Introduction
Ovarian carcinoma is the most frequent cause of
cancer-related death from gynecologic malignancy and is frequently
associated with a poor outcome due to the difficulty of an early
diagnosis and malignancies which are often both large and advanced
at the time of diagnosis (1). Almost
75% of women with ovarian cancer are diagnosed at stage III or IV,
with 10-year survival rates of 21% and less than 5%, respectively
(2). The degree of peritoneal
dissemination and the number of chemotherapy-resistant tumors, as
well, is related to the poor prognosis of patients with
advanced-stage ovarian cancer. Epithelial-mesenchymal-transition
(EMT) is associated with an important step in carcinoma metastasis
via the induction of a highly invasive phenotype (3). We previously showed that the expression
of EMT-related proteins was correlated with the status of tumor
metastasis and the prognostic value in ovarian carcinoma (4). However, the molecular etiology of
ovarian carcinoma remains mostly unknown. Therefore, it is of great
importance to clarify the association of key proteins with ovarian
carcinoma metastasis and invasion (5).
Cisplatin (CDDP) is widely used as an antineoplastic
drug in the clinical treatment of ovarian cancer (6). However, its low aqueous solubility and
high protein binding ability reduce the efficacy of the drug. In
addition, its clinical use may be limited due to intrinsic and
acquired resistance and systemic or nonspecific side effects,
including acute nephrotoxicity and myelosuppresion (7,8).
Furthermore, the understanding of the pathways of cancer
progression and pathogenesis, as well as the development of
multidrug resistance in ovarian cancer, will result in the
discovery of new molecular targets such as microRNA (9), somatic and germline mutations (10), amplifications (11), and structural instability (12). Moreover, new therapies such as drug
delivery systems (DDS) also indicate more effective therapies into
solid tumors while lessening their distribution into normal,
healthy tissue (8). DDSs that can
recognize specific tumors themselves, in order to deliver drugs at
high doses efficiently and practically in vivo, have also
been developed, and liposomes are being widely studied as DSS
because of their amphiphilic characteristics (13).
Cluster of differentiation 24 (CD24) is a small,
mucin-like glycosylphosphatidylinositol (GPI) anchored membrane
molecule over-expressed in a variety of human carcinomas. CD24 is a
glycoprotein expressed on the surface of most B lymphocytes and
differentiating neuroblasts (14).
In neoplasia, the expression of CD24 has not only been described in
haematological malignancy, but also in a large variety of solid
tumors such as nasopharyngeal carcinoma, non-small cell lung
cancer, breast cancer, hepatocellular carcinoma, renal cell
carcinoma, bladder carcinoma, colorectal cancer and epithelial
ovarian cancer (15–17). Moreover, CD24 is involved in the
growth, anchorage-independent reproduction and survival of tumor
cells (18), and this finding
suggests that CD24 functionally enhances the metastatic potential
of cancer cells. In our previous study, we indicated that Caov-3
cells contain CD24-positive cells under normoxia and are involved
in the EMT process, thus showing that CD24 plays a critical role in
regulating the EMT phenomenon (19).
In the present study, we examined whether
CD24-targeted liposomal CDDP specifically killed these
CD24-positive cells, as CD24-positive cells are involved in both
the EMT process and the suppression of tumor growth in Caov-3
×enograft mouse models. This study provides new knowledge on
CD24-targeted therapeutic applications and their promise for future
clinical trials.
Materials and methods
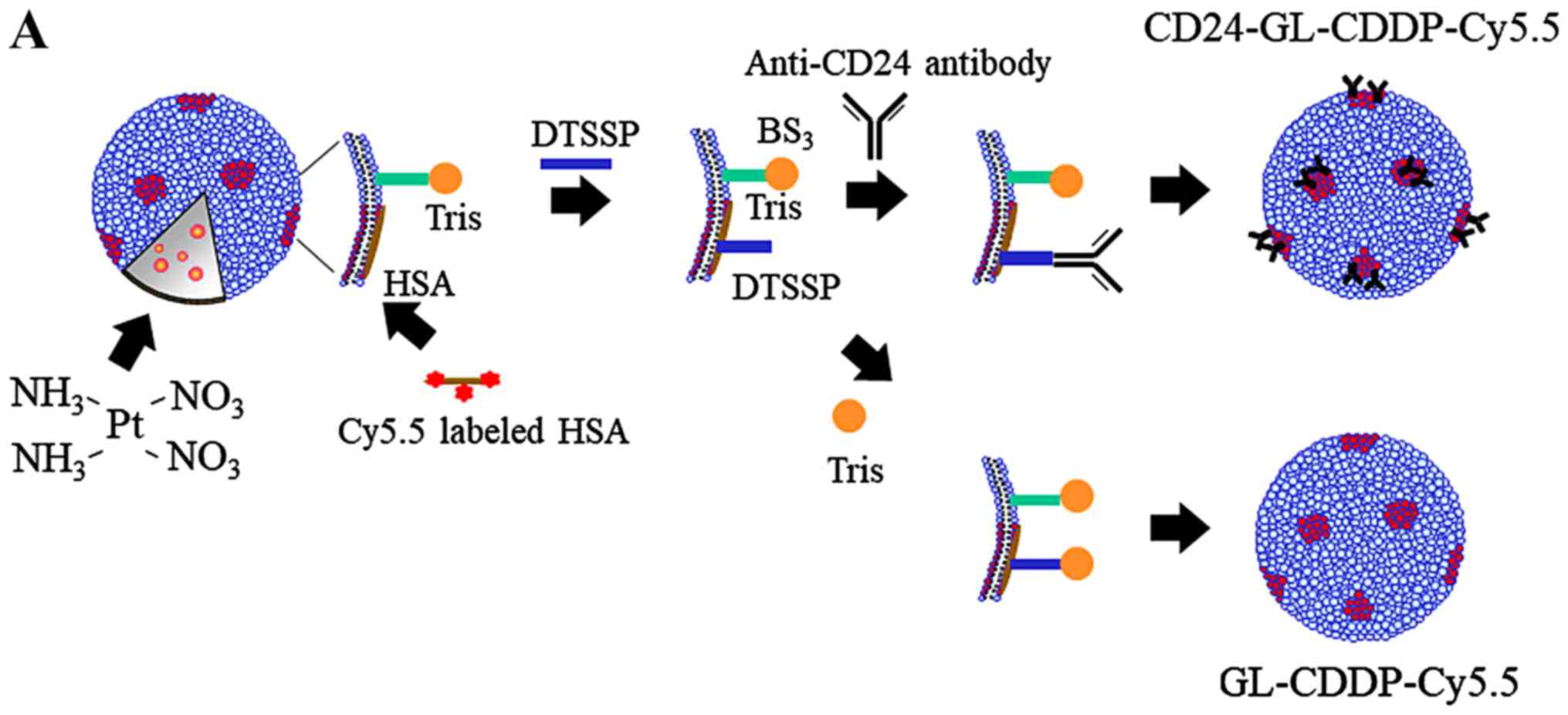
Properties of CD24 targeted liposomal
cisplatin
Preparation of hydrophilic anionic liposomes and
encapsulation of Cy5.5 were carried out as described previously
(20,21). These liposomes (GLYCOLIPO, Katayama
Chemical Industries) are composed of dicetylphosphate,
dipalmitoylphosphatidylcholine,
dipalmitoylphosphatidylethanolamine, cholesterol and ganglioside.
Dicetylphosphate was used to confer a negative charge to the
liposome surface. Briefly, dipalmitoylphosphatidylcholine,
cholesterol, ganglioside dicetylphosphate and
dipalmitoylphosphatidylethanolamine were mixed at different molar
ratios, and cholic acid was added to facilitate micelle formation.
The mixture was dissolved in methanol/chloroform (1:1, v/v), and
the solvent evaporated at 37°C to produce a lipid film which was
dried under vacuum. This film was then dissolved in a 10 mM TAPS
buffer (N-tris(hydroxymethyl)methyl-3-amino-propane sulfonic
acid containing buffer) without NaCl at pH 8.4 and sonicated to
obtain a suspension of uniform micelles. Cy5.5 solution was then
added to the micelle suspension, which was then ultrafiltered
(molecular cutoff 10 000) (Amicon PM10).
Tris(hydroxymethyl)aminomethane (Tris) was crosslinked on the
liposome surface via bis(sulfosuccinimidyl)suberate (BS3) to confer
hydrophilicity, because this process can prevent uptake by the
reticuloendothelial system (RES) in the liver and spleen and by
macrophages and vascular endothelial cells. Human serum albumin
(HSA) and Cy5.5-NHS ester (GE Healthcare) were dissolved in TAPS
(pH 8.4) and stirred at 37°C for 3 h, and the HSA was then binded
to Cy5.5. Using 3,30-dithiobis(sulfosuccinimidylpropionate) (DTSSP,
Pierce), anti-CD24 monoclonal antibody (Gene Copoeia) was
crosslinked to HSA with Cy5.5, which was coupled in advance to the
ganglioside component of the liposomes. DTSSP was used as a
cross-linking reagent. Anti-CD24 was added to a final concentration
of 75 µg/ml and stirred at 25°C for 2 h. Tris-HCl was then added to
reach a final concentration of 132 mg/ml and stirred overnight at
4°C for hydrophilization of the liposome surface. The content of
lipid in the liposomes was determined as total cholesterol in the
presence of 0.5% TritonX-100 with a Determiner TC555 diagnostic kit
(Kyowa Medex). The particle size and zeta-potential were measured
using a Zetasizer Nano-S90 (Malvern Instruments). The amount of
anti-CD24 on the surface of the liposomes was measured by an
enzyme-linked immunosorbent assay using a CD24-immobilized
microplate as described previously (22). The final contents of both
GL-CDDP-Cy5.5 and CD24-GL-CDDP-Cy5.5 had liposome concentrations of
72.0 mg/ml and CDDP concentrations of 2.0 mg/ml, and both had a
median diameter of 158 nm.
Cell culture and cell lines
We used a human ovarian mucinous adenocarcinoma
cancer cell line, Caov-3 cells, which was obtained from the
American Type Culture Collection (ATCC) in this study. The ATCC
routinely authenticate their cell lines by Short Tandem Repeat
(STR) polymorphism profiling analyses. We also performed an STR
polymorphism profiling analysis (Wakennyaku Co.) to confirm the
cell line's identity. Caov-3 cells were grown in phenol DMEM
containing 10% dextran-coated, charcoal-treated fetal calf serum in
a humidified atmosphere of 5% CO2 with 95% air at
37°C.
Flow cytometry
The cultures from the Caov-3 cells were washed with
PBS. For some experiments, single cells dissociated from tumor
spheres were analyzed by the following method. One million
trysinized cells were incubated with 7-amino-actinomycin D (BD
Biosciences) only, or with 7-amino-actinomycin D and a mouse
anti-human monoclonal antibody of CD24-FITC (BD Biosciences) with a
stain buffer (BD Pharmingen™) for 20 min at room temperature in the
dark. After washing, the cells were analyzed using a BD FACS Aria™.
The fluorescence intensity was then analyzed using the BD FACS Diva
software program (BD Biosciences).
Chemotherapeutic uptake and
sensitivity assay in vitro
CDDP was encapsulated in GLYCOLIPO (GL-CDDP-Cy5.5),
which was then conjugated with the anti-CD24 antibody
(CD24-GL-CDDP-Cy5.5). The Caov-3 cells (1×105
cells/well) were seeded in triplicate in a 6-well plate with
GL-CDDP-Cy5.5 or CD24-GL-CDDP-Cy5.5. After 24 h, the cells were
washed with PBS, and we compared the differences in the uptake of
Cy5.5 between GL-CDDP-Cy5.5 and CD24-GL-CDDP-Cy5.5 by fluorescence
microscopy with a Biozero BZ-8100 (Keyence, Osaka, Japan). Flow
cytometry was performed using the Caov-3 cells as previously
described. The cells were then analyzed for FITC and Cy5 by a BD
FACS Diva.
Intraperitoneal xenograft models of
ovarian cancer
Female athymic nude mice (BALB/c Slc-nu/nu) were
purchased from Japan SLC and maintained in accordance with the
institutional guidelines of Osaka Medical College. All of the
animal studies were carried out according to approved experimental
protocols. 1.0×106 Caov-3 cells were suspended in 100 µl
PBS and were intraperitoneally injected into the nude mice
(5-week-old). The ascites by peritoneal dissemination was allowed
to grow to an approximately increased abdominal circumference of
30%.
Platinum concentrations in tissue by
ICP-OES
Thirty-six intraperitoneal xenograft models weighing
15–25 g were randomly divided into 4 groups (n=9/group). CDDP,
GL-CDDP-Cy5.5 and CD24-GL-CDDP-Cy5.5 were injected intravenously
with a single dose of 10 mg/kg body weight, and PBS served as the
control group. The mice for each group were then sacrificed by an
overdose of isoflurane 6, 24 or 48 h (n=3) after injection, and the
disseminated tumors, livers and kidneys were dissected and washed
with saline. The samples were then weighed (50 mg disseminated
tumors, 100 mg liver and 100 mg kidney) and placed into the
digestion vessels. The samples were digested with 1 ml 65% nitric
acid and 0.2 ml hydrogen peroxide. After digestion and evaporation,
the samples were poured into 10 ml volumetric flasks which were
filled up to the mark with bidistilled water. Two samples of
disseminated tumors, liver and kidney were prepared from each
animal. Absolute Platinum (Pt) concentrations in the tissue samples
were measured by ICP-OES (Vista-MPX ICP-OES spectrometer, Seiko
Instruments) as described previously (23). For the standardization of equipment
and measurements, Spectro multi-element and Spectrum 3D standards
were used (24). Standards were
prepared in the same matrix as the samples. The samples were then
measured 3 times, and blank subtraction was applied.
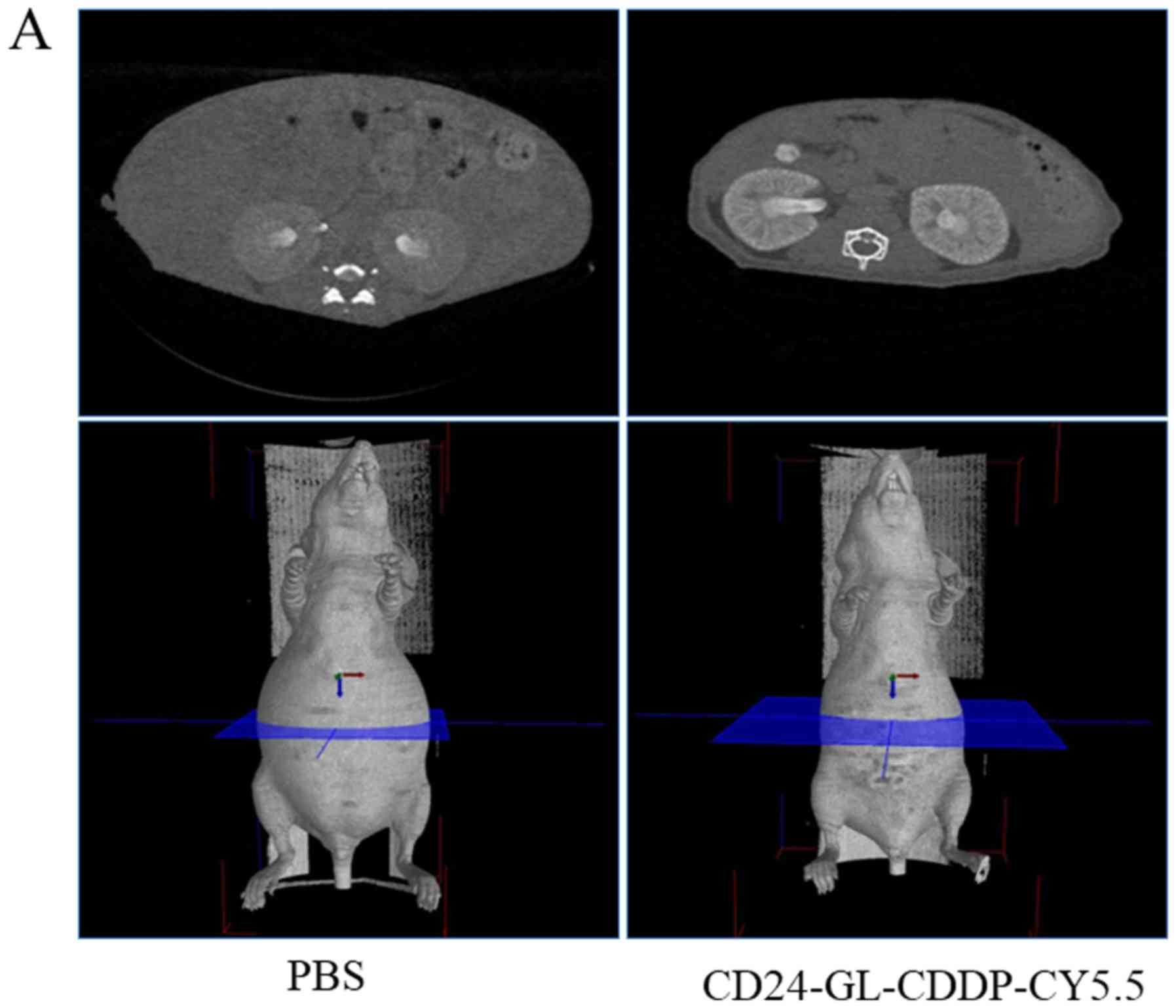
Computed tomography of intraperitoneal
xenograft models to be treated
We employed a 3rd generation computed tomography
scanner - LaTheta LCT-200 (Hitachi-Aloka). The tube voltage was set
at 50 kV, and the current was constant at 0.5 mA. The mice were
scanned in a 48 mm wide specimen holder with a resolution of 96 µm
pixels. Whole abdominal scans in mice under the described
conditions were 150 slices per mouse and a slice thickness of 192
µm. Mean acquisition time for the scan was 10 min and, therefore,
the abdominal regions of the mice were scanned by LCT-200, as
described previously, under isoflurane anesthesia (25).
Anti-tumor efficacy in vivo
Forty intraperitoneal xenograft models weighing
15–25 g were randomly assigned into four groups (n=10 per
group) and intravascularly administered once a week with PBS, CDDP
(10 mg/kg), GL-CDDP-Cy5.5 (10 mg/kg), or CD24-GL-CDDP-Cy5.5 (10
mg/kg) for 3 weeks. Maximum abdominal circumference (MAC), due to
the increased ascites, was measured by a 3D imaging computed
tomography scanner (LCT-200) once a week as a base to evaluate the
therapeutic efficacy. The mice were also measured by LCT-200 once a
week after treatment, and the increase rate of MAC was calculated.
Tumor growth curves were stopped at the exclusion day of the first
mouse in each group. The survival benefit by treatment was measured
with the calculation of overall survival days, and Kaplan-Meier
survival plots were recorded.
In order to determine the survival of the
disseminated tumor-bearing nude mice, the mice were checked daily
for survival and were sacrificed, according to our institutional
guidelines, when the abdominal circumference had reached 10 cm.
Immunohistochemical analysis of
intraperitoneal xenograft models
The disseminated samples with intravenously injected
PBS, CDDP, GL-CDDP-Cy5.5 or CD24-GL-CDDP-Cy5.5 were formalin-fixed
and embedded in paraffin. Deparaffinized and rehydrated sections
(4-µm) were autoclaved in 0.01 mol/l citrate buffer (pH 6.0) for 15
min at 121°C for antigen retrieval. Endogenous peroxidase activity
was blocked with 0.3% solution hydrogen peroxidase in methanol for
30 min. Tumor sections were incubated at 4°C for 12 h with a
CD24-specific antibody (1:50 dilution; Thermo Fisher Scientific,
Inc.), and E-cadherin (1:50 dilution; Cell Signaling Technology)
and Snail antibody (1:100 dilution; ABGENT) were used as described
in a previous study (4,19). The sections were washed with 1X
phosphate-buffered saline (PBS) and incubated with Histofine Simple
Stain MAX PO (Multi; Nichirei) for 30 min at room temperature.
Finally, the sections were washed with 1X PBS, and the signals were
visualized by incubation with
H2O2/diaminobenzidine substrate solution for
5 min. The sections were then counterstained with hematoxylin prior
to dehydration and mounting.
Immunohistochemical evaluations
The expression levels of CD24, E-cadherin and Snail
were assessed using a semiquantitative system, ranging from 0, 1+,
2+ to 3+. Briefly, the expression of CD24 was assessed as carcinoma
cells with positive staining in the membrane and/or cytoplasm and
scored as follows: 0, negative; 1+, <10% positive cells; 2+,
10–50% positive cells; 3+, >50% positive cells. The E-cadherin
expression was scored as follows: 0, negative or <10%
immunoreactivity of tumor cells; 1+, >10% low-intensity
immunoreactivity of tumor cells, 2+, >10% medium-intensity
immunoreactivity of tumor cells, 3+, >10% high-intensity
immunoreactivity of the tumor cells. The Snail expression was
evaluated as being positive only when nuclear staining was
detectable, as follows: 0, negative or <1% positive cells; 1+,
>1% positive cells; 2+, 2–5% positive cells; 3+, >5% positive
cells. Samples with heterogeneous staining were scored based on the
intensity in the largest stained area. Tumor-infiltrating immune
cells were evaluated as described in a previous study (26,27).
Statistical analysis
All statistical analyses were performed using the
JMP software program (SAS Institute). Continuous variables are
expressed as the median and interquartile range or the mean ±
standard deviation. Dunnett's test was used to compare continuous
variables. Statistical differences were evaluated using the
Kruskal-Wallis test prior to Dunnett's post hoc test. The
univariate analyses of overall survival were determined according
to the Kaplan-Meier method using the log-rank test, and the
statistical analysis of post hoc test were used by Dunnett's test.
Descriptive statistics and 95% confidence intervals (CI) were built
to proportions of all groups. P<0.05 was considered to indicate
a statistically significant difference.
Results
Therapeutic effect of CD24-targeted
liposomal cisplatin in vitro
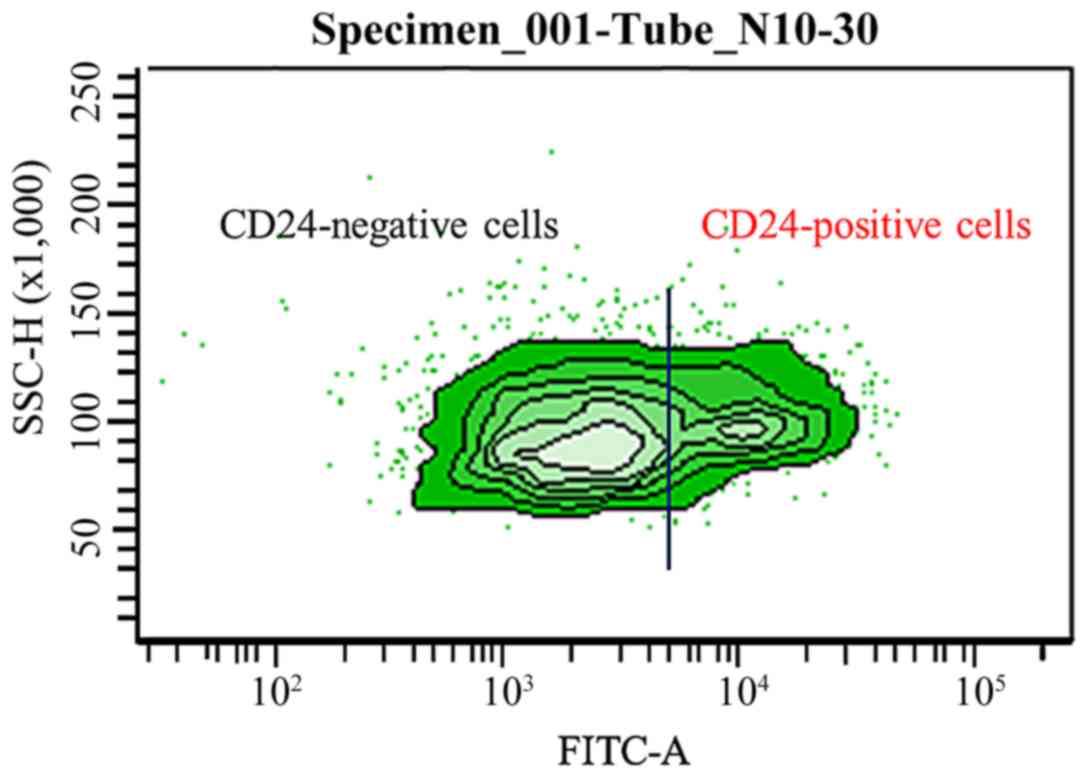
At FITC values, ovarian cancer cell line Caov-3
cells were divided into about 30% CD24-positive cells and about 70%
CD24-negative cells (Fig. 1). We
confirmed that it is quite reasonable to select CD24 to target
ovarian cancer as an application of a specific DDS. To clarify
whether the CD24-targeted liposomal cisplatin (CD24-GL-CDDP-Cy5.5)
has more sensitivity on Caov-3 cells than the non-targeted liposome
containing cisplatin (GL-CDDP-Cy5.5; Fig. 2A), we used BZ-8100 fluorescence
microscopy after the treatment. The uptake of Cy5.5 in Caov-3 cells
was not different between treatment with GL-CDDP-Cy5.5 and with
CD24-GL-CDDP-Cy5.5 (Fig. 2B).
Subsequently, we performed flow cytometry on the Caov-3 cells with
each treatment. Fig. 2C shows that
CD24-GL-CDDP-Cy5.5 accumulates more Cy5 in Caov-3 cells with higher
FITC values; CD24-GL-CDDP-Cy5.5 was uptaken more in CD24-positive
cells than in GL-CDDP-Cy5.5 in vitro. However, we examined
the sensitivity of the cell viability of among CDDP, GL-CDDP-Cy5.5
and CD24-GL-CDDP-Cy5.5 administrations in Caov-3 ovarian cancer
cells, including CD24+ and CD24-, using an MTS assay. CDDP,
GL-CDDP-Cy5.5 and CD24-GL-CDDP-Cy5.5 acted as a concentration
dependent inhibitor of cell proliferation with an IC50 of 72.4,
78.7 and 76.7 µM, respectively. There was no significant difference
in chemotherapeutic sensitivity among CDDP, GL-CDDP-Cy5.5 and
CD24-GL-CDDP-Cy5.5 in Caov-3 ovarian cancer cells (Fig. S1).
Platinum concentrations in various
tissue samples of nude mice by ICP-OES
Progression in tumorigenesis of ovarian cancer is
usually intraperitoneal dissemination. We examined whether
CD24-GL-CDDP-Cy5.5 is specifically uptaken to intraperitoneal
xenograft models by measuring the Pt concentration contained in
cisplatin. 1.0×106 Caov-3 cells were inoculated
intraperitoneally in nude mice, as described in Materials and
Methods. The intraperitoneal xenograft models with peritoneal fluid
were classified into several treatment groups, including PBS, CDDP,
GL-CDDP-Cy5.5 and CD24-GL-CDDP-Cy5.5. Furthermore, each group was
distinguished by the time at which each agent was injected (6h, 24h
or 48 h). Absolute Pt concentrations in disseminated tumors, kidney
and liver of the nude mice after sacrificing were measured by
ICP-OES. The concentration of Pt was below the limit of detection
in the group treated with PBS, while Pt concentration was
detectable in the group treated with CDDP, GL-CDDP-Cy5.5 and
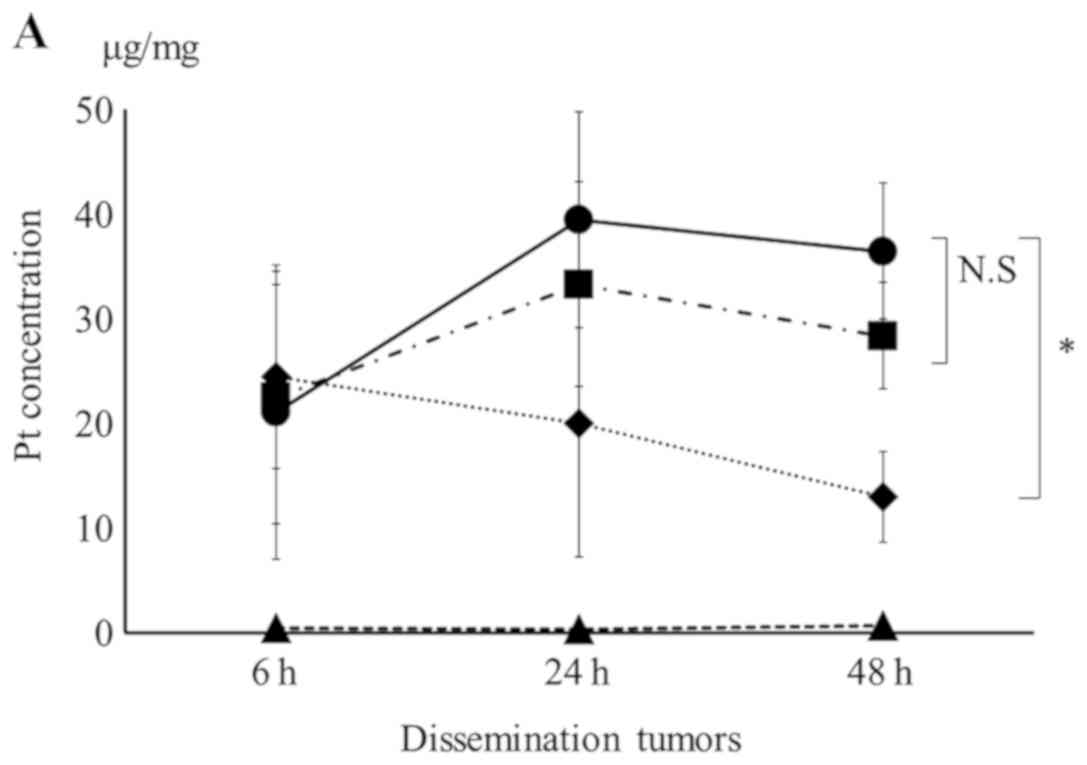
CD24-GL-CDDP-Cy5.5. The Pt concentration in dissemination tumors
increased in treated groups after 6 h. The Pt concentration in
dissemination tumors treated with CD24-GL-CDDP-Cy5.5 after 48 h was
significantly higher than that treated with CDDP (36.5±6.5 µg/mg
vs. 13.2±4.3 µg/mg, P<0.05; Fig.
3A), however, there was no difference in the Pt concentration
between the GL-CDDP-Cy5.5 and CD24-GL-CDDP-Cy5.5 groups. Fig. 3B shows the transition of Pt
concentration over time in the kidney. After 6 and 24 h, the Pt
concentration immediately lower in both the GL-CDDP-Cy5.5 and
CD24-GL-CDDP-Cy5.5 groups than in the CDDP group, and the Pt
concentration in kidneys treated with CDDP after 48 h was
significantly higher than in those treated with CD24-GL-CDDP-Cy5.5
(114.4±36.1 µg/mg vs. 46.5±10.1 µg/mg, P<0.05); however, there
was no difference in the Pt concentration between the GL-CDDP-Cy5.5
and CD24-GL-CDDP-Cy5.5 groups. These finding suggest that the
GL-CDDP-Cy5.5 and CD24-GL-CDDP-Cy5.5 markedly reduced
nephrotoxicity, which is a dose-limiting factor of CDDP. Fig. 3C shows the transition of Pt
concentration over time in the liver. In contrast, the Pt
concentration in livers treated with CD24-GL-CDDP-Cy5.5 after 48 h
was significantly higher than in those treated with CDDP (78.5±21.7
µg/mg vs. 38.5±8.3 µg/mg, P<0.05); however, there was no
difference in the Pt concentration between the GL-CDDP-Cy5.5 and
CD24-GL-CDDP-Cy5.5 groups. Although the Pt concentration in liver
tissues from the GL-CDDP-Cy5.5 and CD24-GL-CDDP-Cy5.5 groups was
higher compared with that in the CDDP group, the liver pathological
changes of microvesicular steatosis and inflammatory infiltrates
were hardly observed (data not shown).
Antitumor efficiency of
CD24-GL-CDDP-Cy5.5 in intraperitoneal xenograft models
A total of 40 nude mice were intraperitoneally
inoculated with Caov-3 cells and followed up for 7 days for tumor
growth. The nude mice were treated with PBS, CDDP (10 mg/kg),
GL-CDDP-Cy5.5 (10 mg/kg) or CD24-GL-CDDP-Cy5.5 (10 mg/kg) once a
week. Each agent was injected via the tail vein on day 8, 15 and
22. On day 29, the MAC was measured by LCT-200 and was
significantly different between the PBS group and the
CD24-GL-CDDP-Cy5.5 group (Fig. 4A).
The mice which were injected with PBS and CDDP showed a fast
increase in ascites volume from the beginning of the therapy
experiment, thus indicating an uninhibited MAC growth (Fig. 4B). On day 22, the MAC growth value
was 1.62 in PBS, 1.65 in CDDP, 1.45 in GL-CDDP-Cy5.5, and 1.26 in
CD24-GL-CDDP-Cy5.5. The group that received CD24-GL-CDDP-Cy5.5
demonstrated the most pronounced delay in MAC growth (vs. PBS:
P<0.01; vs. CDDP: P<0.05; vs. GL-CDDP-Cy5.5: P<0.05). The
antitumor effects of CD24-GL-CDDP-Cy5.5, as well, were also
evaluated by the survival of mice in the same experiments in
vivo (Fig. 4C). The ten mice of
each groups treated with PBS, CDDP, GL-CDDP-Cy5.5 or
CD24-GL-CDDP-Cy5.5 died between day 24 and 96. The median survival
time was 36 days in the PBS group (95% CI=23–48), 37 days in the
CDDP group (95% CI=23–49), 46 days in the GL-CDDP-Cy5.5 group (95%
CI=29–50) and 56 days in the CD24-GL-CDDP-Cy5.5 group (95%
CI=32–60). No significant difference in overall survival could be
observed between the PBS, CDDP and GL-CDDP-Cy5.5 groups. Moreover,
the CD-24-GL-CDDP-Cy5.5 group had a significantly elongated
survival period of mice, as compared with the PBS (P<0.05) and
CDDP (P<0.05) group, but not the GL-CDDP-Cy5.5 group
(P=0.2). Additionally, no weight loss >20% or signs of
distress could be observed at any time during the therapy
experiment. These results supported the superior antitumor efficacy
observed with CD24-GL-CDDP-Cy5.5 in vivo.
CD24-GL-CDDP-Cy5.5 suppress EMT
phenomenon
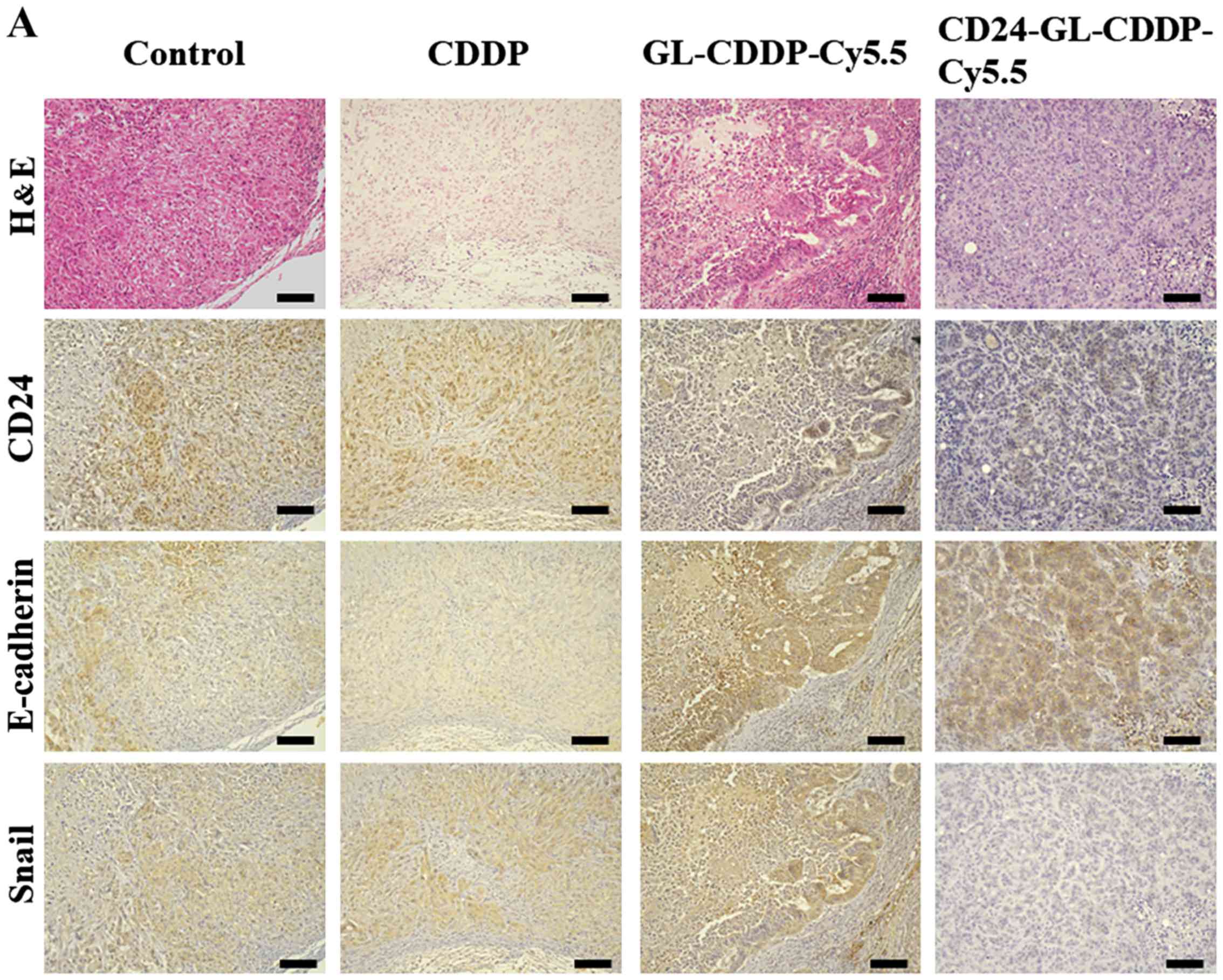
Immunohistochemical staining showed that the number
of CD24-positive cells was significantly lower in the
CD24-GL-CDDP-Cy5.5 group compared to the untreated control group
(P<0.05), CDDP group (P<0.05), or the GL-CDDP-Cy5.5 group
(P<0.05) (Fig. 5A and B). The
expression of E-cadherin was significantly higher in the
CD24-GL-CDDP-Cy5.5 group than in the other groups (P<0.05). The
expression of Snail was also significantly lower in the
CD24-GL-CDDP-Cy5.5 group than that in the other groups (P<0.05;
Fig. 5B). These results supported
CD24 targeted liposomes' ability to suppress the EMT phenomenon
in vivo.
Discussion
In the present study, the possibility of DDS
targeting CD24 for ovarian cancer was assessed. Antibody drug
conjugates use the antibody as a guided missile to deliver
cytotoxic molecules to tumor cells to achieve cancer therapy.
Critical parameters for antibody drug conjugates include the
selection of the tumor target antigen, the antibody against the
target, the cytotoxic molecule, the linker bridging the cytotoxic
molecule, and the antibody which aided the successful development
of DDSs for clinical application. The target antigen is the initial
point at which to design a DDS, and many tumor specific targets
have been evaluated for development. CD24 is an important marker
for the diagnosis and prognosis of various cancers (15–17). We
previously reported that CD24 was expressed in 70.1% of ovarian
cancers and that the expression of CD24 was an independent
predictor of patient survival in patients with ovarian cancer
(19). We also demonstrated that the
overexpression of CD24 in ovarian carcinoma is associated with high
invasiveness and metastatic potential, high tumor proliferation
status, and the activation of both the Akt and ERK pathways.
Moreover, CD24 is a novel predictor for poor prognosis and induces
the EMT phenomenon in ovarian carcinoma (19,28). EMT
is involved in cell invasion, resistance to chemotherapy, and the
formation of side populations of cancer stem-like cells (CSCs)
(29), and we demonstrated that
CD24-positive cells have characteristic of CSCs in a previous study
(19). We have also indicated that
CD24 plays a critical role in regulating the EMT phenomenon in
ovarian cancer (19). Therefore, we
believe that CD24 is the best target of an organotropic drug
delivery system in advanced ovarian cancer and, thus, developed a
CD24-targeted liposomal CDDP incorporating micells. In the present
study, CDDP as the classical chemotherapeutic drug was selected to
be coupled with an anti-CD24 antibody on GLYCOLIPO, and
CD24-GL-CDDP-Cy5.5 showed a targeting potency against CD24-positive
cells in vitro. Based on this, we investigated the
effectiveness of CD24-GL-CDDP-Cy5.5 using a xenograft mouse model
with resistance to CDDP. Studies of tumor inhibition are often done
over a short time interval, which is insufficient to study tumor
invasion in vivo. To avoid this limitation, we investigated
the effect of drug treatments for over a month post treatment. The
intravenous injection of CD24-GL-CDDP-Cy5.5 treatment effectively
decreased tumor growth after treatment. In this study, nude mice
received only three doses of CD24-GL-CDDP-Cy5.5, and it is possible
that additional doses could have further limited tumor growth.
CD24-positive cells have CDDP resistance, but a
certain effect can be obtained with a higher concentration of CDDP
in vitro (19). In this
study, we showed the ability of GL-CDDP-Cy5.5 and
CD24-GL-CDDP-Cy5.5 to promote significantly higher Pt
concentrations in disseminated tumors than free CDDP through
intra-vein injection after 48 h. It is well known that
long-circulating carriers, such as GLYCOLIPO, are able to increase
drug accumulation in tumors due to the enhanced permeability and
retention effect (30). In the
disseminated tumors, there was no significant difference between
GL-CDDP-Cy5.5 and CD24-GL-CDDP-Cy5.5 in terms of Pt concentration.
This result is presumed to be due to the coexistence of
CD24-positive cells and CD24-negative cells in disseminated tumors.
Flow cytometry showed that CD24-GL-CDDP-Cy5.5 increased the
accumulation of Cy5 dye in CD24-positive cells specifically. It was
suggested that CD24-GL-CDDP-Cy5.5 was uptaken with a higher
concentration of CDDP in CD24-positive cells than was
GL-CDDP-Cy5.5. Immunohistochemical evaluation of dissemination
tumors demonstrated that CD24-GL-CDDP-Cy5.5 reduced the expression
of CD24 more than PBS, CDDP and GL-CDDP-Cy5.5. It was also
suggested that CD24-GL-CDDP-Cy5.5 had a higher CDDP concentration
in CD24-positive cells and reduced the number of the cells compared
with the other groups. In this study, we demonstrated that the Pt
concentration in dissemination tumors treated with
CD24-GL-CDDP-Cy5.5 maintained a higher Pt concentration than that
in the CDDP group, and that it was significantly different after 48
h (36.5±6.5 µg/mg vs. 13.2±4.3 µg/mg, P<0.05). In contrast, the
Pt concentration in the kidney immediately lower in the
GL-CDDP-Cy5.5 and CD24-GL-CDDP-Cy5.5 groups than in CDDP group
(43.7±8.3 µg/mg and 46.5±10.1 µg/mg vs. 114.4±36.1 µg/mg,
P<0.05). As the nephrotoxicity of CDDP is considered to depend
on the peak urinary Pt concentration (31), we demonstrated that
CD24-GL-CDDP-Cy5.5 has not only the potential for maintaining a
higher Pt concentration in dissemination tumors but also in
reducing nephrotoxicity. In the other words, CD24-GL-CDDP-Cy5.5 has
the ability for a safer administration in ovarian cancer patients.
However, further pre-clinical studies using other animal models are
necessary.
Snail is a transcriptional repressor of E-cadherin
during the EMT phenomenon (32).
Snail expression in peritoneal dissemination is associated with an
unfavorable prognosis in ovarian cancer (33). CD24-GL-CDDP-Cy5.5 reduced the
expression of Snail and enhanced the expression of E-cadherin. In
previous a study, we suggested that CD24 is a key molecule of
metastatic progression in the EMT phenomenon (19). This study also showed that
CD24-GL-CDDP-Cy5.5 can suppress the EMT phenomenon by decreasing
CD24 expression. Due to this suppression of the EMT phenomenon, the
decrease in MAC in intraperitoneal xenograft models indicates that
the anti-cancer efficacy of CD24-GL-CDDP-Cy5.5 is better in
comparison with PBS, CDDP and GL-CDDP-Cy5.5. In addition,
CD24-GL-CDDP-Cy5.5 significantly prolonged the survival rate of
Caov-3 bearing mice, in comparison with PBS, CDDP and
GL-CDDP-Cy5.5. Thus, our data suggests that CD24-GL-CDDP-Cy5.5
contributes to the suppression of the EMT phenomenon in
intraperitoneal xenograft models transplanted with the Caov-3 cell
line.
In conclusion, the present results indicat that the
CD24-GL-CDDP-Cy5.5 we generated is a selective targeted to
CD24-positive ovarian carcinoma cells. Moreover, CD24-GL-CDDP-Cy5.5
can suppress the EMT phenomenon. Taken together, this study shows
that CD24-GL-CDDP-Cy5.5 is effective for aggressive ovarian cancer
that has acquired a resistant to CDDP, although there are required
that the future studies should examine the effect of CD24 targeted
liposomes in models with different levels of CD24 expression, and
that side effects of treatment with CD24 targeted liposomes must be
assessed in future studies. We consider that our strategy of
targeting CD24 is promising for clinical applications.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by a Japan Society
for the Promotion of Science KAKENHI grant (grant no. 16K11162 to
YTe).
Availability of data and materials
Not appicable.
Authors' contributions
KA, YTe and MO were involved in study conception and
design, data analysis, drafting the article and its final edition.
KA, TT, YTa, SF, KM, ST, HS and MH performed sample collection and
performed all in vivo experiments. KA, TT, KM and MH
analyzed data. KA, YTe and MO wrote the manuscript and checked all
data. All authors gave their final approval of the submitted
version.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Osaka Medical College.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EMT
|
epithelial-mesenchymal-transition
|
|
CDDP
|
cisplatin
|
|
GPI
|
glycosylphosphatidylinositol
|
|
DDS
|
drug delivery system
|
|
RES
|
reticuloendothelial system
|
|
HAS
|
human serum albumin
|
|
STR
|
short tandem repeat
|
|
Pt
|
platinum
|
|
MAC
|
maximum abdominal circumference
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jelovac D and Armstrong DK: Recent
progress in the diagnosis and treatment of ovarian cancer. CA
Cancer J Clin. 61:183–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takai M, Terai Y, Kawaguchi H, Ashihara K,
Fujiwara S, Tanaka T, Tsunetoh S, Tanaka Y, Sasaki H, Kanemura M,
et al: The EMT (epithelial-mesenchymal-transition)-related protein
expression indicates the metastatic status and prognosis in
patients with ovarian cancer. J Ovarian Res. 7:762014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Z, Yun R, Yu X, Hu H, Huang G, Tan B
and Chen T: Overexpression of Notch3 and pS6 is associated with
poor prognosis in human ovarian epithelial cancer. Mediators
Inflamm. 2016:59534982016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Loehrer PJ and Einhorn LH: Cisplatin. Ann
Intern Med. 100:704–713. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kartalou M and Essigmann JM: Mechanisms of
resistance to cisplatin. Mutat Res. 478:23–43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Uchino H, Matsumura Y, Negishi T, Koizumi
F, Hayashi T, Honda T, Nishiyama N, Kataoka K, Naito S and Kakizoe
T: Cisplatin-incorporating polymeric micelles (NC-6004) can reduce
nephrotoxicity and neurotoxicity of cisplatin in rats. Br J Cancer.
93:678–687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu L, Schwartz P, Scarampi L, Rutherford
T, Canuto EM, Yu H and Katsaros D: MicroRNA let-7a: A potential
marker for selection of paclitaxel in ovarian cancer management.
Gynecol Oncol. 122:366–371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cancer Genome Atlas Research Network, .
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Patch AM, Christie EL, Etemadmoghadam D,
Garsed DW, George J, Fereday S, Nones K, Cowin P, Alsop K, Bailey
PJ, et al: Whole-genome characterization of chemoresistant ovarian
cancer. Nature. 521:489–494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lalwani N, Prasad SR, Vikram R, Shanbhogue
AK, Huettner PC and Fasih N: Histologic, molecular, and cytogenetic
features of ovarian cancers: Implications for diagnosis and
treatment. Radiographics. 31:625–646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hwang SY, Cho DY, Kim HK, Cho SH, Choo J,
Yoon WJ and Lee EK: Preparation of targeting proteoliposome by
postinsertion of a linker molecule conjugated with recombinant
human epidermal growth factor. Bioconjug Chem. 21:345–351. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Phillips TM, McBride WH and Pajonk F: The
response of CD24(−/low)/CD44+ breast cancer-initiating cells to
radiation. J Natl Cancer I. 98:1777–1785. 2006. View Article : Google Scholar
|
|
15
|
Kristiansen G, Denkert C, Schlüns K, Dahl
E, Pilarsky C and Hauptmann S: CD24 is expressed in ovarian cancer
and is a new independent prognostic marker of patient survival. Am
J Pathol. 161:1215–1221. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kristiansen G, Winzer KJ, Mayordomo E,
Bellach J, Schlüns K, Denkert C, Dahl E, Pilarsky C, Altevogt P and
Guski H: CD24 expression is a new prognostic marker in breast
cancer. Clin Cancer Res. 9:4906–4913. 2003.PubMed/NCBI
|
|
17
|
Zhu J, Zhang G and Lu H: CD24, COX-2, and
p53 in epithelial ovarian cancer and its clinical significance.
Front Biosci (Elite Ed). 4:2645–2651. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee TK, Castilho A, Cheung VC, Tang KH, Ma
S and Ng IO: CD24(+) liver tumor-initiating cells drive
self-renewal and tumor initiation through STAT3-mediated NANOG
regulation. Cell Stem Cell. 9:50–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamura K, Terai Y, Tanabe A, Ono YJ,
Hayashi M, Maeda K, Fujiwara S, Ashihara K, Nakamura M, Tanaka Y,
et al: CD24 expression is a marker for predicting clinical outcome
and regulates the epithelial-mesenchymal transition in ovarian
cancer via both the Akt and ERK pathways. Oncol Rep. 37:3189–3200.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshida M, Takimoto R, Murase K, Sato Y,
Hirakawa M, Tamura F, Sato T, Iyama S, Osuga T, Miyanishi K, et al:
Targeting anticancer drug delivery to pancreatic cancer cells using
a fucose-bound nanoparticle approach. PLoS One. 7:e395452012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hirai M, Minematsu H, Kondo N, Oie K,
Igarashi K and Yamazaki N: Accumulation of liposome with Sialyl
Lewis X to inflammation and tumor region: Application to in vivo
bio-imaging. Biochem Biophys Res Commun. 353:553–558. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hirai M, Hiramatsu Y, Iwashita S, Otani T,
Chen L, Li YG, Okada M, Oie K, Igarashi K, Wakita H and Seno M:
E-selectin targeting to visualize tumors in vivo. Contrast Media
Mol Imaging. 5:70–77. 2010.PubMed/NCBI
|
|
23
|
Pollmann D, Broekaert JA, Leis F, Tschopel
P and Tolg G: Determination of boron in biological tissues by
inductively-coupled plasma optical-emission spectrometry (ICP-OES).
Fresen J Anal Chem. 346:441–445. 1993. View Article : Google Scholar
|
|
24
|
Duffy M and Thomas R: Benefits of a
dual-view ICP-OES for the determination of boron, phosphorus, and
sulfur in low alloy steels return to document menu. Atom Spectrosc.
17:128–132. 1996.
|
|
25
|
Lubura M, Hesse D, Neumann N, Scherneck S,
Wiedmer P and Schürmann A: Non-invasive quantification of white and
brown adipose tissues and liver fat content by computed tomography
in mice. PLoS One. 7:e370262012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sano A, Kato H, Sakurai S, Sakai M, Tanaka
N, Inose T, Saito K, Sohda M, Nakajima M, Nakajima T and Kuwano H:
CD24 expression is a novel prognostic factor in esophageal squamous
cell carcinoma. Ann Surg Oncol. 16:506–514. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blechschmidt K, Sassen S, Schmalfeldt B,
Schuster T, Höfler H and Becker KF: The E-cadherin repressor Snail
is associated with lower overall survival of ovarian cancer
patients. Br J Cancer. 98:489–495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kawaguchi H, Terai Y, Tanabe A, Sasaki H,
Takai M, Fujiwara S, Ashihara K, Tanaka Y, Tanaka T, Tsunetoh S, et
al: Gemcitabine as a molecular targeting agent that blocks the Akt
cascade in platinum-resistant ovarian cancer. J Ovarian Res.
7:382014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Krantz SB, Shields MA, Dangi-Garimella S,
Munshi HG and Bentrem DJ: Contribution of epithelial-to-mesenchymal
transition and cancer stem cells to pancreatic cancer progression.
J Surg Res. 173:105–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peer D, Karp JM, Hong S, Farokhzad OC,
Margalit R and Langer R: Nanocarriers as an emerging platform for
cancer therapy. Nat Nanotechnol. 2:751–760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Levi FA, Hrushesky WJ, Halberg F, Langevin
TR, Haus E and Kennedy BJ: Lethal nephrotoxicity and hematologic
toxicity of cis-diammine-dichloroplatinum ameliorated by optimal
circadian timing and hydration. Eur J Cancer Clin Oncol.
18:471–477. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taki M, Abiko K, Baba T, Hamanishi J,
Yamaguchi K, Murakami R, Yamanoi K, Horikawa N, Hosoe Y, Nakamura
E, et al: Snail promotes ovarian cancer progression by recruiting
myeloid-derived suppressor cells via CXCR2 ligand upregulation. Nat
Commun. 9:16852018. View Article : Google Scholar : PubMed/NCBI
|