Introduction
The advancement of technology has resulted in highly
accurate radiation therapy for primary solid tumors (1); however, recurrence or distant metastasis
due to residual cancer cells resistant to irradiation remains a
major problem that leads to poor outcome (2) Radioresistance in SAS oral squamous cell
carcinoma cells, HEp-2 laryngeal cancer cells, and lung cancer
cells can be induced by fractionated radiotherapy (3–6). Combining
chemotherapy (e.g. cisplatin, 5-fluorouracil, and paclitaxel) with
targeted inhibitors can potentially overcome radioresistance and
extend progression-free and overall survival (7–12).
However, few studies have examined the efficacy of combined
treatments in preventing distant metastasis (10).
4-Methylumbelliferone (4-MU) is a hyaluronan
synthesis inhibitor (13,14) that has demonstrated anti-tumor and
-invasion/metastasis effects in various cancer cell types and mouse
models of prostate and liver cancer that are exerted via
suppression of hyaluronan synthase (HAS) expression (15,16). 4-MU
was also shown to suppress inflammatory cytokines and chemokines
such as interleukin (IL)-6 and −8 (17). Elevated levels of nuclear factor
(NF)-κB, a cytokine and transcription factor that regulates
proinflammatory molecules such as IL-1β and IL-6 and tumor necrosis
factor (TNF)α induce resistance to apoptosis (18) and radiotherapy (19) in cancer cells. IL-6 is a potential
therapeutic target owing to its close association with cancer stem
cells (20,21).
We speculated that combined administration of 4-MU
which has anti-inflammatory effects and radiotherapy can not only
prevent distant metastasis but also sensitize radioresistant cells
to the effects of X-ray radiation. In our previous study, HT1080
human fibrosarcoma cells were exposed to 2 Gy X-ray radiation in
the presence of 100 µM 4-MU (22);
this inhibited colony-forming ability and metastatic potential,
which was accompanied by downregulation of matrix
metalloproteinases-2 and −9. However, the mechanistic basis of
these effects is unclear. We addressed this in the present study by
performing mRNA profiling to identify factors related to the
anti-tumor and -invasion effects of 4-MU in HT1080 cells.
Materials and methods
Reagents
4-MU was purchased from Nacalai Tesque (Kyoto,
Japan) and diluted in dimethylsulfoxide (Wako Pure Chemical
Industries, Ltd., Osaka, Japan); the working concentration was 500
µM. The reason for using 500 µM 4-MU was that clear effects of 4-MU
could be observed and the cytotoxic effect was low for normal
fibroblast cells at a 500 µM concentration (22). Monoclonal phycoerythin (PE)-conjugated
anti-human cluster of differentiation (CD)126 antibody (cat. no.
352804); mouse monoclonal PE-IgG1, κ isotype control (cat. no.
400114); monoclonal allophycocyanin (APC)-conjugated anti-human
CD130 (gp130) antibody (cat. no. 362005); mouse monoclonal
APC-IgG2a, κ isotype control (cat. no. 400221); fluorescein
isothiocyanate (FITC)-annexin V (cat. no. 640905); and propidium
iodide (PI) (cat. no. 421301) were from BioLegend (San Diego, CA,
USA). PE-conjugated polyclonal anti-human type 1 IL-1 receptor
(IL-1R) antibody (cat. no. FAB269P) and PE-conjugated goat IgG
(cat. no. IC108P) were R&D Systems, Inc., (Minneapolis, MN,
USA).
Cell culture
HT1080 human fibrosarcoma cells from American Type
Culture Collection (Manassas, VA, USA) were cultured in Roswell
Park Memorial Institute 1640 medium (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% heat-inactivated
fetal bovine serum (FBS; Japan Bio Serum, Fukuyama, Japan) and 1%
penicillin/streptomycin (Life Technologies) at 37°C in a humidified
atmosphere of 5% CO2.
Clonogenic potency assay
The clonogenic potency of HT1080 cells was estimated
with the colony formation assay. Appropriate numbers of cells were
seeded and incubated for 2 h, then subjected to X-ray irradiation
at 1–6 Gy in the presence of 500 µM 4-MU followed by incubation for
24 h. After 7–10 days of culture with regular changes of medium
cells were fixed with methanol (Wako Pure Chemical Industries,
Ltd.) stained by Giemsa (Wako Pure Chemical Industries, Ltd.), and
quantified.
Irradiation
Ionizing radiation (IR) was delivered using an X-ray
generator (MBR-1520R-3; Hitachi Medical, Co., Tokyo, Japan) with
0.5-mm aluminum and 0.3-mm copper filters at a distance of 45 cm
between the focus and target (150 kV, 20 mA, 1.0 Gy/min). During
X-ray exposure, the total dose and dose rate were monitored with a
thimble ionization chamber placed next to the sample.
Flow cytometry analysis
To evaluate the expression of the IL-6 receptors
CD126 and CD130, and the IL-1α/β receptor type I IL-1R, cells were
resuspended in 100 µl Dulbecco's phosphate-buffered saline without
CaCl2 and MgCl (Takara Bio, Inc., Otsu, Japan)
containing 5% FBS and PE-conjugated anti-human CD126 antibody (3
µl/106 cells), APC-conjugated anti-human CD130 antibody (3 µl/106
cells), and PE-conjugated anti-human type 1 IL-1R antibody for 15
min at 4°C in the dark. To detect cell death, cells labeled with
FITC-annexin V (3 µl/106 cells) were resuspended in annexin V
binding buffer (cat. no. 422201; BioLegend) and incubated for 15
min at room temperature in dark with PI (6 µl/106 cells), followed
by flow cytometry analysis on FACS Aria instrument (BD Biosciences,
Tokyo, Japan).
RNA extraction and analysis
Total RNA was extracted from HT1080 cells 24 h after
irradiation and/or 4-MU administration using the RNeasy Mini kit
(Qiagen GmbH, Hilden, Germany), and RNA quality was confirmed with
an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Santa
Clara, CA, USA). Cyanine (Cy)3-labeled cRNA samples were
synthesized from total RNA and hybridized to 8×60K format SurePrint
G3 Human GE v2 microarray slides (eArray Design ID=039494)
according to the manufacturer's instructions (Agilent Technologies,
Inc.). Cy3 fluorescence was detected with a DNA microarray scanner
(G2600A SureScan) and processed using Feature Extraction software
(both from Agilent Technologies, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
cDNA was synthesized using a RT kits (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and used as a template
for PCR in a 20-µl reaction mixture containing 2X SYBR Premix Ex
Taq (Takara Bio, Inc.) and 0.5 µM forward and reverse primers
(Table I). The reaction was carried
out on a real-time PCR system (StepOne Plus; Life Technologies)
under the following conditions: 30 sec at 95°C, followed by 40
cycles of 95°C for 5 sec, and 54°C for 30 sec. Target gene
expression levels were calculated relative to that of
glyceraldehyde 3-phosphate dehydrogenase mRNA (internal control)
with the comparative ΔΔCq method (23).
 | Table I.Primer sequences of the target
genes. |
Table I.
Primer sequences of the target
genes.
| Primers | Sequences
(5′-3′) |
|---|
| IL-1α forward |
GGTTGAGTTTAAGCCAATCCA |
| IL-1α reverse |
TGCTGACCTAGGCTTGATGA |
| IL-1β forward |
TACCTGTCCTGCGTGTTGAA |
| IL-1β reverse |
TCTTTGGGTAATTTTTGGGATCT |
| IL-6 forward |
CACTGGGCACAGAACTTATGTTG |
| IL-6 reverse |
AAAATAATTAAAATAGTGTCCTAA CGCTCAT |
| GAPDH forward |
GTGAAGGTCGGAGTCAACG |
| GAPDH reverse |
TGAGGTCAATGAAGGGGTC |
Enzyme-linked immunosorbent assay
(ELISA)
The levels of IL-1α, −1β, and −6 secreted by cells
were measured using the Human IL-1α, IL-1β, and IL-6 DuoSet ELISA
kits (all from R&D Systems), respectively, according to the
manufacturer's protocols. Cells were treated with 500 µM 4-MU and
irradiated with 2 Gy X ray, followed by incubation in serum-free
medium. Culture medium conditioned for 24 h was used for the ELISA
assay. Cytokine concentration was determined per million cells.
Statistical analysis
Statistical analysis of microarray data was
performed using GeneSpring (Agilent Technologies, Inc.). Up- and
downregulated mRNAs transcripts were selected based on fold change
(>2-fold) of irradiated and/or 4-MU-administered samples
relative to control samples. Ingenuity Pathway Analysis (IPA;
Qiagen Silicon Valley, Redwood City, CA, USA) was used for
functional analysis of each transcript. The significance of
differences between control and experimental cultures was evaluated
with one-way analysis of variance and the Tukey-Kramer test.
Statistical analyses were performed using Microsoft Excel 2010
(Microsoft Corporation, Redmond, WA, USA) with the add-on software
Statcel v3 (OMS Publishing, Saitama, Japan). P<0.05 was
considered to indicate a statistically significant difference.
Results
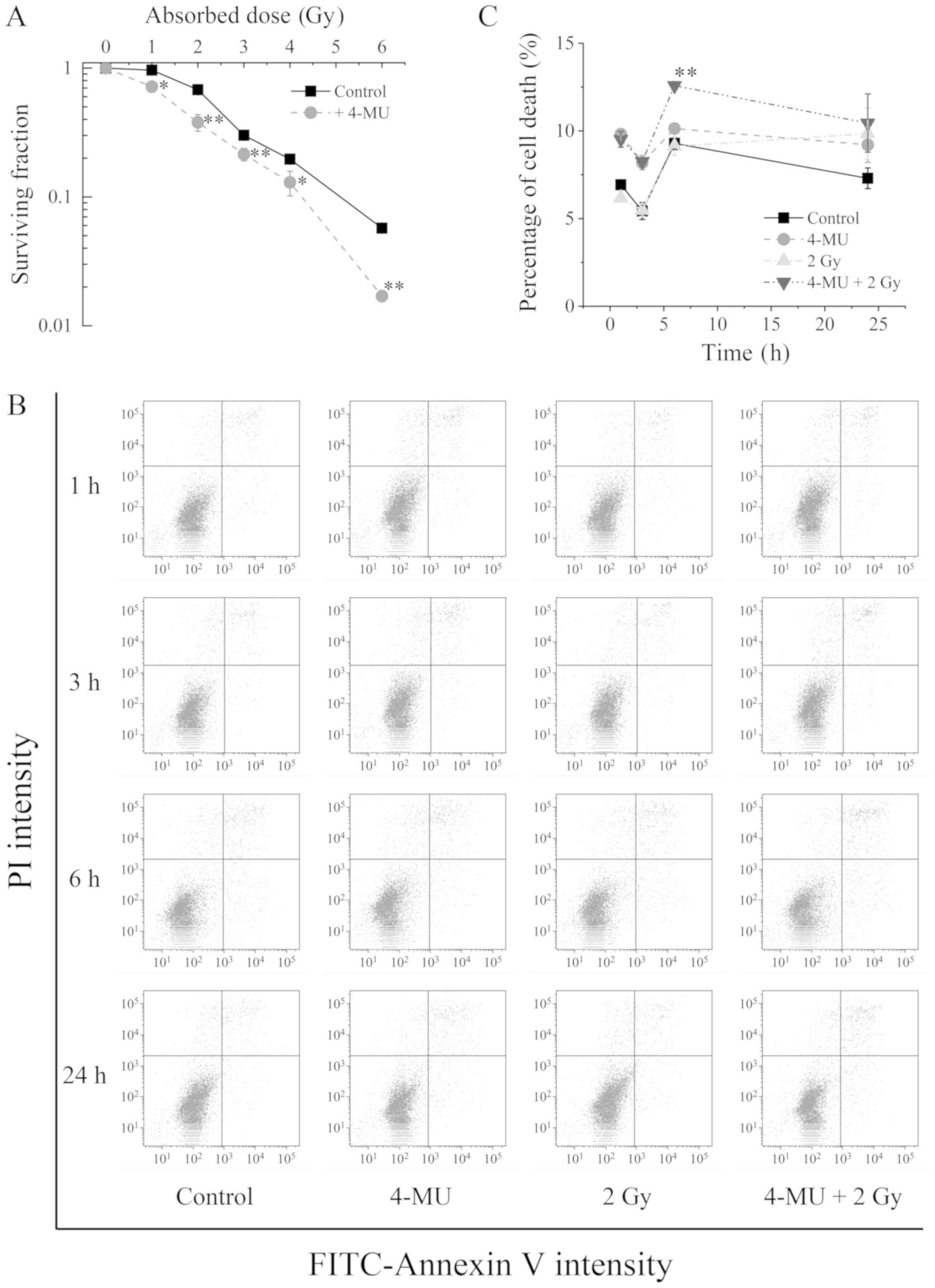
Radiosensitization by 4-MU
To investigate whether the anti-inflammatory effect
of 4-MU enhances radiosensitization, we evaluated the clonogenic
potency of HT1080 cells exposed to 4-MU and/or X-ray radiation with
the colony formation assay. The survival of cells treated with 4-MU
combined with X-ray irradiation was decreased in a radiation
dose-dependent manner compared to X-ray irradiation alone (Fig. 1A). This was confirmed by annexin V and
PI staining. Representative dot plots and quantified data are shown
in Fig. 1B and C. The number of cells
positive for both annexin V and PI was increased 6 h after X-ray
irradiation (9.12±0.52%) (Fig. 1C). A
comparable increase was observed upon treatment with 4-MU alone for
6 h (10.13±0.03%). On the other hand, there were more annexin V and
PI positive cells in the group exposed to both 4-MU and X-ray
radiation as compared to either treatment alone (12.58±0.23%).
These results suggest that 4-MU enhances the sensitivity of HT1080
cells to the lethal effects of X-ray radiation.
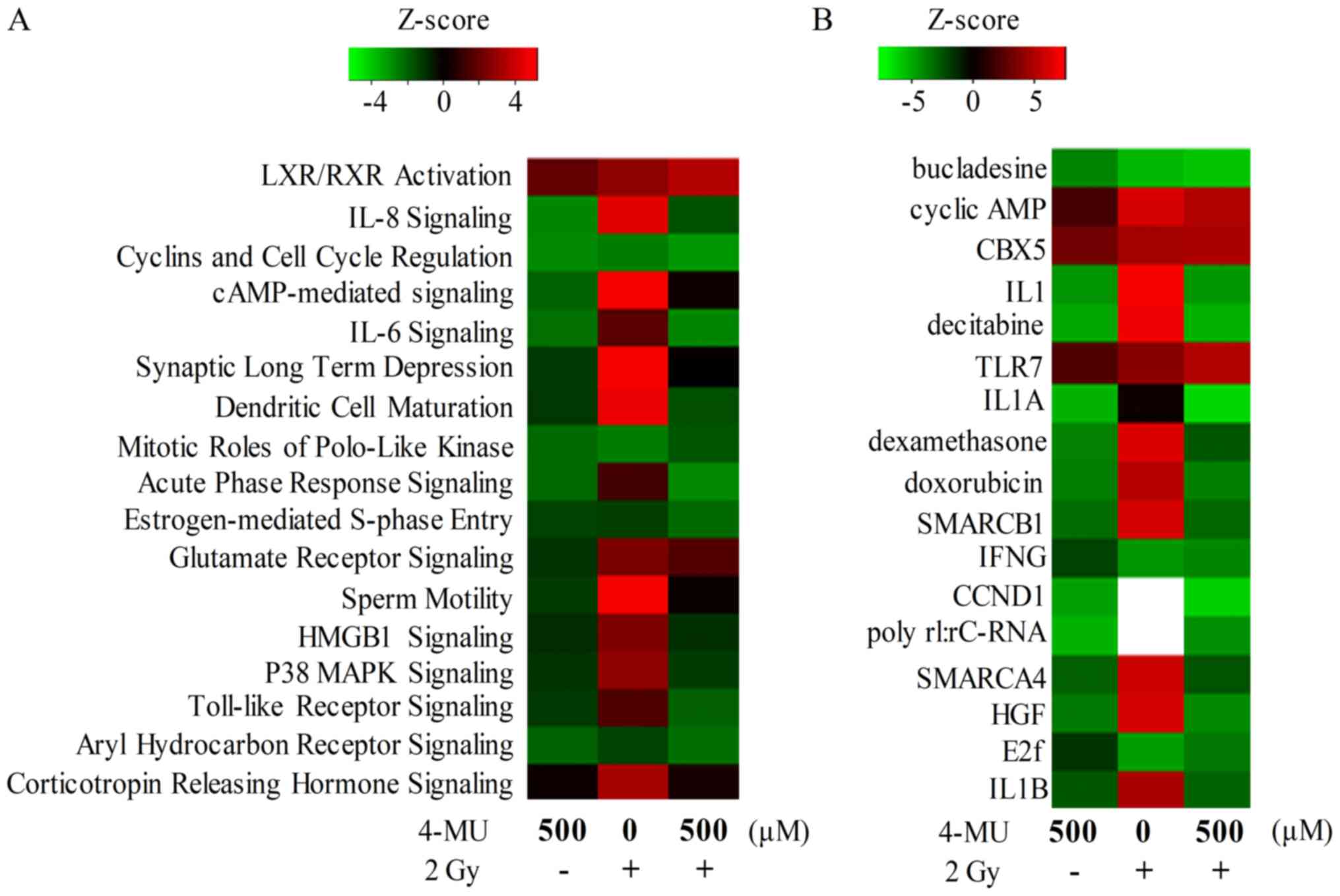
mRNA profiling and IPA
We performed a microarray analysis to determine the
signaling pathways affected by 4-MU treatment combined with X-ray
irradiation. A total of 2873, 4085, and 3179 genes were
differentially expressed in cells treated with 4-MU alone, X-ray
alone, and 4-MU + X-ray, respectively. The IPA z-scores revealed
that signaling pathways associated with inflammation including IL-8
and −6 and Toll-like receptor signaling were altered by treatment
with 4-MU or/and 2 Gy X-ray radiation (Fig. 2A). Moreover, IL-1α and −1β were
inactivated in cells treated with 4-MU only (Fig. 2B).
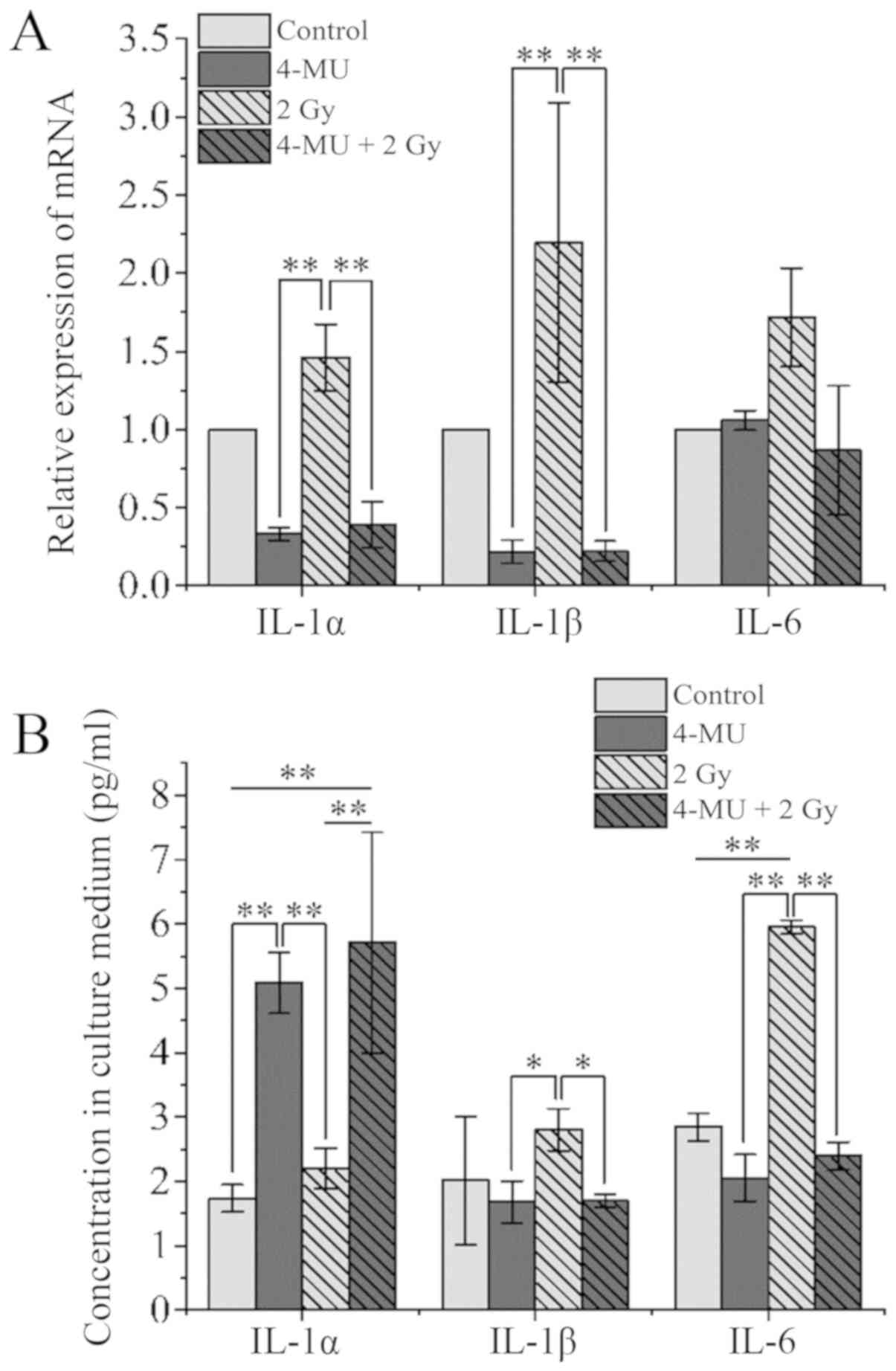
IL-1α, IL-1β, and IL-6 mRNA levels in cells and
IL-1α, IL-1β, and IL-6 concentrations in the cell culture
supernatant were analyzed by RT-qPCR and ELISA, respectively. IL-1α
and −1β transcript levels in cells exposed to X-ray radiation alone
were increased about 2-fold relative to the control, whereas the
levels in cells treated with 4-MU showed the opposite trend
(Fig. 3A). However, IL-1α
concentration in the supernatant was 3-fold higher in 4-MU treated
as compared to control cultures (1.73±0.21 vs. 5.09±0.48 pg/ml),
whereas IL-1α concentration (per 106 cells) in the culture
supernatant of cells exposed to both 4-MU and X-ray radiation was
higher than that in the radiation-only group (5.71±1.73 vs.
2.20±0.31) (Fig. 3B). On the other
hand, the IL-1β concentration in the culture supernatant was
correlated with the mRNA expression. X-ray irradiation increased
IL-6 mRNA level 1.7-fold relative to the control (Fig. 3A); however, the level was decreased
0.8-fold by treatment with 4-MU combined with X-ray irradiation.
IL-6 level in the culture supernatant of cells exposed to radiation
alone was 2-fold higher than that in the control group (2.84±0.21
vs. 5.95±0.11 pg/ml) (Fig. 3B),
whereas concentrations for cells treated with 4-MU with or without
X-ray irradiation were similar to that of control cultures
(2.05±0.36 and 2.39±0.21 pg/ml, respectively). This suggests that
the increase in IL-6 expression and release induced by X-ray
irradiation was reversed by 4-MU treatment which suppressed
inflammation by inhibiting IL-1β production.
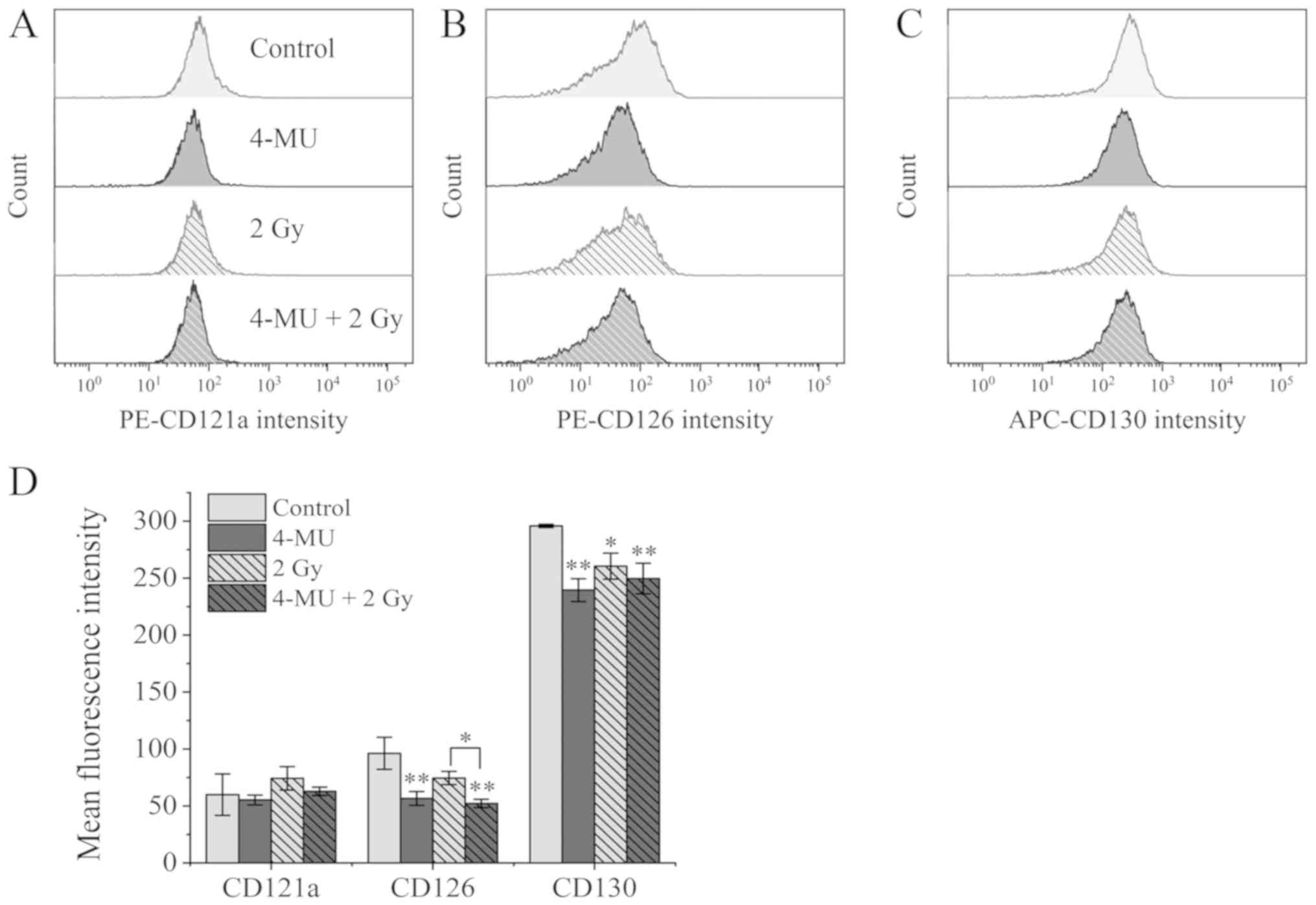
Expression of inflammatory cytokine
receptors
To investigate whether IL-1α, −1β, and −6 receptors
are suppressed by 4-MU, we examined the expression of the cognate
surface receptors [type 1 IL-1R (CD121a), CD126, and CD130,
respectively] by flow cytometry. The mean fluorescence intensity of
CD121a on cells exposed to X-ray radiation alone was higher than
that of control cells (59.88±18.3 vs. 74.29±10.3, respectively)
(Fig. 4A and D). On the other hand,
the mean fluorescence intensity of CD121a in the 4-MU only
(55.22±4.38) and 4-MU/X-ray radiation combination (62.79±3.72)
groups did not differ significantly from that of the control group
(P=0.429, 0.268, respectively), suggesting that the increase in
CD121a expression induced by X-ray irradiation was marginally
suppressed.
The mean fluorescence intensity of CD126, the ligand
binding protein for IL-6, was unaltered by X-ray irradiation
relative to the control (74.48±5.74 vs. 96.17±14.1). 4-MU treatment
reduced the signal intensity to about half that of the control
group (4-MU alone: 56.54±6.12; 4-MU combined with X-ray:
52.11±3.71) (Fig. 4B and D). On the
other hand, the mean fluorescence intensity of CD130, the signal
transducer for IL-6 receptor, was downregulated by 2 Gy X-ray
irradiation as compared to the control (260.40±11.4 vs.
295.76±1.46) and was further decreased by 4-MU administration (4-MU
alone: 239.42±9.99; 4-MU combined: 249.57±13.5) (Fig. 4C and D). These results indicate that
expression of IL-6 receptors were suppressed by 4-MU treatment.
Discussion
Recent some studies have described the anti-tumor
and -invasion effects of 4-MU and the role of HAS in various
malignancies (24–26). HA is closely related to cancer cell
proliferation, invasion, and metastasis (27). 4-MU was reported to inhibit activation
of Akt signaling by modulating the interaction between HA, CD44,
and the receptor for hyaluronan-mediated motility complex (15). On the other hand, angiogenesis was
suppressed by downregulation the proinflammatory cytokines IL-6 and
−8 and the chemokine C-X-C motif chemokine ligand 12 (17). In accordance with these findings, we
found that the increase in IL-1β and −6 expression caused by X-ray
irradiation was abolished by 4-MU treatment. However, whether this
effect leads to radiosensitization effects, needs to be verified
using neutralizing antibodies and gene knockdown experiments. IL-1α
has been reported to consolidate cellular scaffolds in HT1080 cells
(28). Liberation of cells by
processes such as epithelial-mesenchymal transition is known to be
the initiation of invasion into the blood vessel (29). Therefore, it was suggested that 4-MU
prevents liberation of cells by promoting the release of IL-1α.
However, conflicting IL-1α between mRNA and protein levels indicate
that 4-MU might have the effect of stabilizing IL-1α. Since 4-MU
has multiple functions, further verification is necessary.
Inflammatory cytokines have been linked to
radioresistance in cancer stem cells. In particular, IL-6, an
activator of the Janus kinase/signal transducer and activator of
transcription 3 (STAT)3 signaling pathway, has been shown to
inhibit the oxidative stress response (30) and DNA repair in non-small cell lung
cancer cells (31,32) and promote cancer cell resistance to
radio- and chemotherapy. On the other hand, IL-1β promotes
angiogenesis and migration and is implicated in the tumorigenicity
of fibrosarcoma cells (28). Blockade
of IL-1 signaling overcame erlotinib resistance in head and neck
squamous cell carcinoma (33).
Activation of NF-κB, plays an important role in the release of
IL-1β and −6 and TNFα, which triggers a positive feedback loop in
the inflammatory response (34).
NF-κB activation induces anti-apoptotic genes such as B cell
lymphoma (Bcl)-2 and Bcl-extra-large leading to radio- and
chemotherapeutic resistance (35).
Thus, controlling the inflammatory cascade can potentially improve
the outcome of radiotherapy. The expression of CD121a was not
significantly among treatments indicating that 4-MU has no effect
on the IL-1 receptor but suppresses only release of IL-1β. However,
we found that expression of CD126 and CD130 on cell surface was
suppressed in the presence of 4-MU, suggesting that the exchange of
inflammatory response between adjacent cells causing
radioresistance was indirectly suppressed through inhibition of
IL-1β and IL-6 release, and the receptors of IL-6.
HA is synthesized by HAS and is cleaved by
hyaluronidase or oxidative stress, yielding a lower molecular
weight form (36) that activates
macrophages and stimulates the production of proinflammatory
cytokines such as IL-1β through the HA/CD44 interaction thereby
enhancing proliferation and angiogenesis (37). IL-1β and TNFα induced the human
manganese superoxide dismutase gene such as SOD2, which is an
enzyme that degrades active oxygen generated in cells (38). X-ray irradiation has been shown to
produce reactive oxygen species (ROS) or free radicals that
indirectly and/or directly induce DNA strand breakage and exert
cytotoxic effects (39,40). Radiosensitizing effect of 4-MU was
suggested to be due to the accumulation of ROS.
In conclusion, 4-MU increased the sensitivity of
HT1080 cells to X-ray radiation by inhibiting the production of the
proinflammatory cytokines IL-1β and −6. Thus, the efficacy of
radiotherapy can be enhanced by co-administration of 4-MU.
Additional research is needed to determine whether 4-MU can also
prevent distant metastasis of cancer cells; however, our results
highlight the clinical potential of 4-MU as a radiosensitizing
agent that can improve treatment outcome.
Acknowledgements
Not applicable.
Funding
The present study was supported by KAKENHI, Young
Scientists (B) (grant no. 17K16413).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
RS, TN, KO, ET and YH conceived the study,
participated in its design and coordination. RS and KH drafted the
manuscript. RS, KH, KM and MC performed the experiments, and
analyzed and interpreted the data. TN, KO, ET and YH critically
revised the manuscript for important intellectual content. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY,
Kim CH, Park HG, Han SI and Kang HS: Induction of metastasis,
cancer stem cell phenotype, and oncogenic metabolism in cancer
cells by ionizing radiation. Mol Cancer. 16:102017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hara T, Iwadate M, Tachibana K, Waguri S,
Takenoshita S and Hamada N: Metastasis of breast cancer cells to
the bone, lung, and lymph nodes promotes resistance to ionizing
radiation. Strahlenther Onkol. 193:848–855. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuwahara Y, Mori M, Kitahara S and
Fukumoto M, Ezaki T, Mori S, Echigo S, Ohkubo Y and Fukumoto M:
Targeting of tumor endothelial cells combining 2 Gy/day of X-ray
with Everolimus is the effective modality for overcoming clinically
relevant radioresistant tumors. Cancer Med. 3:310–321. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim JS, Chang JW, Yun HS, Yang KM, Hong
EH, Kim DH, Um HD, Lee KH, Lee SJ and Hwang SG: Chloride
intracellular channel 1 identified using proteomic analysis plays
an important role in the radiosensitivity of HEp-2 cells via
reactive oxygen species production. Proteomics. 10:2589–2604. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hazawa M, Hosokawa Y, Monzen S, Yoshino H
and Kashiwakura I: Regulation of DNA damage response and cell cycle
in radiation-resistant HL60 myeloid leukemia cells. Oncol Rep.
28:55–61. 2012.PubMed/NCBI
|
|
6
|
Arechaga-Ocampo E, Lopez-Camarillo C,
Villegas-Sepulveda N, Gonzalez-De la Rosa CH, Perez-Añorve IX,
Roldan-Perez R, Flores-Perez A, Peña-Curiel O, Angeles-Zaragoza O,
Rangel Corona R, et al: Tumor suppressor miR-29c regulates
radioresistance in lung cancer cells. Tumor Biol.
39:10104283176950102017. View Article : Google Scholar
|
|
7
|
Zhou SB, Guo XW, Gu L and Ji SJ:
Influential factors on radiotherapy efficacy and prognosis in
patients with secondary lymph node metastasis after esophagectomy
of thoracic esophageal squamous cell carcinoma. Cancer Manag Res.
10:217–225. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang J, Xu H, Guo X, Zhang J, Ye X, Yang Y
and Ma X: Pretreatment inflammatory indexes as prognostic
predictors for survival in colorectal cancer patients receiving
neoadjuvant chemoradiotherapy. Sci Rep. 8:30442018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsuda T, Sumi Y, Yamashita K, Hasegawa
H, Yamamoto M, Matsuda Y, Kanaji S, Oshikiri T, Nakamura T, Suzuki
S and Kakeji Y: Outcomes and prognostic factors of selective
lateral pelvic lymph node dissection with preoperative
chemoradiotherapy for locally advanced rectal cancer. Int J
Colorectal Dis. 33:367–374. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xia Y, Li YH, Chen Y, Liu Q, Zhang JH,
Deng JY, Ai TS, Zhu HT, Badakhshi H and Zhao KL: A phase II trial
of concurrent chemoradiotherapy with weekly paclitaxel and
carboplatin in advanced oesophageal carcinoma. Int J Clin Oncol.
23:458–465. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fangzheng W, Chuner J, Quanquan S, Zhimin
Y, Tongxin L, Jiping L, Sakamoto M, Peng W, Kaiyuan S, Weifeng Q,
et al: Addition of 5-fluorouracil to docetaxel/cisplatin does not
improve survival in locoregionally advanced nasopharyngeal
carcinoma. Oncotarget. 8:115469–115479. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim HJ, Choi GS, Park JS, Park SY, Cho SH,
Lee SJ, Kang BW and Kim JG: Optimal treatment strategies for
clinically suspicious lateral pelvic lymph node metastasis in
rectal cancer. Oncotarget. 8:100724–100733. 2017.PubMed/NCBI
|
|
13
|
Nakamura T, Takagaki K, Shibata S, Tanaka
K, Higuchi T and Endo M: Hyaluronic-acid-deficient extracellular
matrix induced by addition of 4-methylumbelliferone to the medium
of cultured human skin fibroblasts. Biochem Biophys Res Commun.
208:470–475. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuroda Y, Kasai K, Nanashima N, Nozaka H,
Nakano M, Chiba M, Yoneda M and Nakamura T: 4-Methylumbelliferone
inhibits the phosphorylation of hyaluronan synthase 2 induced by
12-O-tetradecanoyl-phorbol-13-acetate. Biomed Res. 34:97–103. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yates TJ, Lopez LE, Lokeshwar SD, Ortiz N,
Kallifatidis G, Jordan A, Hoye K, Altman N and Lokeshwar VB:
Dietary supplement 4-methylumbelliferone: An effective
chemopreventive and therapeutic agent for prostate cancer. J Natl
Cancer Inst. 107(djv085)2015.PubMed/NCBI
|
|
16
|
Piccioni F, Fiore E, Bayo J, Atorrasagasti
C, Peixoto E, Rizzo M, Malvicini M, Tirado-González I, García MG,
Alaniz L and Mazzolini G: 4-methylumbelliferone inhibits
hepatocellular carcinoma growth by decreasing IL-6 production and
angiogenesis. Glycobiology. 25:825–835. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li F, Hao P, Liu G, Wang W, Han R, Jiang Z
and Li X: Effects of 4-methylumbelliferone and high molecular
weight hyaluronic acid on the inflammation of corneal stromal cells
induced by LPS. Graefes Arch Clin Exp Ophthalmol. 255:559–566.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Piao L, Canguo Z, Wenjie L, Xiaoli C,
Wenli S and Li L: Lipopolysaccharides-stimulated macrophage
products enhance Withaferin A-induced apoptosis via activation of
caspases and inhibition of NF-κB pathway in human cancer cells. Mol
Immunol. 81:92–101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu H, Aravindan N, Xu J and Natarajan M:
Inter- and intra-cellular mechanism of NF-kB-dependent survival
advantage and clonal expansion of radio-resistant cancer cells.
Cell Signal. 31:105–111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mochizuki D, Adams A, Warner KA, Zhang Z,
Pearson AT, Misawa K, McLean SA, Wolf GT and Nör JE: Anti-tumor
effect of inhibition of IL-6 signaling in mucoepidermoid carcinoma.
Oncotarget. 6:22822–22835. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee SO, Yang X, Duan S, Tsai Y, Strojny
LR, Keng P and Chen Y: IL-6 promotes growth and
epithelial-mesenchymal transition of CD133+ cells of non-small cell
lung cancer. Oncotarget. 7:6626–6638. 2016.PubMed/NCBI
|
|
22
|
Saga R, Monzen S, Chiba M, Yoshino H,
Nakamura T and Hosokawa Y: Anti-tumor and anti-invasion effects of
a combination of 4-methylumbelliferone and ionizing radiation in
human fibrosarcoma cells. Oncol Lett. 13:410–416. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lokeshwar VB, Lopez LE, Munoz D, Chi A,
Shirodkar SP, Lokeshwar SD, Escudero DO, Dhir N and Altman N:
Antitumor activity of hyaluronic acid synthesis inhibitor
4-methylumbelliferone in prostate cancer cells. Cancer Res.
70:2613–2623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arai E, Nishida Y, Wasa J, Urakawa H, Zhuo
L, Kimata K, Kozawa E, Futamura N and Ishiguro N: Inhibition of
hyaluronan retention by 4-methylumbelliferone suppresses
osteosarcoma cells in vitro and lung metastasis in vivo. Br J
Cancer. 105:1839–1849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Urakawa H, Nishida Y, Wasa J, Arai E, Zhuo
L, Kimata K, Kozawa E, Futamura N and Ishiguro N: Inhibition of
hyaluronan synthesis in breast cancer cells by
4-methylumbelliferone suppresses tumorigenicity in vitro and
metastatic lesions of bone in vivo. Int J Cancer. 130:454–466.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Li L, Brown TJ and Heldin P:
Silencing of hyaluronan synthase 2 suppresses the malignant
phenotype of invasion breast cancer cells. Int J Cancer.
120:2557–2567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nazarenko I, Marhaba R, Reich E, Voronov
E, Vitacolonna M, Hildebrand D, Elter E, Rajasagi M, Apte RN and
Zöller M: Tumorigenicity of IL-1alpha- and IL-1beta-deficient
fibrosarcoma cells. Neoplasia. 10:549–562. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun Y and Ma L: The emerging molecular
machinery and therapeutic targets of metastasis. Trends Pharmacol
Sci. 36:349–359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tamari Y, Kashino G and Mori H:
Acquisition of radioresistance by IL-6 treatment is caused by
suppression of oxidative stress derived from mitochondria after
γ-irradiation. J Radiat Res. 58:412–420. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen Y, Zhang F, Tsai Y, Yang X, Yang L,
Duan S, Wang X, Keng P and Lee SO: IL-6 signaling promotes DNA
repair and prevents apoptosis in CD133+ stem-like cells of lung
cancer after radiation. Radiat Oncol. 10:2272015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duan S, Tsai Y, Keng P and Chen Y, Lee SO
and Chen Y: IL-6 signaling contributes to cisplatin resistance in
non-small cell lung cancer via the up-regulation of anti-apoptotic
and DNA repair associated molecules. Oncotarget. 6:27651–27660.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stanam A, Gibson-Corley KN, Love-Homan L,
Ihejirika N and Simons AL: Interleukin-1 blockade overcomes
erlotinib resistance in head and neck squamous cell carcinoma.
Oncotarget. 7:76087–76100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grivennikov SI and Karin M: Dangerous
liaisons: STAT3 and NF-kappaB collaboration and crosstalk in
cancer. Cytokine Growth Factor Rev. 21:11–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hei TK, Zhou H, Chai Y, Ponnaiya B and
Ivanov VN: Radiation induced non-targeted response: Mechanism and
potential clinical implications. Curr Mol Pharmacol. 4:96–105.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nagy N, Kuipers HF, Frymoyer AR, Ishak HD,
Bollyky JB, Wight TN and Bollyky PL: 4-methylumbelliferone
treatment and hyaluronan inhibition as a therapeutic strategy in
inflammation, autoimmunity, and cancer. Front Immunol. 6:1232015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hauser-Kawaguchi A, Luyt LG and Turley E:
Design of peptide mimetics to block pro-inflammatory functions of
HA fragments. Matrix Biol. S0945(053X): 30444–30454. 2018.
|
|
38
|
Xu Y, Kiningham KK, Devalaraja MN, Yeh CC,
Majima H, Kasarskis EJ and St Clair DK: An intronic NF-kappaB
element is essential for induction of the human manganese
superoxide dismutase gene by tumor necrosis factor-alpha and
interleukin-1beta. DNA Cell Biol. 18:709–722. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bajinskis A, Natarajan AT, Erixon K and
Harms-Ringdahl M: DNA double strand breaks induced by the indirect
effect of radiation are more efficiently repaired by non-homologous
end joining compared to homologous recombination repair. Mutat Res.
756:21–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vignard J, Mirey G and Salles B:
Ionizing-radiation induced DNA double-strand breaks: A direct and
indirect lighting up. Radiother Oncol. 108:362–369. 2013.
View Article : Google Scholar : PubMed/NCBI
|