|
1
|
Asif HM, Sultana S, Ahmed S, Akhtar N and
Tariq M: HER-2 positive breast cancer-a mini-review. Asian Pac J
Cancer Prev. 17:1609–1615. 2016.PubMed/NCBI
|
|
2
|
Ghislain I, Zikos E, Coens C, Quinten C,
Balta V, Tryfonidis K, Piccart M, Zardavas D, Nagele E,
Bjelic-Radisic V, et al European Organisation for Research and
Treatment of Cancer (EORTC) Quality of Life Group and Breast Cancer
Group; EORTC Headquarters, : Health-related quality of life in
locally advanced and metastatic breast cancer: Methodological and
clinical issues in randomised controlled trials. Lancet Oncol.
17:e294–e304. 2016.PubMed/NCBI
|
|
3
|
DeSantis CE, Ma J, Goding Sauer A, Newman
LA and Jemal A: Breast cancer statistics, 2017, racial disparity in
mortality by state. CA Cancer J Clin. 67:439–448. 2017.PubMed/NCBI
|
|
4
|
Suzawa K, Toyooka S, Sakaguchi M, Morita
M, Yamamoto H, Tomida S, Ohtsuka T, Watanabe M, Hashida S, Maki Y,
et al: Antitumor effect of afatinib, as a human epidermal growth
factor receptor 2-targeted therapy, in lung cancers harboring HER2
oncogene alterations. Cancer Sci. 107:45–52. 2016.PubMed/NCBI
|
|
5
|
Luhtala S, Staff S, Tanner M and Isola J:
Cyclin E amplification, over-expression, and relapse-free survival
in HER-2-positive primary breast cancer. Tumour Biol. 37:9813–9823.
2016.PubMed/NCBI
|
|
6
|
Grosset AA, Poirier F, Gaboury L and
St-Pierre Y: Galectin-7 expression potentiates her-2-positive
phenotype in breast cancer. PLoS One. 11:e01667312016.PubMed/NCBI
|
|
7
|
Yang F, Lyu S, Dong S, Liu Y, Zhang X and
Wang O: Expression profile analysis of long noncoding RNA in
HER-2-enriched subtype breast cancer by next-generation sequencing
and bioinformatics. OncoTargets Ther. 9:761–772. 2016.
|
|
8
|
Gradishar WJ, Anderson BO, Balassanian R,
Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A,
Giordano SH, et al: Breast cancer version 2.2015. J Natl Compr Canc
Netw. 13:448–475. 2015.PubMed/NCBI
|
|
9
|
Ahmed S, Sami A and Xiang J: HER2-directed
therapy: Current treatment options for HER2-positive breast cancer.
Breast Cancer. 22:101–116. 2015.PubMed/NCBI
|
|
10
|
Shi SJ, Wang LJ, Yu B, Li YH, Jin Y and
Bai XZ: LncRNA-ATB promotes trastuzumab resistance and
invasion-metastasis cascade in breast cancer. Oncotarget.
6:11652–11663. 2015.PubMed/NCBI
|
|
11
|
Perez EA, Barrios C, Eiermann W, Toi M, Im
YH, Conte P, Martin M, Pienkowski T, Pivot X, Burris H III, et al:
Trastuzumab emtansine with or without pertuzumab versus trastuzumab
plus taxane for human epidermal growth factor receptor 2-positive,
advanced breast cancer: Primary results from the phase III MARIANNE
study. J Clin Oncol. 35:141–148. 2017.PubMed/NCBI
|
|
12
|
Hendricks WPD, Briones N, Halperin RF,
Facista S, Heaton PR, Mahadevan D and Kim S: Genomic signature of
trastuzumab neoadjuvant therapy predictive of patient survival in
HER2-positive breast cancer. Cancers (Basel).
11:E15662019.PubMed/NCBI
|
|
13
|
Ho GY, Woodward N and Coward JI: Cisplatin
versus carboplatin: Comparative review of therapeutic management in
solid malignancies. Crit Rev Oncol Hematol. 102:37–46.
2016.PubMed/NCBI
|
|
14
|
Theile D: Under-reported aspects of
platinum drug pharmacology. Molecules. 22:3822017.
|
|
15
|
Denkert C, Loibl S, Salat C, Sinn BV,
Schem C, Endris V, Klare P, Schmitt WD, Blohmer JU, Weichert W, et
al: Abstract S1-06: Increased tumor-associated lymphocytes predict
benefit from addition of carboplatin to neoadjuvant therapy for
triple-negative and HER2-positive early breast cancer in the
GeparSixto trial (GBG 66). Cancer Res. 73:(abstract nr S1-06).
2013.
|
|
16
|
Clarke SJ and Rivory LP: Clinical
pharmacokinetics of docetaxel. Clin Pharmacokinet. 36:99–114.
1999.PubMed/NCBI
|
|
17
|
Lyseng-Williamson KA and Fenton C:
Docetaxel: A review of its use in metastatic breast cancer. Drugs.
65:2513–2531. 2005.PubMed/NCBI
|
|
18
|
Leung HW, Chan AL, Muo CH and Leung JH:
Cost-effectiveness of pertuzumab combined with trastuzumab and
docetaxel as a first-line treatment for HER-2 positive metastatic
breast cancer. Expert Rev Pharmacoecon Outcomes Res. 18:207–213.
2018.PubMed/NCBI
|
|
19
|
Echavarria I, Granja M, Bueno C,
Lopez-Tarruella S, Peinado P, Sotelo M, Jerez Y, Moreno F, Torres
G, Lobo M, et al: Multicenter analysis of neoadjuvant docetaxel,
carboplatin, and trastuzumab in HER2-positive breast cancer. Breast
Cancer Res Treat. 162:181–189. 2017.PubMed/NCBI
|
|
20
|
Kataja V and Castiglione M; ESMO
Guidelines Working Group, : Primary breast cancer: ESMO clinical
recommendations for diagnosis, treatment and follow-up. Ann Oncol.
20 (Suppl 4):10–14. 2009.PubMed/NCBI
|
|
21
|
Keil S, Barabasch A, Dirrichs T, Bruners
P, Hansen NL, Bieling HB, Brümmendorf TH and Kuhl CK: Target lesion
selection: An important factor causing variability of response
classification in the Response Evaluation Criteria for Solid Tumors
1.1. Invest Radiol. 49:509–517. 2014.PubMed/NCBI
|
|
22
|
Shintia C, Endang H and Diani K:
Assessment of pathological response to neoadjuvant chemotherapy in
locally advanced breast cancer using the Miller-Payne system and
TUNEL. Malays J Pathol. 38:25–32. 2016.PubMed/NCBI
|
|
23
|
Hornbeck PV: Enzyme-linked immunosorbent
assays. Curr Protoc Immunol. 110:2.1.1–23. 2015.
|
|
24
|
Cho YA, Sung MK, Yeon JY, Ro J and Kim J:
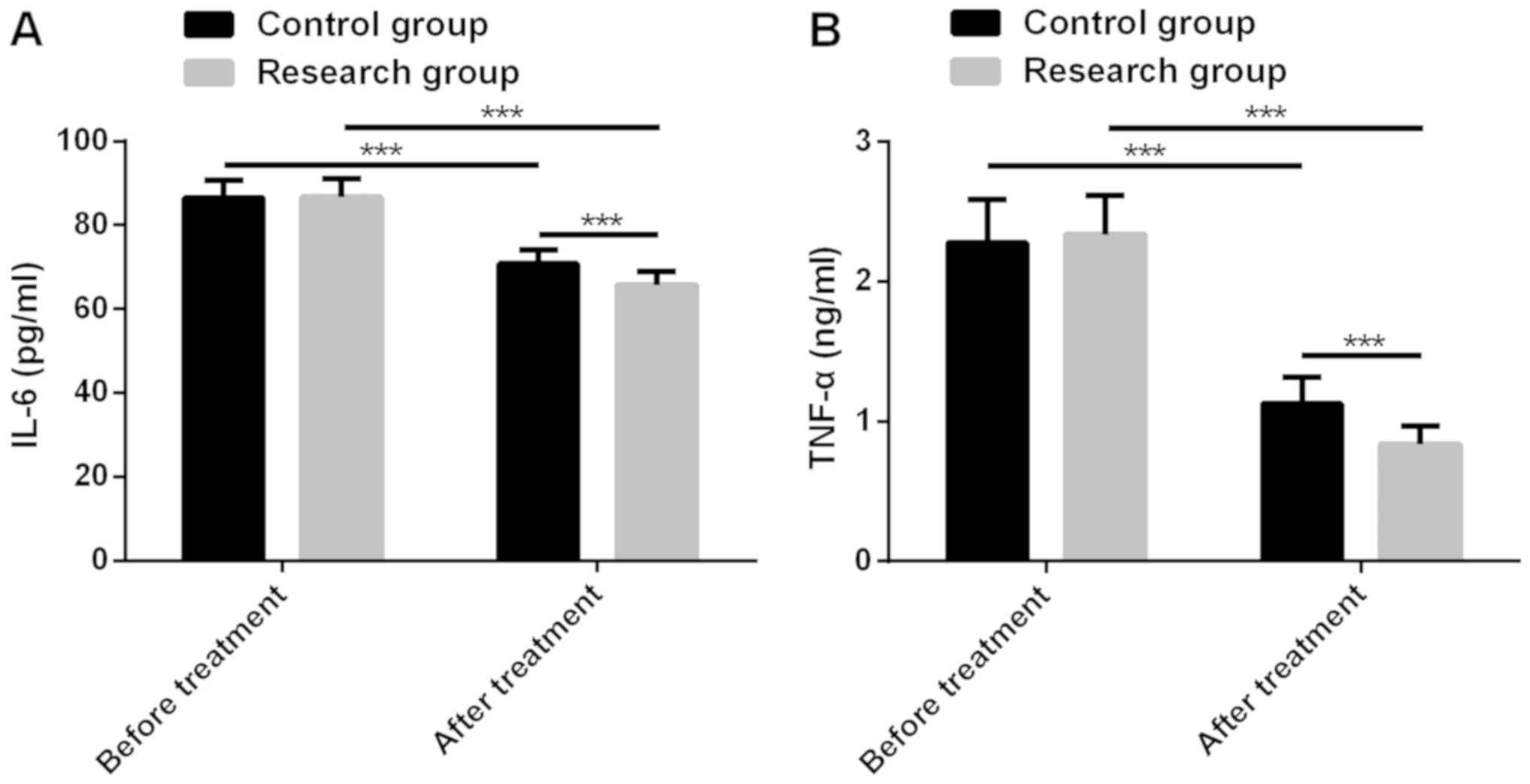
Prognostic role of interleukin-6, interleukin-8, and leptin levels
according to breast cancer subtype. Cancer Res Treat. 45:210–219.
2013.PubMed/NCBI
|
|
25
|
Thriveni K, Raju A, Ramaswamy G,
Shachidevi Krishnamurthy S and Kumar R: Tumor necrosis factor: An
inflammatory microenvironment marker in primary breast cancer
patients. IJMIO. 3:252018.
|
|
26
|
Drygin D, Ho CB, Omori M, Bliesath J,
Proffitt C, Rice R, Siddiqui-Jain A, O'Brien S, Padgett C, Lim JK,
et al: Protein kinase CK2 modulates IL-6 expression in inflammatory
breast cancer. Biochem Biophys Res Commun. 415:163–167.
2011.PubMed/NCBI
|
|
27
|
Muss HB, Bunn JY, Crocker A, Plaut K, Koh
J, Heintz N, Rincon M, Weaver DL, Tam D, Beatty B, et al: Cyclin
D-1, interleukin-6, HER-2/neu, transforming growth factor
receptor-II and prediction of relapse in women with early stage,
hormone receptor-positive breast cancer treated with tamoxifen.
Breast J. 13:337–345. 2007.PubMed/NCBI
|
|
28
|
Tripsianis G, Papadopoulou E,
Anagnostopoulos K, Botaitis S, Katotomichelakis M, Romanidis K,
Kontomanolis E, Tentes I and Kortsaris A: Coexpression of IL-6 and
TNF-α: Prognostic significance on breast cancer outcome. Neoplasma.
61:205–212. 2014.PubMed/NCBI
|
|
29
|
Seo AN, Lee HJ, Kim EJ, Kim HJ, Jang MH,
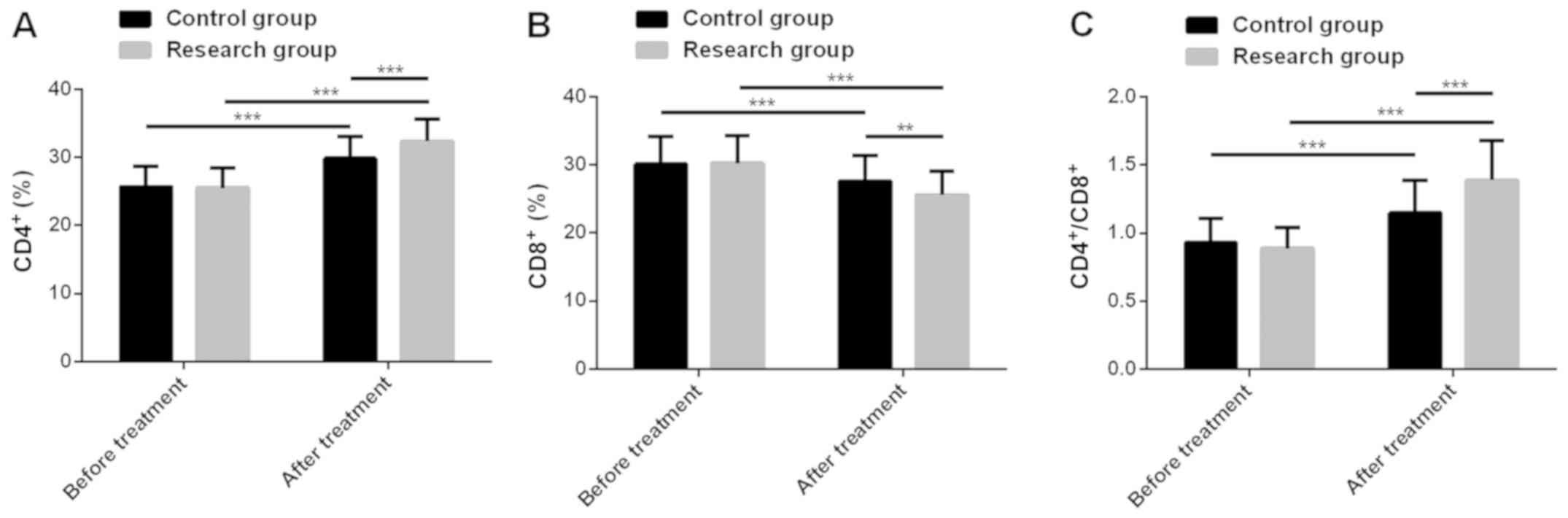
Lee HE, Kim YJ, Kim JH and Park SY: Tumour-infiltrating
CD8+ lymphocytes as an independent predictive factor for
pathological complete response to primary systemic therapy in
breast cancer. Br J Cancer. 109:2705–2713. 2013.PubMed/NCBI
|
|
30
|
Wang B and Pan F: Analysis of T lymphocyte
subsets in peripheral blood of patients with breast cancer and its
clinical value. Int J Lab Med. 39:1230–1232, 1237. 2018.
|
|
31
|
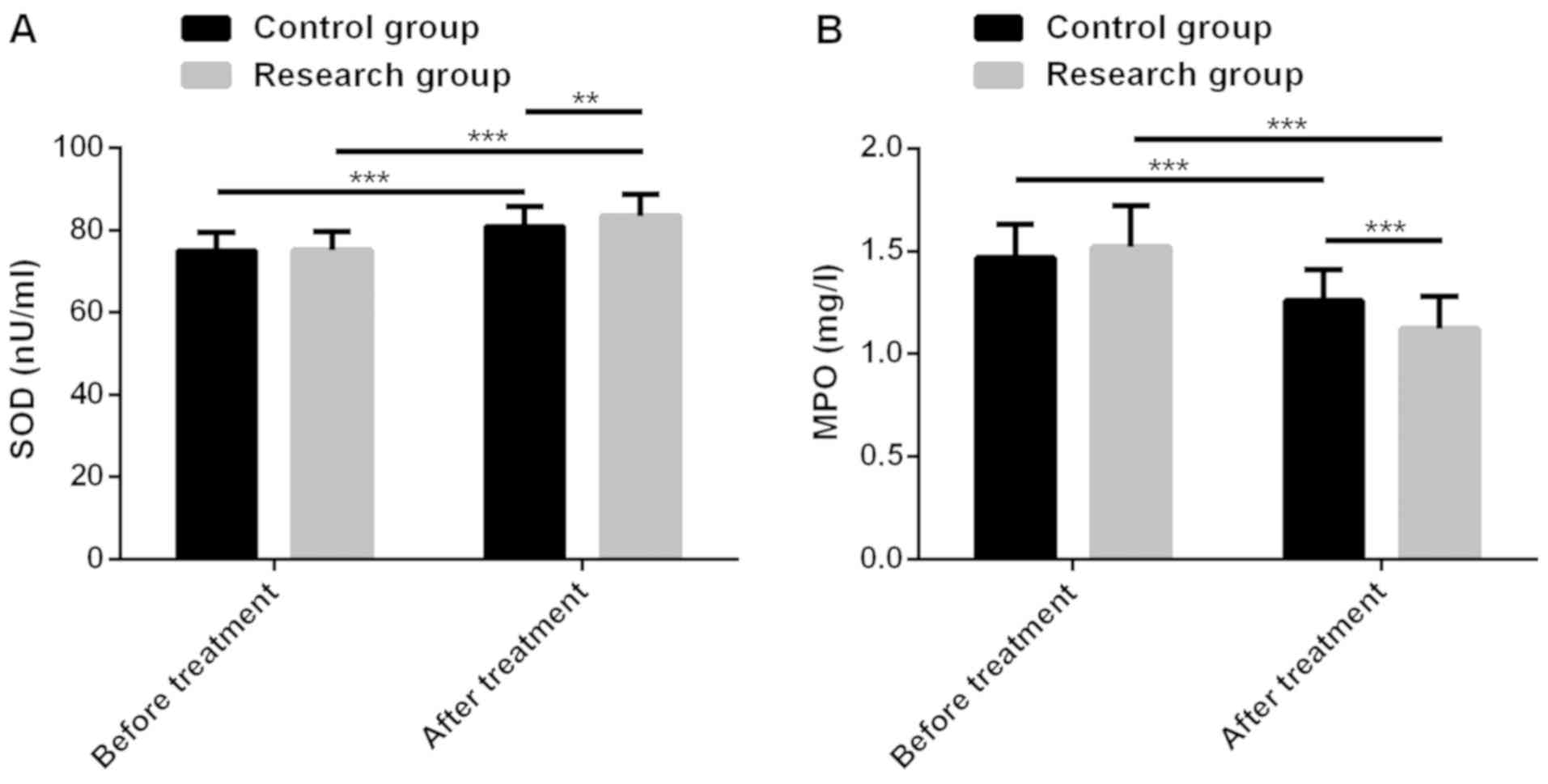
Sahu A, Varma M and Kachhawa K: A
prognostic study of MDA, SOD and catalase in breast Cancer
patients. Int J Sci Res (Ahmedabad). 4:157–159. 2015.
|
|
32
|
Fernandes AS, Saraiva N and Oliveira NG:
Redox therapeutics in breast cancer: Role of SOD mimics.
Redox-Active Therapeutics. Batinic-Haberle I, Reboucas JS and
Spasojevic I: Springer. (Cham, Switzerland). 451–467. 2016.
|
|
33
|
Yang WJ, Wang MY, Pan FZ, Shi C and Cen H:
Association between MPO-463G>A polymorphism and cancer risk:
Evidence from 60 case-control studies. World J Surg Oncol.
15:1442017.PubMed/NCBI
|
|
34
|
Abusoglu S, Eryavuz D, Bal C, Nural C,
Ozcan E, Yildirimel M, Celik S and Unlu A: Assessment of serum
ischemia-modified albumin, prolidase and thiol-disulphide levels in
subjects with breast cancer. Rev Rom Med Lab. 27:25–33. 2019.
|
|
35
|
Zapf I, Moezzi M, Fekecs T, Nedvig K,
Lőrinczy D and Ferencz A: Influence of oxidative injury and
monitoring of blood plasma by DSC on breast cancer patients. J
Therm Anal Calorim. 123:2029–2035. 2016.
|