Introduction
Hepatitis B virus (HBV) is one of the common
infectious diseases, and the liver is the most important organ
infected with HBV. Currently, there are approximately 240 million
infected people and carriers of HBV in the world, and about 620,000
deaths due to HBV-related liver cirrhosis or hepatocellular
carcinoma (HCC) every year (1).
APOBEC3 is a component of innate immunity playing an
important role in resisting viral invasion, including HBV and human
immunodeficiency virus (HIV). In the human body, APOBEC3 contains 7
kinds of proteins, namely APOBEC-3A, −3B, −3C, −3DE, −3F, −3G and
−3H (2). There is one or two
catalytic structural domains with the cytosine deaminase activity
in APOBEC3, which can convert cytosine into uracil in
deoxyribonucleic acid (DNA) (3,4).
APOBEC3, and especially APOBEC3G, inhibit HBV through the
hyper-degeneration-dependent and hyper-degeneration-independent
mechanisms (5,6). APOBEC3G is expressed widely in human
tissues, and its messenger ribonucleic acid (mRNA) level has a
close correlation with the content of lymphocytes (7). As one of the most active deaminases,
APOBEC3G is able to strongly inhibit the replication and editing of
HBV DNA in the body (8–12).
APOBEC3G has extremely low content in normal liver
tissues, but it can exert an effect of resisting HBV infection in
the body. In this study, the correlation between APOBEC3G
expression level and liver function was investigated, and the
influence and antiviral function on the liver was evaluated,
followed by further analysis.
Patients and methods
Sample data
A total of 58 patients carrying HBV admitted in
Weihai Central Hospital were selected, including 34 males and 24
females aged 45–65 years. After admission, the blood biochemical
and blood routine examinations were performed, and other hepatitis
virus infections were excluded. There were 20 cases clinically
diagnosed with chronic hepatitis B, 19 cases with liver cirrhosis
and 19 cases with liver cancer. Partial liver tissues were obtained
from all patients for subsequent studies. The data of patients are
shown in Table I, and there were no
significant differences among groups. The study was approved by the
Ethics Committee of Weihai Central Hospital and informed consents
were signed by the patients and/or guardians.
 | Table I.Specific data of patients. |
Table I.
Specific data of patients.
| Group | n | Male/Female | Minimum age
(years) | Maximum age
(years) | Average age
(years) |
|---|
| Chronic hepatitis
B | 20 | 11/9 | 45 | 63 | 60 |
| Liver cirrhosis | 19 | 11/8 | 47 | 65 | 57 |
| Liver cancer | 19 | 12/7 | 47 | 62 | 57 |
RNA extraction and real-time
quantitative polymerase chain reaction (qPCR)
RNA was extracted from the peripheral blood
mononuclear cells (PBMCs) in the blood or liver tissues, and then
reversely transcribed into cDNA. APOBEC3G expression level was
detected on a real-time qPCR instrument (Applied Biosystems).
Expression of APOBEC3G was quantified with β-actin as an internal
control. Expression levels of APOBEC3G and β-actin were detected
using the following primers: APOBEC3G:
5′-CGGAATTCAAGCCTCACTTCAGAAACAC-3′ and
5′-CGAAGCTTTCTGCCTTCCTTAGAGACTG-3′, β-actin:
5′-GGACTTCGAGCAAGAGATGG-3′ and 5′-AGCACTGTGTTGGCGTACAG-3′. The
expression level was calculated using 2−∆∆Ct method.
Western blotting
The protein was extracted from PBMCs in the blood or
liver tissues (Beyotime), and the protein concentration was
detected using the protein assay kit (Shanghai Sangon). The protein
sample obtained was used for western blotting. Anti-APOBEC3G
monoclonal primary antibody (Cell Signaling Technology) was used in
this study, and β-actin was the internal control.
Immunohistochemical staining
The rabbit anti-human APOBEC3G polyclonal antibody
was purchased from Abcam. Experiments were performed strictly
according to instructions of the kit (Beyotime). The color
development reaction was examined under an optical microscope.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
10.0 software was used for statistical processing. The correlations
among variables were analyzed using Pearson's correlation
coefficient (r). Data are presented as mean ± standard
deviation (SD). P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparisons of liver function indexes
among groups
Liver function indexes in the serum in 58 patients
in hepatitis B group, liver cirrhosis group and liver cancer group
were detected. Results revealed that alanine aminotransferase
(ALT), aspartate aminotransferase (AST), alkaline phosphatase
(ALP), γ-glutamyl transpeptidase (GGT) and total protein (TP) had
significant differences between hepatitis B group and liver cancer
group (P<0.05). AST and GGT had significant differences between
hepatitis B group and liver cirrhosis group (P<0.05). There were
also significant differences in AST and TP between liver cirrhosis
group and liver cancer group (P<0.05). The remaining indexes
were not significantly different among the three groups (Table II).
 | Table II.Comparisons of liver function indexes
among groups. |
Table II.
Comparisons of liver function indexes
among groups.
|
| Group |
|---|
|
|
|
|---|
| Item | Chronic hepatitis B
group | Liver cirrhosis
group | Liver cancer
group |
|---|
| ALT (U/l) | 59.8±7.3 | 73.5±10.2 | 88.6±9.2a |
| AST (U/l) | 35.6±8.8 | 47.5±9.3a |
73.7±12.1a,b |
| ALP (U/l) | 87.3±16.4 | 125.6±21.3 |
128.7±19.6a |
| GGT (U/l) | 24.8±4.43 | 35.7±6.5a | 48.9±7.4a |
| TP (g/l) | 65.1±13.7 | 62.5±12.6 |
53.1±14.7a,b |
| ALB (g/l) | 42.8±10.4 | 35.6±8.9 | 32.1±8.5 |
| T-BIL (µmol/l) | 23.3±6.5 | 28.9±9.1 | 29.2±4.3 |
| D-NIL (µmol/l) | 4.5±1.2 | 5.8±1.4 | 6.1±2.3 |
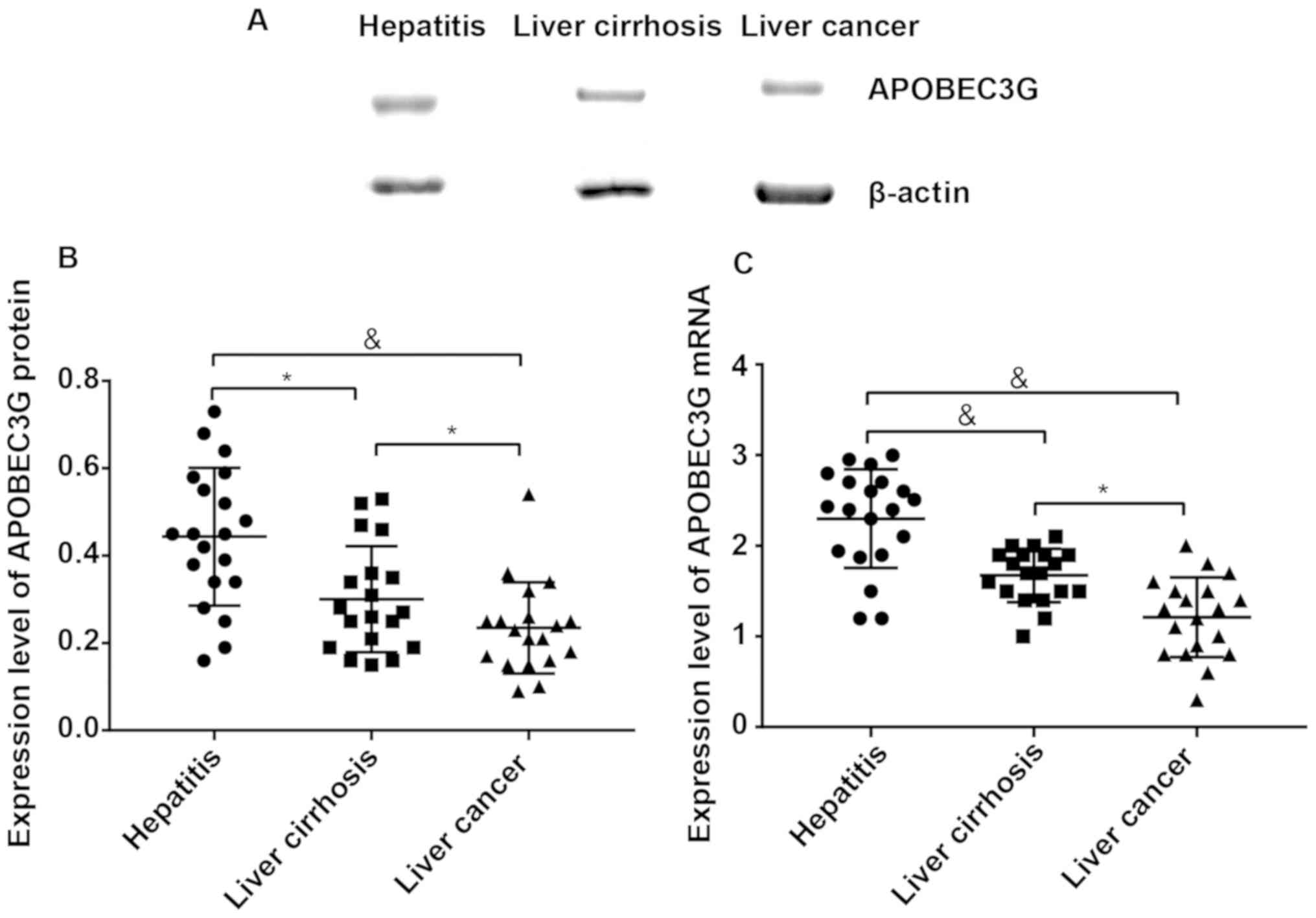
Detection of APOBEC3G expression in
liver tissues via Western blotting and RT-PCR
The expression level of APOBEC3G protein in liver
tissues was (0.44±0.23) in patients with hepatitis B, (0.30±0.17)
in patients with liver cirrhosis and (0.20±0.12) in patients with
liver cancer, respectively, displaying significant differences
among groups (P<0.05) (Fig. 1A and
B). The expression levels of APOBEC3G mRNA in the three groups
were 2.32±0.46, 1.65±0.51 and 1.21±0.37, respectively, also showing
significant differences among groups (P<0.05) (Fig. 1C).
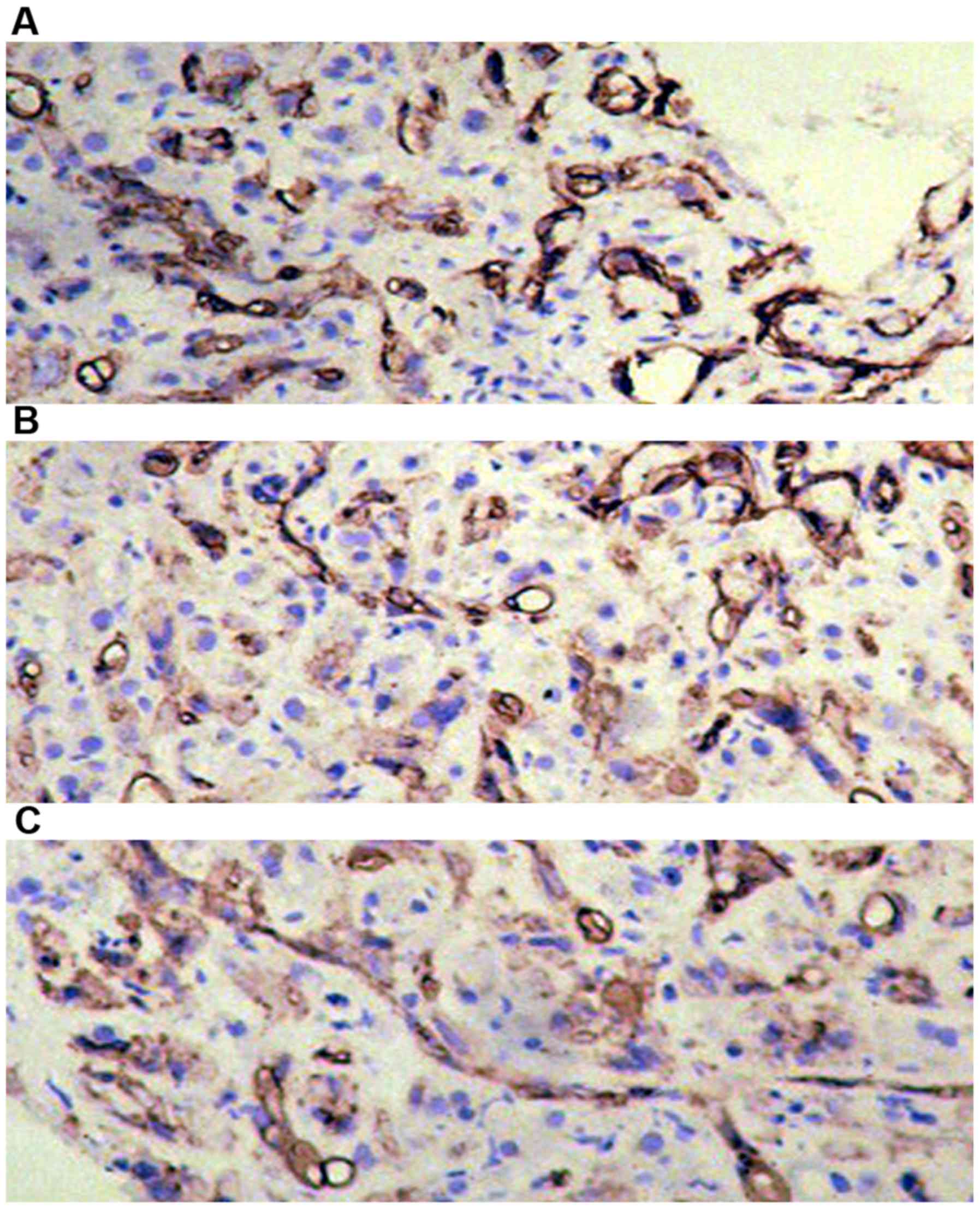
Immunohistochemical detection of
APOBEC3G expression in liver tissues of each group
APOBEC3G was expressed in liver tissues in all the
three groups. The highest expression of APOBEC3G accounted for
approximately 50% in liver tissues of patients with chronic
hepatitis B and only 15% in liver cancer group, and there was a
significant difference between the two groups (P<0.05) (Table III and Fig. 2).
 | Table III.Immunohistochemical detection of
APOBEC3G expression in liver tissues. |
Table III.
Immunohistochemical detection of
APOBEC3G expression in liver tissues.
|
| Expression
intensity/ratio |
|---|
|
|
|
|---|
| Group | Positive (+)/(%) | Positive
(++)/(%) | Positive
(+++)/(%) |
|---|
| Chronic hepatitis B
group | 4/(20.0%) | 6/(30.0%) | 10/(50%) |
| Liver cirrhosis
group | 7/(37%) | 5/(26%) | 7/(37%) |
| Liver cancer
group | 12/(63%)a | 4/(21%) | 3/(15%)a |
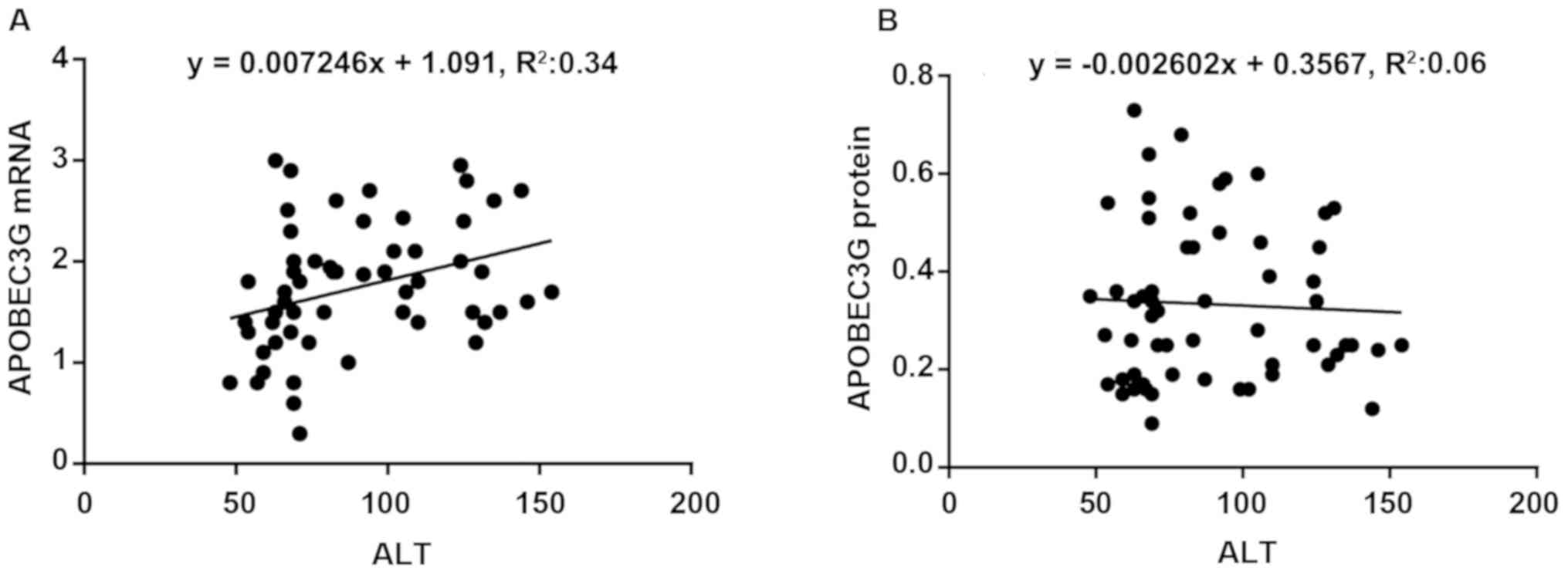
Correlation between APOBEC3G
expression and ALT in patients with chronic hepatitis B
The ALT level was higher than normal in all
patients, and there was a moderate correlation between APOBEC3G
mRNA expression level and ALT content in liver tissues
(r2=0.34, P<0.05) (Fig. 3A). There was no remarkable
correlation between APOBEC3G protein expression and ALT content in
liver tissues (P>0.05) (Fig. 3B).
No significant correlation was found between other liver function
indexes and APOBEC3G expression level (P>0.05).
Discussion
HBV mainly infects liver cells, and its
proliferation and replication in cells will cause damage to liver
cells and lead to persistent hyperplasia of liver cells, resulting
in fibrosis and even carcinogenesis. APOBEC3G, as a component of
innate immunity in the human body, has an antiviral effect, which
can inhibit HBV proliferation and growth in vivo and in
vitro (13–15). Virus removal and pathogenesis of HBV
infection have close correlations with the unique interaction
between virus and infected host (16,17).
According to studies, the risk of liver cirrhosis and HCC is
extremely high for patients with a higher degree of viral
replication (18).
Recently, studies have tried to clarify the
influences of APOBEC3 on HBV replication, core particle association
and HBV DNA editing in vitro (9,11,19).
Cytidine deaminase in the production of protein diversity and
immunity can remove the pathogenic and non-pathogenic exogenous DNA
(20). According to the study of
Stenglein et al, APOBEC3, a member of the cytidine deaminase
family, can restrict the exogenous DNA in human cells (20). APOBEC3G is a member of the APOBEC3
family, which, as a component of innate immune response, can
inhibit the proliferation of DNA viruses, such as HBV (21,22).
Moreover, some studies have demonstrated that APOBEC3G induces C-T
hyper-mutation in the newly-synthesized HBV genomic chain,
resulting in the removal of hepatitis B e-antigen and decline in
HBV DNA. The mode of action of APOBEC3G appears to be inclusion
into HBV particles through direct binding to the hepatitis B core
protein (23). In addition, there
are reports that interferon (IFN) can act on retrovirus and HBV
non-specifically and effectively induce the production of
APOBEC3G18-21 in lymphocytes and hepatocytes, indicating that IFN-α
and IFN-γ increase the transcription of APOBEC3G mRNA in human
liver cell lines and induce high variation of HBV genome (24).
The detection of liver function indexes of patients
in the three groups revealed that there were significant
differences in some liver function indexes between any two groups,
but no obvious rules were found in indexes with significant
differences between two groups in our analysis.
Considering the interaction between APOBEC3G and
HBV, in this study, albumin was selected as an index reflecting the
protein anabolic function of hepatocytes, ALT and AST were selected
as indexes reflecting whether there was damage to hepatocytes and
its severity, and total bilirubin was selected as an index
displaying liver-gallbladder excretion, secretion and
detoxification, and the correlation with APOBEC3G in liver tissues
was analyzed, so as to investigate the correlation between liver
function and APOBEC3G in patients with chronic hepatitis B. Results
manifested that the APOBEC3G mRNA level had a certain correlation
with ALT content in liver tissues (r2=0.34,
P<0.05), but other liver function indexes involved in this study
had no remarkable correlations with APOBEC3G (P>0.05). According
to results of this study, some liver function indexes had a certain
correlation with APOBEC3G, but most indexes had no correlation.
APOBEC3G, as a component of innate immune response, can inhibit HBV
proliferation without direct influence on the liver function, but
the interaction between APOBEC3G and liver function remains to be
further studied.
APOBEC3G content in patients with chronic hepatitis
B, liver cirrhosis and liver cancer was analyzed in this study.
Results revealed that the content of APOBEC3G in liver tissues was
the highest in patients with chronic hepatitis B, slightly lower in
patients with liver cirrhosis and the lowest in patients with liver
cancer, indicating that the expression level of APOBEC3G may
partially display the damage degree of the liver. It was further
confirmed via immunohistochemistry that APOBEC3G was expressed in
liver tissues in all the three groups. The expression intensity of
APOBEC3G was the strongest in liver tissues of patients in chronic
hepatitis B group, accounting for the highest proportion, while it
was slightly weaker in liver cirrhosis group and the weakest in
liver cancer group, accounting for the lowest proportion.
This study suggests that APOBEC3G expression has a
certain correlation with some of the liver function indexes of
patients with chronic hepatitis B. There are significant
differences in the expression level of APOBEC3G in patients with
hepatitis, liver cirrhosis and liver cancer. Larger sample size is
required for a confirmational study to evaluate the correlation of
APOBEC3G with liver function indexes of patients with chronic
hepatitis B.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LN drafted the mansucript. LN and CL collected and
analyzed the general information of patients. LN and YL worked on
qPCR and Western blot analysis. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Weihai Central Hospital and informed consents were signed by the
patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Gerlich WH: Medical virology of hepatitis
B: How it began and where we are now. Virol J.
10:2392013.PubMed/NCBI
|
|
2
|
Teng B, Burant CF and Davidson NO:
Molecular cloning of an apolipoprotein B messenger RNA editing
protein. Science. 260:1816–1819. 1993.PubMed/NCBI
|
|
3
|
Wedekind JE, Dance GS, Sowden MP and Smith
HC: Messenger RNA editing in mammals: New members of the APOBEC
family seeking roles in the family business. Trends Genet.
19:207–216. 2003.PubMed/NCBI
|
|
4
|
Deng Y, Du Y, Zhang Q, Han X and Cao G:
Human cytidine deaminases facilitate hepatitis B virus evolution
and link inflammation and hepatocellular carcinoma. Cancer Lett.
343:161–171. 2014.PubMed/NCBI
|
|
5
|
Nguyen DH, Gummuluru S and Hu J:
Deamination-independent inhibition of hepatitis B virus reverse
transcription by APOBEC3G. J Virol. 81:4465–4472. 2007.PubMed/NCBI
|
|
6
|
Rösler C, Köck J, Kann M, Malim MH, Blum
HE, Baumert TF and von Weizsäcker F: APOBEC-mediated interference
with hepadnavirus production. Hepatology. 42:301–309.
2005.PubMed/NCBI
|
|
7
|
Koning FA, Newman EN, Kim EY, Kunstman KJ,
Wolinsky SM and Malim MH: Defining APOBEC3 expression patterns in
human tissues and hematopoietic cell subsets. J Virol.
83:9474–9485. 2009.PubMed/NCBI
|
|
8
|
Vartanian JP, Henry M, Marchio A, Suspène
R, Aynaud MM, Guétard D, Cervantes-Gonzalez M, Battiston C,
Mazzaferro V, Pineau P, et al: Massive APOBEC3 editing of hepatitis
B viral DNA in cirrhosis. PLoS Pathog. 6:e10009282010.PubMed/NCBI
|
|
9
|
Turelli P, Mangeat B, Jost S, Vianin S and
Trono D: Inhibition of hepatitis B virus replication by APOBEC3G.
Science. 303:18292004.PubMed/NCBI
|
|
10
|
Köck J and Blum HE: Hypermutation of
hepatitis B virus genomes by APOBEC3G, APOBEC3C and APOBEC3H. J Gen
Virol. 89:1184–1191. 2008.PubMed/NCBI
|
|
11
|
Henry M, Guétard D, Suspène R, Rusniok C,
Wain-Hobson S and Vartanian JP: Genetic editing of HBV DNA by
monodomain human APOBEC3 cytidine deaminases and the recombinant
nature of APOBEC3G. PLoS One. 4:e42772009.PubMed/NCBI
|
|
12
|
Suspène R, Guétard D, Henry M, Sommer P,
Wain-Hobson S and Vartanian JP: Extensive editing of both hepatitis
B virus DNA strands by APOBEC3 cytidine deaminases in vitro and in
vivo. Proc Natl Acad Sci USA. 102:8321–8326. 2005.PubMed/NCBI
|
|
13
|
Soros VB and Greene WC: APOBEC3G and
HIV-1: Strike and counterstrike. Curr Infect Dis Rep. 8:317–323.
2006.PubMed/NCBI
|
|
14
|
Chiu YL and Greene WC: Multifaceted
antiviral actions of APOBEC3 cytidine deaminases. Trends Immunol.
27:291–297. 2006.PubMed/NCBI
|
|
15
|
Svarovskaia ES, Xu H, Mbisa JL, Barr R,
Gorelick RJ, Ono A, Freed EO, Hu WS and Pathak VK: Human
apolipoprotein B mRNA-editing enzyme-catalytic polypeptide-like 3G
(APOBEC3G) is incorporated into HIV-1 virions through interactions
with viral and nonviral RNAs. J Biol Chem. 279:35822–35828.
2004.PubMed/NCBI
|
|
16
|
Bonvin M, Achermann F, Greeve I, Stroka D,
Keogh A, Inderbitzin D, Candinas D, Sommer P, Wain-Hobson S,
Vartanian JP, et al: Interferon-inducible expression of APOBEC3
editing enzymes in human hepatocytes and inhibition of hepatitis B
virus replication. Hepatology. 43:1364–1374. 2006.PubMed/NCBI
|
|
17
|
El-Zayadi AR: Hepatitis B virus infection:
The Egyptian situation. Arab J Gastroenterol. 8:94–98. 2007.
|
|
18
|
Guan R, Yap I, Wong L, Tan LH, Oon CJ and
Wee A: Evidence of viral replication in HBsAg positive patients
with hepatocellular carcinoma: Measurement of serum hepatitis B
virus deoxyribonucleic acid (HBV-DNA). Ann Acad Med Singapore.
18:8–11, 21989. 1989.PubMed/NCBI
|
|
19
|
Zhao D, Wang X, Lou G, Peng G, Li J, Zhu
H, Chen F, Li S, Liu D, Chen Z, et al: APOBEC3G directly binds
Hepatitis B virus core protein in cell and cell free systems. Virus
Res. 151:213–219. 2010.PubMed/NCBI
|
|
20
|
Stenglein MD, Burns MB, Li M, Lengyel J
and Harris RS: APOBEC3 proteins mediate the clearance of foreign
DNA from human cells. Nat Struct Mol Biol. 17:222–229.
2010.PubMed/NCBI
|
|
21
|
Vartanian JP, Henry M, Marchio A, Suspene
R, Aynaud MM, Guetard D, Cervantes-Gonzalez M, Battiston C,
Mazzaferro V, Pineau P, et al: Massive APOBEC3 editing of hepatitis
B viral DNA in cirrhosis. PLoS Pathog. 6:e10009282010.PubMed/NCBI
|
|
22
|
Noguchi C, Hiraga N, Mori N, Tsuge M,
Imamura M, Takahashi S, Fujimoto Y, Ochi H, Abe H, Maekawa T, et
al: Dual effect of APOBEC3G on Hepatitis B virus. J Gen Virol.
88:432–440. 2007.PubMed/NCBI
|
|
23
|
Seppen J: Unedited inhibition of HBV
replication by APOBEC3G. J Hepatol. 41:1068–1069. 2004.PubMed/NCBI
|
|
24
|
Tanaka Y, Marusawa H, Seno H, Matsumoto Y,
Ueda Y, Kodama Y, Endo Y, Yamauchi J, Matsumoto T, Takaori-Kondo A,
et al: Anti-viral protein APOBEC3G is induced by interferon-alpha
stimulation in human hepatocytes. Biochem Biophys Res Commun.
341:314–319. 2006.PubMed/NCBI
|