gao CZ, Shan D, Li Y
and Han B: Sensitisation of human lung adenocarcinoma A549 cells to
radiotherapy by Nimotuzumab is associated with enhanced apoptosis
and cell cycle arrest in the G2/M phase. Cell Biol Int. 39:146–151.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Garrido G, Tikhomirov IA, Rabasa A, Yang
E, Gracia E, Iznaga N, Fernández LE, Crombet T, Kerbel RS and Pérez
R: Bivalent binding by intermediate affinity of nimotuzumab: A
contribution to explain antibody clinical profile. Cancer Biol
Ther. 11:373–382. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao L, He LR, Xi M, Cai MY, Shen JX, Li
QQ, Liao YJ, Qian D, Feng ZZ, Zeng YX, et al: Nimotuzumab promotes
radiosensitivity of EGFR-overexpression esophageal squamous cell
carcinoma cells by upregulating IGFBP-3. J Transl Med. 10:2492012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Akashi Y, Okamoto I, Iwasa T, Yoshida T,
Suzuki M, Hatashita E, Yamada Y, Satoh T, Fukuoka M, Ono K and
Nakagawa K: Enhancement of the antitumor activity of ionising
radiation by nimotuzumab, a humanised monoclonal antibody to the
epidermal growth factor receptor, in non-small cell lung cancer
cell lines of differing epidermal growth factor receptor status. Br
J Cancer. 98:749–755. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu H, Yang W, Gao H, Jiang T, Gu B, Dong
Q, Xu W, Wu S and Sun X: Nimotuzumab abrogates acquired
radioresistance of KYSE-150R esophageal cancer cells by inhibiting
EGFR signaling and cellular DNA repair. Onco Targets Ther.
8:509–518. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Berger C, Krengel U, Stang E, Moreno E and
Madshus IH: Nimotuzumab and cetuximab block ligand-independent egf
receptor signaling efficiently at different concentrations. J
Immunother. 34:550–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang X, Ji Y, Kang X, Chen M, Kou W, Jin C
and Lu P: Study on chemotherapeutic sensitizing effect of
nimotuzumab on different human esophageal squamous carcinoma cells.
Oncol Lett. 11:973–978. 2016. View Article : Google Scholar : PubMed/NCBI
|