Introduction
Liver cancer affects millions of patients and is
associated with high incidence and mortality rates worldwide
(1). In certain countries, such as
the US, the incidence rate of liver cancer has declined, whereas
the mortality rate has increased (2). The clinical treatment of this disease
is challenged by the high prevalence of cancer metastasis, lacking
radical treatment options (3).
Chemotherapy, such as the use of carboplatin, is widely applied to
treat patients at advanced stages, however the development of drug
resistance leads to poor outcomes (4,5).
Non-coding RNAs exhibit diverse functions (6). MicroRNAs (miRNAs/miRs) and long
non-coding (lnc)RNAs are two major subgroups of non-coding RNAs
(ncRNAs). lncRNAs and miRNAs are essential players in human
diseases, particularly in cancer (7,8). It has
been widely established that ncRNAs participate in cancer biology
mainly by affecting cancer cell behaviors, such as proliferation,
migration and the sensitivity to chemical drugs (7,8). miR-21
has been found to promote the development of different types of
cancer, including that of the liver (9,10). The
lncRNA cancer susceptibility 11 (CASC11) was found to promote
gastric cancer (11). The role of
these ncRNAs in hepatocellular carcinoma (HCC), a major subtype of
liver cancer, was investigated in the present study. In this study,
the expression of CASC11 and miR-21 in samples from patients with
HCC was measured by reverse transcription-quantitative (RT-q)PCR.
Overexpression experiments were performed to analyze the
interaction between CASC11 and miR-21. MTT assay was used to
analyze the effects of CASC11 and miR-21 expression on the
viability of HCC cells upon carboplatin treatment. The findings of
this study suggest that lncRNA CASC11 mediates the development of
chemoresistance to carboplatin in patients with HCC, via the
upregulation of miR-21.
Materials and methods
Patients, tissues, treatments and cell
lines
A total of 69 patients with advanced HCC, who were
admitted to The Sixth People's Hospital of Qingdao, Qingdao, China,
between September 2015 and May 2018, were enrolled. During biopsy,
adjacent (5 cm around tumors) healthy and tumor tissues were
collected from each participant. Blood (5 ml) was also extracted
from each patient using EDTA tubes before treatment under fasting
conditions for the isolation of plasma. Inclusion criteria: i)
Patients with expected survival time >12 months; ii) patients at
advanced stage (stages III and IV), who were not suitable for
surgical resection; and iii) patients who were treated with
different doses of carboplatin according to their conditions.
Exclusion criteria: i) Patients with other diseases; ii) patients
who were treated before admission; iii) patients who failed to
cooperate with researchers; and iv) patients who died during this
study. Blood was also extracted under fasting conditions at 3 and 6
months after the beginning of carboplatin treatment to extract
plasma. The patients included 38 males and 31 females, with a mean
age of 48.2±5.1 years (age range, 30–66 years). There were 38
patients at stage III and 31 patients at The American Joint
Committee on Cancer stage IV (12).
All patients signed informed consent. The Ethics Committee of The
Sixth People's Hospital of Qingdao approved this study.
HCC cell lines SNU-398 and SNU-182 were purchased
from American Type Culture Collection (ATCC). A mixture composed of
90% RPMI 1640 medium (ATCC) and 10% FBS (ATCC) was used to
cultivate cells at 37°C in a 5% CO2 incubator.
RT-qPCR
In order to detect CASC11, total RNA was extracted
from in vitro cultivated cells, plasma and tissue sample
using RNAzol® RT RNA Isolation reagent (GeneCopoeia,
Inc.) and RT was performed using the Applied Biosystems™
High-Capacity cDNA Reverse Transcription kit (Thermo Fisher
Scientific, Inc.), through the following conditions: 25°C for 10
min, 52°C for 20 min and 80°C for 10 min. Mixtures for qPCR were
prepared using the qScript One-Step RT-qPCR kit (Quantabio). In
order to detect miR-21, the ReliaPrep™ miRNA Cell and Tissue
Miniprep system (Promega Corporation) was used to extract miRNAs,
the miScript II RT kit (Qiagen GmbH) was used to carry out RT, and
qPCR mixtures were prepared using the mirVana qRT-PCR miRNA
Detection kit (Thermo Fisher Scientific, Inc.). All PCR reactions
were performed on the ABI 7500 Real-Time PCR System (Thermo Fisher
Scientific, Inc.). PCR conditions were: 95°C for 1 min, followed by
40 cycles of 95°C for 10 sec and 60°C for 45 sec. Primers of lncRNA
CASC11, miR-21, and endogenous controls GAPDH and U6 were obtained
from Sangon Biotech Co., Ltd. CASC11 forward,
5′-GGACACCAACTATTGCTTCA-3′ and reverse, 5′-TCCAGGCTCCAAATGTAG-3′;
GAPDH forward, 5′-GTCTCCTCTGACTTCAACAGC-3′ and reverse,
5′-ACCACCCTGTTGCTGTAGCCA-3′; miR-21 forward,
5′-TAGCTTATCAGACTGATG-3′ and reverse primer (universal primer) and
U6 primers were from the RT-qPCR miRNA Detection kit (Thermo Fisher
Scientific, Inc.). According to the 2−ΔΔCq method
(13) of quantification, lncRNA
CASC11 expression was normalized to that of GAPDH and miR-21
expression was normalized to that of U6.
Transient transfection
lncRNA CASC11 overexpression vectors and empty
vectors, as well as lncRNA CASC11 small interfering (si)RNA
(5′-UUCUUCACCACCUCCAGUUGC-3′) and negative control siRNA
(5′-UGAACGUACGGGCAUGUCAGC-3′) were purchased from Sangon Biotech
Co., Ltd. Negative miRNA control (5′-UGAACGUACGGGCAUGUCAGC-3′) and
miR-21 mimic (5′-UAGCUUAUCAGACUGAUGUUGA-3′) were purchased from
Sigma-Aldrich; Merck KGaA. Lipofectamine® 2000 reagent
(cat. no. 11668-019; Invitrogen; Thermo Fisher Scientific, Inc.)
was used to transfect 1×106 SNU-398 and SNU-182 cells in
each well of a 6-well cell culture plate (2 ml medium per well)
with vectors at a dose of 10 nM and siRNA or miRNA at a dose of 35
nM. The duration of incubation with transfection reagent and vector
was 6 h. Cells without transfections were considered as the control
cells. The expression of CASC11 and miR-21 was detected by RT-qPCR.
Subsequent experiments were performed 48 h later.
MTT assay
Cells were harvested for an MTT assay when the
overexpression rates of lncRNA CASC11 and miR-21 reached 200% and
the lncRNA CASC11 knockdown rate reached 50%. Briefly, cell
suspensions were prepared and cell density was adjusted to
3×104 cells/ml. Cells suspensions were transferred to a
96-well plate (0.1 ml/well). Carboplatin
[C4H6(CO2)2Pt(NH3)2;
Sigma-Aldrich; Merck KGaA] was added at doses of 200 µg/ml at 4 h
after seeding. Cells were cultured under normal conditions (37°C,
5% CO2) for 24 h, followed by the addition of MTT (10
µl/well). Cells were cultured for an additional 4 h, followed by
the addition of DMSO 10 µl/well) and the measurement of optical
density values at 570 nm.
Statistical analysis
The values of the mean ± SD were calculated from
data of 3 biological replicates. Pearson's correlation analysis was
performed to analyze the correlation between CASC11 and miR-21
levels. Comparisons of lncRNA CASC11 and miR-21 expression levels
between two different types of tissues were performed with paired
Student's t-test. Comparisons among multiple groups were carried
out using one-way ANOVA and Tukey's post hoc test. Any difference
with a P<0.05 was considered to be statistically
significant.
Results
lncRNA CASC11 and miR-21 levels are
upregulated in HCC
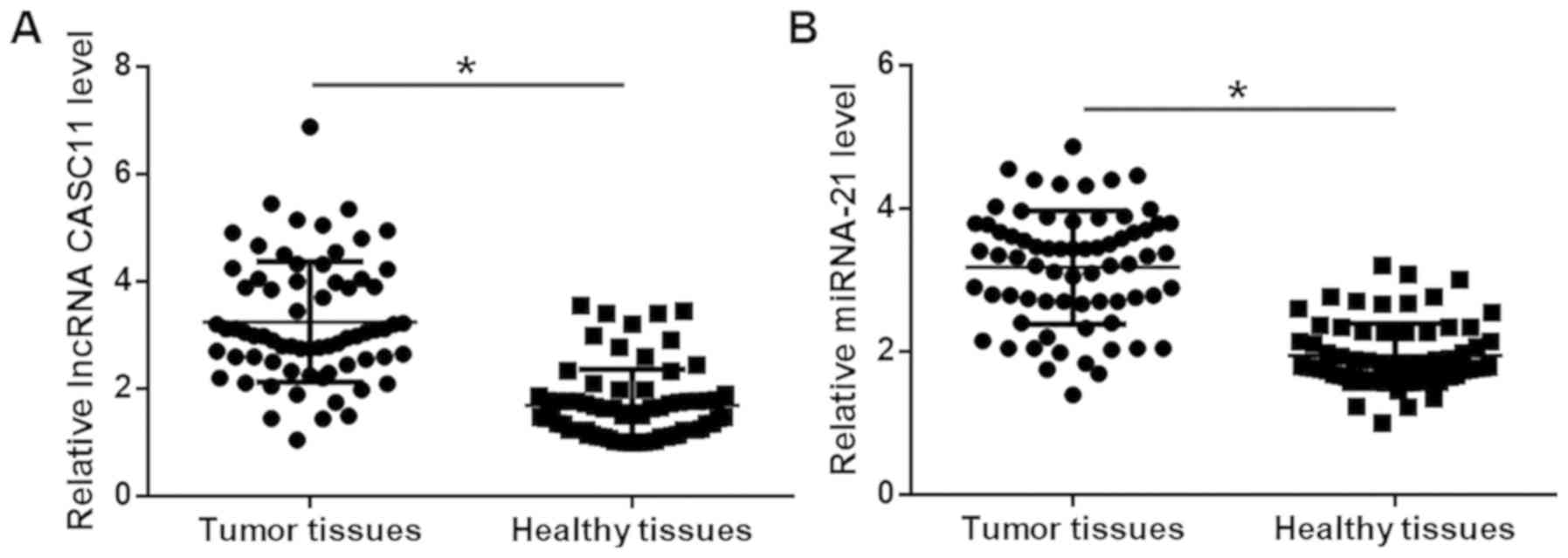
Expression of lncRNA CASC11 and miR-21 was detected
by RT-qPCR. Compared with those in adjacent healthy tissues, the
expression levels of CASC11 (Fig.
1A) and miR-21 (Fig. 1B) were
significantly higher in tumor tissues (both P<0.05).
HCC tumor expression levels of CASC11
and miR-21 are positively correlated
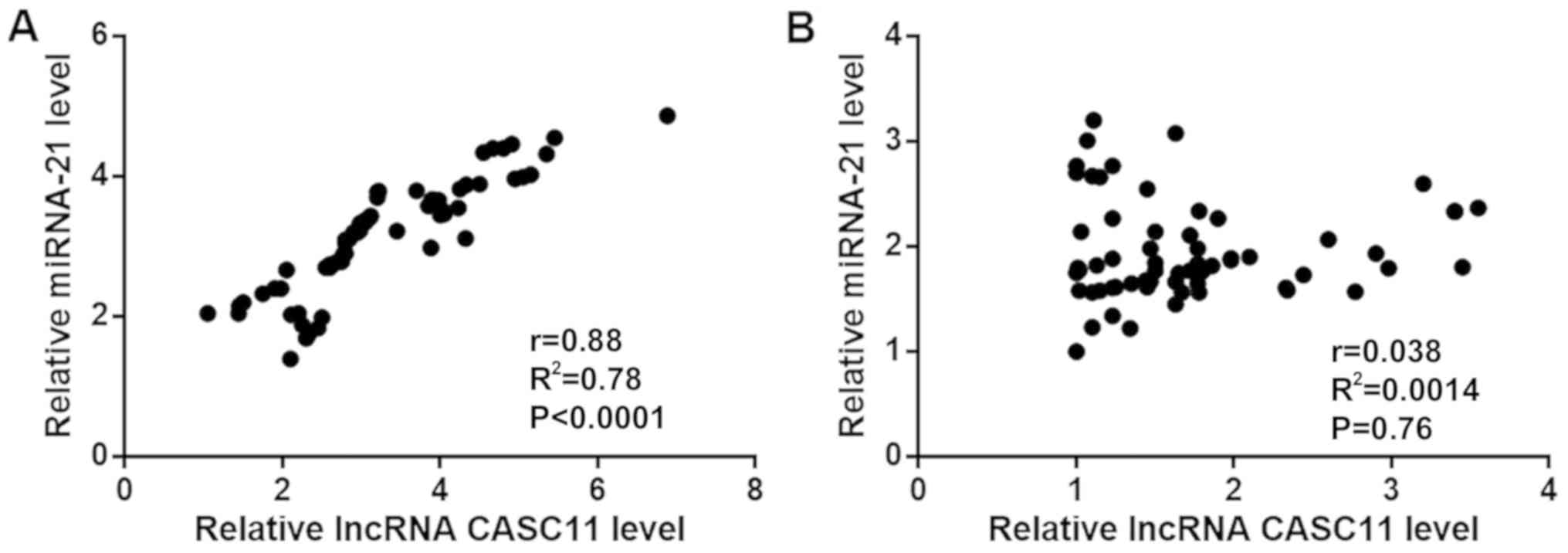
Pearson's correlation analysis was performed to
assess the correlation between the expression levels of lncRNA
CASC11 and miR-21 in tumor tissues and adjacent healthy tissues. As
shown in Fig. 2A, the expression
levels of lncRNA CASC11 and miR-21 were significantly and
positively correlated in tumor tissues (P<0.001). In contrast,
the expression levels of these molecules were not correlated in
adjacent healthy tissues (P=0.76; Fig.
2B).
lncRNA CASC11 functions as an upstream
activator of miR-21 in HCC cells
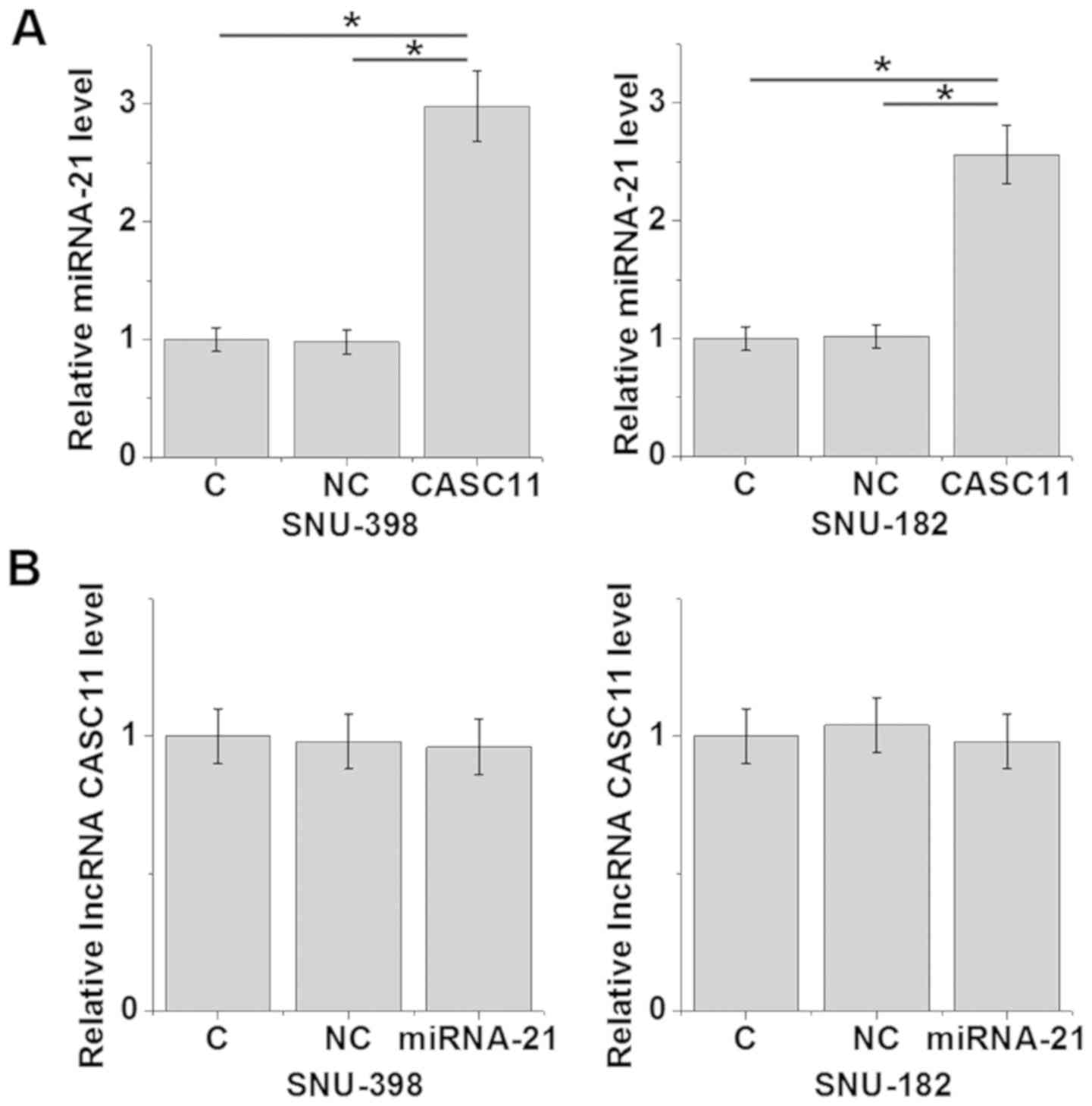
In vitro overexpression experiments were
performed to further investigate the association between lncRNA
CASC11 and miR-21 in HCC cell lines. Compared with the control and
negative control groups, the overexpression of lncRNA CASC11 led to
upregulation of miR-21 in SNU-398 and SNU-182 cells (Fig. 3A; all P<0.05). In contrast, the
overexpression of miR-21 had no significant effect on the
expression of lncRNA CASC11 in these cells (Fig. 3B; all P>0.05).
Plasma levels of lncRNA CASC11 and
miR-21 are upregulated during chemotherapy
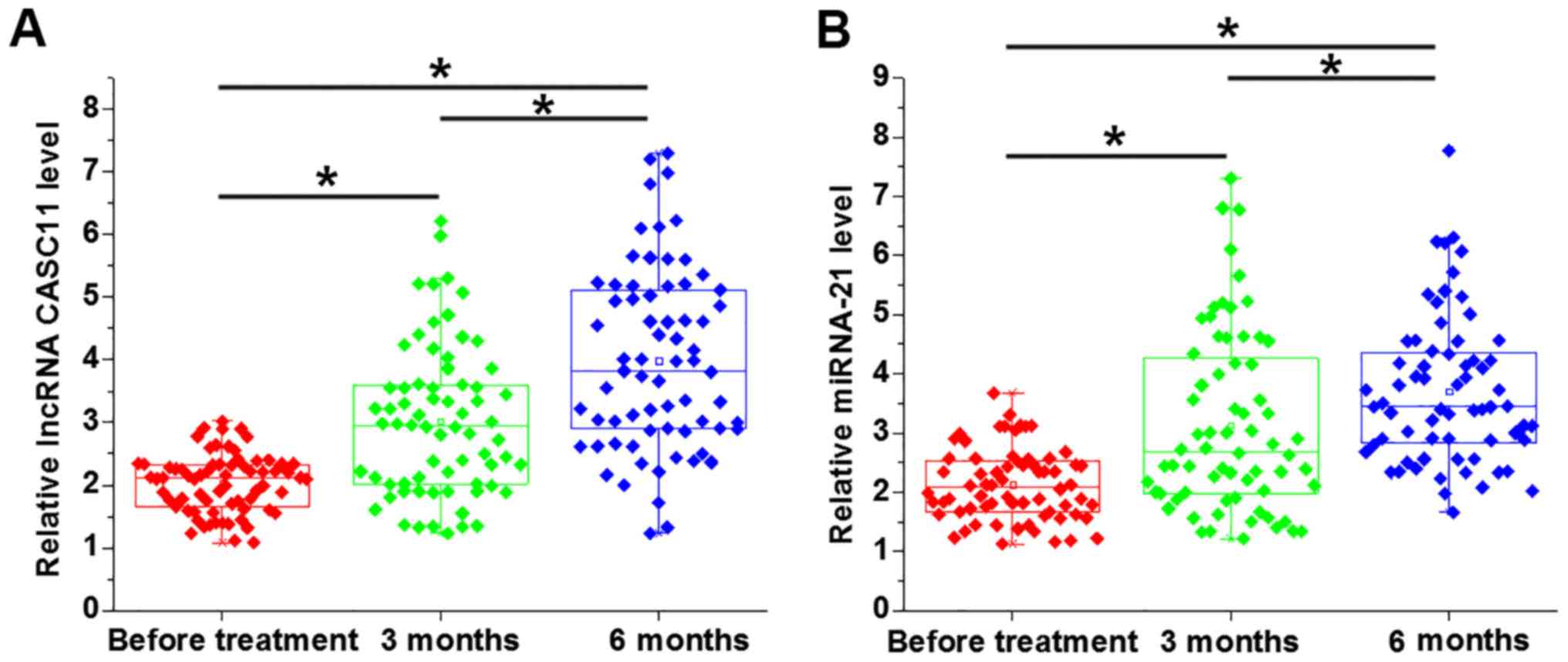
The plasma levels of lncRNA CASC11 and miR-21 were
detected by RT-qPCR at three time-points, including before
treatment and at 3 and 6 months after treatment with carboplatin.
The plasma levels of lncRNA CASC11 (Fig.
4A) and miR-21 (Fig. 4B) were
significantly increased at 3 and 6 months after treatment (all
P<0.05), compared with the levels before treatment. The plasma
levels of lncRNA CASC11 (Fig. 4A)
and miR-21 (Fig. 4B) were also
significantly higher at 6 months after treatment (both P<0.05),
compared with the levels at 3 months.
Carboplatin treatment upregulates
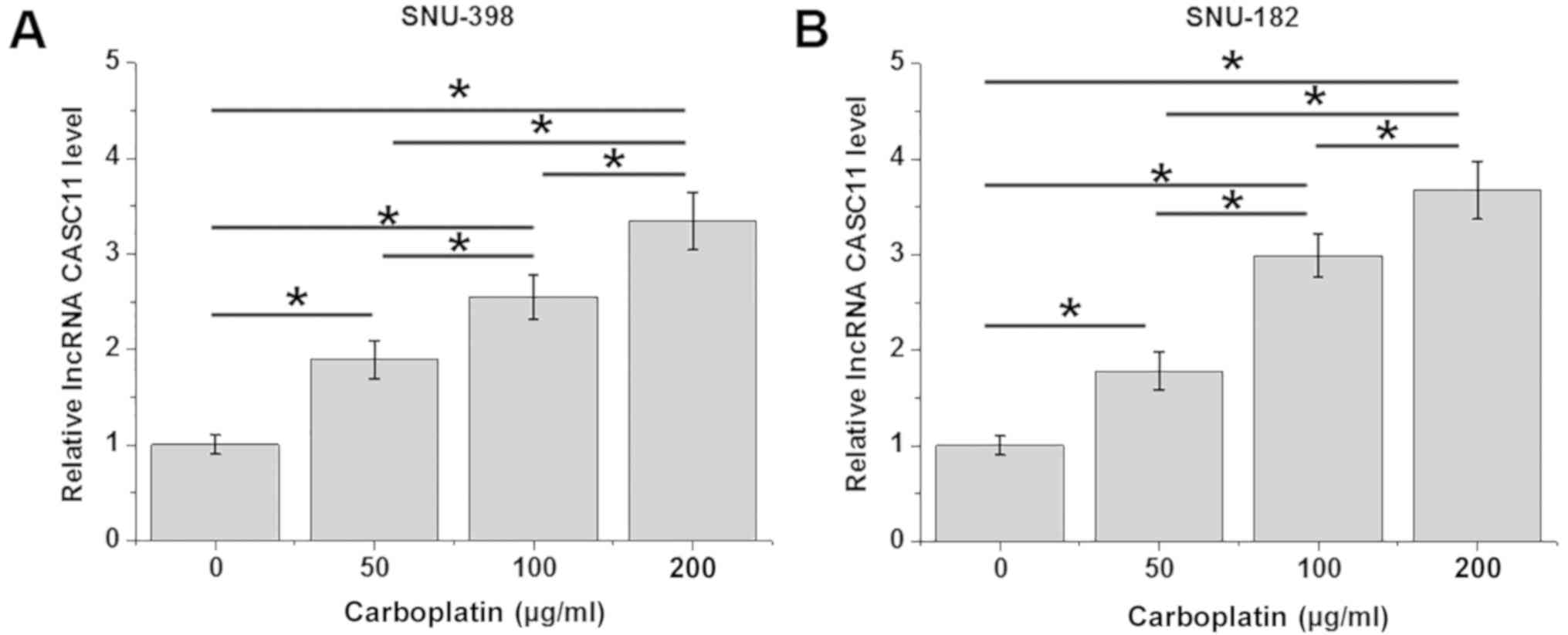
lncRNA CASC11 expression in HCC cells
SNU-398 and SNU-182 cells were treated with 50, 100
and 200 µg/ml carboplatin for 24 h, followed by the detection of
lncRNA CASC11 expression by RT-qPCR. As shown in Fig. 5, carboplatin treatment resulted in a
dose-dependent upregulation of CASC11 in these cells.
lncRNA CASC11 promotes
carboplatin-treated HCC cell viability via miR-21
The cell viability assay was performed under
carboplatin treatment only (200 µg/ml). It is a limitation of this
study that the cell viability assay was performed only under
conditions of carboplatin treatment and no-treatment controls were
not included. Overexpression of lncRNA CASC11 and miR-21 promoted,
whereas lncRNA CASC11 knockdown inhibited, the viability of
carboplatin-treated HCC cells, compared with the control groups
(all P<0.05). In addition, miR-21 overexpression attenuated the
effects of lncRNA CASC11 knockdown on cell viability (P<0.05;
Fig. 6).
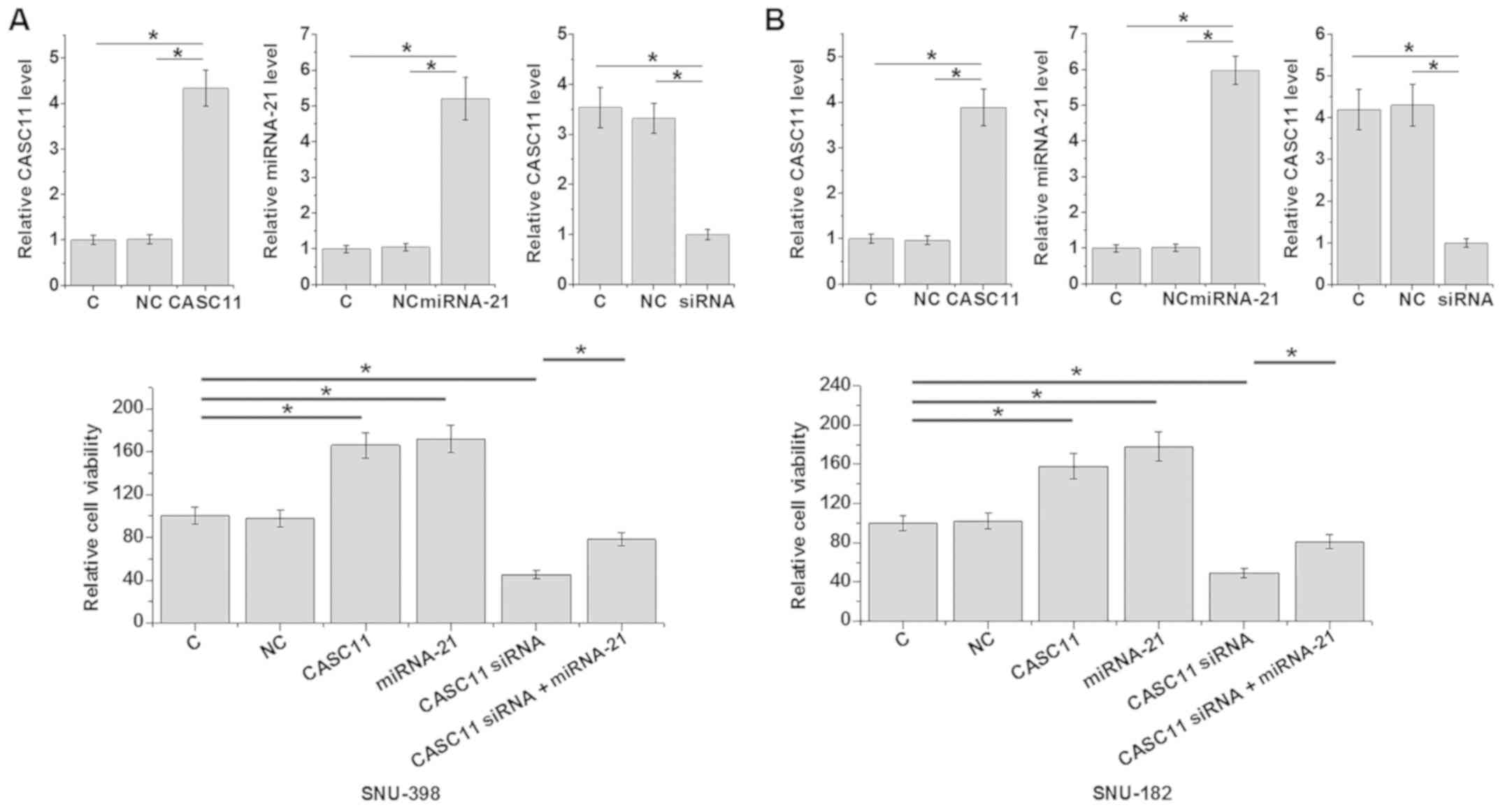 | Figure 6.lncRNA CASC11 promotes hepatocellular
carcinoma cell viability following carboplatin treatment, through
miR-21. Reverse transcription-quantitative analysis confirmed
overexpression of lncRNA CASC11, overexpression of miR-21 and
knockdown of CASC11 in (A, top panel) SNU-398 and (B, top panel)
SNU-182 cells transfected with CASC11 overexpression vector, miR-21
mimic and CASC11 siRNA, respectively. An MTT assay in (A, bottom
panel) SNU-398 and (B, bottom panel) SNU-182 cells demonstrated
that overexpression of lncRNA CASC11 promoted, whereas lncRNA
CASC11 knockdown inhibited the viability of HCC cells following
carboplatin treatment. In addition, miR-21 overexpression
attenuated the effects of lncRNA CASC11 knockdown on cell
viability. Only the empty vector NC group was present as the values
of the overexpression NC, miR mimic NC, siRNA NC and the NC for the
siRNA+mimic combination groups showed similar values, and so, to
simplify the comparisons, all groups were compared with C group.
*P<0.05. C, control cells without transfection; NC, negative
control; lncRNA, long non-coding RNA; miRNA, microRNA; CASC11,
cancer susceptibility 11; siRNA, small interfering RNA. |
Discussion
CASC11 was demonstrated to be an oncogenic lncRNA in
gastric cancer (11). The key
finding of the present study was that lncRNA CASC11 was
overexpressed in HCC, and participates in the development of
chemoresistance to carboplatin in patients with HCC, via the
upregulation of miR-21.
Development of chemoresistance is common during
clinical treatment of liver cancer, providing a major challenge for
clinical management (14). miR-21,
as an oncogenic miRNA, is upregulated in certain types of cancer in
humans, including HCC (15). In the
present study, upregulation of miR-21 was observed in HCC tissues
compared with healthy tissues. Besides its involvement in cancer
development, the expression of miR-21 also determined the
chemosensitivity of cancer cells to chemotherapy (16). In the present study, upregulation of
miR-21 was observed in patients with HCC who underwent carboplatin
treatment. In addition, miR-21 overexpression promoted the
viability of carboplatin-treated HCC cells cultured in
vitro. Therefore, miR-21 appears to inhibit the
chemosensitivity of HCC cells to carboplatin.
The development of chemoresistance in certain cases
also requires the involvement of lncRNAs (17,18). In
the present study the upregulation of lncRNA CASC11 was
demonstrated in HCC, indicating its oncogenic role. Further
investigation revealed the role of lncRNA CASC11 in chemoresistance
to carboplatin in HCC. Therefore, inhibition of lncRNA CASC11 may
serve as a potential therapeutic target. However, further in
vitro and in vivo studies and clinical trials are
required to support this conclusion.
Associations between miRNAs and lncRNAs are
frequently observed during the development of cancer (19,20). The
present study demonstrated lncRNA CASC11 as a likely upstream
activator of miR-21 in HCC. In addition, this association was
suggested to be involved in the development of resistance to
carboplatin in patients. These findings provide new insights into
the mechanism of the development of chemoresistance in patients
with HCC. However, the molecular mechanism mediating the
interaction between CASC11 and miR-21 is unknown. A study by Zhang
et al (21) reported the
interaction of CASC11 with WNT/β-catenin to promote colorectal
cancer. Moreover, it is known that miR-21 can interact with
WNT/β-catenin (22). Therefore,
WNT/β-catenin may mediate the interaction between CASC11 and
miR-21. In addition, CASC11 and miR-21 may regulate the viability
of HCC cells, following carboplatin treatment, via the multidrug
resistance gene, P-glycoprotein, and multidrug
resistance-associated proteins that improve the viability of cancer
cells undergoing chemotherapies. However, preliminary data
demonstrated that the multidrug resistance 1 gene (P-glycoprotein
170) and multidrug resistance-associated proteins 1–3 did not
respond to CASC11 overexpression (unpublished data). Thus, future
studies will explore the involvement of other genes.
In conclusion, the data suggest that lncRNA CASC11
and miR-21 are overexpressed in HCC and that lncRNA CASC11
participates in the development of chemoresistance to carboplatin
in patients with HCC, mediated by the upregulation of miR-21.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL, TL and RW designed the experiments. HL and TL
performed the experiments. YZ and XS collected and analyzed the
data. RW drafted the manuscript. All authors approved the final
version of this manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Sixth People's Hospital of Qingdao (Qingdao, China). All
participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ryerson AB, Eheman CR, Altekruse SF, Ward
JW, Jemal A, Sherman RL, Henley SJ, Holtzman D, Lake A, Noone AM,
et al: Annual Report to the Nation on the Status of Cancer,
1975–2012, featuring the increasing incidence of liver cancer.
Cancer. 122:1312–1337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Altekruse SF, Henley SJ, Cucinelli JE and
McGlynn KA: Changing hepatocellular carcinoma incidence and liver
cancer mortality rates in the United States. Am J Gastroenterol.
109:542–553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang H and Chen L: Tumor microenviroment
and hepatocellular carcinoma metastasis. J Gastroenterol Hepatol.
28 (Suppl 1):S43–S48. 2013. View Article : Google Scholar
|
|
4
|
Lee MH, Kim EJ, Lee H, Kim HM, Chang MJ,
Park SY, Hong KS, Kim JS and Sessler JL: Liposomal texaphyrin
theranostics for metastatic liver cancer. J Am Chem Soc.
138:16380–16387. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao X, Chen Q, Liu W, Li Y, Tang H, Liu X
and Yang X: Codelivery of doxorubicin and curcumin with lipid
nanoparticles results in improved efficacy of chemotherapy in liver
cancer. Int J Nanomedicine. 10:257–270. 2015.PubMed/NCBI
|
|
6
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spizzo R, Almeida MI, Colombatti A and
Calin GA: Long non-coding RNAs and cancer: A new frontier of
translational research? Oncogene. 31:4577–4587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kumarswamy R, Volkmann I and Thum T:
Regulation and function of miRNA-21 in health and disease. RNA
Biol. 8:706–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan X, Wang ZX and Wang R: MicroRNA-21: a
novel therapeutic target in human cancer. Cancer Biol Ther.
10:1224–1232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Kang W, Lu X, Ma S, Dong L and
Zou B: lncRNA CASC11 promoted gastric cancer cell proliferation,
migration and invasion in vitro by regulating cell cycle pathway.
Cell Cycle. 17:1886–1900. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chun YS, Pawlik TM and Vauthey JN: 8th
Edition of the AJCC cancer staging manual: Pancreas and
hepatobiliary cancers. Ann Surg Oncol. 25:845–847. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Briz O, Perez MJ and Marin JJ: Further
understanding of mechanisms involved in liver cancer
chemoresistance. Hepatoma Res. 3:22–26. 2017. View Article : Google Scholar
|
|
15
|
Mao B, Xiao H, Zhang Z, Wang D and Wang G:
MicroRNA-21 regulates the expression of BTG2 in HepG2 liver cancer
cells. Mol Med Rep. 12:4917–4924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei J, Gao W, Zhu CJ, Liu YQ, Mei Z, Cheng
T and Shu YQ: Identification of plasma microRNA-21 as a biomarker
for early detection and chemosensitivity of non-small cell lung
cancer. Chin J Cancer. 30:407–414. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Si X, Zang R, Zhang E, Liu Y, Shi X, Zhang
E, Shao L, Li A, Yang N, Han X, et al: lncRNA H19 confers
chemoresistance in ERα-positive breast cancer through epigenetic
silencing of the pro-apoptotic gene BIK. Oncotarget. 7:81452–8146.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen CJ, Cheng YM and Wang CL: lncRNA PVT1
epigenetically silences miR-195 and modulates EMT and
chemoresistance in cervical cancer cells. J Drug Target.
25:637–644. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou X, Ye F, Yin C, Zhuang Y, Yue G and
Zhang G: The interaction between miR-141 and lncRNA-H19 in
regulating cell proliferation and migration in gastric cancer. Cell
Physiol Biochem. 36:1440–1452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao MX, Jiang YP, Tang YL and Liang XH:
The crosstalk between lncRNA and microRNA in cancer metastasis:
Orchestrating the epithelial-mesenchymal plasticity. Oncotarget.
8:12472–12483. 2017.PubMed/NCBI
|
|
21
|
Zhang Z, Zhou C, Chang Y, Zhang Z, Hu Y,
Zhang F, Lu Y, Zheng L, Zhang W and Li X and Li X: Long non-coding
RNA CASC11 interacts with hnRNP-K and activates the WNT/β-catenin
pathway to promote growth and metastasis in colorectal cancer.
Cancer Lett. 376:62–73. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu D, Shi M and Fan XD: Mechanism of
miR-21 via Wnt/β-catenin signaling pathway in human A549 lung
cancer cells and Lewis lung carcinoma in mice. Asian Pac J Trop
Med. 8:479–484. 2015. View Article : Google Scholar : PubMed/NCBI
|