Metastatic growth relies on the microcirculation to
provide adequate nutrients; as such, tumors require extra vascular
formation by way of angiogenesis and vascular mimicry (VM)
(1–4).
Highly aggressive cancers are dependent on angiogenesis and/or VM,
making them potential targets for future therapies (5–7). However,
to date, anti-angiogenic or anti-VM therapies have elicited only
modest effects, suggesting that this approach alone may be
ineffective (8,9).
It has been suggested that the tumor
microenvironment (TME), which contains various stromal cells as
well as the extracellular matrix (ECM), is critical to
tumorigenesis, tumor neo-angiogenesis and cancer progression
(10,11). Cancer-associated fibroblasts (CAFs),
which are the primary stromal cells within the TME, may contribute
to tumor neo-angiogenesis via proteomic and degradomic alterations
(12,13). As such, understanding how CAFs and
tumor neo-angiogenesis interact may be beneficial for developing
novel tumor treatments. In the present review, blood supply, TME,
CAFs and other research focused on CAF regulation of tumor
neo-angiogenesis are discussed. Also reviewed are the underlying
molecular signaling pathways and potential anti-angiogenic and
anti-VM therapeutic targets.
Tumor angiogenesis is essential for providing
adequate nutrition for tumorigenesis and tumor progression; without
angiogenesis, tumors are unable to grow and metastasize.
Angiogenesis is associated with the ECM, which provides structural
support and delivers molecular signals during all stages of tumor
angiogenesis (5,6,11). Drugs
that are able to block angiogenesis-dependent tumor growth are of
great interest, particularly the use of cleaved proteins,
monoclonal antibodies, synthetic small molecules and natural
products. A number of angiogenic inhibitors, including bevacizumab
[a vascular endothelial growth factor (VEGF) inhibitor], sorafenib,
sunitinib [a VEGF receptor (VEGFR) and platelet-derived growth
factor receptor (PDGFR) inhibitor], erlotinib (an epidermal growth
factor receptor inhibitor), thrombospondin-1 [a VEGF and fibroblast
growth factor (FGF) inhibitor], TNP-470 [a methionine
aminopeptidase-2 inhibitor], SU-5416, endostatin (a VEGFR
inhibitor), celastrol and angiostatin, have been reported to have
antitumor and anti-angiogenic activities (6,14–17). However, previous studies suggest that
anti-angiogenic therapy has limited benefits and that blocking
angiogenesis alone may not be effective (8,9). As such,
combination treatments may provide improved anti-angiogenic and
antitumor activities (6,18,19).
VM, a newly defined pattern for tumor blood supply,
differs from angiogenesis and vasculogenesis as it does not require
endothelial cells (ECs), allowing highly aggressive tumor cell
behavior and the expression of EC-associated genes to form ECM-rich
tubular networks (4). This provides
the required microcirculation for tumor growth and is associated
with poor prognosis in patients with highly aggressive malignant
tumors, such as melanoma and gallbladder cancer (3,7,20–22). The
molecular mechanisms underlying tumor VM formation are associated
with activation of the phosphoinositide 3-kinase (PI3K)/matrix
metalloproteinases (MMPs)/laminin 5γ2 (Ln-5γ2), epithelial cell
kinase/ephrin type A receptor 2 (EphA2)/focal adhesion kinase (FAK)
and VEGF-α signaling pathways (7). VM
formation in human gallbladder was identified to be triggered by
activation of the PI3K/MMPs/Ln-5γ2 and EphA2/FAK/paxillin signaling
pathways in three-dimensional matrices of GBC-SD cells in
vitro and GBC-SD nude mouse xenografts in vivo (23–26).
Anti-angiogenic therapy has been identified to exhibit promising
effects on VM in pancreatic cancer, hepatocellular cancer,
hepatoblastoma, breast cancer, glioblastoma, gastric cancer and
ovarian cancer (27–34). However, owing to individual
differences, these studies have not been applied clinically or, if
they have, have had little effect. Considering the number of cells
involved in angiogenesis and VM, as well as the different molecular
regulation mechanisms, further understanding of the underlying
molecular mechanisms of tumor microcirculation is required in order
to investigate joint targets and develop novel drugs that target
angiogenesis and VM.
The majority of human tumors originate from cancer
epithelial cells, and for years tumors were considered to be
transformed cells with cell-autonomous hyperproliferative and
invasive properties. On this basis, treatments were targeted at the
tumor itself. However, with the emergence of drug resistance and
anti-angiogenic tolerance, tumor occurrence and development are
associated with not only the tumor itself, but also with adjacent
activated stromal cells and the associated chemokine and cytokine
production (10,11). Studies have indicated that tumor
progression is associated with the microenvironment of the
tumor-host interface, which comprises tumor and stromal cells, as
well as genetic mutations and the unlimited proliferation of tumor
cells. Cancer-associated stromal cells, including inflammatory
cells, vascular cells and CAFs, have a complex tumor-stromal
interaction (10,11).
CAFs, which include activated fibroblasts or
myofibroblasts around tumor epithelial cells, are the most
important host stromal cells in the TME and regulate the
microenvironment balance at the tumor-host interface via
cell-to-cell contact, soluble factor secretion, ECM modification
and promotion of malignant transformation of epithelial cells
(10,12,13).
Unlike normal fibroblasts, CAFs express α-smooth muscle actin
(α-SMA), fibroblast activation protein (FAP) and
fibroblast-specific protein-1; they have different gene expression
profiles compared with normal fibroblasts (10,12,13,35).
CAFs mediate paracrine or autocrine factors between tumor and
stromal cells to influence TME and affect tumor dormancy or growth,
invasion, angiogenesis and therapeutic resistance (10,12,13,35–39),
all of which are associated with poor prognosis in patients with
cancer (40,41). Madar et al (42) proposed a novel description of CAFs to
illustrate that they are not a single cell type, rather comprising
various activated cells. Research indicates that CAF inhibition
prolongs the survival of patients with pancreatic cancer compared
with chemotherapy alone, and that anti-CAFs prevent tumor
progression prior to tumor invasion (43–45).
CAFs have a stable genome, are not prone to antigen
loss, are tolerant of chemotherapy, are heterogeneous and account
for between 50 and 90% of solid tumors, as stromal cells are rich
targets and have complex interactions with tumor cells. Therefore,
CAFs and their markers may be effective targets of antitumor
therapy and drug design (42,43). However, crosstalk and interactions
between CAFs and tumor cells and the underlying molecular
mechanisms are not fully understood. It has been identified that
stromal cell-derived factor-1 (SDF-1)/CXC chemokine 12 (CXC12)
promotes angiogenesis in breast cancer (35) and VEGF secreted by CAFs promotes tumor
angiogenesis (46). The use of
conditioned media and human umbilical vein endothelial cells
(HUVECs) in co-culture has suggested that cholangiocarcinoma cells
in hepatic stellate dual-conditioned medium had the most marked
HUVEC lumen formation ability (47).
Furthermore, tumor cells stimulate fibroblasts to produce
angiogenic factors with indirect tumor-stromal cell interaction
patterns (48) and CAFs are the
principal secretors of MMP-2, membrane type 1-MMP (MT1-MMP) and
VEGF. PI3K is involved in VM formation by MMP-2 and MT1-MMP,
whereas activated MMP-2 and MT1-MMP degrade Ln-5γ2 into the
pre-migratory fragments γ2 and γ2×, which are enriched around tumor
cells to promote tumor cell invasion and VM formation. As such,
antibodies against MMP-2 and MT1-MMP, PI3K inhibitors and Ln-5γ2
target short interfering RNA are able to inhibit VM formation
(7). In melanoma cells, VEGF and
reactive oxygen species (ROS) regulate cell formation in the
lumen-like structure, an effect that is reversed by antioxidants
(49). Zinc finger E-box-binding
homologous box (ZEB1) promotes VM formation in colon cancer via
epithelial-mesenchymal transition (EMT) (50). Improving our understanding of the
integrated mechanisms by which CAFs modulate angiogenesis and VM in
human tumors is key to identifying potential novel therapeutic
targets for human tumors.
Cells and non-cellular components are required for
tumor neo-angiogenesis, and diverse molecular signaling pathways
are involved (Table I).
VEGF, which signals via its cognate VEGFR, is
required for angiogenesis under normal conditions and in cancer
(51). It has been identified that
activated stromal cells secrete specific molecules that promote the
expression of VEGF from tumor cells, and that certain factors
secreted by CAFs interact with VEGF to increase tumor angiogenesis
and invasiveness. Esophageal squamous cell carcinoma is a highly
angiogenic tumor type; biochemical studies have identified that the
VEGF signaling pathway derived from activated stromal fibroblasts
induces capillary formation in this disease (52). A study involving lung squamous cancer
cells revealed that podoplanin downregulated VEGF-C and decreased
angiogenic and lymphangiogenic metastases (53). Expression of podoplanin, which
promotes breast cancer angiogenesis and lymphangiogenesis (54), is often reported in CAFs, and
podoplanin has been identified to stimulate angiogenesis and
lymphangiogenesis in invasive ductal carcinoma of the breast via
upregulation of VEGF-C rather than VEGF-A or VEGF-D in cancer cells
(55,56). Galectin-1, a member of the galectin
family of β-galactoside-binding proteins, is involved in cancer
cell invasion and tumor angiogenesis (57–59).
Galectin-1 has been reported to be upregulated in CAFs of gastric
cancer cells (60), and it has been
demonstrated that endogenous CAF-derived galectin-1 is essential
for accelerating angiogenesis by enhancing VEGF expression in
gastric cancer and VEGFR2 phosphorylation in epithelial cells (ECs)
(61). The interaction between
angiomodulin (AGM) and VEGF also facilitates angiogenesis (62). AGM has been reported to be
overexpressed by CAFs in breast, colon, lung and uterus carcinomas
(63), and so is considered to be a
marker of cancer vasculature permeability (64).
Oxidative stress is known to promote tumor
angiogenesis. G-protein-coupled estrogen receptor (GPER),
hypoxia-inducible factor-1α (HIF-1α) and ROS are involved in the
activation of fibroblasts and upregulation of VEGF expression. GPER
knockdown eliminates VEGF expression in activated CAFs under
hypoxic conditions, and it has been identified that breast CAFs
promote hypoxia-dependent tumor angiogenesis in a
HIF-1α/GPER-dependent manner by mediating the expression of VEGF
(65,66). A study of p53-deficient colorectal
xenograft tumor cells revealed that functional loss of p53
significantly increased tube formation and enhanced
neovascularization in a VEGF-dependent manner in vitro and
in vivo by upregulating ROS to activate stromal fibroblasts
(67). Similarly, Jo et al
(68) reported that oxidative stress
stimulates angiogenesis in hepatocellular carcinoma via the protein
kinase B/VEGF pathway.
It has been identified that endothelial progenitor
cells (EPCs) are often located near microvessels in nasopharyngeal
carcinoma, and that VEGF-A is overexpressed in stromal and tumor
cells, indicating that CAFs may enhance angiogenesis in a VEGF-A-
and SDF-1-dependent manner by recruiting EPCs from the bone marrow
into tumor stroma (69).
In conclusion, VEGF is an important factor that
promotes tumor angiogenesis by interacting with the secretome of
CAFs. This suggests that anti-VEGF therapy should target VEGF
itself as well as VEGF-coupled molecules. Such therapies may have
marked anti-angiogenic effects.
SDF-1, or CXC12, is a homeostatic chemokine that
signals via CXCR-4, a G-protein-coupled receptor that is primarily
secreted by hematopoietic progenitor cells and EPCs within injured
sites. The SDF-1/CXCR-4 signaling axis has been reported to serve a
role in recruiting EPCs to tumor stroma (70). A co-implantation tumor xenograft model
revealed that CAFs were essential for angiogenesis-associated tumor
progression in invasive human breast carcinoma because of their
ability to secrete SDF-1 and recruit EPCs to the tumor site via
CXCR-4, which is expressed by carcinoma cells (35). The same functional role of SDF1 was
reported in nasopharyngeal carcinoma (69).
It has been reported that the SDF-1/CXCR-4 axis
derived from CAFs boosts tumor angiogenesis or VM formation;
however, its intrinsic regulatory mechanisms have not been fully
elucidated. It has been suggested that fibroblast-derived SDF-1
enhances the invasiveness of pancreatic cancer cells, whereas SDF-1
promotes angiogenic responses in synergy with CXC chemokine ligand
(CXCL)8 (71). In mouse xenografts of
hepatocellular carcinomas, CAFs could form capillary-like
structures, namely VM, via paracrine transforming growth factor-β
(TGF-β) and SDF-1 (72). In human
fetal lung fibroblast (MRC-5) and human lung cancer (95D) cell
lines, the SDF-1/CXCR-4 axis participated in HUVEC and 95D cell
migration, as well as HUVEC tube formation and angiogenesis, but
not in HUVEC or 95D proliferation (73). An in vitro tube formation assay
of tongue cancer tissues revealed that tumor necrosis factor α
indirectly enhances cancer angiogenesis by increasing activated
fibroblast-derived SDF-1 (74).
Furthermore, cyclo-oxygenase-2 (COX-2) has been reported to
increase tumor stromal formation and angiogenesis by regulating the
pro-angiogenic microenvironment via SDF-1/CXCR-4 signaling
(75). These results suggest a
significant role for the stromal SDF-1/CXCR-4 axis in promoting
tumor growth and enhancing angiogenesis, in part via EPC
recruitment.
TGF-β is a pleiotropic growth factor that is
expressed by cancer and stromal cells. TGF-β/TGF-βR signaling is
required for advanced carcinogenesis via EMT induction,
angiogenesis and modification of the stromal compartment (76,77). It
has been identified that TGF-β and hypoxia work synergistically to
regulate VEGF expression in mRNA biosynthesis (78). However, extracellular TGF-β signaling
pathways that trigger angiogenesis are not well-understood. It has
been reported that TGF-β-primed myofibroblast secretion upregulates
VEGF, which in turn stimulates vascular formation by ECs
co-cultured in a three-dimensional assay (52). VM has been identified to be an
important complement in the tumor microcirculation (4,22,79) as it is a tubular structure that is
formed by tumor cells to transport nutrients, and molecules
including vascular endothelial cadherin (VE-cad), MMPs and laminin
have been reported to be critical for VM formation (7,80,81). TGF-β1 derived from CAFs was
identified to be a central molecular regulator of mesenchymal stem
cells, as well as having a tumor-promoting function in prostate
carcinoma progression (82).
Furthermore, co-implantation of CAFs with tumor cells in mouse
xenografts has been reported to promote the formation of
capillary-like structures in hepatocellular carcinomas. A gene
knockdown assay and gain- and loss-of-function assays revealed that
CAFs secrete TGF-β and SDF-1, which activate VE-cad, MMP2 and
Ln-5γ2 expression via TGF-βR1 and CXCR-4 in tumor cells to promote
the formation of VM. MicroRNA-101 subsequently inhibits VM
formation by suppressing the TGF-β and SDF-1 signaling pathways
(72).
Immunohistochemical data have revealed increased
angiogenesis in mice co-injected with TGF-β1-pretreated
Mc38-luc cells (colon adenocarcinoma cell lines from murine origin)
and CAFs, whereas tumor angiogenesis was significantly decreased in
mice treated with the TGF-β1-inhibitory peptide P17
(83). Similar results were observed
in esophageal squamous epithelial cells (84) and gastric tumor cells (85). Reverse transcription-polymerase chain
reaction and western blot analyses demonstrated that the expression
of angiogenic factor genes (α-SMA and VEGF) was significantly
increased in ovarian cancer cells treated with CAF condition
medium. α-SMA and VEGF downregulation in the intervention group
indicated that the effects of CAF angiogenesis are dependent on the
TGF-β signaling pathway (86).
However, the quantification of cluster of differentiation
(CD)31(+) vessels in breast cancer tissues via
immunostaining indicated no significant differences in angiogenesis
between TGF-β− xenograft and control groups, indicating
that TGF-β− fibroblasts stimulate tumor growth
independently of angiogenesis (87).
Further studies are required to explain the
inconsistencies in experimental results. Owing to the pleiotropy of
TGF-β, few studies have investigated the molecular mechanisms by
which it promotes tumor angiogenesis.
Angiogenesis begins with local degradation of the
basement membrane surrounding capillaries, following which ECs
undergo migration and morphogenesis, leading to new capillary
structures. It has been identified that the pro-angiogenic
properties of ECs were promoted by HGF upregulation (88), and there is crosstalk between the HGF
signaling pathway and VEGF-A in ECs (89).
Diverse stromal cells in the TME release HGF,
whereas cancer cells express its receptor c-MET (90). Conditioned medium genetic knockdown
and overexpression studies have revealed that the HGF/c-MET axis
serves a role in promoting tumorigenesis and invasion in various
types of human cancer (91–94). HGF acts as a significant angiogenic
growth factor in the development of esophageal squamous cell
carcinoma, and an in vitro study revealed that HGF
stimulates VEGF expression to enhance angiogenesis and tumor cell
invasion and migration (95,96). Microvessel density analysis in
esophageal squamous cell carcinoma tissues has revealed that
CAF-secreted TGF-β1 and HGF are associated with the
proliferation of epithelial cells and angiogenesis (84). As such, increasing our understanding
of HGF upregulation in tumor tissues and its interaction with VEGF
or TGF-β1 may be beneficial.
PDGF is a dimeric protein with several variants:
PDGF-AA, PDGF-BB, PDGF-AB, PDGF-CC and PDGF-DD (97). Different tumors may have different
PDGF/PDGFR expression patterns, and PDGF can be expressed by cancer
cells or CAFs (98,99). PDGFR signaling has been reported to be
associated with the progression of carcinoma (100) and the efficacy of chemotherapy
(101,102). In a cervical squamous cell carcinoma
transgenic mouse model, treatment with PDGFR kinase inhibitor
suppressed FGF2 and FGF7 expression in tumor stromal fibroblasts,
which decreased neo-angiogenesis and tumor growth (103). Increased PDGF-C expression in the
stromal cells of chemoresistant tumors contributes to increased
neo-angiogenesis during anti-VEGF treatment (99). Together, the results of these studies
suggest that CAFs may mediate resistance to anti-angiogenic therapy
as well as stimulating tumor angiogenesis.
FAP is a type II cell-surface-bound transmembrane
glycoprotein that is expressed in activated stromal fibroblasts
(104). An in vivo study of
FAP-deficient mouse models indicated a pro-angiogenic effect of FAP
(105). Furthermore, increased VEGF
expression is associated with FAP upregulation in human pancreatic
adenocarcinoma (106), suggesting
that CAF-driven angiogenesis may be regulated by FAP. A recent
study by Koczorowska et al (107) utilized FAP loss- and
gain-of-function systems to investigate the effects of FAP on the
biological function and secreted proteome of CAFs. The results
revealed that increased FAP activity led to a decrease in the
anti-angiogenic protein pigment epithelium-derived factor (PEDF)
and an increase in pro-angiogenic proteins angiopoietin-1 and
VEGF-C in the secretome of CAFs; this suggests that FAP serves a
pro-angiogenic function by affecting the balance of pro- and
anti-angiogenic mediators. A previous study revealed that
FAP-activated protoxin selectively killed FAP-expressing cells and
inhibited tumor growth in xenograft models (108), and a FAP-targeted DNA vaccine
shifted immune polarization from tumor-promoting to suppression of
recruitment of tumor-associated inflammatory cells. This decreased
metastasis, angiogenesis and lymphangiogenesis in a murine breast
cancer model (109). Together, these
previous studies indicate that FAP is associated with the secretion
of pro-angiogenic or anti-vascular proteins. It may therefore be
beneficial to determine the mechanisms responsible for maintaining
the balance of FAP regulatory proteins.
Fibroblasts are an important source of ECM-degrading
proteases, including MMPs, which are zinc-dependent endopeptidases;
this suggests that fibroblasts serve a critical role in ECM
homeostasis (110,111). Recent reports have revealed that
tumor (MMP-1, −2 and −14) and stromal (MMP-9, −13 and −14) MMP
expression is associated with tumor progression and is associated
with squamous cell carcinoma invasion (112). MMP-13 secreted by activated
fibroblasts promotes angiogenesis by releasing ECM-bound VEGF and
increasing the invasive capabilities of squamous cell carcinoma
cells in a mouse xenograft (113).
Zigrino et al (114) used
MMP-13+/+ and null mice to identify that stromal MMP-13
is required for melanoma invasion, angiogenesis and metastasis. In
summary, MMP serves an important function in regulating the
degradation and remodeling of ECM to provide suitable conditions
for tumor angiogenesis.
In order to positively participate in
neo-angiogenesis, human breast CAFs deposit and secrete more
adrenomedullin (AM) compared with normal fibroblasts. AM blockade
is able to impair tumor vascular formation and induce apoptosis
(115). Notch signaling is an
evolutionarily conserved pathway that regulates cell biological
characteristics, whose accommodative dysfunction has been
implicated in various tumors. It was recently reported that oral
squamous cell carcinoma promotes angiogenesis by inducing Notch3
expression in CAFs, whereas other cancer cells have been identified
to induce Notch3 expression in CAFs and promote angiogenesis
(116). Chloride intracellular
channel protein 3 (CLIC3) is a human intracellular Cl−
channel and scaffolding protein that is overexpressed in various
tumor types (117–119). An in vivo study confirmed
that CLIC3, an abundant component of the CAF secretome, increases
the invasive abilities of breast cancer cells as well as improving
functional vascularization, processes which require active
transglutaminase-2 (120). A
previous study revealed that cell division cycle 42 effector
protein 3 (Cdc42EP3) is required in CAFs to allow for the
expression of key angiogenic factors; Cdc42EP3 or SEPT2 depletion
in a mammary tumor model significantly decreased the angiogenic
potential of CAFs in vivo (121). COX-2 and endogenous prostaglandins
(PGs) are important determinants of tumor growth and
tumor-associated angiogenesis. COX-2 and PGE2/EP3/EP4
signaling regulate the tumor stromal pro-angiogenic
microenvironment and enhance stromal formation via CXCL12/CXCR-4
chemokine systems. Conversely, COX-2 inhibitor has been identified
to suppress stromal formation and angiogenesis in a mouse model of
Lewis lung carcinoma by inhibiting CXCL12/CXCR-4 in vivo
(75). The oncogenic potential of
chronic lymphoid leukemia (CLL)-derived exosomes has been
investigated in vitro and in vivo. CLL-derived
exosomes stimulate stromal cells to induce increased angiogenesis,
thus supporting survival and outgrowth (122). FGF-1/-3/FGF receptor (FGFR) 4
signaling derived from CAFs promotes colon cancer cell
proliferation and angiogenesis via activating mitogen-activated
protein kinase/extracellular-signal-regulated kinase (ERK)
kinase/ERK and modulating MMP-7 expression (123). Endoglin (eng) (124), a co-receptor for several members of
the TGF-β family, has also been reported to be associated with the
process of tumor angiogenesis. In a transgenic adenocarcinoma mouse
prostate (TRAMP) mouse model, increased vascularization occurred
was observed in eng+/+ mice compared with in
eng+/− mice, suggesting that eng is required for
multiple aspects of CAF function (125).
Tumor angiogenesis is a complicated and multi-factor
process. In order to improve our understanding of it, the molecular
delivery of each pathway and how multiple pathways are associated
with each other require further investigation.
As angiogenesis is a central process required for
the growth of solid tumors, studies to clarify the molecular basis
of CAFs-associated tumor angiogenesis have identified a number of
potential antitumor agents. Such agents include SU5416 and Z24,
which act as inhibitors of VEGFR and FGFR, respectively (126,127).
An in vitro study of 95D human lung cancer cells revealed
that the addition of exogenous SDF-1 attenuates the anti-angiogenic
effect of vinorelbine (VNR), suggesting that VNR may influence CAFs
to cause SDF-1 and suppress tumor angiogenesis (73). Utilizing the unique enzymatic activity
of FAP and its markedly restricted expression in reactive stroma,
several tumor treatment strategies have been developed, including
the use of small molecules and antibodies to inhibit enzyme
activity, as well as immunosuppression to increase intratumoral
drug concentrations. Potential mechanisms include decreasing blood
vessel density and targeting CAF-associated molecular pathways
(128). In vitro and in
vivo experiments indicated that lenvatinib also exerts an
anti-angiogenic effect by targeting VEGFR1-3 and FGFR1-4 (129). Ω-3 polyunsaturated fatty acids may
suppress MMP-9 expression and tumor angiogenesis (130). Initially, thalidomide was used as an
effective antiemetic drug, but its anti-angiogenic effects were
gradually identified and defined. Thalidomide can inhibit
angiogenesis by targeting the expression of VEGF, FGF, FDGF, HIF
and other molecules. However, clinical studies of thalidomide have
not identified consistent antitumor effects (131).
A number of studies have identified that
anti-pro-angiogenic agents alone are unable to elicit significant
antitumor effects, suggesting that certain factors, such as activin
A receptor like type 1 (ALK1), stabilize angiogenesis or convey
resistance to anti-angiogenic effects. ALK1 is a transmembrane
serine/threonine receptor kinase in the TGF-βR family that is
expressed on ECs (132). Intrinsic
angiostatic drug resistance and extrinsic mechanisms may serve a
role in the resistance of tumor cells to angiostatic therapy
(133). Crawford et al
(99) reported that CAFs from
resistant tumors in a murine lymphoma model secreted more
compensatory PDGF-C to promote sensitive tumor angiogenesis, even
in the presence of anti-VEGF treatment (99). This suggests that tumors are able to
activate stromal fibroblasts via different mechanisms. In a
glioblastoma model, high PDGF-C expression in CAFs was associated
with resistance to anti-VEGF treatment (134). On this basis, therapeutic regimens
targeting VEGF and PDGF were attempted in an advanced renal cell
carcinoma clinical trial; however, the trial had to be terminated
owing to marked toxicity (135). In
an animal experiment, treatment with CAFs-inhibitors plus
oxaliplatin significantly decreased tumor growth and angiogenesis
in colon cancer compared with the use of oxaliplatin or CAF
inhibitors alone (136).
Additional factors have been identified to be
associated with anti-angiogenesis. Kinugasa et al (137) observed that CAFs markedly express
CD44 in hypoxic and avascular areas, and that CD44 was markedly
increased following treatment with angiogenesis inhibitors in
vitro. Similarly, periostin, an ECM protein that is principally
produced by CAFs, was also hypothesized to be a crucial molecular
mediator involved in tumor resistance to anti-angiogenic therapy
(138).
These studies indicate that anti-angiogenesis
strategies must target angiogenic factors as well as internal
molecules that stabilize angiogenesis, while also considering
clinical toxicity. CAFs regulate tumor neo-angiogenesis; the
molecular signaling pathways by which CAFs may promote tumor
neo-angiogenesis and potential therapeutic targets for tumor
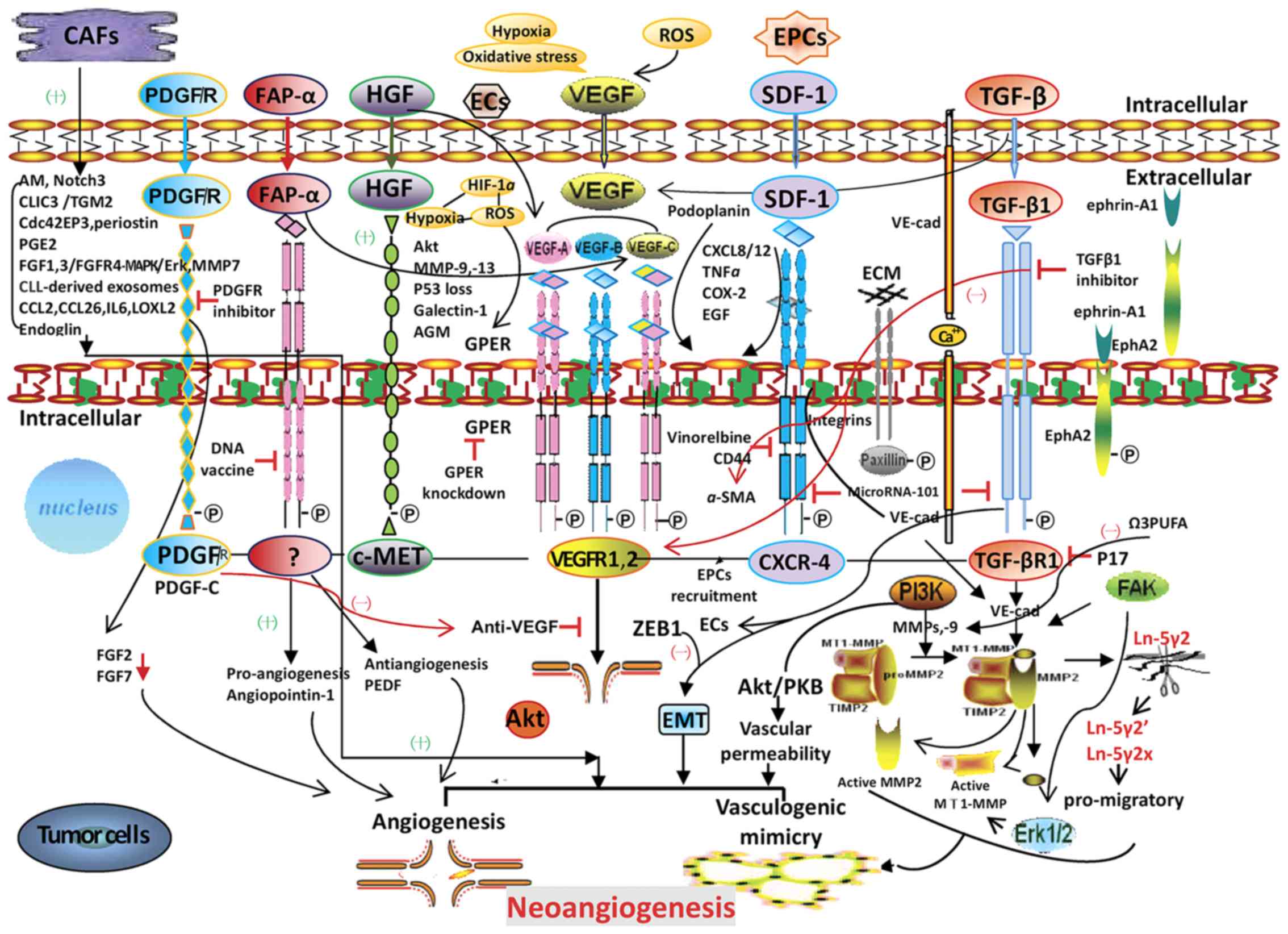
angiogenesis and VM are depicted in Fig.
1. It is hoped that future studies are able to identify
successful therapies utilizing multiple targets and signaling
pathways for tumor treatment.
The TME and neo-angiogenesis are essential for tumor
progression, and so the underlying molecular signaling pathways of
CAFs in human tumors are increasingly being investigated. As
components of tumor stroma, CAFs may serve as potential therapeutic
targets owing to their role in tumor progression. Growth factors
and cytokines have also been investigated as potential drug targets
for impairing interactions between CAFs and cancer cells. To date,
a number of non-clinical studies utilizing anti-angiogenic
strategies with multi-molecular combinations have elicited
promising results. However, clinical investigations have not yet
yielded significant results. Further studies should be performed to
identify effective therapeutic targets and treatment options to
block tumor metastasis and recurrence.
Not applicable.
The present review was supported by the National
Natural Science Foundation of China (grant nos. 30672073 and
81372614).
Not applicable.
FTW and WS wrote the paper. JTZ edited and formatted
the paper. YZF revised the paper and prepared the figure. All
authors have read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Folkman J: Anti-angiogenesis: New concept
for therapy of solid tumors. Ann Surg. 175:409–416. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang G and Chen L: Tumor vasculature and
microenvironment normalization: A possible mechanism of
antiangiogenesis therapy. Cancer Biother Radiopharm. 23:661–667.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Folberg R, Hendrix M and Maniotis A:
Vasculogenic mimicry and tumor angiogenesis. Am J Pathol.
156:361–381. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Senger D and Davis G: Angiogenesis. Cold
Spring Harb Perspect Biol. 3:a0050902011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rhee J and Hoff P: Angiogenesis inhibitors
in the treatment of cancer. Expert Opin Pharmacother. 6:1701–1711.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fan YZ and Sun W: Molecular regulation of
vasculogenic mimicry in tumors and potential tumor-target therapy.
World J Gastrointest Surg. 2:117–127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen HX and Cleck JN: Adverse effects of
anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol.
6:465–477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Higa GM and Abraham J: Biological
mechanisms of bevacizumab-associated adverse events. Expert Rev
Anticancer Ther. 9:999–1007. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liotta LA and Kohn EC: The
microenvironment of the tumour-host interface. Nature. 411:375–379.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reisfeld RA: The tumor microenvironment: A
target for combination therapy of breast cancer. Crit Rev Oncog.
18:115–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Micke P and Ostman A: Tumour-stroma
interaction: Cancer-associated fibroblasts as novel targets in
anti-cancer therapy? Lung Cancer. 45:(Suppl 2). S163–S175. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Franco OE, Shaw AK, Strand DW and Hayward
SW: Cancer associated fibroblasts in cancer pathogenesis. Semin
Cell Dev Biol. 21:33–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
O'Reilly MS: Antiangiogenesis and vascular
endothelial growth factor/vascular endothelial growth factor
receptor targeting as part of a combined-modality approach to the
treatment of cancer. Int J Radiat Oncol Biol Phys. 69:S64–S66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hajitou A, Sounni NE, Devy L,
Grignet-Debrus C, Lewalle JM, Li H, Deroanne C, Lu H, Colige A,
Nusgens BV, et al: Down-regulation of vascular endothelial growth
factor by tissue inhibitor of metalloproteinase-2: Effect on in
vivo mammary tumor growth and angiogenesis. Cancer Res.
61:3450–3457. 2001.PubMed/NCBI
|
|
16
|
Sato M, Arap W and Pasqualini R: Molecular
targets on blood vessels for cancer therapies in clinical trials.
Oncology (Williston Park). 21:1346–1355, 1367, 1370 passim.
2007.PubMed/NCBI
|
|
17
|
Zhang JT, Fan YZ, Chen CQ, Zhao ZM and Sun
W: Norcantharidin: A potential antiangiogenic agent for gallbladder
cancers in vitro and in vivo. Int J Oncol. 40:1501–1514.
2012.PubMed/NCBI
|
|
18
|
Ma J and Waxman DJ: Combination of
antiangiogenesis with chemotherapy for more effective cancer
treatment. Mol Cancer Ther. 7:3670–3684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kamrava M, Bernstein MB, Camphausen K and
Hodge J: Combining radiation, immunotherapy, and antiangiogenesis
agents in the management of cancer: The Three Musketeers or just
another quixotic combination? Mol Biosyst. 5:1262–1270. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frenkel S, Barzel I, Levy J, Lin AY,
Bartsch DJ, Majumdar D, Folberg R and Pe'er J: Demonstrating
circulation in vasculogenic mimicry patterns of uveal melanoma by
confocal indocyanine green angiography. Eye (Lond). 22:948–952.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu Y, Li Q, Li XY, Yang QY, Xu WW and Liu
GL: Short-term anti-vascular endothelial growth factor treatment
elicits vasculogenic mimicry formation of tumors to accelerate
metastasis. J Exp Clin Cancer Res. 31:162012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun W, Fan YZ, Zhang WZ and Ge CY: A pilot
histomorphology and hemodynamic of vasculogenic mimicry in
gallbladder carcinomas in vivo and in vitro. J Exp Clin Cancer Res.
30:462011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu XS, Sun W, Ge CY, Zhang WZ and Fan YZ:
Contribution of the PI3K/MMPs/Ln-5γ2 and EphA2/FAK/Paxillin
signaling pathways to tumor growth and vasculogenic mimicry of
gallbladder carcinomas. Int J Oncol. 42:2103–2115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang JT, Sun W, Zhang WZ, Ge CY, Liu ZY,
Zhao ZM, Lu XS and Fan YZ: Norcantharidin inhibits tumor growth and
vasculogenic mimicry of human gallbladder carcinomas by suppression
of the PI3-K/MMPs/Ln-5γ2 signaling pathway. BMC Cancer. 14:1932014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang H, Sun W, Zhang WZ, Ge CY, Zhang JT,
Liu ZY and Fan YZ: Inhibition of tumor vasculogenic mimicry and
prolongation of host survival in highly aggressive gallbladder
cancers by norcantharidin via blocking the ephrin type a receptor
2/focal adhesion kinase/paxillin signaling pathway. PLoS One.
9:e969822014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu W, Sun W, Zhang JT, Liu ZY, Li XP and
Fan YZ: Norcantharidin enhances TIMP-2 anti-vasculogenic mimicry
activity for human gallbladder cancers through downregulating MMP-2
and MT1-MMP. Int J Oncol. 46:627–640. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han H, Du L, Cao Z, Zhang B and Zhou Q:
Triptonide potently suppresses pancreatic cancer cell-mediated
vasculogenic mimicry by inhibiting expression of VE-cadherin and
chemokine ligand 2 genes. Eur J Pharmacol. 818:593–603. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen J, Zhao M, Zhisheng Z, Lin C, Yayun
Q, Xuanyi W, Feng J, Haibo W, Youyang S, Tadashi H, et al: COE
inhibits vasculogenic mimicry in hepatocellular carcinoma via
suppressing Notch1 signaling. J Ethnopharmacol. 208:165–173. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang F, Zhang CM, Li S, Wang KK, Guo BB,
Fu Y, Liu LY, Zhang Y, Jiang HY and Wu CJ: Low dosage of arsenic
trioxide inhibits vasculogenic mimicry in hepatoblastoma without
cell apoptosis. Mol Med Rep. 17:1573–1582. 2018.PubMed/NCBI
|
|
30
|
Li S, Zhang Q, Zhou L, Guan Y, Chen S,
Zhang Y and Han X: Inhibitory effects of compound DMBT on
hypoxia-induced vasculogenic mimicry in human breast cancer. Biomed
Pharmacother. 96:982–992. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Angara K, Rashid MH, Shankar A, Ara R,
Iskander A, Borin TF, Jain M, Achyut BR and Arbab AS: Vascular
mimicry in glioblastoma following anti-angiogenic and anti-20-HETE
therapies. Histol Histopathol. 32:917–928. 2017.PubMed/NCBI
|
|
32
|
Xue W, Du XS, Wu H, Liu H, Xie T, Tong HP,
Chen X, Guo Y and Zhang WG: Aberrant glioblastoma
neovascularization patterns and their correlation with
DCE-MRI-derived parameters following temozolomide and bevacizumab
treatment. Sci Rep. 7:138942017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zang M, Hu L, Zhang B, Zhu Z, Li J, Zhu Z,
Yan M and Liu B: Luteolin suppresses angiogenesis and vasculogenic
mimicry formation through inhibiting Notchl-VEGF signaling in
gastric cancer. Biochem Biophys Res Commun. 490:913–919. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu W, Lv C, Zhang B, Zhou Q and Cao Z:
MicroRNA-27b functions as a new inhibitor of ovarian
cancer-mediated vasculogenic mimicry through suppression of
VE-cadherin expression. RNA. 23:1019–1027. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Orimo A, Gupta P, Sgroi D,
Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey V, Richardson A
and Weinberg R: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sugimoto H, Mundel T, Kieran M and Kalluri
R: Identification of fibroblast heterogeneity in the tumor
microenvironment. Cancer Biol Ther. 5:1640–1646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Johansson A, Ansell A, Jerhammar F, Lindh
M, Grénman R, Munck-Wikland E, Östman A and Roberg K:
Cancer-associated fibroblasts induce matrix
metalloproteinase-mediated cetuximab resistance in head and neck
squamous cell carcinoma cells. Mol Cancer Res. 10:1158–1168. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Affolter A, Schmidtmann I, Mann WJ and
Brieger J: Cancer-associated fibroblasts do not respond to combined
irradiation and kinase inhibitor treatment. Oncol Rep. 29:785–790.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Al-Ansari MM, Hendrayani SF, Tulbah A,
Al-Tweigeri T, Shehata AL and Aboussekhra A: p16INK4A represses
breast stromal fibroblasts migration/invasion and their
VEGF-A-dependent promotion of angiogenesis through Akt inhibition.
Neoplasia. 14:1269–1277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gao Q, Wang XY, Qiu SJ, Zhou J, Shi YH,
Zhang BH and Fan J: Tumor stroma reaction-related gene signature
predicts clinical outcome in human hepatocellular carcinoma. Cancer
Sci. 102:1522–1531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Herrera M, Herrera A, Domínguez G, Silva
J, García V, García J, Gómez I, Soldevilla B, Muñoz C, Provencio M,
et al: Cancer-associated fibroblast and M2 macrophage markers
together predict outcome in colorectal cancer patients. Cancer Sci.
104:437–444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Madar S, Goldstein I and Rotter V: ‘Cancer
associated fibroblasts’ -more than meets the eye. Trends Mol Med.
19:447–453. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gonda TA, Varro A, Wang TC and Tycko B:
Molecular biology of cancer-associated fibroblasts: Can these cells
be targeted in anti-cancer therapy? Semin Cell Dev Biol. 21:2–10.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mertens J, Fingas CD, Christensen JD,
Smoot RL, Bronk SF, Werneburg NW, Gustafson MP, Dietz AB, Roberts
LR, Sirica AE and Gores GJ: Therapeutic effects of deleting
cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res.
73:897–907. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Olive KP, Jacobetz MA, Davidson CJ,
Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA,
Caldwell ME, Allard D, et al: Inhibition of Hedgehog signaling
enhances delivery of chemotherapy in a mouse model of pancreatic
cancer. Science. 324:1457–1461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fukumura D, Xavier R, Sugiura T, Chen Y,
Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK and Seed B:
Tumor induction of VEGF promoter activity in stromal cells. Cell.
94:715–725. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Okabe H, Beppu T, Hayashi H, Ishiko T,
Masuda T, Otao R, Horlad H, Jono H, Ueda M, Shinriki S, et al:
Hepatic stellate cells accelerate the malignant behavior of
cholangiocarcinoma cells. Ann Surg Oncol. 18:1175–1184. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Guo X, Oshima H, Kitmura T, Taketo M and
Oshima M: Stromal fibroblasts activated by tumor cells promote
angiogenesis in mouse gastric cancer. J Biol Chem. 283:19864–19871.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vartanian AA, Burova OS, Stepanova EV,
Baryshnikov AY and Lichinitser MR: Melanoma vasculogenic mimicry is
strongly related to reactive oxygen species level. Melanoma Res.
17:370–379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu Z, Sun B, Qi L, Li H, Gao J and Leng
X: Zinc finger E-box binding homeobox 1 promotes vasculogenic
mimicry in colorectal cancer through induction of
epithelial-to-mesenchymal transition. Cancer Sci. 103:813–820.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Carmeliet P and Jain R: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Noma K, Smalley KS, Lioni M, Naomoto Y,
Tanaka N, El-Deiry W, King AJ, Nakagawa H and Herlyn M: The
essential role of fibroblasts in esophageal squamous cell
carcinoma-induced angiogenesis. Gastroenterology. 134:1981–1993.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Suzuki H, Onimaru M, Yonemitsu Y, Maehara
Y, Nakamura S and Sueishi K: Podoplanin in cancer cells is
experimentally able to attenuate prolymphangiogenic and
lymphogenous metastatic potentials of lung squamoid cancer cells.
Mol Cancer. 9:2872010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Schoppmann SF, Jesch B, Riegler MF,
Maroske F, Schwameis K, Jomrich G and Birner P: Podoplanin
expressing cancer associated fibroblasts are associated with
unfavourable prognosis in adenocarcinoma of the esophagus. Clin Exp
Metastasis. 30:441–446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pula B, Jethon A, Piotrowska A,
Gomulkiewicz A, Owczarek T, Calik J, Wojnar A, Witkiewicz W, Rys J,
Ugorski M, et al: Podoplanin expression by cancer-associated
fibroblasts predicts poor outcome in invasive ductal breast
carcinoma. Histopathology. 59:1249–1260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Pula B, Wojnar A, Witkiewicz W, Dziegiel P
and Podhorska-Okolow M: Podoplanin expression in cancer-associated
fibroblasts correlates with VEGF-C expression in cancer cells of
invasive ductal breast carcinoma. Neoplasma. 60:516–524. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tang D, Yuan Z, Xue X, Lu Z, Zhang Y, Wang
H, Chen M, An Y, Wei J, Zhu Y, et al: High expression of Galectin-1
in pancreatic stellate cells plays a role in the development and
maintenance of an immunosuppressive microenvironment in pancreatic
cancer. Int J Cancer. 130:2337–2348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wu MH, Hong TM, Cheng HW, Pan SH, Liang
YR, Hong HC, Chiang WF, Wong TY, Shieh DB, Shiau AL, et al:
Galectin-1-mediated tumor invasion and metastasis, up-regulated
matrix metalloproteinase expression, and reorganized actin
cytoskeletons. Mol Cancer Res. 7:311–318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Thijssen VL, Postel R, Brandwijk RJ, Dings
RP, Nesmelova I, Satijn S, Verhofstad N, Nakabeppu Y, Baum L,
Bakkers J, et al: Galectin-1 is essential in tumor angiogenesis and
is a target for antiangiogenesis therapy. Proc Natl Acad Sci USA.
103:15975–15980. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bektas S, Bahadir B, Ucan BH and Ozdamar
SO: CD24 and galectin-1 expressions in gastric adenocarcinoma and
clinicopathologic significance. Pathol Oncol Res. 16:569–577. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tang D, Gao J, Wang S, Ye N, Chong Y,
Huang Y, Wang J, Li B, Yin W and Wang D: Cancer-associated
fibroblasts promote angiogenesis in gastric cancer through
galectin-1 expression. Tumour Biol. 37:1889–1899. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hooper AT, Shmelkov SV, Gupta S, Milde T,
Bambino K, Gillen K, Goetz M, Chavala S, Baljevic M, Murphy A, et
al: Angiomodulin is a specific marker of vasculature and regulates
vascular endothelial growth factor-A-dependent neoangiogenesis.
Circ Res. 105:201–208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Komiya E, Furuya M, Watanabe N, Miyagi Y,
Higashi S and Miyazaki K: Elevated expression of angiomodulin
(AGM/IGFBP-rP1) in tumor stroma and its roles in fibroblast
activation. Cancer Sci. 103:691–699. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Komiya E, Sato H, Watanabe N, Ise M,
Higashi S, Miyagi Y and Miyazaki K: Angiomodulin, a marker of
cancer vasculature, is upregulated by vascular endothelial growth
factor and increases vascular permeability as a ligand of integrin
αvβ3. Cancer Med. 3:537–549. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ren J, Guo H, Wu H, Tian T, Dong D, Zhang
Y, Sui Y, Zhang Y, Zhao D, Wang S, et al: GPER in CAFs regulates
hypoxia-driven breast cancer invasion in a CTGF-dependent manner.
Oncol Rep. 33:1929–1937. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
De Francesco E, Lappano R, Santolla M,
Marsico S, Caruso A and Maggiolini M: HIF-1α/GPER signaling
mediates the expression of VEGF induced by hypoxia in breast cancer
associated fibroblasts (CAFs). Breast Cancer Res. 15:R642013.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hayashi Y, Tsujii M, Kodama T, Akasaka T,
Kondo J, Hikita H, Inoue T, Tsujii Y, Maekawa A, Yoshii S, et al:
p53 functional deficiency in human colon cancer cells promotes
fibroblast-mediated angiogenesis and tumor growth. Carcinogenesis.
37:972–984. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Jo M, Nishikawa T, Nakajima T, Okada Y,
Yamaguchi K, Mitsuyoshi H, Yasui K, Minami M, Iwai M, Kagawa K, et
al: Oxidative stress is closely associated with tumor angiogenesis
of hepatocellular carcinoma. J Gastroenterol. 46:809–821. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang S, Ma N, Kawanishi S, Hiraku Y,
Oikawa S, Xie Y, Zhang Z, Huang G and Murata M: Relationships of
alpha-SMA-positive fibroblasts and SDF-1-positive tumor cells with
neoangiogenesis in nasopharyngeal carcinoma. Biomed Res Int 2014.
5073532014.
|
|
70
|
Orimo A and Weinberg R: Stromal
fibroblasts in cancer: A novel tumor-promoting cell type. Cell
Cycle. 5:1597–1601. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Matsuo Y, Ochi N, Sawai H, Yasuda A,
Takahashi H, Funahashi H, Takeyama H, Tong Z and Guha S: CXCL8/IL-8
and CXCL12/SDF-1 alpha co-operatively promote invasiveness and
angiogenesis in pancreatic cancer. Int J Cancer. 124:853–861. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yang J, Lu Y, Lin YY, Zheng ZY, Fang JH,
He S and Zhuang SM: Vascular mimicry formation is promoted by
paracrine TGF-β and SDF1 of cancer-associated fibroblasts and
inhibited by miR-101 in hepatocellular carcinoma. Cancer Lett.
383:18–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Fang D, Sun L, Lin S, Zhou L, Su N, Yuan S
and Yu B: Vinorelbine inhibits angiogenesis and 95D migration via
reducing hypoxic fibroblast stromal cell-derived factor 1
secretion. Exp Biol Med (Maywood). 237:1045–1055. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhou B, Zhuang XM, Wang YY, Lin ZY, Zhang
DM, Fan S, Li JS and Chen WL: Tumor necrosis factor α induces
myofibroblast differentiation in human tongue cancer and promotes
invasiveness and angiogenesis via secretion of stromal cell-derived
factor-1. Oral Oncol. 51:1095–1102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Katoh H, Hosono K, Ito Y, Suzuki T, Ogawa
Y, Kubo H, Kamata H, Mishima T, Tamaki H, Sakagami H, et al: COX-2
and prostaglandin EP3/EP4 signaling regulate the tumor stromal
proangiogenic microenvironment via CXCL12-CXCR4 chemokine systems.
Am J Pathol. 176:1469–1483. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Massagué J: TGFβ signalling in context.
Nat Rev Mol Cell Biol. 13:616–630. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Meulmeester E and Ten Dijke P: The dynamic
roles of TGF-β in cancer. J Pathol. 223:205–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sánchez-Elsner T, Botella L, Velasco B,
Corbí A, Attisano L and Bernabéu C: Synergistic cooperation between
hypoxia and transforming growth factor-beta pathways on human
vascular endothelial growth factor gene expression. J Biol Chem.
276:38527–38535. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Schnegg C, Yang MH, Ghosh SK and Hsu MY:
Induction of vasculogenic mimicry overrides VEGF-A silencing and
enriches stem-like cancer cells in melanoma. Cancer Res.
75:1682–1690. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Seftor RE, Hess AR, Seftor EA, Kirschmann
DA, Hardy KM, Margaryan NV and Hendrix MJ: Tumor cell vasculogenic
mimicry: From controversy to therapeutic promise. Am J Pathol.
181:1115–1125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kirschmann DA, Seftor EA, Hardy KM, Seftor
RE and Hendrix MJ: Molecular pathways: Vasculogenic mimicry in
tumor cells: Diagnostic and therapeutic implications. Clin Cancer
Res. 18:2726–2732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Barcellos-de-Souza P, Comito G,
Pons-Segura C, Taddei ML, Gori V, Becherucci V, Bambi F, Margheri
F, Laurenzana A, Del Rosso M, et al: Mesenchymal stem cells are
recruited and activated into carcinoma-associated fibroblasts by
prostate cancer microenvironment-derived TGF-β1. Stem Cells.
34:2536–2547. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Gonzalez-Zubeldia I, Dotor J, Redrado M,
Bleau A, Manrique I, de Aberasturi A, Villalba M and Calvo A:
Co-migration of colon cancer cells and CAFs induced by TGFβ1
enhances liver metastasis. Cell Tissue Res. 359:829–839. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Xu Z, Wang S, Wu M, Zeng W, Wang X and
Dong Z: TGFβ1 and HGF protein secretion by esophageal squamous
epithelial cells and stromal fibroblasts in oesophageal
carcinogenesis. Oncol Lett. 6:401–406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Saito H, Tsujitani S, Oka S, Kondo A,
Ikeguchi M, Maeta M and Kaibara N: The expression of transforming
growth factor-beta1 is significantly correlated with the expression
of vascular endothelial growth factor and poor prognosis of
patients with advanced gastric carcinoma. Cancer. 86:1455–1462.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Xu LN, Xu BN, Cai J, Yang JB and Lin N:
Tumor-associated fibroblast-conditioned medium promotes tumor cell
proliferation and angiogenesis. Genet Mol Res. 12:5863–5871. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Guido C, Whitaker-Menezes D, Capparelli C,
Balliet R, Lin Z, Pestell R, Howell A, Aquila S, Andò S,
Martinez-Outschoorn U, et al: Metabolic reprogramming of
cancer-associated fibroblasts by TGF-β drives tumor growth:
Connecting TGF-β signaling with ‘Warburg-like’ cancer metabolism
and L-lactate production. Cell Cycle. 11:3019–3035. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ding S, Merkulova-Rainon T, Han ZC and
Tobelem G: HGF receptor up-regulation contributes to the angiogenic
phenotype of human endothelial cells and promotes angiogenesis in
vitro. Blood. 101:4816–4822. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sulpice E, Ding S, Muscatelli-Groux B,
Bergé M, Han Z, Plouet J, Tobelem G and Merkulova-Rainon T:
Cross-talk between the VEGF-A and HGF signalling pathways in
endothelial cells. Biol Cell. 101:525–539. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Spina A, De Pasquale V, Cerulo G,
Cocchiaro P, Della Morte R, Avallone L and Pavone L: HGF/c-MET axis
in tumor microenvironment and metastasis formation. Biomedicines.
3:71–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Grugan KD, Miller CG, Yao Y, Michaylira
CZ, Ohashi S, Klein-Szanto AJ, Diehl A, Herlyn M, Han M, Nakagawa H
and Rustgi AK: Fibroblast-secreted hepatocyte growth factor plays a
functional role in esophageal squamous cell carcinoma invasion.
Proc Natl Acad Sci USA. 107:11026–11031. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wu X, Chen X, Zhou Q, Li P, Yu B, Li J, Qu
Y, Yan J, Yu Y, Yan M, et al: Hepatocyte growth factor activates
tumor stromal fibroblasts to promote tumorigenesis in gastric
cancer. Cancer Lett. 335:128–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Jia C, Wang T, Liu W, Fu B, Hua X, Wang G,
Li T, Li X, Wu X, Tai Y, et al: Cancer-associated fibroblasts from
hepatocellular carcinoma promote malignant cell proliferation by
HGF secretion. PLoS One. 8:e632432013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Tyan SW, Kuo WH, Huang CK, Pan CC, Shew
JY, Chang KJ, Lee EY and Lee WH: Breast cancer cells induce
cancer-associated fibroblasts to secrete hepatocyte growth factor
to enhance breast tumorigenesis. PLoS One. 6:e153132011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ren Y, Cao B, Law S, Xie Y, Lee PY, Cheung
L, Chen Y, Huang X, Chan HM, Zhao P, et al: Hepatocyte growth
factor promotes cancer cell migration and angiogenic factors
expression: A prognostic marker of human esophageal squamous cell
carcinomas. Clin Cancer Res. 11:6190–6197. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Oshima Y, Yajima S, Yamazaki K, Matsushita
K, Tagawa M and Shimada H: Angiogenesis-related factors are
molecular targets for diagnosis and treatment of patients with
esophageal carcinoma. Ann Thorac Cardiovasc Surg. 16:389–393.
2010.PubMed/NCBI
|
|
97
|
Bergsten E, Uutela M, Li X, Pietras K,
Ostman A, Heldin C, Alitalo K and Eriksson U: PDGF-D is a specific,
protease-activated ligand for the PDGF beta-receptor. Nat Cell
Biol. 3:512–516. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kitadai Y, Sasaki T, Kuwai T, Nakamura T,
Bucana C and Fidler I: Targeting the expression of platelet-derived
growth factor receptor by reactive stroma inhibits growth and
metastasis of human colon carcinoma. Am J Pathol. 169:2054–2065.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Crawford Y, Kasman I, Yu L, Zhong C, Wu X,
Modrusan Z, Kaminker J and Ferrara N: PDGF-C mediates the
angiogenic and tumorigenic properties of fibroblasts associated
with tumors refractory to anti-VEGF treatment. Cancer Cell.
15:21–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ostman A: PDGF receptors-mediators of
autocrine tumor growth and regulators of tumor vasculature and
stroma. Cytokine Growth Factor Rev. 15:275–286. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Pietras K, Rubin K, Sjöblom T, Buchdunger
E, Sjöquist M, Heldin C and Ostman A: Inhibition of PDGF receptor
signaling in tumor stroma enhances antitumor effect of
chemotherapy. Cancer Res. 62:5476–5484. 2002.PubMed/NCBI
|
|
102
|
Pietras K, Gustafson AM, Sjoblom T,
Buchdunger E, McSheehy P, Sjoquist M, Wartmann M, Reed R, Heldin
CH, Rubin K, et al: PDGF receptor inhibition in tumor stroma, with
STI571 or PDGF B-chain aptamers, enhances the effects of
chemotherapy in experimental solid tumors by increasing tumor drug
uptake. Eur J Cancer. 38:S91. 2002. View Article : Google Scholar
|
|
103
|
Pietras K, Pahler J, Bergers G and Hanahan
D: Functions of paracrine PDGF signaling in the proangiogenic tumor
stroma revealed by pharmacological targeting. PLoS Med. 5:e192008.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zi F, He J, He D, Li Y, Yang L and Cai Z:
Fibroblast activation protein α in tumor microenvironment: Recent
progression and implications (review). Mol Med Rep. 11:3203–3211.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Santos AM, Jung J, Aziz N, Kissil JL and
Puré E: Targeting fibroblast activation protein inhibits tumor
stromagenesis and growth in mice. J Clin Invest. 119:3613–3625.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Patsouras D, Papaxoinis K, Kostakis A,
Safioleas MC, Lazaris AC and Nicolopoulou-Stamati P: Fibroblast
activation protein and its prognostic significance in correlation
with vascular endothelial growth factor in pancreatic
adenocarcinoma. Mol Med Rep. 11:4585–4590. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Koczorowska MM, Tholen S, Bucher F, Lutz
L, Kizhakkedathu JN, De Wever O, Wellner U, Biniossek ML, Stahl A,
Lassmann S and Schilling O: Fibroblast activation protein-α, a
stromal cell surface protease, shapes key features of cancer
associated fibroblasts through proteome and degradome alterations.
Mol Oncol. 10:40–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
LeBeau AM, Brennen WN, Aggarwal S and
Denmeade SR: Targeting the cancer stroma with a fibroblast
activation protein-activated promelittin protoxin. Mol Cancer Ther.
8:1378–1386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Liao D, Luo Y, Markowitz D, Xiang R and
Reisfeld RA: Cancer associated fibroblasts promote tumor growth and
metastasis by modulating the tumor immune microenvironment in a 4T1
murine breast cancer model. PLoS One. 4:e79652009. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Chang HY, Chi JT, Dudoit S, Bondre C, van
de Rijn M, Botstein D and Brown PO: Diversity, topographic
differentiation, and positional memory in human fibroblasts. Proc
Natl Acad Sci USA. 99:12877–12882. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Simian M, Hirai Y, Navre M, Werb Z,
Lochter A and Bissell MJ: The interplay of matrix
metalloproteinases, morphogens and growth factors is necessary for
branching of mammary epithelial cells. Development. 128:3117–3131.
2001.PubMed/NCBI
|
|
112
|
Vosseler S, Lederle W, Airola K,
Obermueller E, Fusenig NE and Mueller MM: Distinct
progression-associated expression of tumor and stromal MMPs in
HaCaT skin SCCs correlates with onset of invasion. Int J Cancer.
125:2296–2306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Lederle W, Hartenstein B, Meides A,
Kunzelmann H, Werb Z, Angel P and Mueller M: MMP13 as a stromal
mediator in controlling persistent angiogenesis in skin carcinoma.
Carcinogenesis. 31:1175–1184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zigrino P, Kuhn I, Bäuerle T, Zamek J, Fox
JW, Neumann S, Licht A, Schorpp-Kistner M, Angel P and Mauch C:
Stromal expression of MMP-13 is required for melanoma invasion and
metastasis. J Invest Dermatol. 129:2686–2693. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Benyahia Z, Dussault N, Cayol M, Sigaud R,
Berenguer-Daizé C, Delfino C, Tounsi A, Garcia S, Martin P, Mabrouk
K and Ouafik L: Stromal fibroblasts present in breast carcinomas
promote tumor growth and angiogenesis through adrenomedullin
secretion. Oncotarget. 8:15744–15762. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Kayamori K, Katsube K, Sakamoto K, Ohyama
Y, Hirai H, Yukimori A, Ohata Y, Akashi T, Saitoh M, Harada K, et
al: NOTCH3 is induced in cancer-associated fibroblasts and promotes
angiogenesis in oral squamous cell carcinoma. PLoS One.
11:e01541122016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Tasiopoulou V, Magouliotis D, Solenov EI,
Vavougios G, Molyvdas PA, Gourgoulianis KI, Hatzoglou C and
Zarogiannis SG: Transcriptional over-expression of chloride
intracellular channels 3 and 4 in malignant pleural mesothelioma.
Comput Biol Chem. 59:Pt A. 111–116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Macpherson IR, Rainero E, Mitchell LE, van
den Berghe PV, Speirs C, Dozynkiewicz MA, Chaudhary S, Kalna G,
Edwards J, Timpson P and Norman JC: CLIC3 controls recycling of
late endosomal MT1-MMP and dictates invasion and metastasis in
breast cancer. J Cell Sci. 127:3893–3901. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Dozynkiewicz MA, Jamieson NB, Macpherson
I, Grindlay J, van den Berghe P, von Thun A, Morton JP, Gourley C,
Timpson P, Nixon C, et al: Rab25 and CLIC3 collaborate to promote
integrin recycling from late endosomes/lysosomes and drive cancer
progression. Dev Cell. 22:131–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Hernandez-Fernaud JR, Ruengeler E, Casazza
A, Neilson LJ, Pulleine E, Santi A, Ismail S, Lilla S, Dhayade S,
MacPherson IR, et al: Secreted CLIC3 drives cancer progression
through its glutathione-dependent oxidoreductase activity. Nat
Commun. 8:142062017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Calvo F, Ranftl R, Hooper S, Farrugia AJ,
Moeendarbary E, Bruckbauer A, Batista F, Charras G and Sahai E:
Cdc42EP3/BORG2 and septin network enables mechano-transduction and
the emergence of cancer-associated fibroblasts. Cell Rep.
13:2699–2714. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Paggetti J, Haderk F, Seiffert M, Janji B,
Distler U, Ammerlaan W, Kim YJ, Adam J, Lichter P, Solary E, et al:
Exosomes released by chronic lymphocytic leukemia cells induce the
transition of stromal cells into cancer-associated fibroblasts.
Blood. 126:1106–1117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Bai YP, Shang K, Chen H, Ding F, Wang Z,
Liang C, Xu Y, Sun MH and Li YY: FGF-1/-3/FGFR4 signaling in
cancer-associated fibroblasts promotes tumor progression in colon
cancer through Erk and MMP-7. Cancer Sci. 106:1278–1287. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Núñez-Gómez E, Pericacho M, Ollauri-Ibáñez
C, Bernabéu C and López-Novoa J: The role of endoglin in
post-ischemic revascularization. Angiogenesis. 20:1–24. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Romero D, O'Neill C, Terzic A, Contois L,
Young K, Conley BA, Bergan RC, Brooks PC and Vary CP: Endoglin
regulates cancer-stromal cell interactions in prostate tumors.
Cancer Res. 71:3482–3493. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Mendel DB, Laird AD, Smolich BD, Blake RA,
Liang C, Hannah AL, Shaheen RM, Ellis LM, Weitman S, Shawver LK and
Cherrington JM: Development of SU5416, a selective small molecule
inhibitor of VEGF receptor tyrosine kinase activity, as an
anti-angiogenesis agent. Anticancer Drug Des. 15:29–41.
2000.PubMed/NCBI
|
|
127
|
Wang LL, Li JJ, Zheng ZB, Liu HY, Du GJ
and Li S: Antitumor activities of a novel indolin-2-ketone
compound, Z24: More potent inhibition on bFGF-induced angiogenesis
and bcl-2 over-expressing cancer cells. Eur J Pharmacol. 502:1–10.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Brennen WN, Isaacs JT and Denmeade SR:
Rationale behind targeting fibroblast activation protein-expressing
carcinoma-associated fibroblasts as a novel chemotherapeutic
strategy. Mol Cancer Ther. 11:257–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Yamamoto Y, Matsui J, Matsushima T,
Obaishi H, Miyazaki K, Nakamura K, Tohyama O, Semba T, Yamaguchi A,
Hoshi SS, et al: Lenvatinib, an angiogenesis inhibitor targeting
VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft
models associated with microvessel density and pericyte coverage.
Vasc Cell. 6:182014. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Taguchi A, Kawana K, Tomio K, Yamashita A,
Isobe Y, Nagasaka K, Koga K, Inoue T, Nishida H, Kojima S, et al:
Matrix metalloproteinase (MMP)-9 in cancer-associated fibroblasts
(CAFs) is suppressed by omega-3 polyunsaturated fatty acids in
vitro and in vivo. PLoS One. 9:e896052014. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Wang X, Shen Y, Li S, Lv M, Zhang X and
Yang J, Wang F and Yang J: Importance of the interaction between
immune cells and tumor vasculature mediated by thalidomide in
cancer treatment (Review). Int J Mol Med. 38:1021–1029. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Hu-Lowe DD, Chen E, Zhang L, Watson KD,
Mancuso P, Lappin P, Wickman G, Chen JH, Wang J, Jiang X, et al:
Targeting activin receptor-like kinase 1 inhibits angiogenesis and
tumorigenesis through a mechanism of action complementary to
anti-VEGF therapies. Cancer Res. 71:1362–1373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Huijbers EJ, van Beijnum JR, Thijssen VL,
Sabrkhany S, Nowak-Sliwinska P and Griffioen A: Role of the tumor
stroma in resistance to anti-angiogenic therapy. Drug Resist Updat.
25:26–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
di Tomaso E, London N, Fuja D, Logie J,
Tyrrell JA, Kamoun W, Munn LL and Jain RK: PDGF-C induces
maturation of blood vessels in a model of glioblastoma and
attenuates the response to anti-VEGF treatment. PLoS One.
4:e51232009. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Hainsworth JD, Spigel DR, Sosman JA,
Burris HA III, Farley C, Cucullu H, Yost K, Hart LL, Sylvester L,
Waterhouse DM and Greco FA: Treatment of advanced renal cell
carcinoma with the combination bevacizumab/erlotinib/imatinib: A
phase I/II trial. Clin Genitourin Cancer. 5:427–432. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Li M, Li M, Yin T, Shi H, Wen Y, Zhang B,
Chen M, Xu G, Ren K and Wei Y: Targeting of cancer-associated
fibroblasts enhances the efficacy of cancer chemotherapy by
regulating the tumor microenvironment. Mol Med Rep. 13:2476–2484.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Kinugasa Y, Matsui T and Takakura N: CD44
expressed on cancer-associated fibroblasts is a functional molecule
supporting the stemness and drug resistance of malignant cancer
cells in the tumor microenvironment. Stem Cells. 32:145–156. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Wang W, Ma JL, Jia WD and Xu GL:
Periostin: A putative mediator involved in tumour resistance to
anti-angiogenic therapy? Cell Biol Int. 35:1085–1088. 2011.
View Article : Google Scholar : PubMed/NCBI
|