Introduction
Adenoid cystic carcinoma (ACC) is a rare malignancy
that is able to exist at various sites within the body, although it
is most commonly localized to the salivary gland (1). Salivary ACC displays characteristic
features, including biological behavior diversity and histological
heterogeneity (2). Patients diagnosed
with salivary ACC usually present with a poor prognosis due to
metastasis to distant organs, the high risk of relapse and the
propensity of the tumor to invade the peripheral nerves. The
majority of patients diagnosed with salivary ACC succumb between 5
and 20 years post-diagnosis (3–5).
To understand the course and behavior of the
disease, a number of studies have focused on identifying specific
prognostic factors for salivary ACC. However, few clinical
parameters are associated with a poor prognosis, including advanced
Tumor-Node-Metastasis (TNM) stage (6), perineural invasion and solid
histological pattern (5,7–10).
Moreover, assessment of the clinical parameters alone is not
sufficient to accurately predict the clinical outcome of patients
with salivary ACC. Therefore, molecular prognostic factors may be
beneficial for a more accurate prediction of clinical outcome.
Previous studies have demonstrated that a recurrent
t(6;9)(q22-23;p23-24) chromosomal translocation in salivary ACC
results in the fusion of the MYB proto-oncogene transcription
factor (MYB) gene with the nuclear factor I/B (NFIB)
gene. Notably, the MYB-NFIB gene fusion has not been
observed in any non-ACC carcinomas of the head and neck to date
(11). The fusion gene fragment is
predominantly MYB, leading to the overexpression of
MYB mRNA and a subsequent increase in protein expression
level (12). Clinical and
differential diagnoses of ACC have relied on the detection of
molecular biomarkers, including MYB-NFIB gene fusion or
MYB activation by fluorescence in situ hybridization
(FISH) analysis, or immunohistochemical staining of MYB proteins
(13–15). However, due to the small number of
cases (and the potential possession of multiple primary cancer
sites), the association between MYB rearrangement and
clinical prognosis remains controversial (2,16–21).
The purpose of the present study was to
retrospectively analyze the clinical factors affecting the survival
of patients with salivary ACC, and to identify the frequency of the
rearrangement of MYB using FISH, in order to establish the
association between MYB rearrangement and the survival rate
or prognosis of patients with salivary ACC.
Materials and methods
Participants and recruitment
A total of 97 records of patients with ACC of the
head and neck were analyzed. All patients were treated from January
2007 to December 2014 at the Shanghai Ninth People's Hospital,
Shanghai Jiao Tong University. The inclusion criteria were as
follows: i) Location of the tumor in major or minor salivary gland;
ii) demographic and complete clinicopathological data; iii) radical
surgery without preoperative therapy; and iv) availability of
surgical specimens in formalin-fixed paraffin-embedded blocks. The
study was approved by the Review Board of the Shanghai Ninth
People's Hospital. Clinical, pathological, treatment and follow-up
data were obtained from patient medical records. Young adult (YA)
ACC patients were classed as <40 years old (n=19), middle-aged
(MA) patients were between 41 and 65 years old at disease onset
(n=60) and old-aged (OA) patients were >65 years of age at onset
(n=18). Two experienced pathologists reviewed the histological
specimens. The histological grade was determined according to the
following criteria: Grade 1, no solid component; grade 2, solid
component <30%; and grade 3, solid component ≥30%. Margins were
defined as positive if the tumor was detected at the resection
margin. Patient information is displayed in Table I.
 | Table I.Clinical and histological
characteristics (n=97), and follow-up information (n=94) of
patients with salivary adenoid cystic carcinoma. |
Table I.
Clinical and histological
characteristics (n=97), and follow-up information (n=94) of
patients with salivary adenoid cystic carcinoma.
| Characteristic | Cases, n |
|---|
| Age, years |
|
|
≤40 | 19 |
|
41–65 | 60 |
|
>65 | 18 |
| Sex |
|
|
Male | 52 |
|
Female | 45 |
| Site |
|
|
Major | 34 |
|
Minor | 63 |
| TNM stage |
|
| I | 12 |
| II | 27 |
|
III | 26 |
| IV | 32 |
| Neurological
symptoms |
|
|
Yes | 68 |
| No | 29 |
| Histopathological
grade |
|
| 1 | 66 |
| 2 | 14 |
| 3 | 17 |
| Perineural
invasion |
|
|
Yes | 63 |
| No | 34 |
| Vascular
invasion |
|
|
Yes | 16 |
| No | 81 |
| Margin status |
|
|
Positive | 42 |
|
Negative | 55 |
| Lymph node
metastasis |
|
|
Positive | 5 |
|
Negative | 92 |
| Treatment |
|
|
Surgery | 16 |
| Surgery
and radiation | 62 |
| Surgery
and chemoradiation | 16 |
| New recurrence |
|
|
Yes | 31 |
| No | 63 |
| Distant
metastasis |
|
| Prior
to treatment | 8 |
|
Following initial
treatment | 45 |
| No | 41 |
|
Characteristic | Cases, n |
| Last known vital
status |
|
| Alive
free of tumor | 32 |
| Alive
with tumor | 38 |
|
Succumbed | 24 |
Information collection
The follow-up information for 94 cases was obtained
by case record review and return visits. Follow-up time was defined
as the time from initial presentation at the institution for the
tumor of interest to the date of last contact or mortality for
overall survival (OS) assessments. The endpoint investigated was
OS, which was defined as the time in months from surgery to
mortality from any cause.
FISH analysis
FISH was performed on formalin-fixed
paraffin-embedded sections with a thickness of 5 µm using a
commercially available MYB Dual Color Break-Apart Probe (cat no.
ZTV-Z-2143-200; ZytoVision GmbH, Bremerhaven, Germany). The probe
is designed to detect translocations involving the chromosomal
region 6q23.3 harboring the MYB gene, and has two probes
labeled with green and orange fluorochromes that hybridize at the
5′ and 3′ends of the MYB gene, respectively. Analysis was
conducted as described previously (19). A cell number >30 among 100 isolated
cells indicated MYB abnormalities (16). Cells with a normal pattern presented
as two fusion signals. MYB abnormalities present either with
typical rearrangement (one fusion and another with separate orange
and green signals), deletion (one fusion and only one other orange
or green signal) or amplification (more than two orange and/or
green signals).
Statistical analysis
Statistical analyses were performed using the SPSS
software package (version 13.0; SPSS, Chicago, IL, USA). The
association of MYB with clinical parameters was determined
using the χ2 test. Kaplan-Meier survival analysis and
Cox multivariate analysis were used to evaluate the association
between the clinical factors and OS of the patients with salivary
ACC. The P-values in Table IV were
obtained by log-rank (Mantel-Cox) test during Kaplan-Meier survival
analysis. P<0.05 was considered to indicate a statistically
significant difference.
 | Table IV.Kaplan-Meier survival analysis for OS
time in patients with salivary adenoid cystic carcinoma. |
Table IV.
Kaplan-Meier survival analysis for OS
time in patients with salivary adenoid cystic carcinoma.
| Variable | OS time,
months | P-value |
|---|
| Age, years |
| 0.001 |
|
≤40 | 47.518±6.708 |
|
|
41–65 | 91.381±4.246 |
|
|
>65 | 75.231±8.733 |
|
| Sex |
| 0.035 |
|
Male | 59.205±4.495 |
|
|
Female | 87.080±4.834 |
|
| Site |
| 0.109 |
|
Major | 61.076±4.629 |
|
|
Minor | 83.940±4.931 |
|
| TNM stage |
| 0.001 |
|
I/II | 99.599±3.026 |
|
|
III/IV | 67.537±5.183 |
|
| Neurological
symptoms |
| 0.014 |
|
Yes | 71.842±4.594 |
|
| No | 98.241±3.929 |
|
| Histopathological
grade |
| 0.082 |
| 1 | 83.764±4.783 |
|
|
2/3 | 60.023±5.698 |
|
| Perineural
invasion |
| 0.880 |
|
Yes | 59.356±3.719 |
|
| No | 79.464±5.011 |
|
| Vascular
invasion |
| 0.207 |
|
Yes | 56.333±7.342 |
|
| No | 81.684±4.446 |
|
| Margin status |
| 0.011 |
|
Positive | 61.210±4.887 |
|
|
Negative | 88.098±4.828 |
|
| Treatment |
| 0.143 |
|
Surgery | 67.059±10.782 |
|
| Surgery
and radiation | 73.169±3,222 |
|
| Surgery
and chemoradiation | 72.810±11.052 |
|
| MYB
rearrangement |
| 0.029 |
|
Yes | 55.582±3.842 |
|
| No | 96.615±7.095 |
|
Results
Clinicopathological features and
follow-up information
The clinical data and histological features of 97
patients with salivary ACC are presented in Table I. Notably, among the 97 patients,
follow-up information could only be collected from 94 patients. The
cohort included 45 females (46%) and 52 males (54%), resulting in a
male-to-female (M:F) ratio of 1:0.87. The age of the patients at
the time of diagnosis ranged from 20 to 83 years, with a mean age
of 53.9 years. YA patients with salivary ACC (n=19; 19.6%) were
defined as those <40 years of age, MA patients (n=60; 61.9%) as
between 41 and 65 years of age at onset and OA patients (n=18;
18.6%) as >65 years of age at onset. The incidence of ACC in the
minor salivary glands (n=63; 64.9%) was higher compared with that
in the major salivary glands (n=34; 35.1%). The palate was the most
frequent location for salivary ACC (n=28; 28.9%). Overall, the
tumor evaluation according to the TNM staging system identified 12
cases (12.4%) of T1, 27 cases (27.8%) of T2, 26 cases (26.8%) of T3
and 32 cases (33.0%) of T4 cancer at diagnosis.
The median time to presentation for treatment was 6
months (range, 0.5–125 months). There were 7 cases in which the
presentation was delayed for over 60 months, including certain
patients presenting with slow-growing tumors, 1 patient initially
diagnosed with trigeminal neuralgia and another patient treated for
glossopharyngeal neuralgia.
The majority of patients (n=68; 70.1%) presented
with neurological symptoms, including pain, paresthesia, tongue
deviation and facial paralysis, while the remainder (n=29; 29.9%)
had no apparent symptoms or presented with other symptoms,
including rapid growth of a mass, nasal obstruction or bleeding,
and ulceration. The lesions of the palate frequently manifested as
ulcers with numbness or pain. Parotid lesions frequently appeared
as facial skin needle- or electric shock-like pain, in addition to
facial paralysis. The floor of the mouth, tongue and sublingual
gland lesions displayed different symptoms, including tongue
numbness, pain and tongue deviation. The lesions of the maxillary
sinus were frequently accompanied by epistaxis and diplopia.
Histopathological evaluation of the tumor specimens identified 66
cases at grade 1, 14 cases at grade 2 and 17 cases at grade 3. A
total of 63 cases (64.9%) displayed evidence of perineural
invasion, while vascular infiltration was observed in 16 cases
(16.5%). Moreover, 42 cases (43.3%) presented with positive
surgical margins. Metastases to the regional lymph nodes were noted
in 5 cases (5.2%). All the cervical lymph node metastases occurred
in T3 and T4 tumors, with 4 originating from the floor of the mouth
and 1 case from the buccal mucosa.
All patients underwent surgery, and of the 94
patients with available follow-up information, 62 (66%) received
postoperative radiotherapy. Patients with metastasis or positive
margins (n=16; 17.0%) received concomitant chemotherapy. The mean
follow-up time was 45.1 months (range, 4–104 months). At the end of
the follow-up, 24 patients (25.5%) succumbed due to distant
metastasis and/or local recurrence (LR), while of the remaining 70
patients (74.5%), 38 possessed residual disease. There were 53
patients (56.4%) with distant metastasis; 46 cases (48.9%)
metastasized to the lung, 2 cases to the lungs and brain, 2 cases
to the lungs, liver and bone, 1 case to the lungs and sternum, 1
case to the lungs and liver, and 1 case to lungs, kidney and bone.
The median time for metastasis identification was 11 months (range,
0–101 months). There were no cases of lymph node metastasis
development following surgery. The LR rate was 33.0% (31 out of 94
cases). The median time until LR was 12 months (range, 1–48
months).
MYB rearrangement
Using a dual color MYB break-apart FISH probe,
MYB rearrangements were detected in 83 of the 97 cases. The
typical patterns of MYB rearrangements with an intact signal
(fused orange/green signals) (Fig.
1A) and a split signal (separated orange and green signals),
indicating a breakpoint within the MYB gene (Fig. 1B), compared with the intact signal,
were observed in 71 tumors. Gain of one or more green signals was
apparent in 5 cases, indicating selective amplification of the
5′end of MYB (Fig. 1C), and
multiple pairs of fusion signals were observed in one case,
indicating gain of MYB copies (Fig.
1D) (Table II). A typical
pattern with one fusion signal and one green/ orange signal,
indicating the loss of the 3′ end, was observed in 4 cases
(Fig. 1E) and the loss of the 5′end
was observed in 2 cases (Fig.
1F).
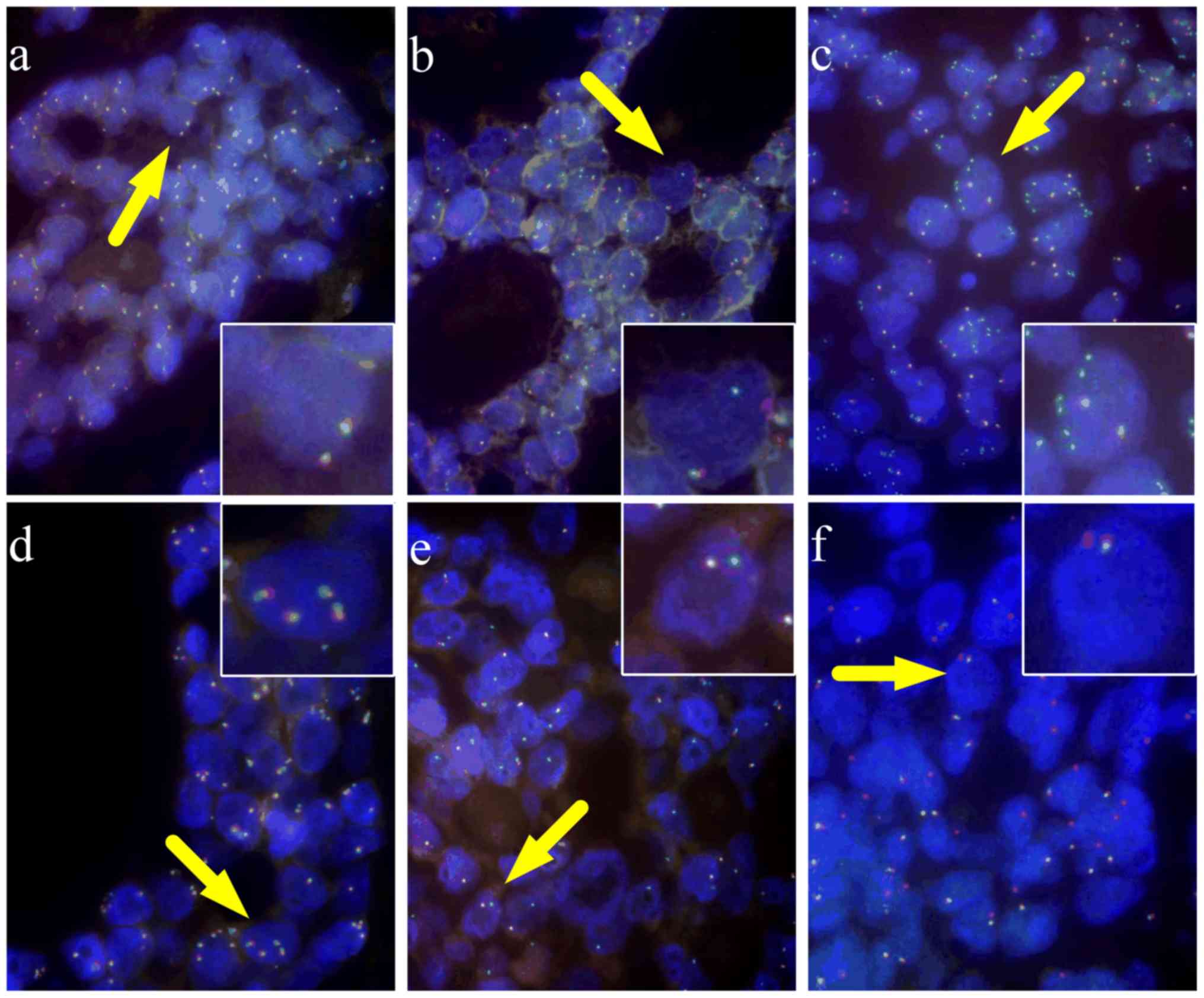 | Figure 1.Rearrangements of MYB detected by
fluorescence in situ hybridization. (A) Normal (×1,000, oil
mirror), (B) split (×1,000, oil mirror), (C) 5′-part amplification
(×1,000, oil mirror), (D) copy number amplification (×1,000, oil
mirror), (E) loss of 3′-part and (F) loss of 5′-part (×1,000, oil
mirror). Cells with a normal pattern present as two fusion signals.
MYB abnormalities present either with typical rearrangement (one
fusion and another separate orange and green signal), deletion (one
fusion and only one other orange or green signal) or amplification
(more than two orange and/or green signals). MYB, MYB
proto-oncogene transcription factor. |
 | Table II.Rearrangements of MYB detected
by fluorescence in situ hybridization. |
Table II.
Rearrangements of MYB detected
by fluorescence in situ hybridization.
| MYB
rearrangements | Number |
|---|
| Split signal | 71 |
| Split and 5′-part
amplification | 5 |
| Loss of
3′-part | 4 |
| Loss of
5′-part | 2 |
| Copy number
amplification | 1 |
| Normal | 14 |
The statistical significance between
clinicopathological characteristics and MYB rearrangement in
patients with salivary ACC patients was subsequently assessed
(summarized in Table III). Only TNM
stage was significantly associated with MYB rearrangement,
wherein the late TNM stage was associated with higher MYB
rearrangement (P=0.033). These results suggest that MYB
rearrangement is associated with the progression of salivary ACC,
which may be interpreted as an indicator of poor prognosis.
Notably, MYB gene rearrangement was not statistically
associated with any other clinicopathological variable.
 | Table III.Associations between
clinicopathological parameters and MYB rearrangement. |
Table III.
Associations between
clinicopathological parameters and MYB rearrangement.
|
| MYB
rearrangement, n (%) |
|
|---|
|
|
|
|
|---|
| Parameter | With (n=81) | Without (n=13) | P-value |
|---|
| Age, years |
|
| 0.157 |
|
≤40 | 18 (100.0) | 0 (0.0) |
|
|
41–65 | 48 (82.8) | 10 (17.2) |
|
|
>65 | 15 (83.3) | 3 (16.7) |
|
| Sex |
|
| 0.372 |
|
Male | 38 (90.5) | 4 (9.5) |
|
|
Female | 43 (82.7) | 9 (17.3) |
|
| Site |
|
| 0.788 |
|
Major | 28 (87.5) | 4 (12.5) |
|
|
Minor | 53 (85.5) | 9 (14.5) |
|
| TNM stage |
|
| 0.033 |
|
I/II | 29 (76.3) | 9 (23.7) |
|
|
III/IV | 52 (92.9) | 4 (7.1) |
|
| Neurological
symptoms |
|
| 0.995 |
|
Yes | 56 (86.2) | 9 (13.8) |
|
| No | 25 (86.2) | 4 (13.8) |
|
| Histopathological
grade |
|
| 0.332 |
| 1 | 54 (83.1) | 111 (16.9) |
|
|
2/3 | 27 (93.1) | 2 (6.9) |
|
| Perineural
invasion |
|
| 0.764 |
|
Yes | 53 (86.9) | 8 (13.1) |
|
| No | 28 (84.8) | 5 (15.2) |
|
| Vascular
invasion |
|
| 0.119 |
|
Yes | 15 (100.0) | 0 (0.0) |
|
| No | 66 (86.2) | 13 (13.8) |
|
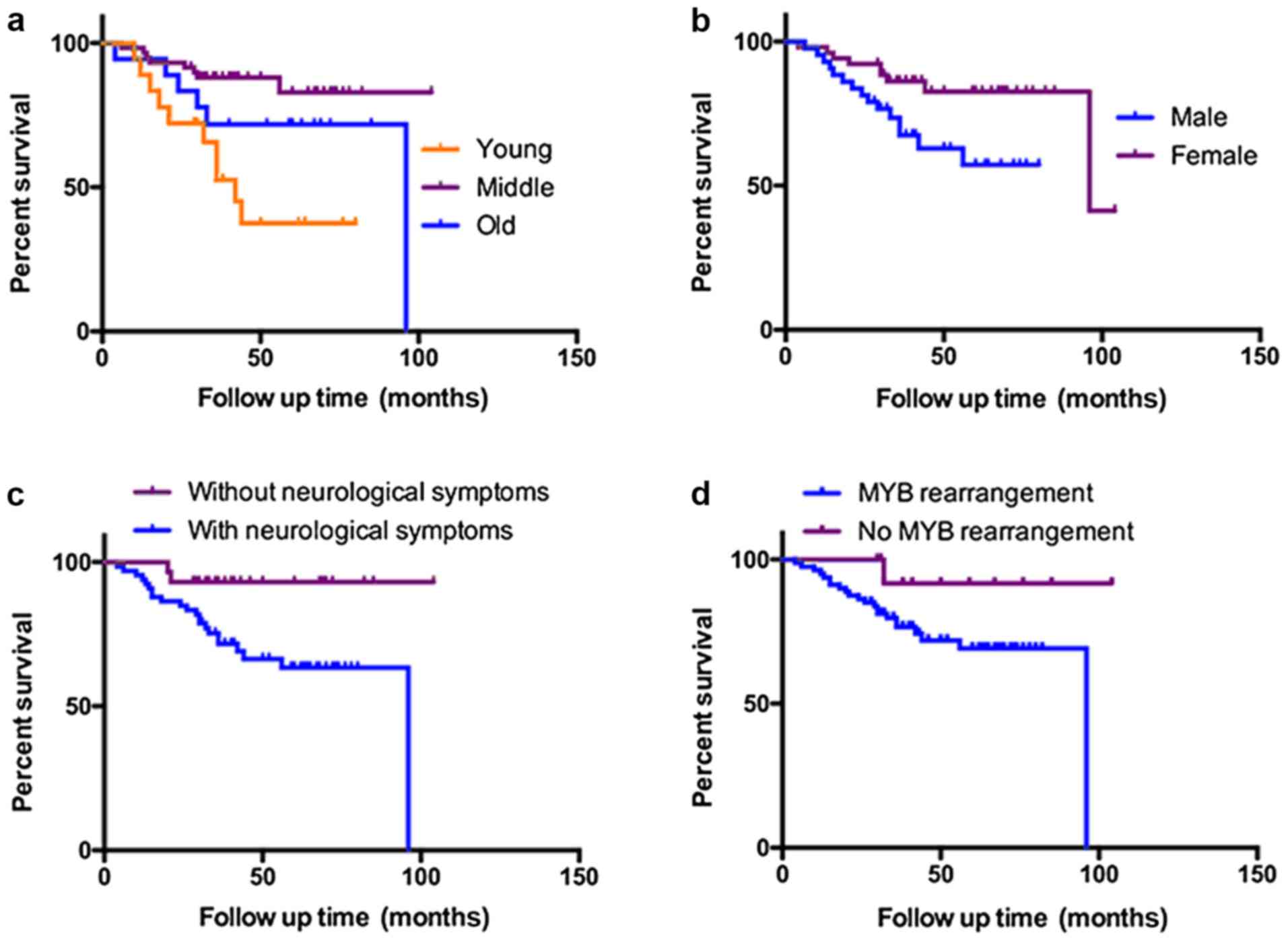
Survival analyses
Kaplan-Meier analysis revealed significant
differences in OS, dependent on age, sex, TNM stage, neurological
symptoms, margin status and MYB rearrangement (Fig. 2; Table
IV). For specific groups, the median survival times were
absent, as until the end of the follow-up period, the overall
survival rate in these groups remained at >50%. Therefore, the
method used to determine survival analysis could not provide the
hazard ratio in the case of >2 groups. In multivariate analysis,
YA was indicated to be significantly associated with a shorter OS
time (Table V).
 | Table V.Univariate and Multivariate Cox
analysis of variables considered for overall survival of salivary
adenoid cystic carcinoma patients. |
Table V.
Univariate and Multivariate Cox
analysis of variables considered for overall survival of salivary
adenoid cystic carcinoma patients.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (≤40 vs.
>40) | 3.884 | 1.676–9.003 | 0.002a | 2.921 | 1.191–7.142 | 0.019a |
| Margin status
(positive vs. negative) | 3.014 | 1.228–7.396 | 0.016a | 2.677 | 1.049–6.830 | 0.039a |
| Neurological
symptoms (yes vs. no) | 5.137 | 1.202–21.966 | 0.027a | 3.357 | 0.757–14.895 | 0.111 |
| TNM stage (III/IV
vs. I/II) | 8.904 | 2.087–37.994 | 0.003a | 3.947 | 0.854–18.248 | 0.079 |
| Histopathology
grade (2/3 vs. 1) | 2.585 | 1.053–6.347 | 0.038a | 2.483 | 0.925–6.661 | 0.071 |
| Sex (male vs.
female) | 0.676 | 0.274–1.688 | 0.395 |
|
|
|
| Site (major vs.
minor) | 0.968 | 0.545–1.720 | 0.912 |
|
|
|
| Perineural invasion
(yes vs. no) | 1.056 | 0.429–2.956 | 0.906 |
|
|
|
| Vascular invasion
(yes vs. no) | 1.880 | 0.692–5.180 | 0.215 |
|
|
|
| MYB rearrangement
(yes vs. no) | 0.035 | 0.000–4.223 | 0.170 |
|
|
|
Discussion
Salivary ACC is one of the most common malignant
tumors of the oral and maxillofacial region. In agreement with
previous studies (5,22,23), the
present study indicated that salivary ACC originates more
frequently from minor rather than major glands, and predominantly
affects the palate.
Although previous studies have suggested that
salivary ACC affects a wide age distribution and has no sex
predilection (5,24), the present results indicate that
salivary ACC has a minor male predominance. Consistently, analysis
using Kaplan-Meier survival curves revealed significant differences
between men and women, with women having improved survival
outcomes. Moreover, salivary ACC typically occurs in the fifth or
sixth decade of life, although it may also present in YA patients
(25,26). Certain reports suggest that older age
is a risk factor for lower survival rates in patients with salivary
ACC (5,10,27,28).
Accordingly, the present study revealed that the peak incidence of
salivary ACC was amongst MA patients. The incidence of salivary ACC
in YA patients was relatively low and represented a minority of
cases. The association between YA and prognosis is a well-described
phenomenon reported across multiple cancer types (29,30). For
example, in head and neck squamous cell carcinoma, young cancer
patients presented with a worse prognosis (31). However, the prognosis of the YA
patients in the context of salivary ACC has yet to be evaluated.
The present results indicated that YA patients presented with a
worse prognosis compared with MA and OA patients.
Patients with ACC frequently develop neurological
symptoms in the early stages of the disease (32,33).
Neural spread away from a tumor frequently results in specific
symptoms, including pain, muscle weakness and atrophy, depending on
the nerves involved. Occasionally, pain may occur in the early
stage of the disease until there is a noticeable swelling. In
certain cases, the treatment of patients is delayed due to the
initial diagnosis of trigeminal or glossopharyngeal neuralgia
(22). Although the most common
initial presentation is unclear, almost one-third of patients
present with dysaesthesia. This symptom is important in the absence
of clinically visible symptoms, as it may indicate an underlying
neoplastic variation and warrant further investigation. Despite
these observations, the association between histopathological
perineural invasion and disease prognosis remains controversial
(28,34,35).
Moreover, the connection between neurological symptoms and
prognosis in patients with cancer is yet to be addressed. By
contrast, the present results indicate that patients with
neurological symptoms had a comparatively poor prognosis, but did
not exhibit histopathological perineural invasion. These results
suggested that patients with salivary ACC, but without neurological
symptoms, had a relatively good prognosis, and were more likely to
be misdiagnosed with other salivary gland tumors.
The present study confirmed previous studies
indicating that TNM stage at diagnosis is the primary factor for
survival prediction in patients with cancer (5,7,8,36). Owing
to the slow-growing nature and protracted clinical presentation of
salivary ACC, patients are not always aware of the cancer until the
rapid growth or metastasis stage. In the present study, there were
8 patients who presented with pulmonary metastasis during the time
of diagnosis of the primary lesion. Moreover, lymph node
involvement in the salivary ACC is uncommon. In the study by Min
et al (24), 62 out of 616
(10.1%) cases of lymph node metastasis were identified, with the
most common primary site originating from the base of the tongue,
mobile tongue and floor of the mouth. In the present study,
metastases to the regional lymph nodes were determined in only 5
cases, which all occurred in T3 and T4 tumors, with 4 cases
originating from the floor of the mouth and 1 case from the buccal
mucosa.
Salivary ACC has notable heterogeneous morphology
and contains tubular, cribriform, solid or mixed patterns. However,
these histopathological features are not specific to salivary ACC
and may also be present in other salivary gland tumors, including
pleomorphic adenoma, basal cell adenoma, basal cell adenocarcinoma
and polymorphous low-grade adenocarcinoma. Therefore, novel
molecular biomarkers may encourage more accurate prediction of
clinical outcome in patients with salivary ACC. Genetic alterations
detected in tumors may be associated with the oncogenic process and
potentially serve as diagnostic biomarkers. MYB-NFIB gene
fusion and MYB activation have frequently been utilized as
molecular biomarkers in clinical and differential diagnoses
(13–15). However, studies investigating the
expression of MYB in pleomorphic adenoma suggest that
alterations in the MYB gene are not a definitive diagnostic
biomarker (37). Moreover, as a
potential diagnostic biomarker for ACC, the specific association of
MYB rearrangement with clinical features and the prognosis
of salivary ACC have not been completely elucidated (11,21).
FISH studies by Perrson et al (38) indicated that MYB-NFIB gene
fusion occurred in 86% of tumors, and that gene rearrangement due
to chromatin instability frequently led to a loss of 6q24.1–6q27,
more commonly in high histological grade cancer. Roden et al
(20) detected 35 cases of lung ACC
with the MYB break-apart probe, showing 100% specificity of
MYB rearrangement in 41% of cases; however, there was no
correlation between MYB rearrangement and clinical features
or prognosis. Broz et al (2)
detected 23 cases of salivary ACC, with 15 cases positive for
rearrangement and 8 cases with no rearrangement (2). There were no significant differences in
age, sex, perineural invasion, the presence of hematogenic and
nodal metastases, or degree of histopathological grading between
patients with and without MYB rearrangement. Survival
analysis revealed no statistically significant differences in
survival between patients with and without MYB
rearrangement; however, a lack of MYB alteration was
associated with a better prognosis (2). In the present study, the vast majority
of cases exhibited MYB isolation, and few cases displayed
deletion or amplification of MYB; there was no significant
association between the different gene alterations and the
histological phenotype of ACC. Survival analysis indicated
significantly improved OS times in patients with no alterations in
MYB compared with those in patients with MYB
rearrangements, which is in agreement with the conclusions of Broz
et al (2), despite their
findings not being statistically significant.
Moreover, the present results suggested that YA
patients with salivary ACC have a worse prognosis, which is
significantly different from the majority of patients presenting
with salivary ACC. Detection of MYB alterations by FISH
could achieve a high positive detection rate in salivary ACC, which
would facilitate clinical diagnosis, and the lack of MYB
rearrangement may be associated with a better prognosis. Therefore,
establishing the association between MYB rearrangement and
prognosis may be beneficial for a greater understanding and the
improved treatment of salivary ACC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Fund of China (grant nos. 81372910 and 81302360).
Availability of data and material
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JH and CYZ were responsible for the study conception
and design. TG and XY conducted experimental testing and follow-up.
LWH and ZT collected the data and revised the manuscript. Data
analysis and interpretation was conducted by JL and CPZ. All
authors contributed to writing the manuscript and provided final
approval.
Ethics approval and consent to
participate
The present study was approved by the Review Board
of the Ninth People's Hospital and informed consent was obtained
from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Coca-Pelaz A, Rodrigo JP, Bradley PJ,
Vander Poorten V, Triantafyllou A, Hunt JL, Strojan P, Rinaldo A,
Haigentz M Jr, Takes RP, et al: Adenoid cystic carcinoma of the
head and neck--An update. Oral Oncol. 51:652–661. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Broz M, Steiner P, Salzman R, Hauer L and
Starek I: The incidence of MYB gene breaks in adenoid cystic
carcinoma of the salivary glands and its prognostic significance.
Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 160:417–22.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fordice J, Kershaw C, El-Naggar A and
Goepfert H: Adenoid cystic carcinoma of the head and neck:
Predictors of morbidity and mortality. Arch Otolaryngol Head Neck
Surg. 125:149–52. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spiro RH: Distant metastasis in adenoid
cystic carcinoma of salivary origin. Am J Surg. 174:495–498. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang CY, Xia RH, Han J, Wang BS, Tian WD,
Zhong LP, Tian Z, Wang LZ, Hu YH and Li J: Adenoid cystic carcinoma
of the head and neck: Clinicopathologic analysis of 218 cases in a
Chinese population. Oral Surg Oral Med Oral Pathol Oral Radiol.
115:368–375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM classification of malignant tumours (8th edition).
(Chichester, West Sussex, UK). Wiley-Blackwell. ISBN
978-1-4443-3241-4. 2017.
|
|
7
|
Khafif A, Anavi Y, Haviv J, Fienmesser R,
Calderon S and Marshak G: Adenoid cystic carcinoma of the salivary
glands: A 20-year review with long-term follow-up. Ear Nose Throat
J. 84:662–664, 667. 2005.PubMed/NCBI
|
|
8
|
Dodd RL and Slevin NJ: Salivary gland
adenoid cystic carcinoma: A review of chemotherapy and molecular
therapies. Oral Oncol. 42:759–769. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ciccolallo L, Licitra L, Cantú G and Gatta
G; EUROCARE Working Group, : Survival from salivary glands adenoid
cystic carcinoma in European populations. Oral Oncol. 45:669–674.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhayani MK, Yener M, El-Naggar A, Garden
A, Hanna EY, Weber RS and Kupferman ME: Prognosis and risk factors
for early-stage adenoid cystic carcinoma of the major salivary
glands. Cancer. 118:2872–2878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stenman G: Fusion oncogenes in salivary
gland tumors: Molecular and clinical consequences. Head Neck
Pathol. 7 Suppl 1:S12–S19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Persson M, Andrén Y, Mark J, Horlings HM,
Persson F and Stenman G: Recurrent fusion of MYB and NFIB
transcription factor genes in carcinomas of the breast and head and
neck. Proc Natl Acad Sci USA. 106:18740–18744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pusztaszeri MP, Sadow PM, Ushiku A,
Bordignon P, McKee TA and Faquin WC: MYB immunostaining is a useful
ancillary test for distinguishing adenoid cystic carcinoma from
pleomorphic adenoma in fine-needle aspiration biopsy specimens.
Cancer Cytopathol. 122:257–265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tian Z, Li L, Zhang CY, Gu T and Li J:
Differences in MYB expression and gene abnormalities further
confirm that salivary cribriform basal cell tumors and adenoid
cystic carcinoma are two distinct tumor entities. J Oral Pathol
Med. 45:698–703. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hudson JB and Collins BT: MYB gene
abnormalities t(6;9) in adenoid cystic carcinoma fine-needle
aspiration biopsy using fluorescence in situ hybridization. Arch
Pathol Lab Med. 138:403–409. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
West RB, Kong C, Clarke N, Gilks T,
Lipsick JS, Cao H, Kwok S, Montgomery KD, Varma S and Le QT: MYB
expression and translocation in adenoid cystic carcinomas and other
salivary gland tumors with clinicopathologic correlation. Am J Surg
Pathol. 35:92–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
von Holstein SL, Fehr A, Persson M,
Therkildsen MH, Prause JU, Heegaard S and Stenman G: Adenoid cystic
carcinoma of the lacrimal gland: MYB gene activation, genomic
imbalances, and clinical characteristics. Ophthalmology.
120:2130–2138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
D'Alfonso TM, Mosquera JM, MacDonald TY,
Padilla J, Liu YF, Rubin MA and Shin SJ: MYB-NFIB gene fusion in
adenoid cystic carcinoma of the breast with special focus paid to
the solid variant with basaloid features. Hum Pathol. 45:2270–2280.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rettig EM, Tan M, Ling S, Yonescu R,
Bishop JA, Fakhry C and Ha PK: MYB rearrangement and
clinicopathologic characteristics in head and neck adenoid cystic
carcinoma. Laryngoscope. 125:E292–E299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roden AC, Greipp PT, Knutson DL,
Kloft-Nelson SM, Jenkins SM, Marks RS, Aubry MC and García JJ:
Histopathologic and cytogenetic features of pulmonary adenoid
cystic carcinoma. J Thorac Oncol. 10:1570–1575. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen TY, Keeney MG, Chintakuntlawar AV,
Knutson DL, Kloft-Nelson S, Greipp PT, Garrity JA, Salomao DR and
Garcia JJ: Adenoid cystic carcinoma of the lacrimal gland is
frequently characterized by MYB rearrangement. Eye (Lond).
31:720–725. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
DeAngelis AF, Tsui A, Wiesenfeld D and
Chandu A: Outcomes of patients with adenoid cystic carcinoma of the
minor salivary glands. Int J Oral Maxillofac Surg. 40:710–714.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guzzo M, Locati LD, Prott FJ, Gatta G,
McGurk M and Licitra L: Major and minor salivary gland tumors. Crit
Rev Oncol Hematol. 74:134–148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Min R, Siyi L, Wenjun Y, Ow A, Lizheng W,
Minjun D and Chenping Z: Salivary gland adenoid cystic carcinoma
with cervical lymph node metastasis: A preliminary study of 62
cases. Int J Oral Maxillofac Surg. 41:952–957. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kokemueller H, Eckardt A, Brachvogel P and
Hausamen JE: Adenoid cystic carcinoma of the head and neck--a 20
years experience. Int J Oral Maxillofac Surg. 33:25–31. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Triantafillidou K, Dimitrakopoulos J,
Iordanidis F and Koufogiannis D: Management of adenoid cystic
carcinoma of minor salivary glands. J Oral Maxillofac Surg.
64:1114–1120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ellington CL, Goodman M, Kono SA, Grist W,
Wadsworth T, Chen AY, Owonikoko T, Ramalingam S, Shin DM, Khuri FR,
et al: Adenoid cystic carcinoma of the head and neck: Incidence and
survival trends based on 1973–2007 Surveillance, Epidemiology, and
End Results data. Cancer. 118:4444–4451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
da Cruz Perez DE, de Abreu Alves F, Nobuko
Nishimoto I, de Almeida OP and Kowalski LP: Prognostic factors in
head and neck adenoid cystic carcinoma. Oral Oncol. 42:139–146.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kataoka A, Iwamoto T, Tokunaga E, Tomotaki
A, Kumamaru H, Miyata H, Niikura N, Kawai M, Anan K, Hayashi N, et
al: Young adult breast cancer patients have a poor prognosis
independent of prognostic clinicopathological factors: A study from
the Japanese Breast Cancer Registry. Breast Cancer Res Treat.
160:163–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu S, Feng F, Xu G, Liu Z, Tian Y, Guo M,
Lian X, Cai L, Fan D and Zhang H: Clinicopathological features and
prognosis of gastric cancer in young patients. BMC Cancer.
16:4782016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sgaramella N, Lindell Jonsson E, Boldrup
L, Califano L, Coates PJ, Tartaro G, Lo Muzio L, Fåhraeus R,
Colella G, Dell'Aversana Orabona G, et al: High expression of
podoplanin in squamous cell carcinoma of the tongue occurs
predominantly in patients ≤40 years but does not correlate with
tumour spread. J Pathol Clin Res. 2:3–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou YE, O'Rourke JP, Edwards JS and Ness
SA: Single molecule analysis of c-myb alternative splicing reveals
novel classifiers for precursor B-ALL. PLoS One. 6:e228802011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Glasspool RM, Brown R, Gore ME, Rustin GJ,
McNeish IA, Wilson RH, Pledge S, Paul J, Mackean M, Hall GD, et al:
A randomised, phase II trial of the DNA-hypomethylating agent
5-aza-2′-deoxycytidine (decitabine) in combination with carboplatin
vs carboplatin alone in patients with recurrent, partially
platinum-sensitive ovarian cancer. Br J Cancer. 110:1923–1929.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Barrett AW and Speight PM: Perineural
invasion in adenoid cystic carcinoma of the salivary glands: A
valid prognostic indicator? Oral Oncol. 45:936–940. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gondivkar SM, Gadbail AR, Chole R and
Parikh RV: Adenoid cystic carcinoma: A rare clinical entity and
literature review. Oral Oncol. 47:231–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gomez DR, Hoppe BS, Wolden SL, Zhung JE,
Patel SG, Kraus DH, Shah JP, Ghossein RA and Lee NY: Outcomes and
prognostic variables in adenoid cystic carcinoma of the head and
neck: A recent experience. Int J Radiat Oncol Biol Phys.
70:1365–1372. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mendoza PR, Jakobiec FA and Krane JF:
Immunohistochemical features of lacrimal gland epithelial tumors.
Am J Ophthalmol. 156:1147–1158.e1. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Persson M, Andrén Y, Moskaluk CA, Frierson
HF Jr, Cooke SL, Futreal PA, Kling T, Nelander S, Nordkvist A,
Persson F and Stenman G: Clinically significant copy number
alterations and complex rearrangements of MYB and NFIB in head and
neck adenoid cystic carcinoma. Genes Chromosomes Cancer.
51:805–817. 2012. View Article : Google Scholar : PubMed/NCBI
|