Introduction
Lung cancer is one of the leading causes of
cancer-associated mortality globally, with a poor prognosis and a
5-year survival rate of <10% in patients with advanced-stage
cancer, according to an international surveillance published in
2016 (1). Recent advancements in
molecular targeted therapies for oncogenic driver mutations of
advanced non-small cell lung cancer (NSCLC) have improved the
prognosis in those individuals with tumors that express the
appropriate molecular targets for inhibitory agents (2). However, the majority of patients with
advanced NSCLC do not possess any molecular aberrations that can be
targeted by any current agents. Therefore, further studies are
required to identify and establish novel agents and concepts for
molecular targeted therapy.
Antibody-mediated blockade of the interaction
between programmed cell death-1 (PD-1) and activated cytotoxic T
lymphocytes (CTLs), and between programmed cell death ligand-1
(PD-L1) and tumor cells, has exhibited significant clinical
efficacy in a number of types of cancer, including NSCLC.
Antibody-mediated blockade inactivates the tumoricidal activity of
CTLs and therefore allows tumor cell immune evasion. Immune
checkpoint inhibitors (ICIs) nivolumab, pembrolizumab and
atezolizumab are currently approved for treating advanced-stage
NSCLC. The CheckMate-017 (3),
CheckMate-057 (4), KEYNOTE-010
(5) and OAK (6) trials demonstrated the superiority of
these agents over docetaxel, which was the standard care for
second-line therapy. However, the response to ICIs is only ~20%. In
immunohistochemistry, despite the fact that PD-L1 has been approved
as a biomarker, it is not sufficient for predicting the response to
ICIs.
Efficacy of ICIs could be influenced not only by the
intrinsic factors of patients, but also by extrinsic factors.
Increasing focus has been placed on the role of gut microbiota in
shaping systemic immune responses (7–9).
Antibiotics cause changes in the gut microbiota (10–12) that
may influence the efficacy of ICIs (13,14). A
recent study indicated that prior use of antibiotics negatively
influenced the efficacy of ICIs in the clinical settings (15). Using a prospective observational
database, the present study performed a retrospective analysis to
examine the influence of antibiotics on the clinical outcomes of
patients treated with nivolumab for advanced NSCLC.
Materials and methods
Database acquisition
Clinical data from 90 patients with advanced NSCLC
were retrospectively analyzed. Patients were treated with nivolumab
as the second or later line of chemotherapy at the Tokyo
Metropolitan Cancer and Infectious Diseases Center Komagome
Hospital (Tokyo, Japan) between January 2016 and April 2017. The
database of a prospective observational study [University hospital
Medical Information Network (UMIN) registry: UMIN000021694] was
used. The following clinical factors of the patients were examined:
Age, sex, Eastern Cooperative Oncology Group performance status
(ECOG-PS) (16), histological
subtype, oncogenic driver mutation status (EGFR mutations and
anaplastic lymphoma kinase gene rearrangement),
Tumor-Node-Metastasis (TNM) staging (1), lines of chemotherapy, use of
antibiotics, use of proton pump inhibitors (PPIs) or histamine
H2-blockers (H2B) and use of
antiflatulents.
Patients treated with antibiotics for ≥3 days within
30 days of nivolumab therapy were defined as those who were treated
with antibiotics, regardless of the spectrums or the dosages of the
antibiotics, the administration routes (intravenous or oral) or the
purpose of antibiotic use. The same criteria were employed for
defining patients who used PPIs or H2B, and
antiflatulents.
Statistical analysis
Descriptive statistics were used to summarize the
baseline characteristics of the patients. Progression-free survival
(PFS) time was defined as the period from the date of initial
nivolumab administration to the date of clinical disease
progression, mortality from any cause or the last follow-up.
Overall survival (OS) time was defined as the period from the date
of initial nivolumab administration to the date of mortality from
any cause or the last follow-up. The Kaplan-Meier method was used
to assess PFS and OS time. Data of patients who were lost to
follow-up were censored at the time of last contact. The log-rank
test was used for identifying prognostic indicators using
univariate and multivariate analyses. The candidate variables
analyzed included ECOG-PS, driver mutations, use of antibiotics,
use of PPIs or H2B, and use of antiflatulents. P<0.05
using the Cox proportional hazard model was considered to indicate
a statistically significant difference. All statistical analyses
were performed using the JMP 11.0 software (SAS Institute, Inc.,
Cary, NC, USA).
The study protocol was approved by the Ethics
Committee of the Tokyo Metropolitan Cancer and Infectious diseases
Center Komagome Hospital (approval no., 1469) and was conducted
according to the Declaration of Helsinki. The study was registered
with the UMIN Clinical Trials Registry (ID no., UMIN000021694).
Results
Baseline characteristics
A total of 90 patients with NSCLC (57 male and 33
female) were treated with nivolumab as the second or later line of
chemotherapy. All patients were treated with nivolumab monotherapy
at the recommended dose (2 mg/kg, day 1, every 2 weeks). The median
age of the patients was 68 years (range, 36–87 years). At the time
of nivolumab initiation, according to 8th Edition of TNM
Classification for Lung Cancer, 12 patients (13.3%) presented with
stage IVA disease, 38 (42.2%) with stage IVB disease and 40 (44.4%)
with recurrent disease. Overall, 55 patients (61.1%) had
adenocarcinoma and 21 (23.3%) had squamous cell carcinoma. A total
of 21 patients (23.3%) exhibited oncogenic driver mutations. During
the 30 days prior to nivolumab therapy, 13 patients (14.4%) were
treated with antibiotics, 47 (52.2%) with PPIs or H2B,
and 11 (12.2%) with antiflatulents. Other patient characteristics
are presented in Table I. The details
of the patients with prior antibiotic use are summarized in
Table II.
 | Table I.Baseline characteristics of enrolled
patients divided into those treated with (n=13) and without (n=77)
antibiotics. |
Table I.
Baseline characteristics of enrolled
patients divided into those treated with (n=13) and without (n=77)
antibiotics.
| Characteristics | Abx+
group | Abx−
group |
|---|
| Median age (range),
years | 67 (47–78) | 68 (36–87) |
| Sex, n (%) |
|
|
|
Male | 9 (69.2) | 48 (62.3) |
|
Female | 4 (30.8) | 29 (37.7) |
| ECOG-PS, n (%) |
|
|
|
0/1 | 4 (30.8) | 60 (77.9) |
| 2 | 3 (23.1) | 10 (13.0) |
| 3 | 6 (46.2) | 7 (9.1) |
| Histological
subtypes, n (%) |
|
|
|
Adenocarcinoma | 11 (84.6) | 44 (57.1) |
|
SQC | 2 (15.4) | 19 (24.7) |
| NSCLC,
NOS | 0 (0.0) | 9 (11.7) |
|
ADSQC | 0 (0.0) | 2 (2.6) |
|
LCNEC | 0 (0.0) | 2 (2.6) |
|
NEC | 0 (0.0) | 1 (1.3) |
| Driver mutations, n
(%) |
|
|
|
None | 12 (92.3) | 57 (74.0) |
| EGFR
exon19 del | 0 (0.0) | 6 (7.8) |
| EGFR
exon20 | 0 (0.0) | 1 (1.3) |
| EGFR
exon21 L861Q | 0 (0.0) | 1 (1.3) |
| EGFR
exon21 L858R | 1 (7.7) | 10 (13.0) |
|
KRAS | 0 (0.0) | 1 (1.3) |
|
ROS-1 | 0 (0.0) | 1 (1.3) |
| Staging, n (%) |
|
|
|
IVA | 3 (23.1) | 9 (11.7) |
|
IVB | 6 (46.2) | 32 (41.6) |
|
Recurrent | 4 (30.8) | 36 (46.8) |
| Median
number of chemotherapy lines (range) | 2 (2–5) | 2 (2–5) |
| Use of PPIs or
H2Bs, n (%) |
|
|
|
Yes | 12 (92.3) | 35 (45.5) |
| No | 1 (7.7) | 42 (54.5) |
| Use of
antiflatulents, n (%) |
|
|
|
Yes | 4 (30.8) | 7 (9.1) |
| No | 9 (69.2) | 70 (90.9) |
 | Table II.Cases of antibiotic use prior to
nivolumab therapy (n=13). |
Table II.
Cases of antibiotic use prior to
nivolumab therapy (n=13).
| Patient no. | Reasons for Abx
use | Duration, days | Types of Abx | Administration
routes |
|---|
| 1 | Prophylaxis
(steroid use) | 8 | TMP/SMX | Oral |
| 2 | Prophylaxis
(steroid use) | 22 | TMP/SMX | Oral |
| 3 | Prophylaxis
(steroid use) | 31 | TMX/SMX | Oral |
| 4 | Prophylaxis
(steroid use) | 35 | TMX/SMX | Oral |
| 5 | Lung infection | 11 | AMPC/CVA | Oral |
| 6 | Lung infection | 13 | CTRX, MEPM | Intravenous |
| 7 | Lung infection | 14 | AMPC/CVA | Oral |
| 8 | Lung infection | 18 | PIPC/TAZ | Intravenous |
| 9 | Obstructive
pneumonia | 10 | ABPC/SBT | Intravenous |
| 10 | Obstructive
pneumonia | 60 | AMPC/CVA | Oral |
| 11 | Pyelonephritis | 21 | CEZ, TMP/SMX | Oral |
| 12 | Fever | 5 | LVFX | Oral |
| 13 | Fever | 10 | AMPC/CVA | Oral |
Clinical outcomes of nivolumab
therapy
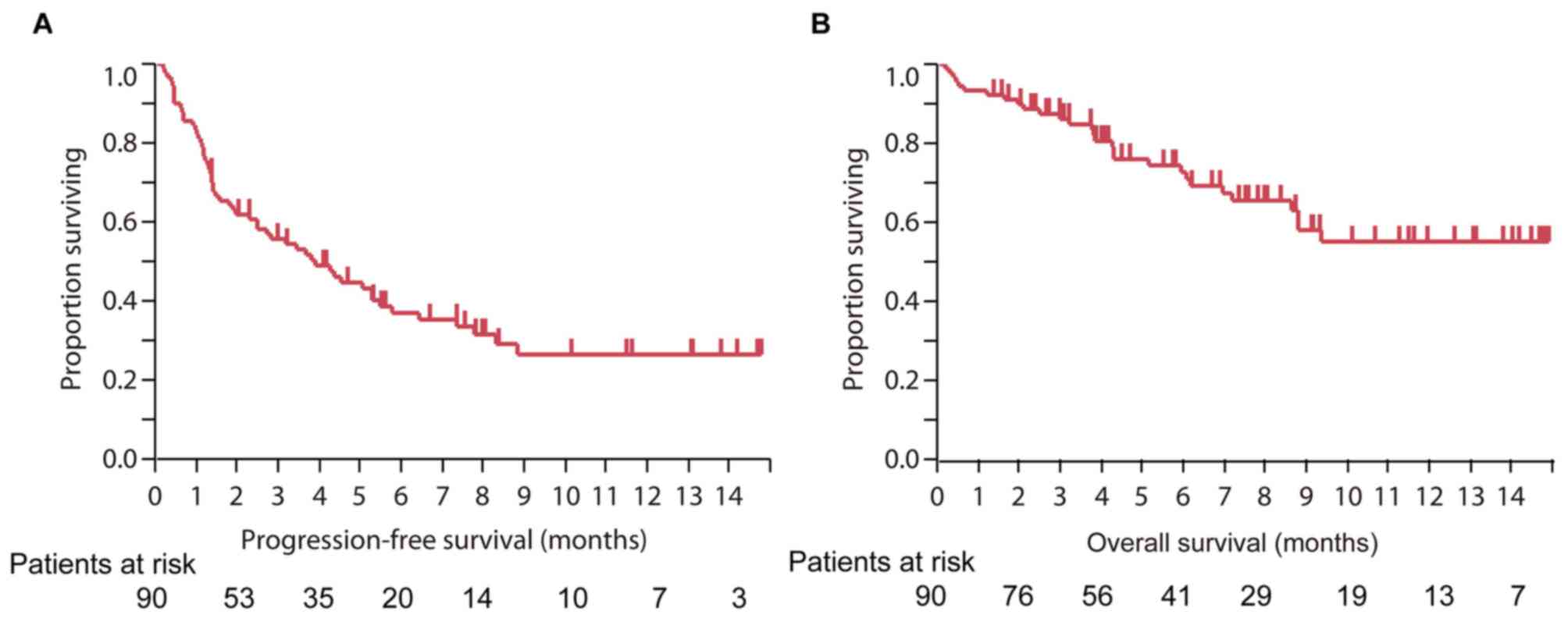
The median PFS time of all patients treated with
nivolumab was 3.9 months [95% confidence interval (CI), 2.3–5.5],
and the median OS time was not reached (Fig. 1).
Clinical outcomes of nivolumab therapy
in the subgroups previously treated or not treated with
antibiotics, H2B or PPIs, and antiflatulents
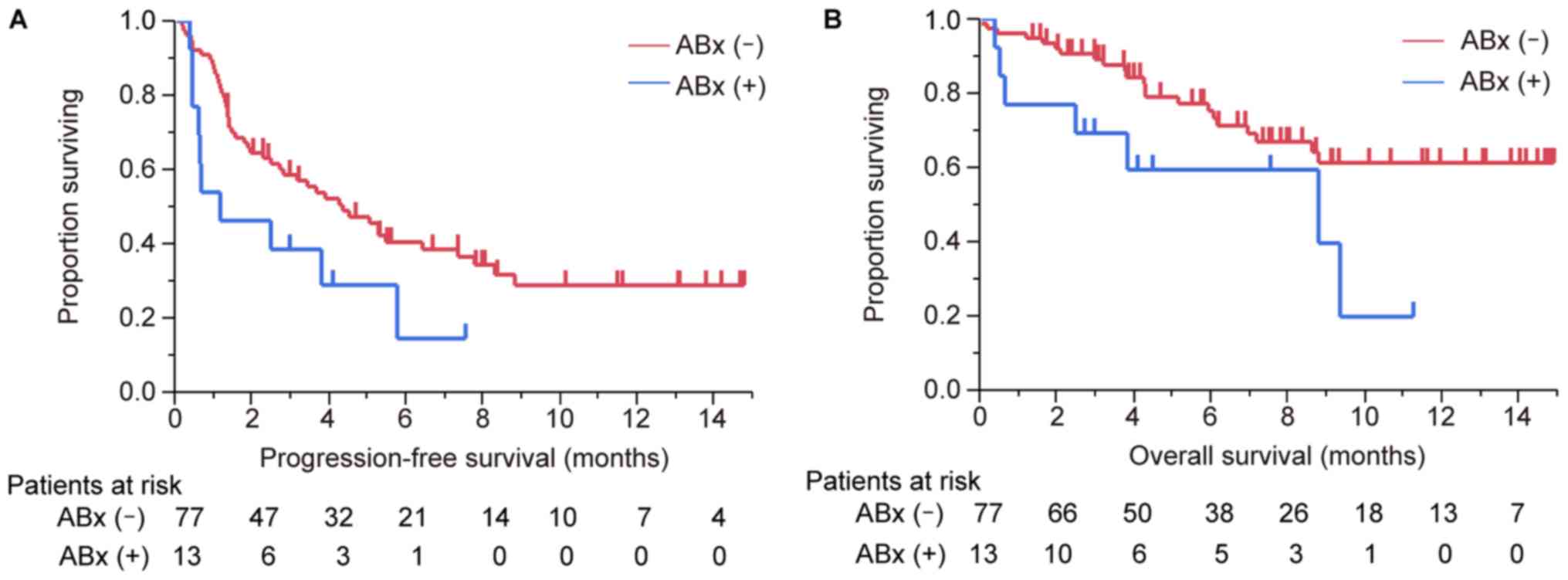
The median PFS time of patients treated with
antibiotics was 1.2 months (95% CI, 0.5–5.8) and the median PFS
time of patients not treated with antibiotics was 4.4 months (95%
CI, 2.5–7.4). The median OS of patients treated and those not
treated with antibiotics was 8.8 months and not reached,
respectively (Fig. 2). The
differences between the survival curves with regard to PFS and OS
were statistically significant (P=0.04 and P=0.037,
respectively).
Univariate and multivariate
analyses
Univariate analysis revealed that ECOG-PS, oncogenic
driver mutations, use of antibiotics, and use of PPIs or
H2B were significantly associated with OS (Table III). Multivariate analysis indicated
that driver mutations were significantly associated with patient
survival, whereas significant associations were not observed
between OS and use of antibiotics, PPIs or H2Bs
(Table IV).
 | Table III.Univariate analysis of survival in
patients treated with nivolumab. |
Table III.
Univariate analysis of survival in
patients treated with nivolumab.
| Variants | n | MST (95% CI),
months | P-value |
|---|
| Age, years |
|
|
|
|
<70 | 56 | NR (7.0-NR) | 0.64 |
|
≥70 | 34 | NR (7.2-NR) |
|
| Sex |
|
|
|
|
Male | 57 | NR (8.8-NR) | 0.19 |
|
Female | 33 | 9.4 (4.3-NR) |
|
| ECOG-PS |
|
|
|
|
<2 | 64 | NR (8.8-NR) | 0.01a |
| ≥2 | 26 | 7.0 (3.8-NR) |
|
| Histology |
|
|
|
|
Adenocarcinoma | 55 | 8.8 (5.9-NR) | 0.06 |
|
Squamous cell carcinoma | 21 | NR (NR-NR) |
|
|
Other | 14 | NR (7.0-NR) |
|
| Driver
mutations |
|
|
|
|
Yes | 21 | 4.3 (2.1-NR) |
<0.001a |
| No | 69 | NR (8.8-NR) |
|
| Lines of
chemotherapy |
|
|
|
| 2 | 54 | NR (8.8-NR) | 0.14 |
| ≥3 | 36 | 8.6 (5.2-NR) |
|
| Use of
antibiotics |
|
|
|
|
Yes | 13 | 8.8 (0.7-NR) | 0.04a |
| No | 77 | NR (8.6-NR) |
|
| Use of PPIs or
H2Bs |
|
|
|
|
Yes | 47 | 8.8 (5.9-NR) | 0.04a |
| No | 43 | NR (NR-NR) |
|
| Use of
antiflatulents | 11 | NR (2.5-NR) | 0.64 |
|
Yes | 11 | NR (2.5-NR) | 0.64 |
| No | 79 | NR (8.8-NR) |
|
 | Table IV.Multivariate analysis of survival in
patients treated with nivolumab. |
Table IV.
Multivariate analysis of survival in
patients treated with nivolumab.
| Variants | HR | 95% CI | P-value |
|---|
| ECOG-PS (poor vs.
good) | 2.17 | 0.89–5.25 | 0.09 |
| Driver mutations
(yes vs. no) | 4.82 | 2.05–11.3 |
<0.001a |
| Use of antibiotics
(yes vs. no) | 2.02 | 0.70–5.83 | 0.19 |
| Use of PPIs or
H2Bs (yes vs. no) | 1.90 | 0.80–4.51 | 0.15 |
Discussion
In recent years, clinical responses to ICIs have
been observed to be more favorable in patients with an indicative
active endogenous T-cell response in the tumor microenvironment
(16–19). However, the underlying mechanisms that
govern the presence or absence of this phenotype remain unclear. In
the present study, a retrospective analysis of 90 patients treated
with nivolumab for NSCLC was performed. A statistically significant
association between survival and prior antibiotic use was not
indicated, although a certain trend toward the negative influence
of antibiotic use was suggested.
The gut microbiota serves an important role in
shaping systemic immune responses (7–9). A number
of studies have indicated that certain types of bacteria or
bacterial products can modulate systemic inflammation and antitumor
immunity. Numerous families of bacteria and metabolites from the
bacterial breakdown of indigestible dietary components have been
indicated to interact with specific immune components that
influence the synthesis of regulatory cytokines (20).
The associations between the gut microbiota and the
responsiveness to anticancer therapy have been extensively
investigated. Previous studies have mainly focused on patients with
colorectal cancer and have demonstrated the role of gut microbiota
in carcinogenesis and the response to cytotoxic chemotherapy
(21–32). However, it remains unclear whether
commensal microbiota influence spontaneous immune responses against
tumors, affecting the therapeutic activity of ICIs regardless of
the type of cancer.
Preclinical and clinical data support the hypothesis
that the gut microbiota shapes the innate and adaptive immune
system, influencing the CTL-associated protein 4 (CTLA-4) and
PD-1/PD-L1 axis, thereby affecting the efficacy of ICIs (13,14). The
abundance of Bifidobacterium species in the intestine has
been indicated to improve anti-PD-L1 therapy in a tumor-bearing
mouse model. In patients with metastatic melanoma, analysis of
fecal samples indicated that bacterial diversity and relative
abundance of bacteria of the Ruminococcaceae family were
fecal microbial predictors of an anti-PD-1 therapy response.
Metagenomic studies revealed functional differences in responders,
including enrichment of anabolic pathways (33). An improved response to anti-PD-L1
therapy was observed in germ-free mice receiving fecal microbiota
transplantation from responsive patients compared with that in the
mice colonized with feces from non-responsive patients (34,35). The
aforementioned observations suggest that a comprehensive analysis
of the gut microbiota may prove valuable for detecting novel
biomarkers or therapeutic targets for cancer patients treated with
ICIs.
The effect of antibiotics on the efficacy of ICIs
has also been investigated due to their impact on the gut
microbiota, however the causal relationship is still unclear in a
clinical setting (Table V).
Anti-CTLA-4 antibody loses its therapeutic efficacy in mice that
are reared under germ-free conditions or are treated with
broad-spectrum antibiotics. In a clinical setting, a retrospective
study indicated that prior antibiotic use negatively influenced the
survival of patients treated with ICIs for metastatic renal cell
carcinoma and NSCLC (15). This
result may implicate the disruption of gut microbiota to interfere
with the efficacy of ICIs. However, another study indicated that
the administration of antibiotics did not influence the outcomes in
patients with NSCLC (36). In the
present analysis, no statistically significant association was
observed between survival and prior antibiotic use, but a certain
trend toward the negative influence of antibiotic use was conveyed.
The fact that certain medical conditions require the use of
antibiotics should be taken into consideration, as they themselves
could affect patient survival.
 | Table V.Comparison of studies examining the
association of antibiotics and the efficacy of immune check point
inhibitors in patients with non-small cell lung cancer. |
Table V.
Comparison of studies examining the
association of antibiotics and the efficacy of immune check point
inhibitors in patients with non-small cell lung cancer.
| Variables | Derosa et al
(n=239)a | Kaderbhai et
al (n=74)b | Present study
(n=90) |
|---|
| Abx use, n (%) | 48 (20.1) | 15 (20.3) | 13 (14.4) |
| Time of Abx
treatment prior to ICI use, days | 30 | 90 | 30 |
| Reasons for Abx, n
(%) |
|
Prophylaxis | 15 (31.2) | 0 (0.0) | 4 (30.8) |
|
Therapy | 33 (68.8) | 15 (100.0) | 9 (69.2) |
| Duration of Abx
treatment, n (%) |
| ≤7
days | 35 (72.9) | 7 (46.7) | 2 (15.3) |
| >7
days | 13 (27.1) | 8 (53.3) | 11 (84.6) |
| Administration
routes, n (%) |
|
Oral | 42 (87.5) | 11 (73.3) | 10 (76.9) |
|
Intravenous/muscular | 5 (10.4) | 4 (26.7) | 3 (23.1) |
| Not
reported | 1 (2.1) | 0 (0.0) | 0 (0.0) |
| Median PFS
(Abx+ vs. Abx−), months | 1.9 vs. 3.8 | NA | 1.2 vs. 4.4 |
| Median OS
(Abx+ vs. Abx−), months | 7.9 vs. 24.6 | NA | 8.8 vs. NR |
Considering the differences between the
aforementioned studies, the timing of antibiotic use prior to the
start of nivolumab therapy may serve an important role, since the
composition of the microbiota changes with the passage of time
following the discontinuation of antibiotics. Previous studies have
mainly focused on eradication treatment for Helicobacter
pylori and have indicated that the microbiota returns to its
baseline within 1 week to 3 months after the discontinuation of
antibiotics, whereas the effect of antibiotics for a number of
other bacteria may remain for years. It may be difficult to set the
optimal cutoff point for the ‘prior antibiotics use’ considering
its effect on the efficacy of following ICIs. However, studies such
as that by Derosa et al (15)
may be of assistance. In this study, the associations of antibiotic
use (within 30 or 60 days) and the efficacy of ICIs were examined.
The impact of antibiotics prior to 60 days was not as potent as
that within the first 30 days prior to ICIs. In another study, in
which the prior use of antibiotics was defined as antibiotics
administered in the last 3 months prior to nivolumab (36), no association between antibiotic use
and the efficacy of ICIs was observed. Further interpretation of
these results is required. Furthermore, future studies focusing on
how the antibiotic spectrum, the administration routes and the
co-administration of corticosteroids may affect the efficacy of
ICIs are also required.
Recently, non-antibiotic drugs, including antacids,
corticosteroids, non-steroidal anti-inflammatory drugs and
antipsychotics, have been associated with changes in the gut
microbiota (37–40). Regarding antacids, a previous study
demonstrated the effect of PPIs on the gut microbiota (41), but the association between antacid use
and the efficacy of ICIs requires further investigation. In the
present retrospective analysis, the prior use of PPIs or
H2B exhibited a trend towards being negatively
influential on ICI efficacy in the same way as antibiotics. The
influence of antiflatulents on the efficacy of ICIs was also
examined, due to potential benefits of probiotics or prebiotics
suggested in previous studies (42).
In the present analysis, however, no association between survival
and prior antibiotic use was observed.
The present study has a number of limitations.
First, the serial changes in the gut microbiota to confirm the
influence of antibiotics and antacids was not assessed. Considering
the retrospective nature of the study, it was reasonable to use
clinical outcomes, including PFS or OS, as surrogate indicators of
these influences. Second, the influence of the use of antibiotics
and antacids during nivolumab therapy was not investigated. Third,
this was a retrospective, nonrandomized study that was performed at
a single institution, with a relatively small number of patients
who used antibiotics for a number of conditions. To the best of our
knowledge, the present study is the first to examine the
association among non-antibiotics drugs, antacids and
antiflatulents and the efficacy of ICIs. Therefore, future focus on
the experimental measures to control confounding factors with
regard to the complex medications used by patients is required, and
further studies are warranted to confirm the findings of the
present study. Additional research is being conducted to
investigate changes in the gut microbiome by obtaining stool
samples to determine changes in the microbiome, or the types of
microbiome that may predict responses to ICIs.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TH and MO acquired the clinical data. TH, YO, MO and
YH were responsible for the interpretation of the data. TH and YO
drafted the manuscript. All authors have read and approved the
current version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Committee of Tokyo Metropolitan Cancer and Infectious
diseases Center Komagome Hospital (Tokyo, Japan) (approval number:
1952). Due to the retrospective nature of the study, written
informed consent was not required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Detterbeck FC, Boffa DJ and Tanoue LT: The
new lung cancer staging system. Chest. 136:260–271. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gonzalvez F, Schug ZT, Houtkooper RH,
MacKenzie ED, Brooks DG, Wanders RJ, Petit PX, Vaz FM and Gottlieb
E: Cardiolipin provides an essential activating platform for
caspase-8 on mitochondria. J Cell Biol. 183:681–696. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus Docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus Docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: Atezolizumab versus docetaxel in patients with
previously treated non-small-cell lung cancer (OAK): A phase 3,
open-label, multicentre randomised controlled trial. Lancet.
389:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hooper LV, Littman DR and Macpherson AJ:
Interactions between the microbiota and the immune system. Science.
336:1268–1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ivanov II and Honda K: Intestinal
commensal microbes as immune modulators. Cell Host Microbe.
12:496–508. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McAleer JP and Kolls JK: Maintaining
poise: Commensal microbiota calibrate interferon responses.
Immunity. 37:10–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jernberg C, Löfmark S, Edlund C and
Jansson JK: Long-term ecological impacts of antibiotic
administration on the human intestinal microbiota. ISME J. 1:56–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Korpela K, Salonen A, Virta LJ, Kekkonen
RA, Forslund K, Bork P and de Vos WM: Intestinal microbiome is
related to lifetime antibiotic use in Finnish pre-school children.
Nat Commun. 7:104102016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Becattini S, Taur Y and Pamer EG:
Antibiotic-induced changes in the intestinal microbiota and
disease. Trends Mol Med. 22:458–478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sivan A, Corrales L, Hubert N, Williams
JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B,
Alegre ML, et al: Commensal Bifidobacterium promotes antitumor
immunity and facilitates anti-PD-L1 efficacy. Science.
350:1084–1089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vétizou M, Pitt JM, Daillére R, Lepage P,
Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong
CP, et al: Anticancer immunotherapy by CTLA-4 blockade relies on
the gut microbiota. Science. 350:1079–1084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Derosa L, Routy B, Enot D, Baciarello G,
Massard C, Loriot Y, Fizazi K, Escudier BJ, Zitvogel L and Albiges
L: Impact of antibiotics on outcome in patients with metastatic
renal cell carcinoma treated with immune checkpoint inhibitors. J
Clin Oncol. 35:462. 2017. View Article : Google Scholar
|
|
16
|
Tumeh PC, Harview CL, Yearley JH, Shintaku
IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu
V, et al: PD-1 blockade induces responses by inhibiting adaptive
immune resistance. Nature. 515:568–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spranger S, Spaapen RM, Zha Y, Williams J,
Meng Y, Ha TT and Gajewski TF: Up-regulation of PD-L1, IDO, and
T(regs) in the melanoma tumor microenvironment is driven by CD8(+)
T cells. Sci Transl Med. 5:200ra1162013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ji RR, Chasalow SD, Wang L, Hamid O,
Schmidt H, Cogswell J, Alaparthy S, Berman D, Jure-Kunkel M,
Siemers NO, et al: An immune-active tumor microenvironment favors
clinical response to ipilimumab. Cancer Immunol Immunother.
61:1019–1031. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gajewski TF, Louahed J and Brichard VG:
Gene signature in melanoma associated with clinical activity: A
potential clue to unlock cancer immunotherapy. Cancer J.
16:399–403. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Belkaid Y and Hand TW: Role of the
microbiota in immunity and inflammation. Cell. 157:121–141. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kostic AD, Gevers D, Pedamallu CS, Michaud
M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J, et
al: Genomic analysis identifies association of Fusobacterium with
colorectal carcinoma. Genome Res. 22:292–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rowland IR: The role of the
gastrointestinal microbiota in colorectal cancer. Curr Pharm Des.
15:1524–1527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu Q, Gao R, Wu W and Qin H: The role of
gut microbiota in the pathogenesis of colorectal cancer. Tumour
Biol. 34:1285–1300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen HM, Yu YN, Wang JL, Lin YW, Kong X,
Yang CQ, Yang L, Liu ZJ, Yuan YZ, Liu F, et al: Decreased dietary
fiber intake and structural alteration of gut microbiota in
patients with advanced colorectal adenoma. Am J Clin Nutr.
97:1044–1052. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang
X, Jia W, Cai S and Zhao L: Structural segregation of gut
microbiota between colorectal cancer patients and healthy
volunteers. ISME J. 6:320–329. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peek RM Jr and Blaser MJ: Helicobacter
pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer.
2:28–37. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Y, Wang X, Huycke T, Moore DR,
Lightfoot SA and Huycke MM: Colon macrophages polarized by
commensal bacteria cause colitis and cancer through the bystander
effect. Transl Oncol. 6:596–606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Castellarin M, Warren RL, Freeman JD,
Dreolini L, Krzywinski M, Strauss J, Barnes R, Watson P,
Allen-Vercoe E, Moore RA and Holt RA: Fusobacterium nucleatum
infection is prevalent in human colorectal carcinoma. Genome Res.
22:299–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McIntosh GH, Royle PJ and Playne MJ: A
probiotic strain of L. acidophilus reduces DMH-induced large
intestinal tumors in male Sprague-Dawley rats. Nutr Cancer.
35:153–159. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sonnenberg GF and Artis D: Innate lymphoid
cell interactions with microbiota: Implications for intestinal
health and disease. Immunity. 37:601–610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu S, Shin J, Zhang G, Cohen M, Franco A
and Sears CL: The Bacteroides fragilis toxin binds to a specific
intestinal epithelial cell receptor. Infect Immun. 74:5382–5390.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu S, Rhee KJ, Albesiano E, Rabizadeh S,
Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, et al: A
human colonic commensal promotes colon tumorigenesis via activation
of T helper type 17 T cell responses. Nat Med. 15:1016–1022. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matson V, Fessler J, Bao R, Chongsuwat T,
Zha Y, Alegre ML, Luke JJ and Gajewski TF: The commensal microbiome
is associated with anti-PD-1 efficacy in metastatic melanoma
patients. Science. 359:104–108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gopalakrishnan V, Spencer CN, Nezi L,
Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman
K, Wei SC, et al: Gut microbiome modulates response to anti-PD-1
immunotherapy in melanoma patients. Science. 359:97–103. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Routy B, Le Chatelier E, Derosa L, Duong
CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C,
Roberti MP, et al: Gut microbiome influences efficacy of PD-1-based
immunotherapy against epithelial tumors. Science. 359:91–97. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kaderbhai C, Richard C, Fumet JD, Aarnink
A, Foucher P, Coudert B, Favier L, Lagrange A, Limagne E, Boidot R
and Ghiringhelli F: Antibiotic use does not appear to influence
response to nivolumab. Anticancer Res. 37:3195–3200.
2017.PubMed/NCBI
|
|
37
|
Jackson MA, Goodrich JK, Maxan ME,
Freedberg DE, Abrams JA, Poole AC, Sutter JL, Welter D, Ley RE and
Bell JT: Proton pump inhibitors alter the composition of the gut
microbiota. Gut. 65:749–756. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang EY, Inoue T, Leone VA, Dalal S, Touw
K, Wang Y, Musch MW, Theriault B, Higuchi K, Donovan S, et al:
Using corticosteroids to reshape the gut microbiome: Implications
for inflammatory bowel diseases. Inflamm Bowel Dis. 21:963–972.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rogers MAM and Aronoff DM: The influence
of non-steroidal anti-inflammatory drugs on the gut microbiome.
Clin Microbiol Infect. 22:178 e171–178 e179. 2016. View Article : Google Scholar
|
|
40
|
Flowers SA, Evans SJ, Ward KM, McInnis MG
and Ellingrod VL: Interaction between atypical antipsychotics and
the gut microbiome in a bipolar disease cohort. Pharmacotherapy.
37:261–267. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Imhann F, Bonder MJ, Vich Vila A, Fu J,
Mujagic Z, Vork L, Tigchelaar EF, Jankipersadsing SA, Cenit MC,
Harmsen HJ, et al: Proton pump inhibitors affect the gut
microbiome. Gut. 65:740–748. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Derosa L, Hellmann MD, Spaziano M,
Halpenny D, Fidelle M, Rizvi H, Long N, Plodkowski AJ, Arbour KC,
Chaft JE, et al: Negative association of antibiotics on clinical
activity of immune checkpoint inhibitors in patients with advanced
renal cell and non-small-cell lung cancer. Ann Oncol. 29:1437–1444.
2018. View Article : Google Scholar : PubMed/NCBI
|