Introduction
In July 2017, the International Agency for Research
on Cancer published the new edition of the World Health
Organisation (WHO) Endocrine Organ Tumor Classification as an
update to the 2004 WHO Endocrine Organ Pathology and Genetic Tumor
Classification (1,2). The new edition reflects the latest
developments in research in this field over the past 13 years.
The current World Health Organisation (WHO)
classification of pituitary neuroendocrine tumours or pituitary
adenomas is based on pituitary cell lineages defined by the
immunohistochemical expression of anterior pituitary hormones and
pituitary specific transcription factors (TFs) in tumour cells
(2). The transcription factors
regulating differentiation of the hormone producing
adenohypophysial cells have been known for a long time (3–7).
However, their practical immunohistochemical application has only
recently been introduced (2),
enabling more precise classification of pituitary neuroendocrine
tumours (PitNETs) with sparse or no hormone expression. Pituitary
transcription factor 1 (Pit-1) governs the differentiation of
somatotroph, lactotroph and thyrotroph cells and is preserved in
tumours derived from these cell types (2). T-box family member TBX19 (T-Pit) acts
as a TF for corticotroph cells (6)
and is a marker of corticotroph pituitary tumours (8,9).
Steroidogenic factor 1 (SF-1) determines development of gonadotroph
cells (4) and is also expressed in
gonadotroph PitNETs (3). By contrast
to Pit-1 and T-Pit, which are specific to the anterior pituitary,
SF-1 is also active in the adrenal glands and reproductive system
(10). Estrogen receptor alpha (ERα)
is other TF not limited to the pituitary gland that influence the
differentiation of gonadothroph, lactotroph and thyrotroph cells
and are expressed in pituitary tumours originating from these cells
(11). According to their expression
of adenohypophysial hormones and pituitary-associated transcription
factors, Pit −1, SF −1 and T -Pit, PitNETs can be divided into six
categories (somatotroph, lactotroph, thyrotroph, corticotroph,
gonadotroph and plurihormonal tumours). Each of the tumours may
result in metabolic disorders due to hypersecretion of anterior
pituitary hormones, or behave as non-functioning or hormonally
silent tumours, resulting in pituitary insufficiency and/or other
symptoms associated with the intrasellar tumour mass (2). However, ~80% of clinically
non-functioning or silent pituitary tumours are of the gonadotroph
subtype, expressing follicle stimulating hormone (FSH) and/or
luteinising hormone (LH), corticotroph tumours are the second most
prevalent and the other hormonal subtypes are rarely reported
(12). Hormonally inactive sellar
tumours, negative for both anterior pituitary hormones and
pituitary-specific TFs, are designated null cell adenomas (2). Occasionally, pituitary tumours are
composed of two or even three distinct components, consistent with
double or triple adenomas (13).
Pituitary adenoma (PA) is a relatively common
intracranial tumour, with the incidence being third only to
meningioma and glioma, accounting for 10–16.7% of all intracranial
tumours in an epidemiological study of the UK population (14,15). In
autopsy cases, the incidence of PA is 3.2–27% (15). As PAs occur in the adenohypophysis
cells that secrete functional hormones (such as somatotroph,
lactotroph, thyrotroph and corticotroph adenomas), the majority of
clinical symptoms manifest as endocrine system symptoms (such as
acromegaly, amenorrhea, lactation, Cushing's syndrome and
hyperthyroidism), and some patients experience compression symptoms
(such as hypopituitarism, headache, dizziness and visual field
changes) (14). Although PA is a
benign tumour, it can show invasive biological characteristics,
pituitary apoplexy and recurrence. In the new 2017 edition of the
WHO guidelines, PAs and their histological grading were
reclassified into adenohypophysis cell lines, which can be
diagnosed by pituitary hormones, pituitary-specific transcription
factors and immunohistochemistry (IHC) features, without
complicated and expensive ultrastructural analysis used in the 2004
edition of the guidelines.
Few studies on the clinical and pathological
features of PAs have been conducted using the 2017 WHO
classification guidelines (16–18).
Therefore the present study retrospectively analysed the clinical
and pathological data of 250 patients classified with PAs using the
2017 WHO guidelines, that were surgically resected and confirmed by
pathology in Tianjin Huanhu Hospital (Tianjin, China).
Materials and methods
Patient selection
Patients with a pathologically confirmed diagnosis
of PA were identified from the electronic medical records system of
Tianjin Huanhu Hospital (Tianjin, China), and those with incomplete
clinical and radiological data were excluded. Overall, 250 patients
were included, and all underwent surgery between November 2017 and
November 2018 in the Department of Neurosurgery in Tianjin Huanhu
Hospital. Patient information, such as sex, age at diagnosis,
clinical manifestation and relevant medical events was
retrospectively collected. Any information that might reveal the
identity of the patient was removed before analysis. This study was
ethically approved by the Tianjin Huanhu Hospital Ethics Committee
of Tianjin Huanhu Hospital, and written informed consent was
obtained from each patient prior to the study.
Histopathological evaluation
All biological specimens were sliced into 5 µm
sections and fixed with formalin (37%) at 26°C and embedded in
paraffin for further pathological diagnosis. The block was
incubated in an oven at 45°C for 20 min to allow complete embedding
of the grafted tissue cylinders in the paraffin of the recipient
block, and then stored at 4°C until microtome sectioning. Tumor
slices were verified using routine hematoxylin and eosin (H&E)
staining at 80°C for 30 min and further IHC staining with specific
antibodies for follicle-stimulating hormone (FSH; prediluted; cat.
no. ZM-0114), luteinizing hormone (LH; prediluted; cat. no.
ZA-0344), thyroid-stimulating hormone (TSH; prediluted; cat. no.
ZA-0640), adrenocorticotropic hormone (ACTH; prediluted; cat. no.
ZM-0004), growth hormone (GH; prediluted; cat. no. ZA-0531), and
prolactin (PRL; prediluted; cat. no. ZA-0596) (all OriGene
Technologies, Inc.). The antibodies were incubated at a dilution of
1:200 (37°C for 2 h). Endogenous peroxidase was inhibited by
incubation with freshly prepared 3% hydrogen peroxide with 0.1%
sodium azide. Tissues were incubated with biotinylated antibodies
(prediluted; anti-mouse; cat. no. AP31512BT-N) and horseradish
peroxidase (prediluted; anti-mouse; cat. no. AM06196SU-N) at 37°C
for 1.5 h. Staining was conducted using diaminobenzidine substrate
and sections were counterstained with hematoxylin at 37°C for 15
min (all OriGene Technologies, Inc.) Slides were scored by two
pathologists who were blinded to the patients' characteristics.
According to the 2017 edition of the WHO guidelines,
PAs were categorized into somatotroph, lactotroph, corticotroph,
gonadotroph, null cell and plurihormonal adenomas (2). High-risk PA subtypes included sparsely
granulated somatotroph adenoma, lactotroph adenoma in men, silent
corticotroph adenoma, and Crooke's cell adenoma. For Ki-67
staining, the following methods were used in the present study:
Immunohistochemical staining was conducted (as above), and the
cells that stained positive for the nuclear antigen Ki-67
(prediluted; cat. no. ZA-0502; OriGene Technologies, Inc.) were
visually and quantitatively evaluated under a light microscope
(magnification, ×400). The Ki-67 percentage score was defined as
the percentage of positively-stained tumour cells among the total
number of cells assessed (19).
Additionally, only positive staining was of interest, independent
of the intensity of coloration.
Neuroradiological evaluation
All patients underwent pre- and postoperative
magnetic resonance imaging (MRI) using standard 3.0 T scanners,
including T1-weighted and T2-weighted 2 mm sagittal and coronal
scans with contrast enhancement. Invasion of the cavernous sinus
was notably higher for grades 3 or 4 on the Knosp classification
(20). Sphenoid sinus invasion was
defined as lesions growing into the sphenoid sinus on preoperative
MRI or with visual confirmation during the surgical procedure
(21). Therefore, an invasive PA was
considered in tumours with cavernous sinus or sphenoid sinus
invasion. According to the 2017 edition of the WHO guidelines, the
term ‘invasive (aggressive)’ has been used by clinicians to
designate radiologically invasive adenomas, which grow rapidly,
tend to recur or progress, and are resistant to combined treatment
including surgery and radiation (22). The degree of surgical resection was
determined using MRI examinations within 3 days of and at 3 months
after the initial surgery. Follow-up MRI was performed annually (or
on the return of symptoms) to determine whether there is
recurrence.
Recurrence
MRI of the pituitary for surgical follow-up was
performed 6 months after the surgical procedure. Recurrence was
defined as progression of a residual tumour or new tumour growth
after total resection. Endocrine examinations (1 month, 3 month, 6
months and 1 year postoperatively) were performed to assess the
reappearance of hormonal hypersecretion after normalization.
Clinical symptoms that were monitored include the reappearance of
tumour compression or endocrine symptoms after surgery.
Pituitary apoplexy
Patients were monitored for pituitary apoplexy for
two weeks preoperatively via observation of the following: i)
Typical clinical symptoms, including severe headaches, nausea and
vomiting, and visual disorders; ii) signs of haemorrhage on
computed tomography or MRI scans; and iii) intraoperative findings
indicative of a haemorrhagic focus in the tumour (23).
Statistical analysis
SPSS v19.0 statistical software (IBM Corp.) was used
for data analysis. Data are expressed as the mean ± standard
deviation. The association between the Ki-67 indexes was
statistically analysed using the paired Student's t-test. Fisher's
exact test was used for comparisons of categorical variables, and
paired Student's t-test were used for continuous variables.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient and tumour
characteristics
A total of 250 patients were included, comprising
106 males (42.4%) and 144 females (57.6%), with a male-to-female
ratio of 0.74:1. The mean age of all patients was 51.71±12.87
years, the mean age of the male patients was 52.02±13.95 years, and
the mean age of the female patients was 51.49±12.06 years. The PAs
in the present study predominantly manifested as compression
symptoms, especially gonadotropin adenomas (80.6%), corticotroph
adenomas (78.8%) and lactotroph adenomas (69.2%). Based on the most
recent histopathological classification, patients most frequently
had gonadotropin adenomas (n=93; 37.2%), followed by corticotroph
adenomas (n=61; 24.4%), somatotroph adenomas (n=45; 18.0%),
lactotroph adenomas (n=26; 10.4%), null cell adenomas (n=15 cases;
6.0%) and plurihormonal adenomas (n=9; 3.6%), and one patient had a
thyrotroph adenoma (n=1; 0.4%). Details of each classification and
subtype are listed in Tables
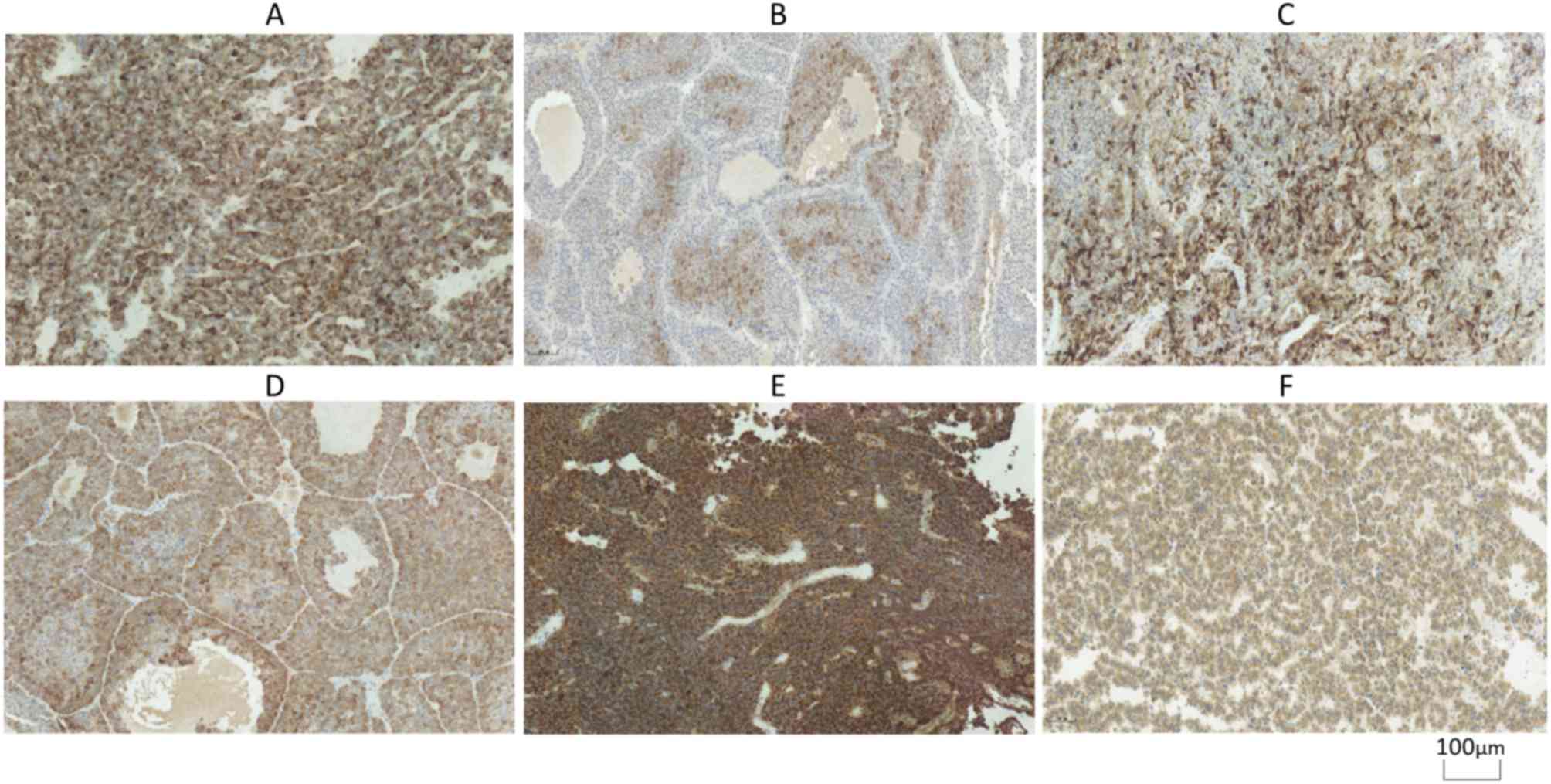
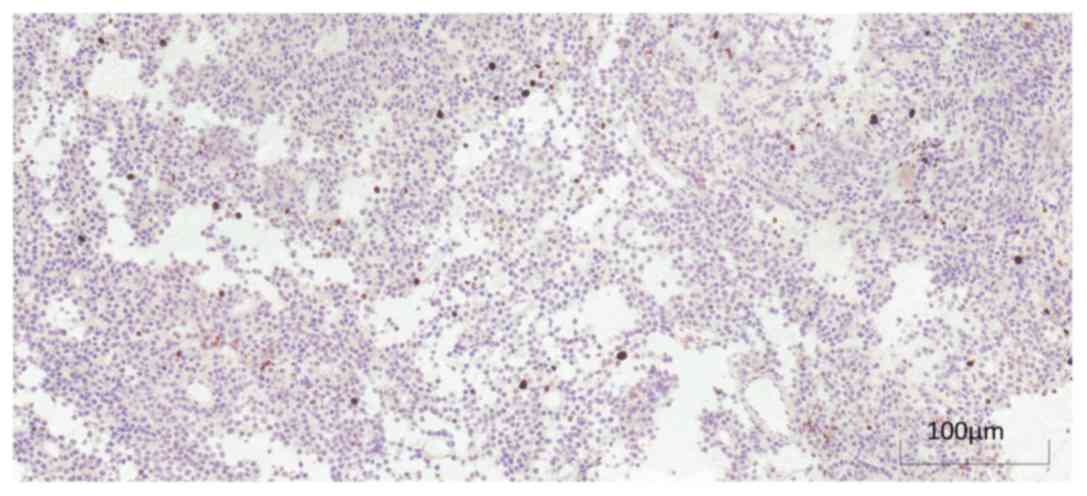
I–III. Immunohistochemical and
Ki-67 staining of different types of pituitary adenoma are
exhibited in Figs. 1 and 2.
 | Table I.Classification of the 250 patients
with pituitary adenoma (Percentage of Somatotroph adenomas patients
in total male pituitary adenomas). |
Table I.
Classification of the 250 patients
with pituitary adenoma (Percentage of Somatotroph adenomas patients
in total male pituitary adenomas).
| Adenoma
subtype | Total number,
n | Total
percentage | Number of Males,
n | Percentage of total
males, % | Number of females,
n | Percentage of total
females |
|---|
| Somatotroph
adenomas | 45 | 18.0 | 15 | 14.2% | 30 | 20.8% |
| Densely
granulated | 15 | 6.0 | – | – | – | – |
| Sparsely
granulated | 17 | 6.8 | – | – | – | – |
| Mixed
somatotroph-lactotroph | 13 | 5.2 | – | – | – | – |
| Lactotroph
adenomas | 26 | 10.4 | 11 | 10.4% | 15 | 10.4% |
| Densely
granulated | 19 | 7.6 | – | – | – | – |
| Sparsely
granulated | 7 | 2.8 | – | – | – | – |
| Thyrotroph
Adenoma | 1 | 0.4 | 0 | 0 | 1 | 0.7% |
| Corticotroph
adenomas | 61 | 24.4 | 5 | 4.7% | 56 | 38.9% |
| Gonadotropin
adenomas | 93 | 37.2 | 62 | 58.5% | 31 | 21.5% |
| Plurihormonal
adenomas | 9 | 3.6 | 4 | 3.8% | 5 | 3.5% |
| PIT-1 positive | 3 | 1.2 | – | – | – | – |
| Other types | 6 | 2.4 | – | – | – | – |
| Null cell
adenoma | 15 | 6.0 | 9 | 8.5% | 6 | 4.2% |
 | Table III.Distribution of high-risk pituitary
adenoma types. |
Table III.
Distribution of high-risk pituitary
adenoma types.
| Tumour types | Total number,
n | Number of males,
n | Number of females,
n |
|---|
| Sparsely granulated
somatotroph adenoma | 17 | 5 | 12 |
| Lactotroph adenoma
in men | 11 | 11 | 0 |
| Plurihormonal
PIT-1-positive adenoma | 3 | 0 | 3 |
| Silent corticotroph
adenoma | 42 | 3 | 39 |
| Crooke's cell
adenoma | 0 | 0 | 0 |
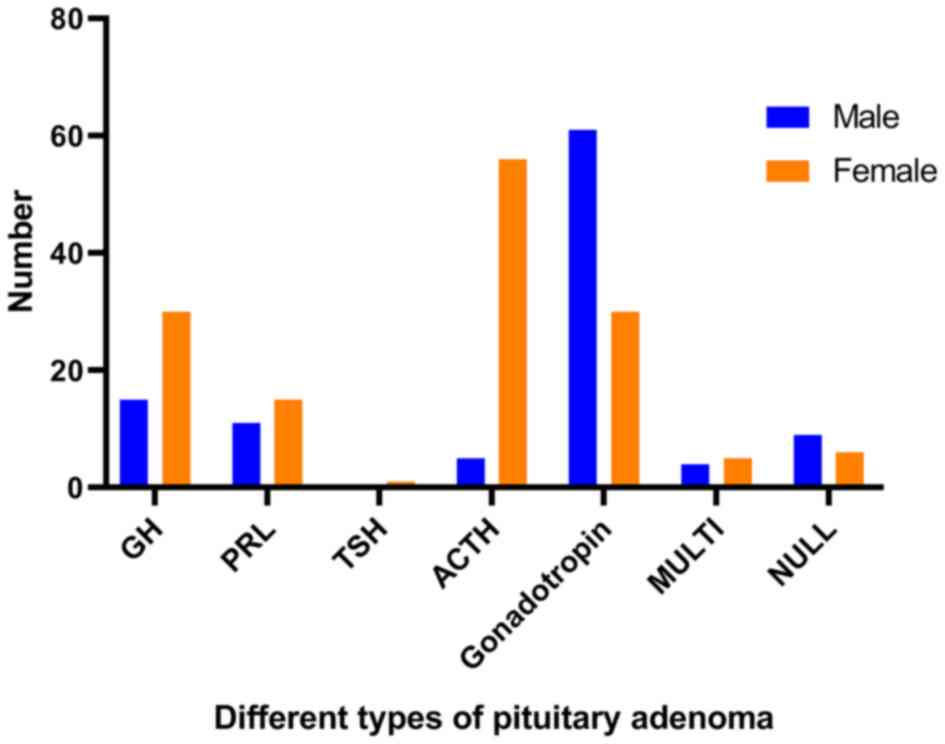
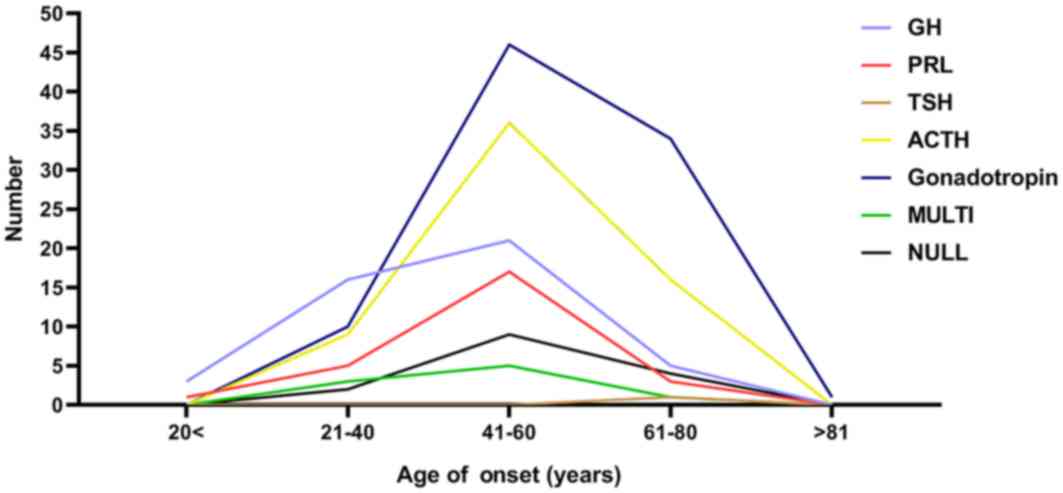
Sex and age distribution of patients
with different types of PAs
In terms of sex distribution, somatotroph adenomas
and corticotroph adenomas were observed most often in female
patients, while gonadotropin adenomas were mostly observed in male
patients. In terms of age distribution, the age groups between 21
and 60 years accounted for 72% of all patients, and the overall
incidence peak of the various types of PAs was between 41 and 60
years. The details are exhibited in Figs. 3 and 4.
Clinical manifestations of different
types of PAs
A total of 26 patients (10.4%) in the present study
exhibited endocrine symptoms (increase or decrease of menstrual
blood volume or changes in menstrual cycle, lactation, decreased
sexual function and acromegaly), 180 patients (72.0%) exhibited
compressive symptoms (headache, dizziness and decreased vision), 11
patients (4.4%) exhibited both endocrine symptoms and tumour
compression symptoms, and 33 patients (13.2%) did not exhibit any
obvious symptoms. The details are shown in Table IV.
 | Table IV.Clinical manifestations of each type
of pituitary adenoma. |
Table IV.
Clinical manifestations of each type
of pituitary adenoma.
| Adenoma type | Endocrine symptoms,
n (%) | Tumor mass effects,
n (%) | Both, n (%) | No symptoms
(%) |
|---|
| Somatotroph
adenomas | 13 (28.9) | 22 (48.9) | 5 (11.1) | 5 (11.1) |
| Lactotroph
adenomas | 3 (11.5) | 18 (69.2) | 0 (0.0) | 5 (19.3) |
| Thyrotroph
Adenoma | 0 (0.0) | 1 (100) | 0 (0.0) | 0 (0.0) |
| Corticotroph
adenomas | 5 (8.2) | 48 (78.8) | 4 (6.5) | 4 (6.5) |
| Gonadotropin
adenomas | 1 (1.1) | 75 (80.6) | 2 (2.2) | 15 (16.1) |
| Plurihormonal
adenomas | 4 (44.4) | 5 (55.6) | 0 (0.0) | 0 (0.0) |
| Null cell
adenoma | 0 (0.0) | 11 (73.3) | 0 (0.0) | 4 (26.7) |
| Total | 26 (10.4) | 180 (72) | 11 (4.4) | 33 (13.2) |
Comparison of the incidence of
invasion, recurrence and apoplexy between high-risk and low-risk
PAs
A significant difference in the incidence of
invasive PAs was found. The incidence of invasive PAs in the
high-risk group was higher compared with that in the low-risk group
(χ2=60.627; P<0.001). In addition, the recurrence
rate of high-risk PAs was higher compared with that in low-risk PAs
(χ2=67.903; P<0.001). Finally, the incidence of
pituitary apoplexy was significantly higher in the high-risk group
compared with that in the low-risk group (χ2=38.942;
P<0.001). The details are shown in Table V [WHO Classification of Tumors of
Endocrine Organs (2017) clarified 5 high risk pituitary adenomas
as: Sparsely granulated somatotroph adenoma (17/250), lactotroph
adenoma in men (11/250), plurihormonal PIT-1-positive adenoma
(3/250), silent corticotroph adenoma (42/250) and Crooke's cell
adenoma (0/250)].
 | Table V.Comparison of the incidence of
invasion, recurrence and apoplexy between the high- and the
low-risk groups. |
Table V.
Comparison of the incidence of
invasion, recurrence and apoplexy between the high- and the
low-risk groups.
|
| Invasion | Recurrence | Apoplexy |
|---|
|
|
|
|
|
|---|
| Group | Total number,
n | % | χ2 | P-value | Total number,
n | % | χ2 | P-value | Total number,
n | % | χ2 | P-value |
|---|
| High-risk | 21 | 28.8 | 60.627 | <0.001 | 13 | 17.8 | 67.903 | <0.001 | 6 | 8.2 | 38.942 | <0.001 |
| Low-risk | 16 | 9.0 | – | – | 10 | 5.6 | – | – | 8 | 4.5 | – | – |
Relationship between invasive and
recurrent PAs and the Ki-67 index
In the present study, there were 37 cases (14.8%) of
invasive PAs and 213 cases (85.2%) of non-invasive PAs. The Ki-67
index of the invasive group was significantly higher compared with
that in the non-invasive group, (t=3.268; P<0.01). There were 23
cases (9.2%) of recurrent PAs and 213 cases (90.8%) of
non-recurrent PAs. The Ki-67 index of the recurrent group was
significantly higher compared with that in the non-recurrent group
(t=3.974; P<0.001). The details are shown in Tables VI and VII.
 | Table VI.Comparison of the Ki-67 index between
the invasive and the non-invasive groups. |
Table VI.
Comparison of the Ki-67 index between
the invasive and the non-invasive groups.
|
| Group |
|
|
|---|
|
|
|
|
|
|---|
| Tumour type | Invasive
(n=37) | Non-invasive
(n=213) | t | P-value |
|---|
| Somatotroph
adenoma | 6.2±3.4 | 2.7±1.1 | 4.581 | <0.001 |
| Lactotroph
adenoma | 3.0±0.8 | 2.1±1.1 | 1.838 | 0.078 |
| Thyrotroph
adenoma | 0.0 | 2.7 | – | – |
| Corticotroph
adenoma | 3.6±1.5 | 2.7±1.1 | 2.068 | 0.043 |
| Gonadotropin | 2.9±1.1 | 2.8±1.1 | 0.066 | 0.947 |
| Plurihormonal
adenoma | 4.2±1.1 | 2.6±1.5 | 1.409 | 0.202 |
| Null cell
adenoma | 4.3±2.6 | 3.7±2.4 | 0.500 | 0.625 |
| Total | 3.5±1.8 | 2.8±1.3 | 3.268 | 0.001 |
 | Table VII.Comparison of the Ki-67 index between
the recurrence and the non-recurrence group. |
Table VII.
Comparison of the Ki-67 index between
the recurrence and the non-recurrence group.
|
| Group |
|
|
|---|
|
|
|
|
|
|---|
| Tumour type | Recurrent
(n=23) | Non-recurrent
(n=227) | t | P-value |
|---|
| Somatotroph
adenoma | 4.4±1.2 | 2.8±1.5 | 1.816 | 0.076 |
| Lactotroph
adenoma | 1.5±1.3 | 2.4±1.1 | −1.079 | 0.291 |
| Thyrotroph
adenoma | 0.0 | 2.7 | – | – |
| Corticotroph
adenoma | 3.6±1.5 | 2.8±1.2 | 1.575 | 0.120 |
| Gonadotropin | 3.9±1.4 | 2.7±1.1 | 2.783 | 0.007 |
| Plurihormonal
adenoma | 5.0 | 2.7±1.4 | 1.504 | 0.176 |
| Null cell
adenoma | 10.0 | 3.7±1.9 | 3.263 | 0.006 |
| Total | 3.9±1.9 | 2.8±1.3 | 3.974 | <0.001 |
Discussion
In addition to clinical symptoms, serum hormone
levels and imaging examinations, the postoperative pathological
diagnosis of pituitary tumours is also very important.
Histopathology includes histochemical staining and IHC. In 1892,
according to H&E staining, Tapar et al (24) reported that Schoneman divided PAs
into chromophobe, eosinophilic, basophilic and mixed pituitary
adenomas. Among them, the most common was chromophobe cellular
adenoma, accounting for 75–80% of total PA diagnoses.
Unfortunately, these pathological classifications cannot accurately
reflect the tumour function (for example, some eosinophilic cell
tumours secrete GH and some secrete PRL) (25).
The 2004 edition classified pituitary endocrine
tumours into only three categories using the WHO Classification of
Tumors (typical adenoma 8272/0, atypical adenoma 8272/1 and
pituitary adenoma 8272/3) (25).
Hormone-producing adenomas were stratified into subtypes according
to their pathological immunoreactivities for the anterior hormones:
ACTH, GH, PRL, TSH and FSH/LH (26).
The fourth edition of the WHO classification of
tumours of the pituitary gland (2)
was published in 2017, bringing several changes to the
classification of tumours of the anterior pituitary gland. It
introduces a more precise cell lineage-based classification using
IHC based on transcription factors and hormones produced. In
addition, in this new edition, anterior pituitary tumours and their
histological grading are reclassified into adenohypophysis cell
lines, which can be diagnosed by pituitary hormones,
pituitary-specific transcription factors and standard IHC, without
complicated or expensive ultrastructural analysis. For 35–45% of
PAs, the surrounding structure is involved (2). The classification system collectively
refers to sparsely granulated somatotroph adenoma, lactotroph
adenoma in men, Crooke's cell adenoma, silent corticotroph adenoma,
and plurihormonal POU class 1 homeobox 1-positive adenoma as
‘invasive adenoma’ or ‘high-risk adenoma’ (12). This type of adenoma grows rapidly,
tends to recur or progress and is resistant to surgery and
radiotherapy. Silent adenomas are clinically asymptomatic, have low
levels of serum hormones and are immunohistochemically positive for
certain hormones [thyroid stimulating hormone (TSH) growth hormone
(GH) luteinising hormone (LH) prolactin (PRL), adrenocorticotropic
hormone (ACTH), follicle stimulating hormone (FSH)] (27). PA with definite metastasis and
cerebrospinal cord dissemination is considered a carcinoma. The new
edition does not include the term ‘atypical adenoma’ under
pituitary adenoma and no longer recommends the concept of
‘hormone-producing pituitary adenoma’. Null cell adenoma was
redefined as an adenoma composed of anterior pituitary cells, and
IHC, pituitary hormone detection and transcription factor detection
showed no evidence of cell-specific differentiation. The present
study applies the new classification method to the diagnosis of
patients.
The present study retrospectively analysed the
clinical and pathological data of 250 patients with PAs using the
2017 WHO classification system. The results showed that the
proportion of patients with gonadotropin adenomas was the highest
of any subtype, followed by corticotroph adenomas. Moreover,
thyrotroph adenoma was found to have the lowest proportion, and the
proportion of null cell adenoma was lower compared with that
reported previously (28). Although
surgical treatment is the first choice for the majority of
pituitary tumours, a number of drugs that can be used for the
treatment of pituitary tumours are published in the European
Guidelines (2018) (29). A consensus
has been reached on the preferred drug therapy for lactotroph
adenomas: First-line drugs are dopamine receptor agonists, mainly
bromocriptine and cabergoline (26).
Drug therapy is also important for the other three types of
functional pituitary tumours and for cases in which patients are
unable to undergo surgical treatment, are unwilling to undergo
surgical treatment, or cannot achieve complete remission after
surgery. For somatotroph adenomas, somatostatin analogues are
preferred, including octreotide and landrapeptide (26). For corticotroph adenomas, clinical
studies have shown that the application of parapeptide in patients
with Cushing disease who do not undergo surgery can significantly
reduce the level of urinary free cortisol and effectively improve
the symptoms of hypercortisolism (30). Somatostatin analogues may also be
preferred for patients with thyrotroph adenomas (31). Therefore, it is important to clarify
the pathological properties of pituitary tumours for the drug
treatment of patients with pituitary tumours. A previous study
revealed that null cell adenomas, somatotroph adenomas and
lactotroph adenomas are more common (P<0.01) (32). As the 2017 classification standard
was adopted in the current study, the number of cases of
gonadotropin adenoma and corticotroph adenoma was increased due to
the recognition of partially stationary PAs in non-functional
adenomas. The classification of PAs in the 2004 edition of the WHO
guidelines mainly used the cellular morphology of tumour tissues,
intracellular hormone secretory components and ultrastructural
electron microscopy to classify PAs. However, using the 2004 WHO
Classification of Tumors were prone to misdiagnose non-functional
PAs, especially for null cell adenomas. If the pituitary hormone
immunohistochemistry staining is negative, a tumour may be
diagnosed as null cell adenoma. However, some null cell adenomas
were weakly expressed in focal subunits or do not secrete other
hormones (TSH, GH, LH, PRL, FSH and ACTH), and most of the
hormone-negative adenomas were derived from differentiated
adenohypophysis cells by staining with associated transcription
factors (PIT-1, ERα, T-PIT and SF-1) (33). In addition, according to the 2004 WHO
classification of Endocrine Organ Pathology and Genetic Tumours,
~30% of tumours can be diagnosed as null cell adenomas. However,
using the 2017 edition, <5% of adenomas are diagnosed as null
cell adenomas. In the present study, there were only 15 cases of
null cell adenoma, accounting for 6% of the total cases, which was
consistent with a previous report using the 2017 classification
system (2). Therefore, we
hypothesize that the 2004 classification standard increased the
diagnostic rate of null cell adenoma, resulting in a lower
diagnostic rate of other non-functional adenomas.
The PAs in the present study mainly manifested as
compression symptoms. All PAs of different pathological types
showed clinical manifestations caused by tumour compression,
especially gonadotropin adenomas (80.6%), corticotroph adenomas
(78.8%), and lactotroph adenomas (69.2%). The majority of the
patients with endocrine symptoms were women; the clinical symptoms
(menstrual changes and galactorrhoea) caused by hormonal changes
may be more obvious in women, and most of them can be detected and
treated immediately. In men, however, there are often no obvious
clinical symptoms, so the tumour is not diagnosed until compression
symptoms are noted.
In addition, female patients account for the
majority of somatotroph adenomas and corticotroph adenomas, while
male patients account for the majority of gonadotropin adenomas in
the present study. This finding is consistent with a previous study
using the 2004 classification system (34), which suggests that some types of PAs
are significantly associated with sex. The number of patients in
the 21–60 years age groups was the highest, and the incidence was
the highest in the 41–60 years age group. As previously reported,
the prevalence of PAs increases with age, and the peak age of
diagnosis is 30–60 years (35).
The 2017 classification system removed the diagnosis
of ‘atypical PAs’ and explicitly proposed five types of ‘high-risk
PAs’, suggesting that these five subtypes are likely to have a
poorer prognosis (36). At present,
there is a lack of specific diagnostic criteria for aggressive
pituitary tumors, which is considered only a clinical combination
of various pituitary tumours showing refractory behaviour. In the
present study, the proportion of invasive adenomas in high-risk PAs
reached 28.8%, the recurrence rate was 17.8%, and the incidence of
apoplexy was 8.2%. The results showed that the incidence of
invasion, recurrence and apoplexy of high-risk PAs was higher
compared with that in non-high-risk PAs. This finding indicates
that these specific types of adenomas have more inherent biological
characteristics of highly invasive behaviour, high risk of
recurrence and apoplexy, and neurosurgeons should attach great
importance to the diagnosis of these adenomas. In a European study,
Crooke's cell adenoma is a subtype of high-risk PA, which is very
rare and accounts for >1% of Pas (37); it was not identified in the present
study.
Currently, surgery is still the preferred treatment
for invasive PAs. However, due to the similar malignant biological
behaviour, total surgical resection of invasive PAs is difficult;
the rate of total resection is lower compared with that of
non-invasive tumours, and the postoperative recurrence rate is
higher, requiring multiple surgical treatments (38). Thus, patients with invasive PAs often
need adjuvant drug treatment. Although the WHO guidelines do not
currently include invasive tumours in the PAs classification, the
2017 classification also emphasizes that invasive adenoma can be
used as an important feature for identifying clinically refractory
adenomas and determining prognosis. Invasive growth can be
identified using preoperative MRI and intraoperative findings of
tumour spread to the dura mater, bone or nasal mucosa (39). The relationship between the invasion
of PAs and the Ki-67 index has also been discussed in different
ways (40). It has been postulated
that there is no relationship between invasiveness and a high Ki-67
index (41). The results of the
present study indicated that the Ki-67 index was related to the
invasion and recurrence of PAs. Invasive PAs have a higher Ki-67
index, and the differences in the Ki-67 index between the invasive
group and the non-invasive group in somatotroph adenomas and
corticotroph adenomas are statistically significant. It is
suggested that the Ki-67 index can objectively reflect the
proliferation potential of PAs and has good clinical value in
predicting the invasion of Pas (42). There were statistically significant
differences in the Ki-67 index between the recurrent group and the
non-recurrent group in gonadotropin adenoma and null cell adenoma.
It is suggested that the Ki-67 index has a certain guiding
significance in predicting the prognosis of patients to evaluate
the tendency of tumour recurrence after surgery by reflecting the
number of proliferating tumour cells (43). According to the Ki-67 index of
postoperative pathological diagnosis, combined with preoperative
imaging findings and intraoperative findings, the individualized
selection of postoperative treatment options is conducive to the
postoperative recovery of patients with invasive pituitary tumours,
reducing postoperative hormone abnormalities, delaying tumour
recurrence and improving the quality of life of patients (44–46).
However, the present study has several limitations.
The follow-up period was too short to detect the reported
recurrences, despite using the 2017 WHO classification standard. In
addition, a further case follow-up study is currently being
performed and the results of which will be published in the near
future. The numbers of invasive pituitary adenomas of each subtype
were small, thus the results may not reflect the effect of Ki-67 on
the invasiveness of pituitary adenomas in each subtype. For
example, the sample size of invasive pituitary adenoma in the TSH
and PRL groups was >5. Therefore, future studies should include
a higher number of invasive pituitary adenomas for each subtype, to
ensure more reliable data.
Furthermore analysis focusing on categorising
tumours based on functional compared with non-functional tumours
was not performed. Further research is needed to explore why many
functional pituitary adenomas do not have the endocrine symptoms,
invasiveness and recurrence of other subgroups.
In conclusion, the new 2017 classification is
practical and reasonable from both a molecular and clinical
pathology perspective, as this classification is based on IHC
studies of transcription factors, which are intuitively
understandable and are technically and diagnostically helpful
(26). The present study is not the
first clinicopathological analysis of pituitary adenomas based on
the new classification (26,47–50),
however previous studies have also showed that the new WHO 2017
classification categorizes less null cell adenomas compared with
previous classifications (26,47). In
the present study, patients with pituitary tumour diagnosed after
2017 were systematically classified according to the 2017
classification criteria and provides a theoretical basis for the
accurate diagnosis of PAs. An accurate understanding and
application of the latest classification system will contribute to
better clinical diagnosis and treatment as well as advanced
prediction of tumour outcomes and patient prognosis. Although the
number of cases in the present study is not sufficiently large, the
new classification should be further analysed and used in
combination with clinical practice in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JL and YH made substantial contributions to the
conception and design of the work, drafting the work and revising
it critically for important intellectual content, final approval of
the version to be published and agreement to be accountable for all
aspects of the work. YHH, XZ and XY contributed to the acquisition,
analysis and interpretation of data for the work, drafting the work
and revising it critically for important intellectual content, and
also gave final approval of the version to be published and
agreement to be accountable for all aspects of the work.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Tianjin Huanhu Hospital, and all patients provided
written informed consent to participate.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kovacs K: The 2004 WHO classification of
pituitary tumors: Comments. Acta Neuropathol. 111:62–63. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lopes MBS: The 2017 world health
organization classification of tumors of the pituitary gland: A
summary. Acta Neuropathol. 134:521–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asa SL, Bamberger AM, Cao B, Wong M,
Parker KL and Ezzat S: The transcription activator steroidogenic
factor-1 is preferentially expressed in the human pituitary
gonadotroph. J Clin Endocrinol Metab. 81:2165–2170. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee SL, Sadovsky Y, Swirnoff AH, Polish
JA, Goda P, Gavrilina G and Milbrandt J: Luteinizing hormone
deficiency and female infertility in mice lacking the transcription
factor NGFI-A (Egr-1). Science. 273:1219–1221. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao L, Bakke M, Krimkevich Y, Cushman LJ,
Parlow AF, Camper SA and Parker KL: Steroidogenic factor 1 (SF1) is
essential for pituitary gonadotrope function. Development.
128:147–154. 2001.PubMed/NCBI
|
|
6
|
Lamolet B, Pulichino AM, Lamonerie T,
Gauthier Y, Brue T, Enjalbert A and Drouin J: A pituitary
cell-restricted T box factor, Tpit, activates POMC transcription in
cooperation with Pitx homeoproteins. Cell. 104:849–859. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scully KM and Rosenfeld MG: Pituitary
development: Regulatory codes in mammalian organogenesis. Science.
295:2231–2235. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vallette-Kasic S, Figarella-Branger D,
Grino M, Pulichino AM, Dufour H, Grisoli F, Enjalbert A, Drouin J
and Brue T: Differential regulation of proopiomelanocortin and
pituitary-restricted transcription factor (TPIT), a new marker of
normal and adenomatous human corticotrophs. J Clin Endocrinol
Metab. 88:3050–3056. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sjöstedt E, Bollerslev J, Mulder J,
Lindskog C, Pontén F and Casar-Borota O: A specific antibody to
detect transcription factor T-Pit: A reliable marker of
corticotroph cell differentiation and a tool to improve the
classification of pituitary neuroendocrine tumours. Acta
Neuropathol. 134:675–677. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo X, Ikeda Y and Parker KL: A
cell-specific nuclear receptor is essential for adrenal and gonadal
development and sexual differentiation. Cell. 77:481–490. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dasen JS, O'Connell SM, Flynn SE, Treier
M, Gleiberman AS, Szeto DP, Hooshmand F, Aggarwal AK and Rosenfeld
MG: Reciprocal interactions of Pit1 and GATA2 mediate signaling
gradient-induced determination of pituitary cell types. Cell.
97:587–598. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Manojlovic-Gacic E, Engstrom BE and
Casar-Borota O: Histopathological classification of non-functioning
pituitary neuroendocrine tumors. Pituitary. 21:119–129. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kontogeorgos G, Scheithauer BW, Horvath E,
Kovacs K, Lloyd RV, Smyth HS and Rologis D: Double adenomas of the
pituitary: A clinicopathological study of 11 tumors. Neurosurgery.
31:840–849. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raappana A, Koivukangas J, Ebeling T and
Pirilä T: Incidence of pituitary adenomas in northern Finland in
1992–2007. J Clin Endocrinol Metab. 95:4268–4275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Buurman H and Saeger W: Subclinical
adenomas in postmortem pituitaries: Classification and correlations
to clinical data. Eur J Endocrinol. 154:753–758. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eremkina AK, Dzeranova LK, Pigarova EK,
Mokrysheva NG and Dedov II: Morphofunctional features of
non-functioning pituitary adenomas. Arkh Patol. 81:71–78. 2019.(In
Russian). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
García-Sáenz M, Uribe-Cortés D,
González-Virla B, Mendoza-Zubieta V and Vargas-Ortega G: Silent
pituitary plurihormonal adenoma: Clinical relevance of
immunohistochemical analysis. Rev Med Inst Mex Seguro Soc.
57:48–55. 2019.(In English and Spanish). PubMed/NCBI
|
|
18
|
Pappy AL II, Savinkina A, Bicknese C,
Neill S, Oyesiku NM and Ioachimescu AG: Predictive modeling for
pituitary adenomas: Single center experience in 501 consecutive
patients. Pituitary. 22:520–531. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Zhang X, Yan X, Sun M, Fan Y and
Huang Y: Significance of TERT and ATRX mutations in glioma. Oncol
Lett. 17:95–102. 2019.PubMed/NCBI
|
|
20
|
Micko AS, Wohrer A, Wolfsberger S and
Knosp E: Invasion of the cavernous sinus space in pituitary
adenomas: Endoscopic verification and its correlation with an
MRI-based classification. J Neurosurg. 122:803–811. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Andujar-Plata P, Villar-Taibo R,
Ballesteros-Pomar MD, Vidal-Casariego A, Pérez-Corral B,
Cabezas-Agrícola JM, Álvarez-Vázquez P, Serramito R and Bernabeu I:
Long-term outcome of multimodal therapy for giant prolactinomas.
Endocrine. 55:231–238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chatzellis E, Alexandraki KI, Androulakis
II and Kaltsas G: Aggressive pituitary tumors. Neuroendocrinology.
101:87–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mou C, Han T, Zhao H, Wang S and Qu Y:
Clinical features and immunohistochemical changes of pituitary
apoplexy. J Clin Neurosci. 16:64–68. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tapar K, Kovach K and Khorvat E:
Classification, pathology and molecular biology of pituitary
adenoma. Arkh Patol. 59:7–17. 1997.(In Russian). PubMed/NCBI
|
|
25
|
Klimstra DS: Pathology reporting of
neuroendocrine tumors: Essential elements for accurate diagnosis,
classification, and staging. Semin Oncol. 40:23–36. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Inoshita N and Nishioka H: The 2017 WHO
classification of pituitary adenoma: Overview and comments. Brain
Tumor Pathol. 35:51–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cooper O and Melmed S: Subclinical
hyperfunctioning pituitary adenomas: The silent tumors. Best Pract
Res Clin Endocrinol Metab. 26:447–460. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chalkley MD, Kiupel M and Draper AC:
Pituitary null cell adenoma in a domestic llama (Lama glama). J
Comp Pathol. 151:51–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Raverot G, Burman P, McCormack A, Heaney
A, Petersenn S, Popovic V, Trouillas J and Dekkers OM; European
Society of Endocrinology, : European society of endocrinology
clinical practice guidelines for the management of aggressive
pituitary tumours and carcinomas. Eur J Endocrinol. 178:G1–G24.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Colao A, Petersenn S, Newell-Price J,
Findling JW, Gu F, Maldonado M, Schoenherr U, Mills D, Salgado LR
and Biller BM; Pasireotide B2305 Study Group, : A 12-month phase 3
study of pasireotide in Cushing's disease. N Engl J Med.
366:914–924. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamada S, Fukuhara N, Horiguchi K,
Yamaguchi-Okada M, Nishioka H, Takeshita A, Takeuchi Y, Ito J and
Inoshita N: Clinicopathological characteristics and therapeutic
outcomes in thyrotropin-secreting pituitary adenomas: A
single-center study of 90 cases. J Neurosurg. 121:1462–1473. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mortini P, Losa M, Barzaghi R, Boari N and
Giovanelli M: Results of transsphenoidal surgery in a large series
of patients with pituitary adenoma. Neurosurgery. 56:1222–1233.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nishioka H, Inoshita N, Mete O, Asa SL,
Hayashi K, Takeshita A, Fukuhara N, Yamaguchi-Okada M, Takeuchi Y
and Yamada S: The complementary role of transcription factors in
the accurate diagnosis of clinically nonfunctioning pituitary
adenomas. Endocr Pathol. 26:349–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Arafah BM and Nasrallah MP: Pituitary
tumors: Pathophysiology, clinical manifestations and management.
Endocr Relat Cancer. 8:287–305. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Agustsson TT, Baldvinsdottir T, Jonasson
JG, Olafsdottir E, Steinthorsdottir V, Sigurdsson G, Thorsson AV,
Carroll PV, Korbonits M and Benediktsson R: The epidemiology of
pituitary adenomas in Iceland, 1955–2012: A nationwide
population-based study. Eur J Endocrinol. 173:655–664. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mete O, Gomez-Hernandez K, Kucharczyk W,
Ridout R, Zadeh G, Gentili F, Ezzat S and Asa SL: Silent subtype 3
pituitary adenomas are not always silent and represent poorly
differentiated monomorphous plurihormonal Pit-1 lineage adenomas.
Mod Pathol. 29:131–142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Manojlovic-Gacic E, Bollerslev J and
Casar-Borota O: Invited Review: Pathology of pituitary
neuroendocrine tumours: Present status, modern diagnostic approach,
controversies and future perspectives from a neuropathological and
clinical standpoint. Neuropathol Appl Neurobiol. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dworakowska D and Grossman AB: Aggressive
and malignant pituitary tumours: State-of-the-art. Endocr Relat
Cancer. 25:R559–R575. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sav A, Rotondo F, Syro LV, Di Ieva A,
Cusimano MD and Kovacs K: Invasive, atypical and aggressive
pituitary adenomas and carcinomas. Endocrinol Metab Clin North Am.
44:99–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pizarro CB, Oliveira MC, Coutinho LB and
Ferreira NP: Measurement of Ki-67 antigen in 159 pituitary adenomas
using the MIB-1 monoclonal antibody. Braz J Med Biol Res.
37:235–243. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Honegger J, Prettin C, Feuerhake F,
Petrick M, Schulte-Mönting J and Reincke M: Expression of Ki-67
antigen in nonfunctioning pituitary adenomas: Correlation with
growth velocity and invasiveness. J Neurosurg. 99:674–679. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Marques P, Mafra M, Calado C, Martins A,
Monteiro J and Leite V: Aggressive pituitary lesion with a
remarkably high Ki-67. Arq Bras Endocrinol Metabol. 58:656–660.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Del Basso De Caro M, Solari D, Pagliuca F,
Villa A, Guadagno E, Cavallo LM, Colao A, Pettinato G and
Cappabianca P: Atypical pituitary adenomas: Clinical
characteristics and role of ki-67 and p53 in prognostic and
therapeutic evaluation. A series of 50 patients. Neurosurg Rev.
40:105–114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Klibanski A: Clinical practice.
Prolactinomas. N Engl J Med. 362:1219–1226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sheehan J, Rainey J, Nguyen J, Grimsdale R
and Han S: Temozolomide-induced inhibition of pituitary adenoma
cells. J Neurosurg. 114:354–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen XY, Wang Z, Li B, Zhang YJ and Li YY:
Pim-3 contributes to radioresistance through regulation of the cell
cycle and DNA damage repair in pancreatic cancer cells. Biochem
Biophys Res Commun. 473:296–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Batista RL, Trarbach EB, Marques MD,
Cescato VA, da Silva GO, Herkenhoff CGB, Cunha-Neto MB and Musolino
NR: Nonfunctioning pituitary adenoma recurrence and its
relationship with sex, size, and hormonal immunohistochemical
profile. World Neurosurg. 120:e241–e246. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee JYK, Cho SS, Zeh R, Pierce JT,
Martinez-Lage M, Adappa ND, Palmer JN, Newman JG, Learned KO, White
C, et al: Folate receptor overexpression can be visualized in real
time during pituitary adenoma endoscopic transsphenoidal surgery
with near-infrared imaging. J Neurosurg. 129:390–403. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mitrofanova LB, Vorobeva OM and Gorshkov
AN: Analysis of pituitary adenoma expression patterns suggests a
potential role for the NeuroD1 transcription factor in
neuroendocrine tumor-targeting therapies. Oncotarget. 10:289–312.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang S, Ding C, Xiao D, Wu Z and Wei L:
Evaluation of a novel general pituitary hormone score to evaluate
the function of the residual anterior pituitary (adenohypophysis)
in patients following surgery for pituitary adenoma. Med Sci Monit.
24:7944–7951. 2018. View Article : Google Scholar : PubMed/NCBI
|