Introduction
In 2018, renal cancers were reported to be among the
10 most common types of cancer in men and women; in addition,
65,340 newly diagnosed cases renal cancer and 14,970 cases of
associated mortality are predicted to occur in the United States in
2018 (1). Renal cell carcinoma (RCC)
is the most common and lethal among urological cancers, with a
mortality rate of ~90%. Localized RCC can be successfully managed
with surgery, whereas up to 30% of patients develop metastasis
(2) and ~40% of patients relapse
(3), due to high resistance to
conventional chemotherapy. Therefore, the development of effective
therapy for RCC is crucial.
Gastric carcinoma high expressed transcript 1
(GHET1) is a long non-coding RNA (lncRNA), which is upregulated in
gastric cancer. Non-coding RNAs account for ~98% of the human
genome, including microRNAs and a large class of lncRNAs (4,5).
Increasing evidence has demonstrated that lncRNAs may serve an
important role in the progression of numerous types of carcinoma
(6–8).
Yang et al demonstrated that high expression levels of GHET1
are correlated with tumor size, tumor invasion and poor survival,
and that GHET1 promotes cancer cell proliferation by increasing
c-Myc stability and expression (9).
Zhou et al confirmed the inhibitory effects of GHET1 on
colorectal cancer (10). In this
study, authors demonstrated that GHET1 is overexpressed in
colorectal cancer, and that GHET1 silencing suppresses cell
proliferation, cell cycle arrest, cell migration and cell invasion.
GHET1 may therefore represent a novel therapeutic target for the
treatment of colorectal cancer. Epithelial-mesenchymal transition
(EMT) has been demonstrated to be essential for development and
physiological response in carcinogenesis, particularly during the
complex initial processes of tissue invasion and extravasation
(11,12). Furthermore, EMT is characterized by
the loss of epithelial markers, including E-cadherin, and the
upregulation of mesenchymal markers, such as Fibronectin and
Vimentin (13). However, to the best
of our knowledge, the expression and function of GHET1 in RCC
remain unknown.
The aim of the present study was to investigate the
role of GHET1 in RCC. It was demonstrated that RCC tissues and cell
lines presented high expression levels of GHET1. In addition, GHET1
knockdown suppressed RCC cell proliferation and migration, thus
suggesting that GHET1 may act as an oncogene. The underlying
mechanisms of GHET1 in RCC were further investigated.
Materials and methods
Tissue samples
This study was approved by the Human Ethics
Committee of The First Affiliated Hospital of Nanchang University
(Nanchang, China). A total of 40 RCC tissues and paired adjacent
healthy tissues were obtained from patients undergoing primary RCC
resection between April 2010 and August 2015. No chemotherapy was
administered to patients prior to sample collection.
Clinicopathological characteristics were also collected. All
patients provided written informed consent. All samples were
identified by histopathological evaluation and stored at −80°C. The
overall survival (OS) of patients was defined as the time interval
between surgery and either mortality or the latest follow-up
examination.
Cell culture
The human RCC cell lines 786-O and A498, and 293
cells were obtained from the American Type Culture Collection
(Manassas, VA, USA). All cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), supplemented with 10% (v/v) fetal bovine serum
(Gibco; Thermo Fisher Scientific), 1% 100 U/ml penicillin and 1%
100 mg/ml streptomycin sulfate (Sigma-Aldrich: Merck KGaA,
Darmstadt, Germany) at 37°C in a humidified atmosphere containing
5% CO2.
Cell treatment
Small interfering RNA (siRNA) specifically targeting
GHET1 was provided by Shanghai GenePharma Co., Ltd. (Shanghai,
China). The interference sequence was
5′-CGGCAGGCATTAGAGATGAACAGCA-3′. A negative control siRNA was
purchased from Shanghai GenePharma Co. Ltd. (Cat. No. A06001),
which was used as a negative control (NC). Cells were seeded in
6-well plates at 50–70% confluence and transfected with either the
negative control siRNA or GHET1-siRNA (200 nM) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. After
48 h transfection, cells were harvested for subsequent
analyses.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from RCC or adjacent tissues,
and cells using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). RNA concentration was measured by reading
the absorbance at 260/280 nm using a Nanodrop Spectrophotometer
(ND-100; NanoDrop; Thermo Fisher Scientific, Inc., Wilmington, DE,
USA). cDNA was generated using a PrimeScript™ RT kit (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. RT-qPCR reactions were performed as follows: 2 min at
50°C, 10 min at 95°C, followed by 40 cycles at 95°C for 15 sec and
1 min at 60°C, and an extension step at 72°C for 5 min using the
ABI 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA,
USA). Each sample was analyzed at least three times. The relative
expressions levels were normalized to endogenous controls and were
expressed as 2−ΔΔCq (14).
GHET1 and GAPDH primers were designed as follows: GHET1, forward
5′-TACCACACCCTTTCTTGCCC-3′, reverse 5′-GGGAGCCAAAAGGGTCA-3′; and
GAPDH, forward 5′-GGGAGCCAAAAGGGTCAT-3′ and reverse
5′-GAGTCCTTCCACGATACCAA-3′.
Western blot analysis
Cells were lysed using radioimmunoprecipitation
assay buffer (Beyotime Institute of Biotechnology, Shanghai, China)
and the protein concentration was measured using Bradford Protein
Assay kit (Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. Proteins (50 µg) were prepared in 1X
sodium dodecyl sulfate buffer, separated by 8–12% SDS-PAGE and
transferred onto polyvinylidene fluoride membranes. The membranes
were blocked with 5% nonfat milk in Tris-buffered saline-Tween (25
mm Tris, pH 8.0, 150 mm NaCl, and 0.05% Tween-20) for 2 h at 37°C,
then incubated with primary antibodies overnight at 4°C: E-cadherin
(Cat. No. 14472S; 1:1,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA), Fibronectin (Cat. No. F0916; 1:1,000;
Sigma-Aldrich), Vimentin (Cat. No. 49636; 1:1,000, Cell Signaling
Technology, Inc.) and GAPDH (Cat. No. 97166; 1:10,000; Cell
Signaling Technology, Inc.), and with horseradish
peroxidase-conjugated goat anti-mouse immunoglobulin G antibody
(Cat. No. 7076; 1:10,000; Cell Signaling Technology) for 1 h at
37°C. Enhanced chemiluminescence reagent (Merck KGaA) was used to
detect the signal on the membrane. The data were analyzed via
densitometry using Image-Pro Plus software 6.0 (Media Cybernetics,
Rockville, MD, USA) and normalized to the expression of the
internal control (β-actin).
Cell Counting Kit-8 (CCK-8) cell
proliferation assay
The proliferation of 786-O and A498 cells was
assessed using the CCK-8 assay, according to the manufacturer's
protocol. Cells in the logarithmic growth phase were seeded into a
96-well culture plate at 3.5×104/well, and at 12 h, the
cells were transfected with either the negative control siRNA or
the GHET1-siRNA for 12 h. After 0, 24, 48 or 72 h of transfection,
10 µl CCK-8 reagents (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) were added to each well, and absorbance was
measured at 450 nm using an enzyme immunoassay analyzer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Each experiment was
repeated at least three times.
Colony formation assay
786-O and A498 cells (500/well) in the logarithmic
growth phase were transfected with the control siRNA or GHET1
siRNA, and were plated in 6-well plates. After 2 weeks, cells were
washed twice with phosphate-buffered saline (PBS, Sigma-Aldrich),
then fixed with 4% paraformaldehyde (Sigma-Aldrich) for 15 min and
stained with 0.5% crystal violet (Sigma-Aldrich) for 20 min at
37°C. The number of colonies was calculated by use of ImageJ
software V.1.48 (National Institutes of Health, Bethesda, MD, USA).
The experiment was performed in triplicate.
Cell migration assay
A total of 1×105 786-O and A498 cells
were transfected with GHET1-siRNA or control siRNA for 6 h, and a
scratch was made in the cell monolayer. Cell debris was washed by
PBS and cells were incubated at 37°C for 48 h. Images of the cells
were captured under an inverted microscope (×10 magnification,
Leica Microsystems GmbH, Wetzlar, Germany) 0 and 24 h after
scratching.
Statistical analysis
GraphPad Prism 5 software (GraphPad Software, Inc.,
La Jolla, CA, USA) was used for statistical analysis, and data are
presented as the means ± standard deviation from three independent
experiments. All P-values were calculated using unpaired Student's
t-test or one-way analysis of variance with Tukey's post hoc test.
Paired Student's t-test was applied to analyze the differences of
GHET1 expression levels between RCC tissues and adjacent normal
tissues. The Pearson's χ2 test was used to determine the
difference between GHET1 expression levels and clinicopathological
factors. P<0.05 was considered to indicate a statistically
significant difference.
Results
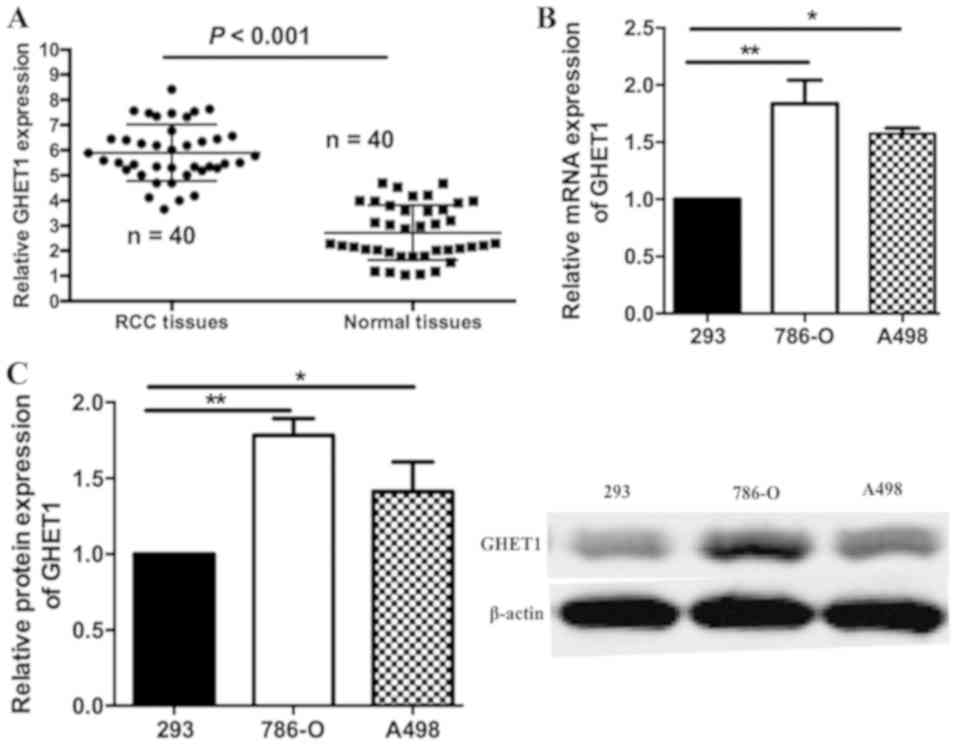
GHET1 is upregulated in RCC tissues
and cell lines
In order to investigate the biological function of
GHET1 in RCC, the expression levels of GHET1 were assessed in 40
RCC tissues and adjacent normal tissues by RT-qPCR. As illustrated
in Fig. 1A, GHET1 expression was
significantly increased in the RCC samples compared with the
adjacent tissues (P<0.001). The expression levels of GHET1 in
293, 786-O and A498 cell lines were also measured. When normalized
to 293 levels, GHET1 was overexpressed in 786-O and A498 cells
(Fig. 1B). Relative protein
expression levels of GHET1 were similar, as determined by western
blotting (Fig. 1C). These results
indicated that GHET1 may act as an oncogene in RCC progression.
Association between GHET1 expression
and clinical characteristics in RCC
The possible association between the expression
levels of GHET1 and the clinicopathological characteristics of
patients was then measured. A total of 40 RCC tissues were
classified into two groups, based on the median ratio of relative
GHET1 expression (6.2), as follows: The high-GHET1 group (n=29)
with GHET1 expression ratio≥median ratio; and the low-GHET1 group
(n=11) with GHET1 expression ratio≤median ratio. As demonstrated in
Table I, upregulated GHET1 expression
was associated with histological grade, clinical stage and
metastasis, but not with age and sex. Notably, a higher number of
patients with increased GHET1 expression levels were in the III–IV
phases (P<0.05) or suffered from cancer metastasis (P<0.05).
These results suggested that GHET1 may serve an important role in
RCC development.
 | Table I.Association between GHET1 expression
and clinical characteristics in renal cell carcinoma. |
Table I.
Association between GHET1 expression
and clinical characteristics in renal cell carcinoma.
|
|
| Relative expression
of GHET1 |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
characteristic | Number | High (n) | Low (n) | χ2 | P-valuea |
|---|
| Sex |
|
|
| 1.189 | 0.916 |
| Male | 28 | 21 | 7 |
|
|
|
Female | 12 | 8 | 4 |
|
|
| Age (years) |
|
|
| 1.332 | 0.818 |
|
<60 | 24 | 15 | 9 |
|
|
| ≥60 | 16 | 14 | 2 |
|
|
| Histological
grade |
|
|
| 7.122 | 0.035a |
|
I–II | 7 | 5 | 2 |
|
|
|
III–IV | 33 | 24 | 9 |
|
|
| Clinical stage |
|
|
| 8.322 | 0.029a |
|
I–II | 10 | 6 | 4 |
|
|
|
III–IV | 30 | 23 | 7 |
|
|
| Metastasis |
|
|
| 6.977 | 0.034a |
|
Yes | 31 | 22 | 9 |
|
|
| No | 9 | 7 | 2 |
|
|
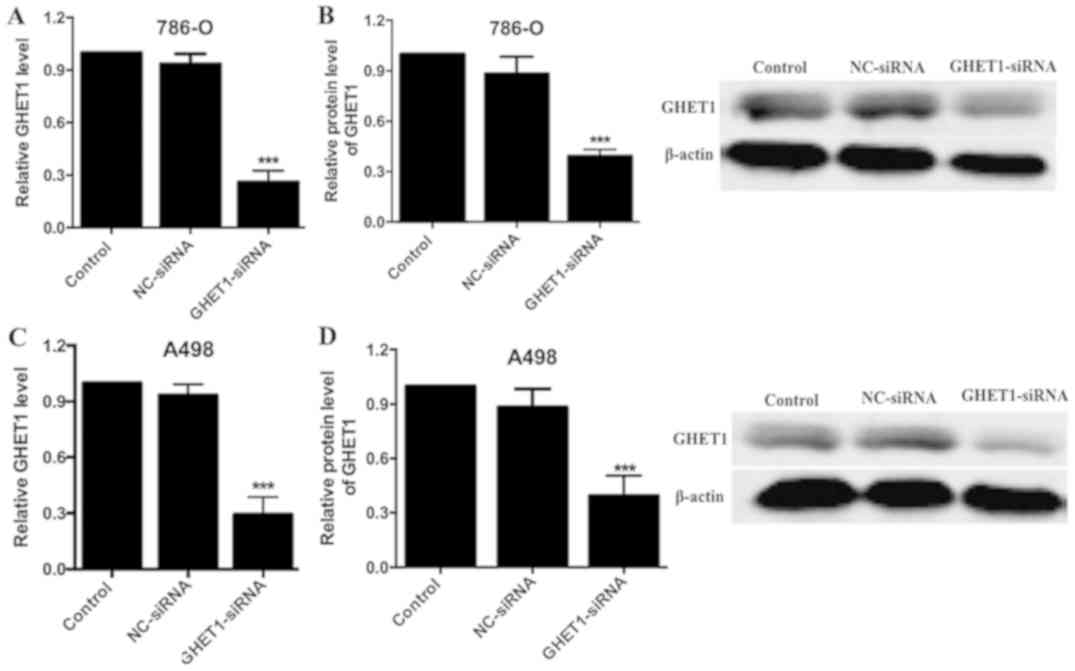
GHET1-siRNA induces effective
silencing of GHET1
To investigate the role of GHET1 in RCC cells,
GHET1-siRNA or control siRNA (NC group) plasmids were transfected
into 786-O and A498 cells (Fig. 2).
After 48 h, the interference efficiency was demonstrated to be
significant in the GHET1-siRNA group compared with in the NC group
(P<0.05; Fig. 2A and C). Similar
results were detected with regards to GHET1 protein expression
(P<0.05; Fig. 2B and D),
confirming effective siRNA silencing.
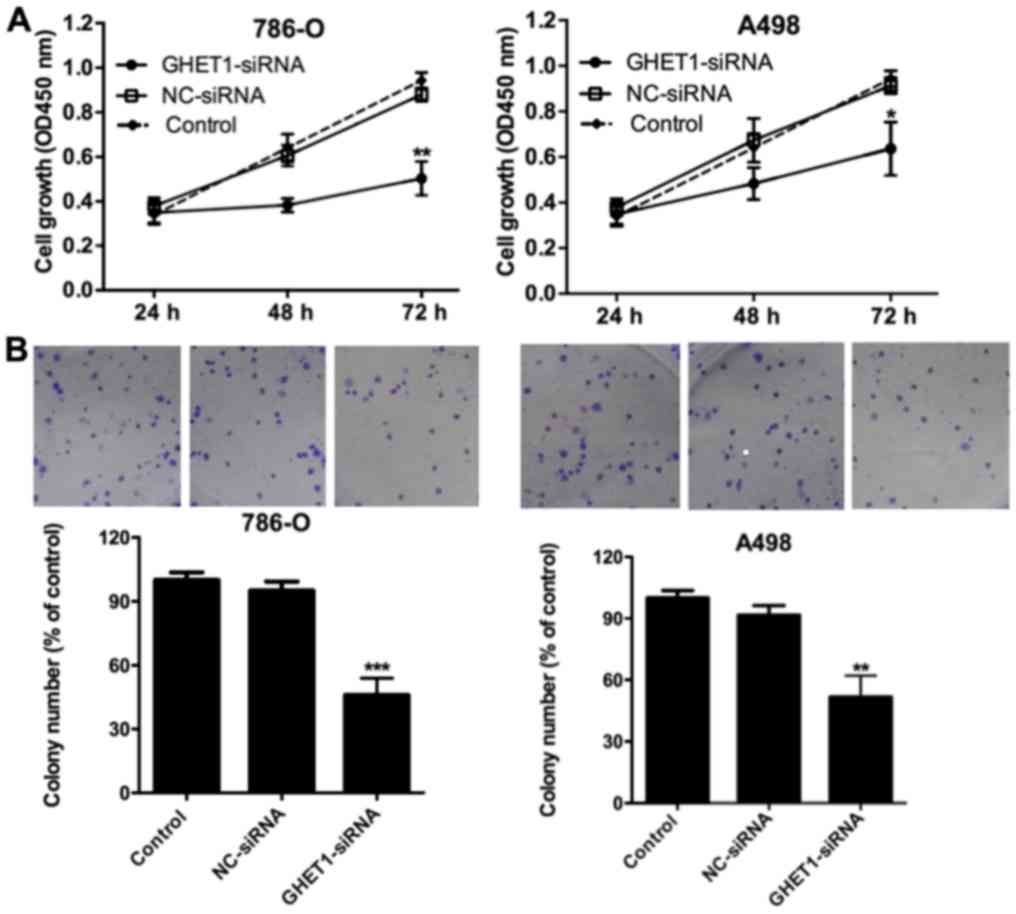
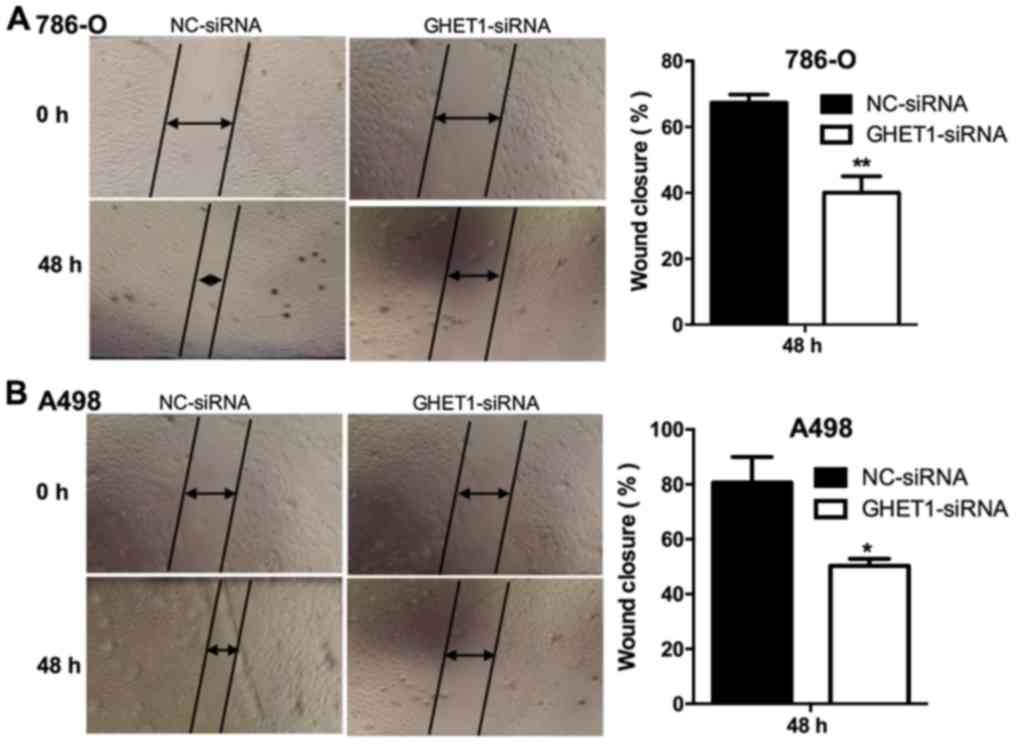
GHET1 knockdown inhibits cell
proliferation and migration
The CCK-8 assay was used to detect cell
proliferation. After 48 h GHET1-siRNA transfection, proliferation
was significantly inhibited in the 786-O and A498 cell lines
(P<0.05; Fig. 3A). GHET1 silencing
had a similar effect on the colony formation of 786-O and A498 cell
lines (P<0.01; Fig. 3B). The
scratch assay demonstrated that cell migratory ability was
significantly decreased in the GHET1-siRNA group compared with the
NC group (P<0.01 Fig. 4A;
(P<0.05, Fig. 4B).
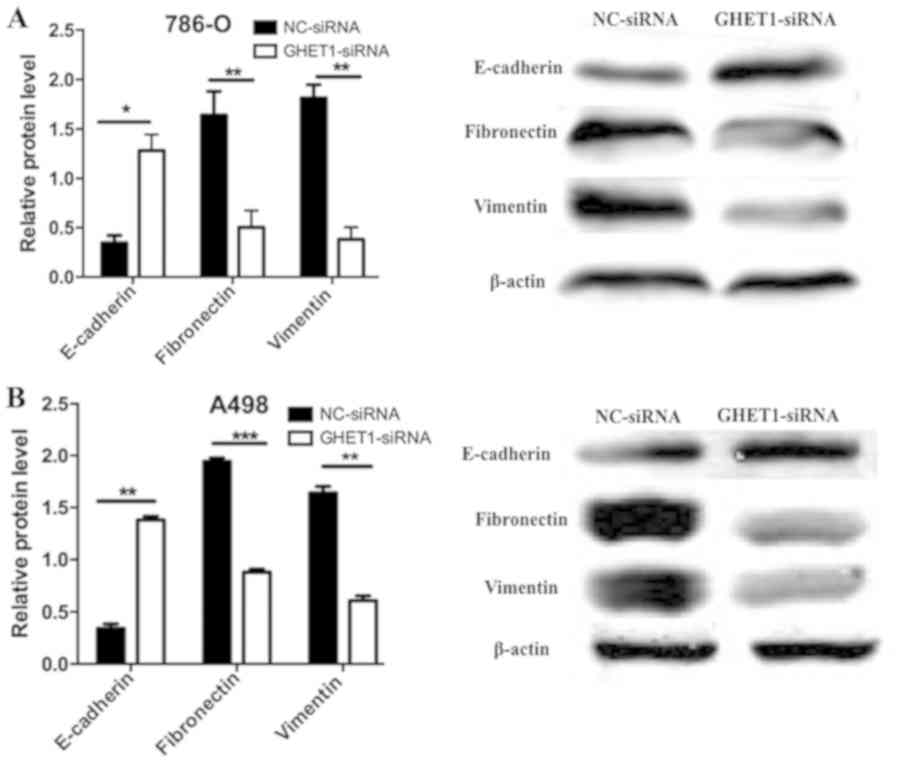
GHET1 regulates
epithelial-mesenchymal-transition (EMT)-associated protein
expression
The EMT has been reported to serve a crucial role in
cancer metastasis and expansion of the cancer stem cell population
(15). In the present study, the
possible effects of GHET1 on EMT were therefore assessed. Western
blotting confirmed that GHET1 knockdown induced a significant
increase in E-cadherin expression, whereas fibronectin and vimentin
protein levels were significantly reduced (P<0.05; Fig. 5A and B).
Discussion
Although various treatment methods are available for
RCC, including surgery, chemotherapy and minor biotherapy, the
prognosis of advanced or metastatic RCC remains poor. In the past
20 years, cytokine treatment has become a standard therapy for
metastatic RCC. Interferon-α, interleukin-2, sunitinib, sorafenib
and bevacizumab have demonstrated favorable results in clinical
trials involving patients with metastatic RCC, although the drug
toxicity has not been established (16–19). A
better understanding of the mechanisms underlying RCC and the
identification of novel therapeutic targets are therefore a
priority to develop novel metastatic RCC treatments and improve
prognosis. The incidence and development of RCC being very complex,
various factors, such as lncRNAs, have been considered to serve a
role in RCC diagnosis and therapy.
The analysis of extensive gene expression and copy
number variation of lncRNAs has demonstrated that alteration of
their expression is associated with tumor development. Cao et
al reported that downregulation of cancer susceptibility
candidate 2 (CASC2) lncRNA by microRNA-21 increases RCC
proliferation and migration, thus suggesting that CASC2 may be a
tumor suppressor gene in RCC (20).
In addition, the suppressing androgen receptor in renal cell
carcinoma (SARCC) lncRNA has been demonstrated to attenuate RCC
cell invasion, migration and proliferation in vitro and
in vivo via altering androgen receptor miRNA-143-3p signals;
SARCC may therefore be associated with a better prognosis in
patients with RCC (21). HOX
transcript antisense RNA (HOTAIR) is another lncRNA involved in
RCC. Its expression has been demonstrated to be increased in RCC,
thus promoting cell proliferation, migration and the EMT process,
and inhibiting cell apoptosis via microRNA-217/hypoxia-inducible
factor 1-α/AXL receptor tyrosine kinase signaling (22). In addition, GHET1 has been identified
as a tumor promoter and prognostic biomarker in various types of
cancer, including hepatocellular carcinoma (23), pancreatic ductal adenocarcinoma
(24), non-small cell lung cancer
(25) and breast cancer (26).
The present study aimed to characterize the role of
GHET1 in RCC. GHET1 was significantly overexpressed in RCC tissues
and 786-O and A498 cell lines, compared with in adjacent normal
tissues and 293 cells, respectively. Furthermore, the knockdown of
GHET1 in 786-O and A498 cells significantly inhibited cell
proliferation and migration. These findings suggested that
downregulation of GHET1 may inhibit the development and progression
of RCC.
Although EMT was originally defined in the context
of developmental stages, its evolution from normal to transformed
cell phenotype has been associated with carcinoma progression
(27). Essential hallmarks of EMT
include loss of E-cadherin, and increased vimentin and fibronectin
(28). In addition, numerous
transcription factors including snail, slug, zing finger E-box
binding homeobox 1 and twist have been demonstrated to be involved
in the EMT process (29). A previous
study identified that some lncRNAs serve a role in the regulation
of EMT. For example, the suppression of HOTAIR can reverse EMT in
gastric cancer and reduce invasiveness, thus suggesting that HOTAIR
may be a novel target in the diagnosis and treatment of gastric
cancer (30). In the present study,
the expression of hallmarks and transcription factors associated
with EMT were examined following GHET1 silencing in RCC cells.
Knockdown of GHET1 was revealed to downregulate vimentin and
fibronectin expression, and to upregulate E-cadherin. These results
suggested that GHET1 knockdown was associated with EMT
modifications.
In conclusion, to the best of our knowledge, the
present study is the first to explore the expression and biological
functions of GHET1 in RCC. The results demonstrated that GHET1 was
highly expressed in RCC tissues and cells, which was positively
associated with the histological grade and clinical stage of
cancer, and the presence of metastasis. In addition, inhibition of
GHET1 expression decreased cell proliferation and migration. This
inhibitory effect on tumor progression may be mediated by the EMT
process, and may lead to the development of a novel diagnostic
marker and therapeutic strategy for RCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WX, XL and MM performed RT-qPCR and western-blot
assays. XY and BG performed the cells proliferation and migration
experiments. JC conceptualised the study and wrote the original
draft. TS and QC collected and curated the data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Human Ethics
Committee of The First Affiliated Hospital of Nanchang University
(Nanchang, China). All patients provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lam JS, Leppert JT, Belldegrun AS and
Figlin RA: Novel approaches in the therapy of metastatic renal cell
carcinoma. World J Urol. 23:202–212. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Janzen NK, Kim HL, Figlin RA and
Belldegrun AS: Surveillance after radical or partial nephrectomy
for localized renal cell carcinoma and management of recurrent
disease. Urol Clin North Am. 30:843–852. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kugel JF and Goodrich JA: Non-coding RNAs:
Key regulators of mammalian transcription. Trends Biochem Sci.
37:144–151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fachel AA, Tahira AC, Vilella-Arias SA,
Maracaja-Coutinho V, Gimba ER, Vignal GM, Campos FS, Reis EM and
Verjovski-Almeida S: Expression analysis and in silico
characterization of intronic long noncoding RNAs in renal cell
carcinoma: Emerging functional associations. Mol Cancer.
12:1402013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bhan A and Mandal SS: LncRNA HOTAIR: A
master regulator of chromatin dynamics and cancer. Biochim Biophys
Acta 1856. 151–164. 2015.
|
|
7
|
Cui Y, Zhang F, Zhu C, Geng L, Tian T and
Liu H: Upregulated lncRNA SNHG1 contributes to progression of
non-small cell lung cancer through inhibition of miR-101-3p and
activation of Wnt/β-catenin signaling pathway. Oncotarget.
8:17785–17794. 2017.PubMed/NCBI
|
|
8
|
She K, Yan H, Huang J, Zhou H and He J:
miR-193b availability is antagonized by LncRNA-SNHG7 for
FAIM2-induced tumour progression in non-small cell lung cancer.
Cell Prolif. 51:2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang F, Xue X, Zheng L, Bi J, Zhou Y, Zhi
K, Gu Y and Fang G: Long non-coding RNA GHET1 promotes gastric
carcinoma cell proliferation by increasing c-Myc mRNA stability.
FEBS J. 281:802–813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou J, Li X, Wu M, Lin C, Guo Y and Tian
B: Knockdown of long noncoding RNA GHET1 inhibits cell
proliferation and invasion of colorectal cancer. Oncol Res.
23:303–309. 2016. View Article : Google Scholar
|
|
11
|
Hugo H, Ackland ML, Blick T, Lawrence MG,
Clements JA, Williams ED and Thompson EW: Epithelial-mesenchymal
and mesenchymal-epithelial transitions in carcinoma progression. J
Cell Physiol. 213:374–383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weischenfeldt J, Simon R, Feuerbach L,
Schlangen K, Weichenhan D, Minner S, Wuttig D, Warnatz HJ, Stehr H,
Rausch T, et al: Integrative genomic analyses reveal an
androgen-driven somatic alteration landscape in early-onset
prostate cancer. Cancer Cell. 23:159–170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abell AN and Johnson GL: Implications of
mesenchymal cells in cancer stem cell populations: Relevance to
EMT. Curr Pathobiol Rep. 2:21–26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thiounn N, Mathiot C, Dorval T, Flam TA,
Tartour E, Mosseri V, Zerbib M, Fridman WH and Debre B: Lack of
efficacy of low-dose subcutaneous recombinant interleukin-2 and
interferon-alpha in the treatment of metastatic renal cell
carcinoma. Br J Urol. 75:586–589. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abel EJ and Wood CG: Cytoreductive
nephrectomy for metastatic RCC in the era of targeted therapy. Nat
Rev Urol. 6:375–383. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Faris JE and Michaelson MD: Targeted
therapies: Sunitinib versus interferon-alpha in metastatic RCC. Nat
Rev Clin Oncol. 7:7–8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grünwald V, Weikert S, Seidel C, Busch J,
Johannsen A, Fenner M, Reuter C, Ganser A and Johannsen M: Efficacy
of sunitinib re-exposure after failure of an mTOR inhibitor in
patients with metastatic RCC. Onkologie. 34:310–314. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao Y, Xu R, Xu X, Zhou Y, Cui L and He X:
Downregulation of lncRNA CASC2 by microRNA-21 increases the
proliferation and migration of renal cell carcinoma cells. Mol Med
Rep. 14:1019–1025. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhai W, Sun Y, Guo C, Hu G, Wang M, Zheng
J, Lin W, Huang Q, Li G, Zheng J and Chang C: LncRNA-SARCC
suppresses renal cell carcinoma (RCC) progression via altering the
androgen receptor(AR)/miRNA-143-3p signals. Cell Death Differ.
24:1502–1517. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong Q, Li O, Zheng W, Xiao WZ, Zhang L,
Wu D, Cai GY, He JC and Chen XM: LncRNA HOTAIR regulates HIF-1α/AXL
signaling through inhibition of miR-217 in renal cell carcinoma.
Cell Death Dis. 8:e27722017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jin L, He Y, Tang S and Huang S: LncRNA
GHET1 predicts poor prognosis in hepatocellular carcinoma and
promotes cell proliferation by silencing KLF2. J Cell Physiol.
233:4726–4734. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou HY, Zhu H, Wu XY, Chen XD, Qiao ZG,
Ling X, Yao XM and Tang JH: Expression and clinical significance of
long-non-coding RNA GHET1 in pancreatic cancer. Eur Rev Med
Pharmacol Sci. 21:5081–5088. 2017.PubMed/NCBI
|
|
25
|
Guan ZB, Cao YS, Li Y, Tong WN and Zhuo
AS: Knockdown of lncRNA GHET1 suppresses cell proliferation,
invasion and LATS1/YAP pathway in non small cell lung cancer.
Cancer Biomark. 21:557–563. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song R, Zhang J, Huang J and Hai T: Long
non-coding RNA GHET1 promotes human breast cancer cell
proliferation, invasion and migration via affecting epithelial
mesenchymal transition. Cancer Biomark. 22:565–573. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Savagner P: The epithelial-mesenchymal
transition (EMT) phenomenon. Ann Oncol. 21 Suppl 7:vii89–vii92.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tam WL and Weinberg RA: The epigenetics of
epithelial-mesenchymal plasticity in cancer. Nat Med. 19:1438–1449.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Djebali S, Davis CA, Merkel A, Dobin A,
Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F,
et al: Landscape of transcription in human cells. Nature.
489:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu ZY, Yu QM, Du YA, Yang LT, Dong RZ,
Huang L, Yu PF and Cheng XD: Knockdown of long Non-coding RNA
HOTAIR suppresses tumor invasion and reverses
Epithelial-mesenchymal transition in gastric cancer. Int J Biol
Sci. 9:587–597. 2013. View Article : Google Scholar : PubMed/NCBI
|