Introduction
Globally, lung cancer is ranked as the second most
prevalent malignancy among all types of cancer and is a common
cause of cancer-associated mortalities (1). It has been reported that >80% of
diagnosed lung-cancer cases are non-small cell lung cancers
(NSCLCs) (2). Lung-cancer treatment
is promising if the disease is detected at its early stage, and
less beneficial at advanced stage, though a relatively high risk of
recurrence occurs even after exposure to available potent treatment
(3). NSCLC migration and invasion
contribute to poor treatment outcome and lung-cancer mortality
(4). NSCLC cells aggressively
proliferate and invade adjacent tissues, cross the basement
membrane and migrate to establish colonies in distant organs of the
body via either the vascular system or the lymphatic system
(5). With advancements in molecular
biology tools and the biological processes identified in the past
decades, the basic biology of tumors is now well understood
(6). Several oncogenes have been
identified, including passenger and driver genes, such as KRAS,
ERBB2 and BRAF (7). Various
oncogenes modulating cancer angiogenesis and metastasis have been
identified in recent years, providing novel potential therapeutic
targets such as aquaporin (AQP), which was investigated in the
present study.
AQPs are water channels, and are ubiquitous integral
membrane proteins that control extra- and intra-cellular fluid
passage (8). AQPs play a regulatory
role in human carcinogenesis (9).
The expression of human AQP1 has been found to be unaffected in
brain tumors, renal cell carcinoma, colon cancer and pancreatic
cancer, whereas AQP5 expression was identified to be increased in
lung, salivary glands and kidney cancer (10). In addition, AQP5 is increased in
ovarian and cervical cancer, esophageal squamous cell carcinoma,
and hepatocellular carcinoma; however, lymphocyte activation
stimulates the transcription of AQP1, AQP3 and AQP5 (11–14).
AQPs play an important functional role in cell migration in various
cell types, including brain, pancreas and colon cancer cells
(15,16). A previous study examining NSCLC
revealed that AQP5 level is associated with the progression of lung
cancer (4). AQP5 upregulation was
also observed in adenocarcinoma cells, but it is relatively less
expressed in non-mucinous bronchioloalveolar carcinoma (11). The H1299 cell line is a human NSCLC
cell line, which is derived from the lymph node and has been widely
used to investigate a number of disease-associated mechanisms, such
as tumor necrosis and cancer cells apoptosis (17,18). A
previous study indicated that AQP5 activates the EGFR1/2 and ERK1/2
pathway, which regulates cancer cell migration and proliferation
(19,20). In the present study, the influence of
AQP5 on H1299 cell migration and regulation of angiogenesis was
investigated, and the present study suggested that AQP5
downregulation significantly decreased both migration and
angiogenesis regulation of NSCLC H1299 cells.
Materials and methods
Cell culture
The NSCLC cell lines H1299 and 16HBE were purchased
from the American Type Culture Collection. Cells were cultured in
RPMI-1640 medium (Hyclone; GE Healthcare Life Sciences)
supplemented with 10% FBS (Thermo Fisher Scientific, Inc.), 100
U/ml penicillin, and 100 µg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc.). Cells were passaged 3–5 times before collection.
HUVECs were obtained from The Dalian Medical University, and
cultured in M199 medium (Gibco; Thermo Fisher Scientific, Inc.).
Both cultures were incubated at 37°C with 5% CO2.
Total mRNA extraction
H1299 cells were grown until they reached 80–90%
confluency and were washed twice with sterile PBS and harvested
using RNAiso Plus (Takara Bio, Inc.) according to the
manufacturer's protocol. Cells were resuspended in RNAiso Plus, put
on ice for 5 min and centrifuged at 4°C at 12,000 × g for 15 min.
The supernatant was added to an equal volume of chloroform,
incubated on ice for 10 min and centrifuged at 4°C at 12,000 × g.
In total, 550 µl isopropanol was added to the aqueous phase, and
the solution was mixed gently and centrifuged at 12,000 × g for 15
min at 4°C. The sediment was washed in 1 ml ethanol (75%) in
diethyl pyrocarbonate (DEPC)-treated H2O, and
centrifuged for 10 min at 4°C at 12,000 × g. The pellet was
air-dried, dissolved in 10 µl RNase-free H2O, and the
concentration was determined using a NanoDrop 2000 (Thermo Fisher
Scientific, Inc.).
Reverse transcription PCR
(RT-PCR)
RNA (~1 µg) was converted to cDNA using the
PrimeScript first strand cDNA Synthesis kit (Takara Bio, Inc.)
according to the manufacturer's protocol. A mixture containing
oligo dT primer, dNTP mixture (Takara Bio, Inc.), RNase-free
ddH2O and the extracted RNA was incubated at 65°C for 5
min and immediately cooled on ice. The buffer 5X PrimeScript
(Takara Bio, Inc.), RNase inhibitor, reverse transcriptase and
RNase-free ddH2O (Takara Bio, Inc.) were added to the
previous mixture in a total volume of 20 µl, according to the
manufacturer's protocol. The mixture was homogenized gently using a
micro-centrifuge at 6,000 × g at room temperature for 5 min, and
incubated for 60 min at 42°C. The enzyme was inactivated by
incubating the samples at 95°C for 5 min. Subsequently, the samples
were cooled on ice. PCR was performed using PCR premix (Takara Bio,
Inc.) as follows: 30 cycles of 94°C for 5 min, 94°C for 30 sec,
56°C for 30 sec, 72°C for 1 min, 72°C for 7 min and final hold at
12°C, the PCR product was loaded in 2% agarose gel and visualized
using EthidiumBromide, and GAPDH was used as an internal control to
evaluate cDNA synthesis efficiency. The RT bands were determined by
IMAGEJ software (version 1.46; National Insitutes of Health). The
primers used in the present study were purchased from Takara Bio,
Inc. (Table I).
 | Table I.Primer sequences used for cDNA
amplification. |
Table I.
Primer sequences used for cDNA
amplification.
| Gene | Sequences
(5′→3′) |
|---|
| AQP1 | F:
TCTGGAGGCTGTGGTGGCT |
|
| R:
AAGTGAGTTCTCGAGCAGGGA |
| AQP3 | F:
AGCGAGTTTGGATGAGCAGCAGA |
|
| R:
AAGGAGACGGCAAGCAGGGTGTA |
| AQP4 | F:
ATGGTGGCTTTCAAAGGGGT |
|
| R:
GATGGGCCCAACCCAATATAT |
| AQP5 | F:
TGGGTCTTCTGGGTAGGGCCTATTGT |
|
| R:
GCCGGCTTTGGCACTTGAGATACT |
| AQP8 | F:
TCATTGGAGATGGGAAG ACC |
|
| R:
TGAGAAGCAAGGAAGTG GC |
| AQP9 | F:
CTCAGTCCCAGGCTCTTCAC |
|
| R:
CTCAGTCCCAGGCTCTTCAC |
| VEGF | F:
TTCTGGGCTGTTCTCGCTTC |
|
| R:
CTCTCCTCTTCCTTCTCTTCTTCC |
| GAPDH | F:
TGACCACAGTCCATGCCATCAC |
|
| R:
CGCCTGCTTCACCACCTTCTT |
Protein extraction
H1299 cells cultured to 100% confluence were scraped
using ice-cold PBS and a cold plastic scraper and harvested
following a centrifugation at 12,000 × g for 3 min at 4°C. RIPA
lysis buffer (Nanjing KeyGen Biotech Co., Ltd.) containing a
proteinase inhibitor, phosphate inhibitor and PMSF, and cells were
lysed according to the manufacturer's protocol. The mixture was
then vortexed for 40 min at room temperature and centrifuged at
12,000 × g for 15 min at 4°C. The supernatant was used as the total
protein extract. Total protein concentration was measured using a
bicinchoninic acid assay (Nanjing KeyGen Biotech Co., Ltd.)
according to the manufacturer's protocol. A standard absorbance
curve was established using known concentrations of protein, and
the protein concentration in the samples was extrapolated from the
standard curve. Total protein (~20 µg) was used for SDS-PAGE and
western blot analysis.
Western blot analysis
Total protein (~20 µg/lane) extracted from H1299 or
16HBE cells was loaded on 12% SDS-PAGE, transferred to Hybond-C
nitrocellulose membranes (GE Healthcare Life Sciences), and blocked
with 5% skimmed milk in 0.5% TBS-Tween 20 (TBST) at room
temperature for 1 h. The membranes were incubated with rabbit
anti-AQP5 (cat. no. ab78486), EGFR (cat. no. ab52894), p-EGFR (cat.
no. ab5644), ERK (cat. no. ab54230) and p-ERK (cat. no. ab65142),
(dilution, 1:1,000) and β-actin (dilution, 1:1,000; cat no. ab6276)
(all from Abcam) at 4°C overnight. After the membranes were washed
three times with TBST, they were incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibodies
(dilution 1:100, catalog no. ab205718; Abcam) for 1 h at room
temperature. The protein was visualized using an enhanced
chemiluminescence detection system (Bio-Rad Laboratories, Inc.).
Band density was quantified using the Image Lab software (version
4.0; Bio-Rad Laboratories, Inc.).
AQP5 gene silencing
Small interfering (si)RNAs against AQP5, negative
(NC) and positive (PC) controls were purchased from Shanghai
GenePharma Co., Ltd. siRNA sequences are presented in Table II. The siRNAs were dissolved in 125
µl DEPC-treated water (Shanghai GenePharma Co., Ltd.) and
transfected into H1299 cells at 70–90% confluence. According to the
manufacturer's protocol, cells were transfected with 20 nmol of
siRNA using Lipofectamine™ 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The effect of the transfection was confirmed by
RT-PCR, western blot analysis and fluorescence microscopy 48 h
after transfection.
 | Table II.Sequences of siRNA used to knockdown
AQP5 expression. |
Table II.
Sequences of siRNA used to knockdown
AQP5 expression.
| siRNA | Sequence
(5′→3′) |
|---|
| AQP5-560 | F:
CCGUGUUCGCAGAGUUCUUTT |
|
| R:
AAGAACUCUGCGAACACGGTT |
| AQP5-883 | F:
GCGUGUGGCCAUCAUCAAATT |
|
| R:
UUGUGUUGUUGUUGAGCGCTT |
| AQP5-1224 | F:
GCGUGUGGCCAUCAUCAAATT |
|
| R:
UUUGAUGAUGGCCACACGCTT |
| Negative siRNA | F:
UUCUCCGAACGUGUCACGUTT |
|
| R:
ACGUGACACGUUCGGAGAATT |
| Positive siRNA | F:
UGACCUCAACUACAUGGUUTT |
|
| R:
AACCAUGUAGUUGAGGUCATT |
Transwell migration assay
Transwell migration assay was performed to measure
tumor cell migration. Transwell inserts (8-µm pores; Corning, Inc.)
in 24-well plates were used. H1299 cells (~100 µl; 1×105
cells/ml) in 100 µl serum-free RPMI, were added to the upper
chambers, and the lower chambers were filled with 350 µl RPMI
medium containing 20% FBS as the attracting agent. After 24 h of
incubation at 37°C with 5% CO2, the non-migrated cells
were removed from the upper side, whereas the migrated cells were
fixed with 70% methanol for 10 min at room temperature, and stained
with 0.1% crystal violet at room temperature for 10 min. The cells
were visualized using a light inverted microscope (Olympus 1X71;
Olympus Corporation) at ×10 magnification.
Wound-healing assay
H1299 cells were cultured into six-well plates for
24 h until they reached 100% confluence, and then the middle of
each well was scratched using a 200 µl pipette tip. The scratched
layers were washed with PBS to remove non-adherent cells, and the
medium was replaced with serum-free RPMI. Wound healing was
observed using light inverted microscope (Olympus 1X71; Olympus
Corporation) at ×10 magnification, and evaluated by comparing the
cell-free area with the initial wound region.
HUVEC tube formation assay
HUVEC tube formation was evaluated using a
previously described protocol (12).
The supernatant of H1299 cells was collected and stored at −20°C
before use. Growth factor-reduced Matrigel (BD Biosciences) was
added into 48-well plates (100 µl each) and allowed to solidify at
37°C for 30 min. (1×102 cells/ml) HUVECs with serum-free
RPMI medium were resuspended in H1299 culture supernatants with 1%
FBS RPMI (total 200 µl) at a 1:1 ratio, added onto a Matrigel layer
and incubated at 37°C with 5% CO2. Following cell
seeding, tube formation was observed every hour using light
inverted microscope (Olympus 1X71; Olympus Corporation) using ×10
magnification, and cells were imaged after 8 h.
Statistical analysis
GraphPad Prism (version 6.07; GraphPad Software,
Inc.) was used for all statistical analyses. Data are presented as
mean ± standard deviation. Student's t-test was used to compare
differences between two groups, and one-way ANOVA was employed to
compare ≥3 groups, and the multiple comparisons were performed
using one-way analysis, followed by Tukeys post hoc test. All
experiments were performed in triplicate and P<0.05 was
considered to indicate a statistically significant difference.
Results
AQP5 is upregulated in H1299
cells
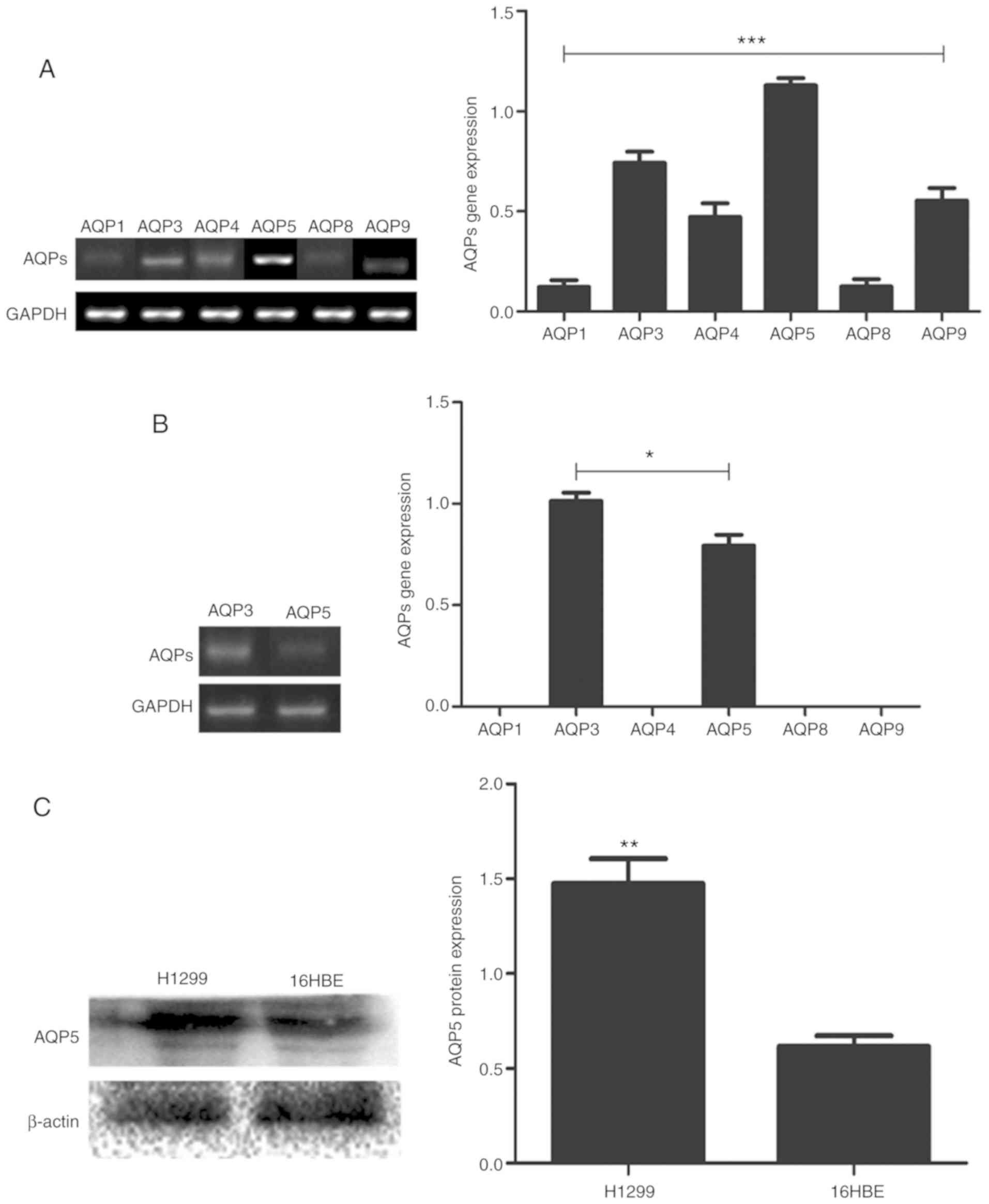
To assess the pattern of AQP expression in NSCLC,
the expression of six AQPs genes, including AQP 1, 3, 4, 5, 8 and
9, was evaluated in H1299 cells via RT-PCR. Among the six genes
investigated, AQP5 was upregulated in H1299 cells (Fig. 1A). The expression of AQP5 was also
assessed in the normal human bronchial epithelial cells 16HBE, and
it was observed that AQP5 gene was expressed at a lower level
compared with AQP3, with no detectable expression levels of the
other AQPs genes (1, 4, 8 and 9), (Fig.
1B). The protein levels of AQP5 were assessed in both H1299 and
16HBE cells by western blot analysis. AQP5 protein was
significantly upregulated in H1299 cells compared with 16HBE cells
(Fig. 1C). AQP5 was therefore
selected as a target for gene silencing.
AQP5 gene silencing
H1299 cells were transfected with various siRNAs
against AQP5. In total, three different siRNA sequences were tested
to determine the optimal siRNA to knockdown AQP5 expression.
siRNA-560 exhibited the highest downregulation of AQP5 gene, as
evidenced by fluorescence imaging for determine the transfection
efficiency using (FAM) conjugated to the 5′ end of the siRNAs
(Fig. 2A), RT-PCR (Fig. 2B) and western blot analysis (Fig. 2C).
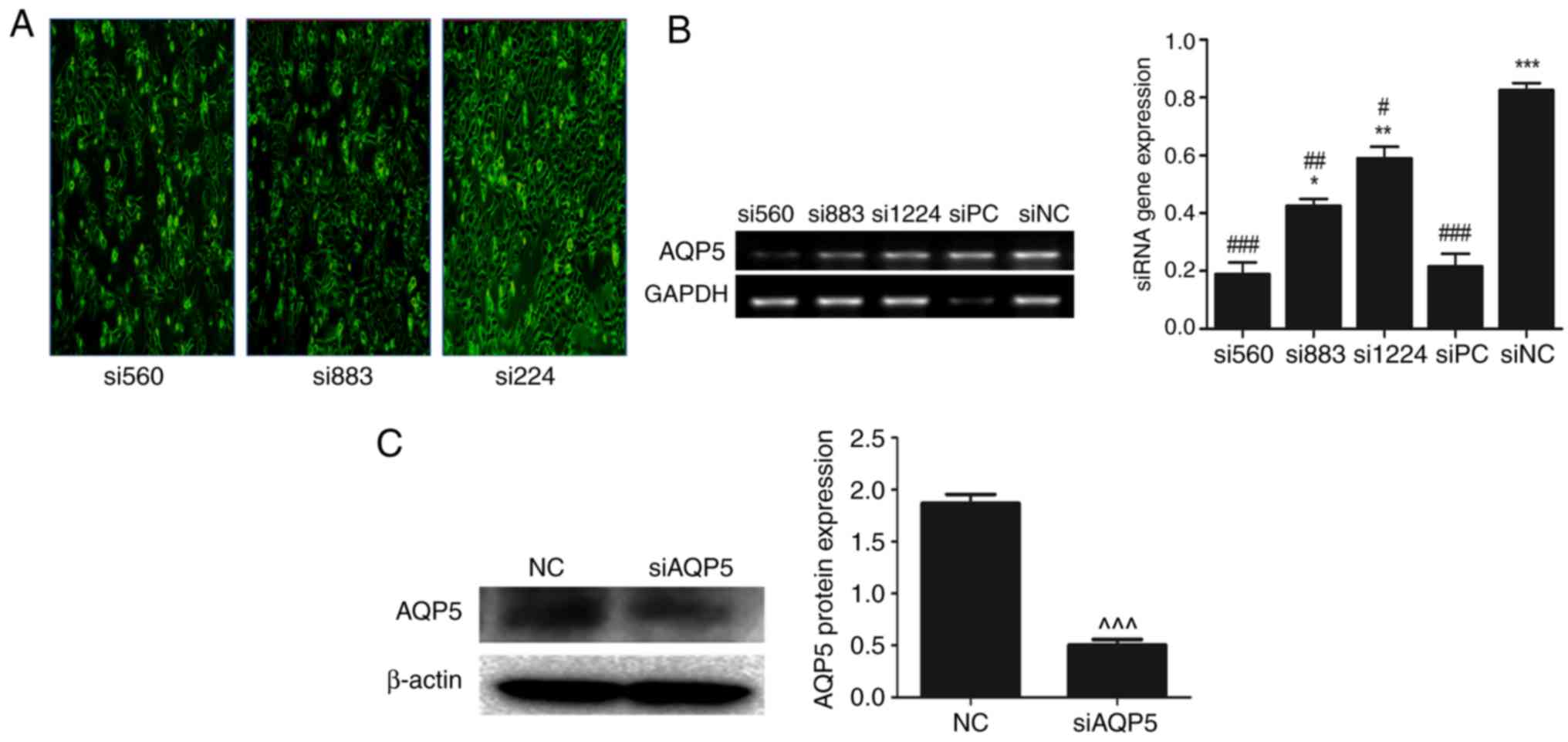 | Figure 2.Knockdown of AQP5 in H1299 cells and
AQP5 expression. (A) H1299 cells were transfected with different
siRNAs. Images were obtained using a fluorescence microscope,
magnification, ×10. (B) Gene expression of AQP5 following
transfection with different siRNAs. (C) Protein expression of AQP5
in H1299 cells with and without transfection. GAPDH was used as
internal control for gene expression, and β-actin was used as
reference protein. Results shown are representative of three
independent experiments. *P<0.05, **P<0.001, ***P<0.0001
vs. siPC; #P<0.05, ##P<0.001,
###P<0.0001 vs. siNC; ^^^P<0.0001 vs.
NC. siRNA, small interfering RNA; siPC, siRNA positive control
targeting GAPDH; siNC, siRNA negative control; NC, untransfected
cells; AQP, aquaporin. |
AQP5 is involved in lung cancer cell
migration
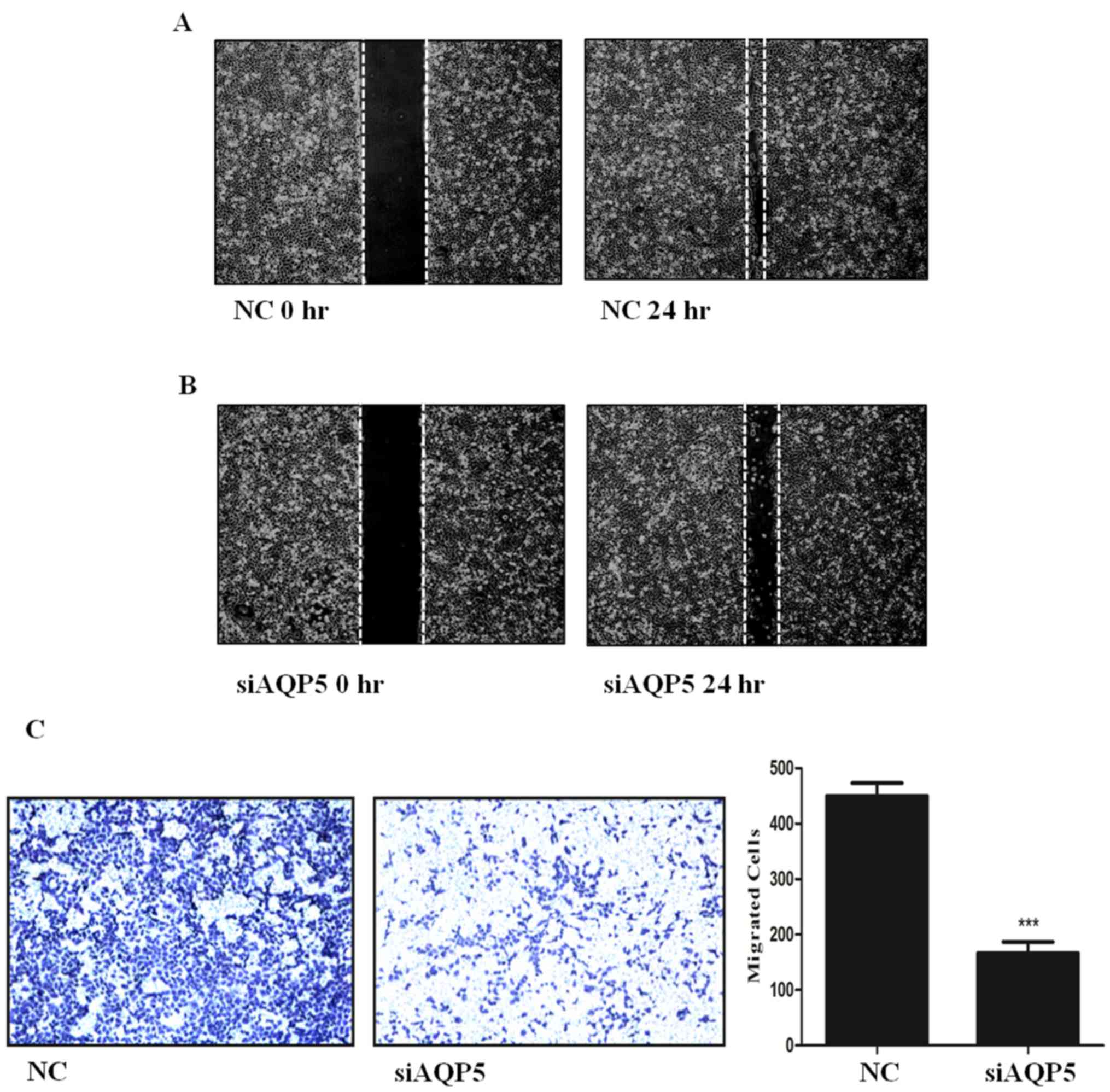
To evaluate the migration of H1299 cells following
AQP5 downregulation, Transwell migration and wound closure assays
were performed. A significant reduction in the closure of the wound
gap was observed after AQP5 knockdown (siAQP5) compared with the NC
cells (Figs. 3A and 3B). Similarly, the Transwell assay
indicated that siAQP5 significantly decreased H1299 cell migration
compared with the NC cells (Fig.
3C). Collectively, the present results suggested that AQP5 may
be involved in NSCLC cell migration, and reducing its expression
may represent a potential therapeutic treatment.
Downregulation of AQP5 inhibits
angiogenesis and decreases activation of the epidermal growth
factor receptor (EGFR)/ERK pathway
Vascular supply is required for cancer cells to
proliferate and survive (21).
Therefore, the role of AQP5 in tumor vascularisation was
investigated using HUVECs. These cells exhibit the ability to grow
and form tube-like structures, depending on the growth factors
contained in the supernatant (22).
HUVECs were treated with the supernatant of NC cultures as control
medium (Fig. 4A). The supernatant of
H1299 cells transfected with siAQP5 was used as the experimental
sample. Increased tube formation was observed in HUVECs treated
with the supernatant collected from NC H1299 cells compared with
siAQP5-treated H1299 cells (Fig.
4A). The present results suggested that AQP5 knockdown reduced
H1299 cell vascularisation. The expression of vascular endothelial
growth factor (VEGF) in NC and siAQP5-treated H1299 cells was also
assessed. The present results suggested that the expression level
of VEGF was reduced in siAQP5 cells compared with NC cells
(Fig. 4B). NC cells showed
significantly increased EGFR and ERK1/2 phosphorylation, whereas
AQP5 downregulation significantly decreased EGFR/ERK pathway
activation in H1299 cells (Fig.
4C).
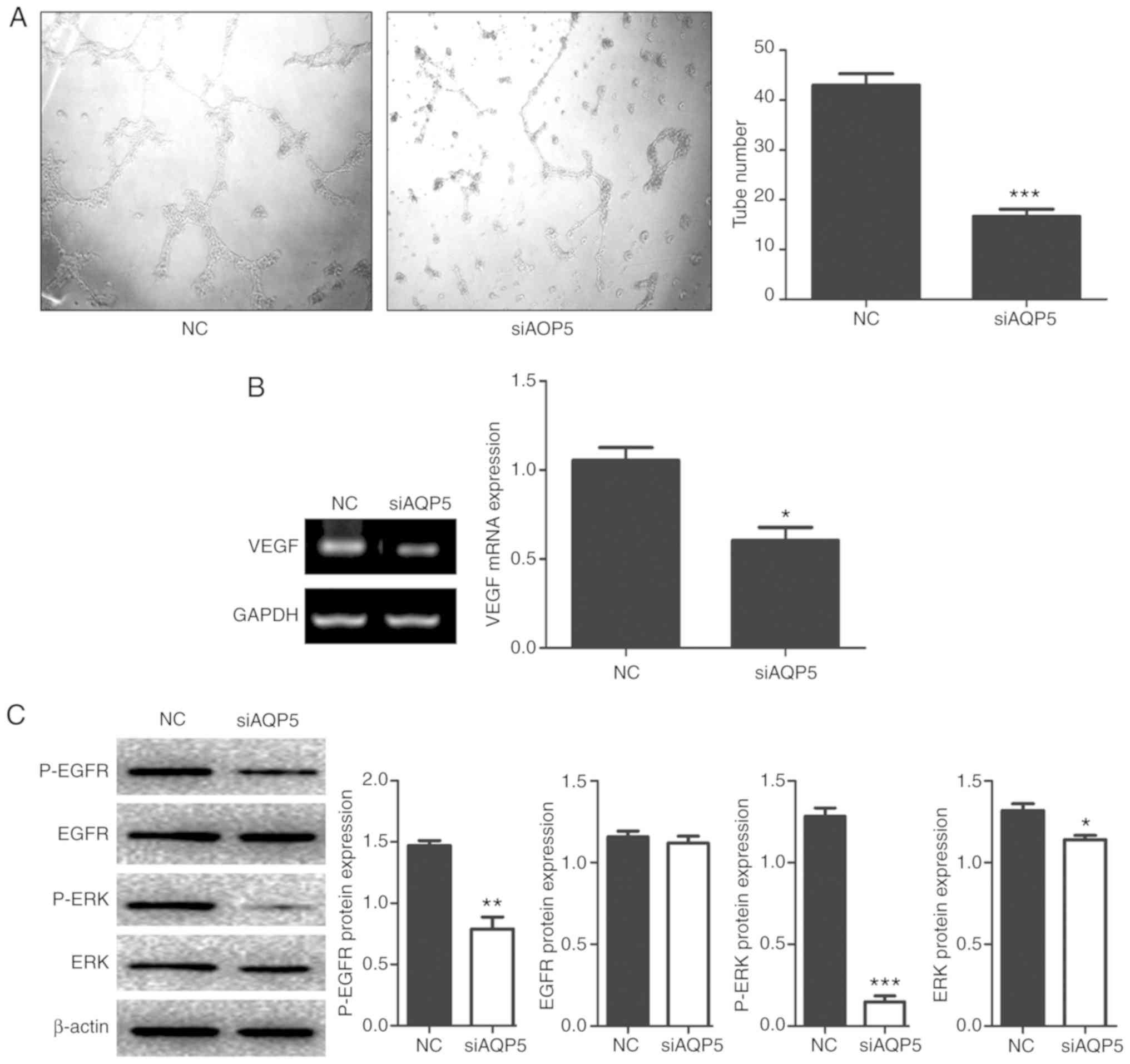 | Figure 4.AQP5 downregulation inhibits
angiogenesis and the activity of the EGFR/ERK signaling pathway.
(A) HUVEC cultured in conditioned medium isolated from
untransfected and transfected H1299 cells, magnification, ×10. (B)
VEGF mRNA levels in untransfected and transfected H1299 cells. (C)
Knockdown of AQP5 caused a significantly decreased activity in the
expression levels of protein associated with the EGFR/ERK pathway
in H1299 cells. GAPDH was used as internal control for gene
expression and β-actin was used as reference protein. *P<0.05,
**P<0.001, ***P<0.0001 vs. NC. siRNA, small interfering RNA;
HUVEC, human umbilical vein endothelial cell; NC, untransfected
cells; AQP, aquaporin; EGFR, epidermal growth factor receptor; p-,
phosphorylated; VEGF, vascular endothelial growth factor. |
Discussion
AQPs serve important roles in vascular permeability
and interstitial fluid pressure in tumors (23). An increased expression of AQP genes
was found in different types of tumors, suggesting their potential
to influence tumor activities in various tissues (8,23,24). Low
AQP expression can inhibit the outgrowth of capillaries by
decreasing VEGF expression in NSCLC (21). Therefore, AQPs may be partly
responsible for tumor metastasis, and reducing their biological
activity in tumors may be a promising therapeutic option (25–27). The
growth, development, invasion and migration of NSCLC depend on an
efficient supply of nutrients and metabolic activity (11). Water molecules are indispensable in
cellular activities, especially in tumors, and an increased
nutrition and water supply is required by cancerous cells.
Consistent with previous findings (21), six different types of AQPs were found
to be expressed in NSCLC and H1299 cells in the present study.
Specifically, AQP1, 3, 4, 5, 8 and 9 were identified to be
expressed in NSCLC cells. However, only AQP3 and 5 were detected in
the normal lung cell line 16HBE, in contrast to other studies
reporting that multiple AQPs are expressed in normal lung cells
(28–30). In normal lung cells, AQP3 was
significantly increased compared with AQP5; however, AQP3 and AQP5
exhibited opposite trends in NSCLCs. The present study did not
investigate whether knockdown of AQP3 in NSCLC could increase the
expression level of AQP5 in tumours. However, Machida et al
(11) have reported that the
expression of AQP3 and AQP5 in lung cancer cells is generally
associated with cellular differentiation.
The role of AQP5 in lung cancer migration in
vitro has been investigated in the present study. Tumor
migration is a major feature of cancer, and suppressing this
process is essential to reduce the spread of tumors (31). Both AQP3 and AQP5 are involved in
malignant tumors (23). Woo et
al (13) observed that AQP5 is
involved in promoting tumor cells proliferation, whereas other
researchers have reported the role of elevated AQP5 in the
metastasis of colorectal, hepatocellular, squamous cell, cervical
and early breast cancer (8,9,13,23,25,27,32).
A high level of AQP5 has been observed in NSCLC, which positively
correlates with lymph node metastasis; in addition, AQP5 expression
is significantly higher in stages III and IV NSCLC (2), compared with that in stages I and II,
suggesting its role in lung cancer progression (2). Woo et al (13) reported that AQP5 is a promising
therapeutic target and may be involved in tumor establishment and
progression more predominantly than other AQPs. AQP5 upregulation
is associated with cellular differentiation and serves a major role
in invading lung-cancer cells (19).
In the present study, it was observed that AQP5 was
highly expressed in H1299, a NSCLC cell line. This observation is
in line with previous findings suggesting the upregulation of AQP5
in lung adenocarcinoma cells (14).
In clinical settings, AQP5 upregulation is considered as a sign of
poor cancer prognosis (2,19). Patients with NSCLC exhibiting AQP5
upregulation present high rates of recurrence and AQP5 upregulation
is associated with early disease progression, supporting the
oncogenic roles of AQP5 in tumor cells (10). The EGFR/ERK signalling pathway is
crucial in lung cancer metastasis (19). AQP5 expression levels are directly
associated with the activity of the EGFR/ERK signalling pathway, in
addition, p-ERK activates hypoxia inducible factor (HIF)-1α, which
leads to the degradation of inhibitors of AQP5 transcription, and
enhances the transcriptional activators of AQP5, modulating AQP5
transcription (33). An association
between AQP5 and mucin 5 subtype AC gene expression has been
observed, and increased AQP5 expression results in mucin
hypersecretion in human pulmonary tracts (19). The present study identified a reduced
migration of H1299 cells following AQP5 knockdown, suggesting that
AQP5 may serve a regulatory role in the expression of
migration-associated genes.
The present study suggested that AQP5 knockdown
decreased tube formation in HUVECs, suggesting a role for AQP5 in
angiogenesis. AQPs are water channels and exhibit significant
diagnostic and prognostic potential, particularly in tumours
(34). AQP3 and AQP5 were found to
play crucial roles in tumour vascularisation, and AQP3 knockdown
reduces the density of microvessels in NSCLC via HIF-2α (26). Inhibition of AQP5 significantly
decreases the expression of VEGF, which is critical in tumour
angiogenesis, suggesting that increased expression of VEGF is
positively associated with angiogenesis (21). In a previous study, AQP5
downregulation in colorectal cancer cells resulted in decreased
expression of VEGF and a corresponding inhibition of angiogenesis
in HUVEC (35). In line with this
previous study, the present study found that supernatant collected
from H1299 cells following AQP5 knockdown exhibited reduced HUVEC
angiogenesis. Tumors exhibit an increased vascularisation compared
with normal tissues, the increased vascularisation in tumors can
reduce the nutrition supply of normal tissues, decreasing their
metabolic activities (21). This
effect suggests that suppressing tumour-specific angiogenesis may
facilitate cancer treatment. As identified in the present study,
downregulation of AQP5 may repress tumor-specific vascularization.
The present study did not investigate whether concomitant knockdown
of AQP3 and AQP5 may synergistically decrease tumor
vascularization, particularly in NSCLC. Collectively, the present
results suggested that AQP5 may be involved in the regulation of
lung cancer cells migration and angiogenesis via the EGFR/ERK1/2
signaling pathway, and that AQP5 knockdown may be used as a
potential target for the prevention of lung cancer metastasis and
angiogenesis. However, the association between AQP5 and NSCLC
remains elusive, and further studies are required to verify the
role of AQP5 in vivo.
Acknowledgements
Not applicable.
Funding
The present study was funded by The National Natural
Science Foundation of China (grant no. 81671606), The Special Grant
for Translational Medicine (Dalian Medical University; grant no.
2015010), The College Scientific Research Project of Education
Department of Liaoning Province (grant no. LQ2017004), Liao Ning
Science and Technology Department (grant no. 20180550789) and the
Key Laboratory of Human Microecology, Homeostasis and Disease
Immune Mechanisms (Dalian).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
AE, MA and WW designed and performed the
experiments, analyzed the data and wrote the manuscript. HL, XO,
XS, YT and BW interpreted the experiment and analyzed the data. XL
and JW planned the experiments, analyzed data, modified the paper
and approved the final version of the manuscript submitted for
publication. All authors read and approved the final version of
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dela Cruz CS, Tanoue LT and Matthay RA:
Lung cancer: Epidemiology, etiology, and prevention. Clin Chest
Med. 32:605–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song T, Yang H, Ho JC, Tang SC, Sze SC,
Lao L, Wang Y and Zhang KY: Expression of aquaporin 5 in primary
carcinoma and lymph node metastatic carcinoma of non-small cell
lung cancer. Oncol Lett. 9:2799–2804. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grunnet M and Sorensen JB:
Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung
Cancer. 76:138–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ge XJ, Zheng LM, Feng ZX, Li MY, Liu L,
Zhao YJ and Jiang JY: H19 contributes to poor clinical features in
NSCLC patients and leads to enhanced invasion in A549 cells through
regulating miRNA-203-mediated epithelial-mesenchymal transition.
Oncol Lett. 16:4480–4488. 2018.PubMed/NCBI
|
|
5
|
O'Flaherty JD, Gray S, Richard D, Fennell
D, O'Leary JJ, Blackhall FH and O'Byrne KJ: Circulating tumour
cells, their role in metastasis and their clinical utility in lung
cancer. Lung Cancer. 76:19–25. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lemjabbar-Alaoui H, Hassan OU, Yang YW and
Buchanan P: Lung cancer: Biology and treatment options. Biochim
Biophys Acta. 1856:189–210. 2015.PubMed/NCBI
|
|
7
|
Sholl LM, Aisner DL, Varella-Garcia M,
Berry LD, Dias-Santagata D, Wistuba II, Chen H, Fujimoto J, Kugler
K, Franklin WA, et al: Multi-institutional oncogenic driver
mutation analysis in lung adenocarcinoma: The lung cancer mutation
consortium experience. J Thorac Oncol. 10:768–777. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishimoto S, Wada K, Usami Y, Tanaka N,
Aikawa T, Okura M, Nakajima A, Kogo M and Kamisaki Y: Differential
expression of aquaporin 5 and aquaporin 3 in squamous cell
carcinoma and adenoid cystic carcinoma. Int J Oncol. 41:67–75.
2012.PubMed/NCBI
|
|
9
|
Moon C, Soria JC, Jang SJ, Lee J, Obaidul
Hoque M, Sibony M, Trink B, Chang YS, Sidransky D and Mao L:
Involvement of aquaporins in colorectal carcinogenesis. Oncogene.
22:6699–6703. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chae YK, Woo J, Kim MJ, Kang SK, Kim MS,
Lee J, Lee SK, Gong G, Kim YH, Soria JC, et al: Expression of
aquaporin 5 (AQP5) promotes tumor invasion in human non small cell
lung cancer. PLoS One. 3:e21622008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Machida Y, Ueda Y, Shimasaki M, Sato K,
Sagawa M, Katsuda S and Sakuma T: Relationship of aquaporin 1, 3,
and 5 expression in lung cancer cells to cellular differentiation,
invasive growth, and metastasis potential. Hum Pathol. 42:669–678.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mobasheri A, Airley R, Hewitt SM and
Marples D: Heterogeneous expression of the aquaporin 1 (AQP1) water
channel in tumors of the prostate, breast, ovary, colon and lung: A
study using high density multiple human tumor tissue microarrays.
Int J Oncol. 26:1149–1158. 2005.PubMed/NCBI
|
|
13
|
Woo J, Lee J, Chae YK, Kim MS, Baek JH,
Park JC, Park MJ, Smith IM, Trink B, Ratovitski E, et al:
Overexpression of AQP5, a putative oncogene, promotes cell growth
and transformation. Cancer Lett. 264:54–62. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jo YM, Park TI, Lee HY, Jeong JY and Lee
WK: Prognostic significance of aquaporin 5 expression in non-small
cell lung cancer. J Pathol Transl Med. 50:122–128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Papadopoulos MC and Saadoun S: Key roles
of aquaporins in tumor biology. Biochim Biophys Acta.
1848:2576–2583. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu S, Zhang S, Jiang H, Yang Y and Jiang
Y: Co-expression of AQP3 and AQP5 in esophageal squamous cell
carcinoma correlates with aggressive tumor progression and poor
prognosis. Med Oncol. 30:6362013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park JY and Juhnn YS: cAMP signaling
increases histone deacetylase 8 expression via the Epac2-Rap1A-Akt
pathway in H1299 lung cancer cells. Exp Mol Med. 49:e2972017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang A, Zhao C, Liu X, Su W, Duan G, Xie
Z, Chu S and Gao Y: Knockdown of TBRG4 affects tumorigenesis in
human H1299 lung cancer cells by regulating DDIT3, CAV1 and RRM2.
Oncol Lett. 15:121–128. 2018.PubMed/NCBI
|
|
19
|
Zhang Z, Chen Z, Song Y, Zhang P, Hu J and
Bai C: Expression of aquaporin 5 increases proliferation and
metastasis potential of lung cancer. J Pathol. 221:210–220. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wee P and Wang Z: Epidermal growth factor
receptor cell proliferation signaling pathways. Cancers (Basel).
9:E522017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nico B and Ribatti D: Aquaporins in tumor
growth and angiogenesis. Cancer Lett. 294:135–138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun X, Wei J, Tang Y, Wang B, Zhang Y, Shi
L, Guo J, Hu F and Li X: Leptin-induced migration and angiogenesis
in rheumatoid arthritis is mediated by reactive oxygen species.
FEBS Open Bio. 7:1899–1908. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo X, Sun T, Yang M, Li Z, Li Z and Gao
Y: Prognostic value of combined aquaporin 3 and aquaporin 5
overexpression in hepatocellular carcinoma. Biomed Res Int.
2013:2065252013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Watanabe T, Fujii T, Oya T, Horikawa N,
Tabuchi Y, Takahashi Y, Morii M, Takeguchi N, Tsukada K and Sakai
H: Involvement of aquaporin-5 in differentiation of human gastric
cancer cells. J Physiol Sci. 59:113–122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee SJ, Chae YS, Kim JG, Kim WW, Jung JH,
Park HY, Jeong JY, Park JY, Jung HJ and Kwon TH: AQP5 expression
predicts survival in patients with early breast cancer. Ann Surg
Oncol. 21:375–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xia H, Ma YF, Yu CH, Li YJ, Tang J, Li JB,
Zhao YN and Liu Y: Aquaporin 3 knockdown suppresses tumour growth
and angiogenesis in experimental non-small cell lung cancer. Exp
Physiol. 99:974–984. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang T, Zhao C, Chen D and Zhou Z:
Overexpression of AQP5 in cervical cancer: Correlation with
clinicopathological features and prognosis. Med Oncol.
29:1998–2004. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu YL, Matsuzaki T, Nakazawa T, Murata S,
Nakamura N, Kondo T, Iwashina M, Mochizuki K, Yamane T, Takata K
and Katoh R: Expression of aquaporin 3 (AQP3) in normal and
neoplastic lung tissues. Hum Pathol. 38:171–178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mobasheri A and Marples D: Expression of
the AQP-1 water channel in normal human tissues: A semiquantitative
study using tissue microarray technology. Am J Physiol Cell
Physiol. 286:C529–C537. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Funaki H, Yamamoto T, Koyama Y, Kondo D,
Yaoita E, Kawasaki K, Kobayashi H, Sawaguchi S, Abe H and Kihara I:
Localization and expression of AQP5 in cornea, serous salivary
glands, and pulmonary epithelial cells. Am J Physiol.
275:C1151–C1157. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu J and Verkman AS: Increased migration
and metastatic potential of tumor cells expressing aquaporin water
channels. FASEB J. 20:1892–1894. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jung HJ, Park JY, Jeon HS and Kwon TH:
Aquaporin-5: A marker protein for proliferation and migration of
human breast cancer cells. PLoS One. 6:e284922011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kawedia JD, Yang F, Sartor MA, Gozal D,
Czyzyk-Krzeska M and Menon AG: Hypoxia and hypoxia mimetics
decrease aquaporin 5 (AQP5) expression through both hypoxia
inducible factor-1α and proteasome-mediated pathways. PLoS One.
8:e575412013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Verkman MS: Role of aquaporin water
channels in kidney and lung. Am J Med Sci. 316:310–320. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang W, Li Q, Yang T, Li D, Ding F, Sun H
and Bai G: RNA interference-mediated silencing of aquaporin (AQP)-5
hinders angiogenesis of colorectal tumor by suppressing the
production of vascular endothelial growth factor. Neoplasma.
65:55–65. 2018. View Article : Google Scholar : PubMed/NCBI
|