Introduction
Mesothelin (MSLN) is a glycophosphatidylinositol
(GPI)-linked cell surface protein that is usually expressed in
mesothelial cells. MSLN encodes a precursor protein of 71
kDa that is processed to a shed 31 kDa protein, megakaryocyte
potentiating factor (MPF), and 40-kDa membrane-bound MSLN protein
(1). Based on normal growth and
reproduction in a Msln-deficient mouse model, the biological
function of MSLN is not fully understood (2).
In neoplastic conditions, MSLN was reported to be
highly expressed in several types of malignant tumors, including
malignant pleural mesothelioma, ovarian cancer, pancreatic
adenocarcinoma, and gastric cancer (3–10).
Recently, our group observed significant expression of MSLN in
colorectal tumors, with up to 60% of cases demonstrating positivity
(11).
Prognostication using MSLN immunohistochemistry has
been reported in several types of tumors. In breast and lung
adenocarcinoma, high-level MSLN expression was reported to be
associated with poor prognosis (8,9,12). In contrast, prolonged survival in
patients with MSLN-expressing tumors was noted in ovarian serous
and thymic carcinomas as well as malignant pleural mesothelioma
(11,13,14). In
colorectal carcinomas, the clinicopathological profiles and
prognosis of MSLN-positive CRC patients have not been fully
studied. Furthermore, the biological significance of MSLN
expression in CRC development remains to be elucidated (15–17).
Anti-MSLN immunotherapies using anti-MSLN monoclonal
antibodies, antibody drug conjugates, and chimeric antigen
receptors (CAR) T cells have been developed and are believed to be
promising therapeutics for patients with MSLN-expressing tumors
(18). MSLN expression in
immunohistochemistry is envisaged to be a suitable biomarker for
predicting clinical response to these therapeutics, but its
efficacy has never been fully evaluated.
The aim of the present study was to evaluate the
clinicopathological, prognostic, and biological significance of
MSLN expression in colorectal cancer (CRC) to elucidate the
usefulness of MSLN immunohistochemistry for determining the
prognosis of CRC patients and to identify potential candidates for
MSLN immunotherapy.
Materials and methods
Tissue samples
The Institutional Ethical Review Board of Aichi
Medical University Hospital approved this project to perform
without collecting patient consent by giving them the opportunity
for opt out. Two hundred and seventy primary colorectal tumors,
resected at Aichi Medical University Hospital during 2009–2012,
were collected according to the availability of tissue samples and
clinical information. Fifty-two normal colonic mucosae samples
adjacent to tumors as well as 44 metastases from 27 patients were
also collected. After surgery, patients were followed up for up to
90 months. All the tumors were diagnosed to be invasive and naïve
to chemotherapy or radiotherapy. Tumors with glandular formation
(>50%) were defined as differentiated histology. Single 4.5-mm
core tumor tissue samples derived from surgical specimens were
assembled into tissue arrays containing up to 30 samples. The size
of tumor tissue samples was estimated to exceed the size of a
single 0.6 mm2 core by a factor of 8–9.
Antibodies
The antibodies used in this study are as follows:
MSLN (Rockland Immunochemicals Inc.), MLH1 (Clone G168-728; BD
Biosciences), MSH2 (Clone G219-1129; BD Biosciences) MSH6 (Clone
44; BD Biosciences) PMS2 (Clone A16-4; BD Biosciences) CCNA
(sc-751; Santa Cruz Biothechnology, Inc.), GAPDH (Clone 6C5; Santa
Cruz Biothechnology), ERK (Clone 137F5; Cell Signaling Technology,
Inc.), P-ERK (Clone 20G11; Cell Signaling Technology, Inc.).
Immunohistochemistry
Immunohistochemistry was performed using the Ventana
BenchMark XT automated immunostainer (Roche Diagnostics). The
conditions for immunohistochemistry are summarized in Table I. Signals were visualized by
3,3-diaminobenzidine (DAB) staining. MSLN immunoreactivity
(luminal/membranous) was evaluated with a detection cut-off of 5%
for any signal intensity, as described in our previous report
(11). Cyclin A (CCNA) labeling
indices were determined by counting more than 500 tumor cells per
case.
 | Table I.Antibodies and conditions for
immunohistochemistry and immunoblot analysis. |
Table I.
Antibodies and conditions for
immunohistochemistry and immunoblot analysis.
|
|
Immunohistochemistry | Immunoblot |
|
|---|
|
|
|
|
|
|---|
| Genes | Reagent | Dilution | Dilution | Antibodies |
|---|
| MSLN | OV | 2,000 | 20,000 | Rockland
Immunochemicals Inc. |
| MLH1 | OV | 200 | – | Clone G168-728, BD
Biosciences |
| MSH2 | OV | 200 | – | Clone G219-1129, BD
Biosciences |
| MSH6 | OV | 400 | – | Clone 44/MSH6, BD
Biosciences |
| PMS2 | OV+Linker | 50 | – | Clone A16-4, BD
Biosciences |
| CCNA | IV | 100 | 500 | sc-751, Santa Cruz
Biothechnology, Inc. |
| ERK | – | – | 1,000 | Clone 137F5, Cell
Signaling Technology, Inc. |
| P-ERK | – | – | 500 | Clone 20G11, Cell
Signaling Technology, Inc. |
| GAPDH | – | – | 3,000 | Clone 6C5, Santa
Cruz Biothechnology, Inc. |
Statistical analyses
All statistical analyses were performed with EZR
version 1.32. software (19).
Chi-squared or Student's t-test were performed to investigate the
statistical association. The Bonferroni-corrected P-value for
significance was P=0.0042 (0.05/12). The Spearman's rank
correlation coefficient was used to analyze positivity (% positive
cells) between primary tumors and their metastases. According to
previous reports (11,14), the impact of diffuse (100% positive
cells on the lumen/cell membrane) MSLN expression on overall
survival was analyzed using Kaplan-Meier survival estimates with
log-rank tests. Cox proportional hazards regression analysis was
used to analyze the association of MSLN with survival and other
factors. The initial model included age (<70 years vs. ≥70
years), sex (male vs. female), primary tumor location (right-sided
colon vs. left-sided colon vs. rectum), tumor size (<5 cm vs. ≥5
cm), T stage (2 vs. 3 vs. 4), surgical status (complete resection
vs. residual tumor), tumor histology (well to moderately vs. poorly
differentiated), lymph node metastasis (positive vs. negative),
distant organ metastasis (positive vs. negative), peritoneal
metastasis (positive vs. negative), mismatch repair (MMR) system
status (deficient vs. preserved), and data from MSLN
immunohistochemistry (diffuse MSLN expression: 100% positive cells
on the lumen/cell membrane vs. negative or partial expression in
any location). Backward elimination with a threshold of P=0.05 was
used to select variables in the final model. The Mann-Whitney U or
Kruskal-Wallis with post-hoc test (Dunnett's test) was used for the
statistical analyses in molecular experiments.
In vitro molecular experiments
The origins of other colon cancer cell lines were
described previously (20). The
human colon cancer cell lines, COLO205, CW-2, HCT116 and LoVo were
obtained from the RIKEN BioResource Center. SW480 and Caco2 were
from American Type Culture Collection (ATCC). The human colon
cancer cell line SW48 was kindly provided by Dr. Yutaka Kondo
(Nagoya University, Aichi, Japan). Cells were maintained in
Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10%
fetal bovine serum (FBS). For β-estradiol stimulation experiments,
activated charcoal-treated FBS was used. β-estradiol stimulation
was performed for 24 h at a concentration of 10 µM, as defined in
our previous studies (21–23).
CW-2 and HCT-116 cell lines expressing exogenous
MSLN or its control LacZ were established by stable transfection
with pcDNA 3.1 vectors (Invitrogen; Thermo Fisher Scientific, Inc.)
containing MSLN or LacZ followed by IRES2 and
puromycin resistance genes. The 21-nucleotide duplex siRNAs were
synthesized as follows: siMSLN−1,
5′-CCCGUUUCUUCUCCCGCAUTT-3′ and 5′-AUGCGGGAGAAGAAACGGGTT-3′;
siMSLN−2, 5′-GCCUCAUCUUCUACAAGAATT-3′ and
5′-UUCUUGUAGAAGAUGAGGCTT-3′; siControl, 5′-GACGUAUGACUAACUAACATT-3′
and 5′-UGUUAGUUAGUCAUACGUCTT-3′ (Nippon Gene Material Co., Ltd.).
Transient transfection of siRNAs was performed using Lipofectamine
RNAiMAX (Thermo Fisher Scientific, Inc.).
Immunoblot analyses were performed as previously
reported (21–23). In short, whole cell lysates were
prepared using 1× Sodium Dodecyl Sulfate (SDS) sample buffer,
containing 50 mM Tris-HCl and 2% SDS. The SDS polyacrylamide gel
electrophoresis was performed using 12% polyacrylamide gel and
separated proteins were transferred to a PVDF membrane. Antibody
dilutions are summarized in Table I.
Each immunoblot panel was made from one membrane. For sequential
detection, antibody stripping buffer (0.1 M Glycine-HCl pH 2.5) was
used. Signal intensity was measured by ImageJ software (National
Institutes of Health).
Cellular proliferation activity was measured using
CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega
Corporation) in accordance with the manufacturer's instructions.
Anchorage-independent cell proliferation in soft agar was measured
using a Cytoselect 96-well cell transformation assay kit (Cell
Biolabs Inc.) according to the manufacturer's protocol.
Results
MSLN expression in 52 normal colonic
mucosae and 270 primary colorectal tumors
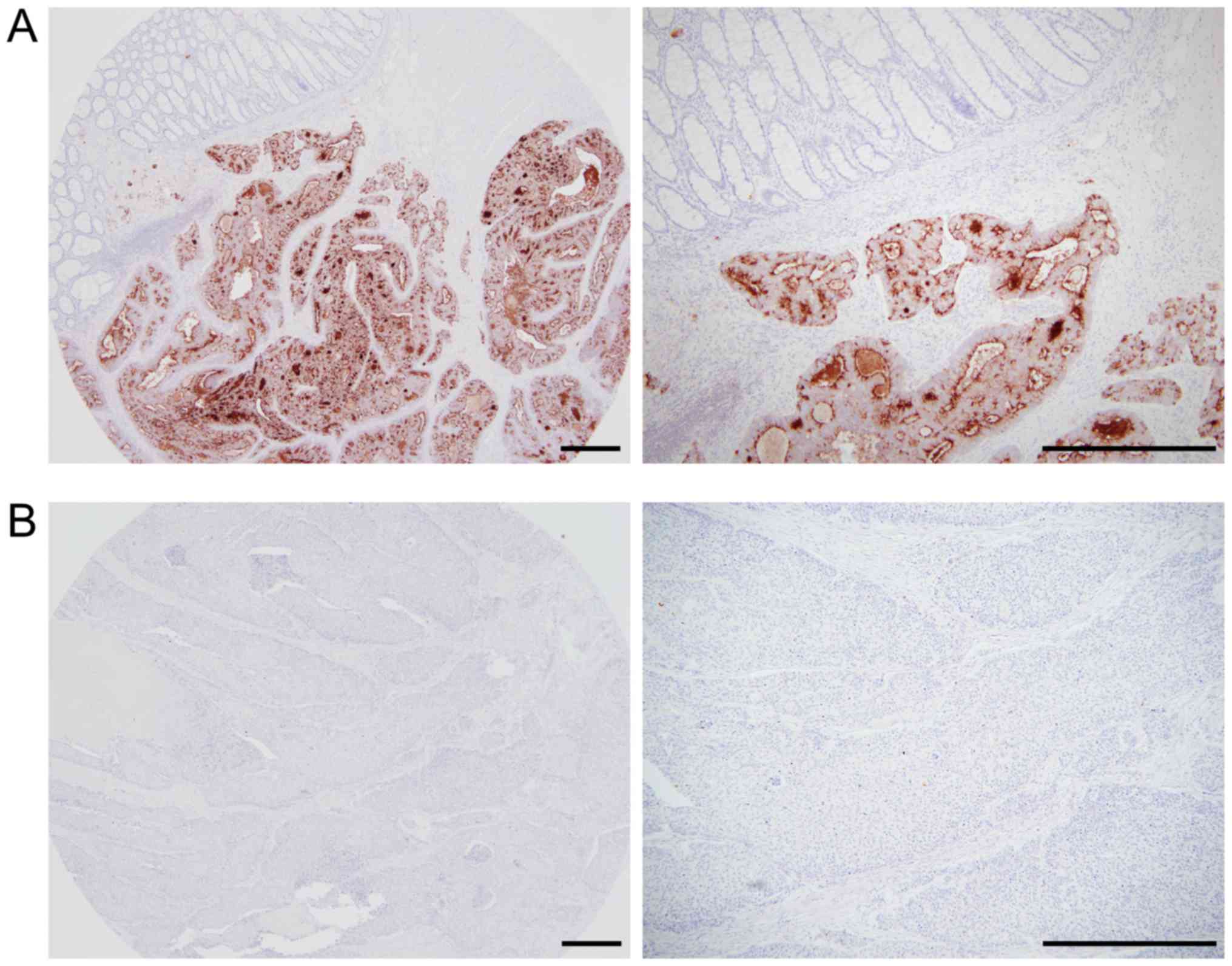
None of the normal colonic mucosae evaluated in the
present study expressed MSLN. Representative images for MSLN
immunohistochemistry are shown in Fig.
1. The results of MSLN immunohistochemistry in primary
colorectal tumors are summarized in Table II. MSLN expression was detected in
53% (142/270) of CRC cases. Significantly higher MSLN-positivity
was observed in tumors from female patients (P=0.0042).
 | Table II.Characteristics of colorectal
carcinomas with or without MSLN expression. |
Table II.
Characteristics of colorectal
carcinomas with or without MSLN expression.
|
|
| MSLN expression, n
(%) |
|
|---|
|
|
|
|
|
|---|
| Variables | Total, n (%)
(n=270) | Positive (n=142;
53%) | Negative (n=128;
47%) | P-value |
|---|
| Sex |
|
|
| 0.0042a |
|
Male | 143 (53) | 63 (45) | 80 (63) |
|
|
Female | 127 (47) | 79 (56) | 48 (38) |
|
| Age, years (mean ±
SD) | 68.6±12.6 | 67.4±14.3 | 69.9±10.3 | 0.093b |
| Size, cm (mean ±
SD) | 4.99±2.6 | 4.9±2.3 | 5.1±2.8 | 0.52b |
| Tumor location |
|
|
| 0.77a |
|
Right-sided colon | 125 (46) | 64 (45) | 61 (48) |
|
|
Left-sided colon | 86 (32) | 45 (32) | 41 (32) |
|
|
Rectum | 58 (22) | 33 (23) | 25 (20) |
|
| T stage |
|
|
| 0.14a |
| T2 | 37 (14) | 14 (10) | 23 (18) |
|
| T3 | 189 (70) | 105 (74) | 84 (66) |
|
| T4 | 44 (17) | 23 (16) | 21 (16) |
|
| Histological
differentiation |
|
|
| 0.94a |
| Well to
moderate | 242 (90) | 128 (90) | 114 (89) |
|
|
Poor | 28 (10) | 14 (10) | 14 (11) |
|
| Solid/sheet-like
proliferation |
|
|
| 0.014a |
|
Positive | 13 (5) | 2 (1) | 11 (9) |
|
|
Negative | 257 (95) | 140 (99) | 117 (91) |
|
| Lymph node
metastasis |
|
|
| 0.64a |
|
Positive | 124 (49) | 70 (52) | 54 (45) |
|
|
Negative | 130 (51) | 64 (48) | 66 (55) |
|
| Omental
metastasis |
|
|
| 0.49a |
|
Positive | 50 (19) | 29 (20) | 21 (16) |
|
|
Negative | 220 (81) | 113 (80) | 107 (84) |
|
| Distant organ
metastasis |
|
|
| 0.65a |
|
Positive | 44 | 25 | 19 |
|
|
Negative | 226 | 117 | 109 |
|
| Operation
status |
|
|
| 0.80a |
|
Complete resection | 238 (88) | 124 (87) | 114 (89) |
|
|
Residual disease | 32 (12) | 18 (13) | 14 (11) |
|
| MMR system
status |
|
|
| 0.066a |
|
Deficient | 31 (11) | 11 (8) | 20 (16) |
|
|
Preserved | 239 (89) | 131 (92) | 108 (84) |
|
Tumors presenting solid/sheet-like proliferation
tended to show a lower rate of MSLN expression than those with
tubular structures (P=0.014).
In the present study, 11% (31/270) showed
MMR-deficient phenotypes. Similar to our previous report (11), however, no significant correlation
was detected between MSLN expression and MMR system status.
MSLN expression in 44 CRC
metastases
The results of MSLN immunohistochemistry in
metastatic tumors are summarized in Table III. Among the metastases analyzed,
lymph node (39%) and liver (30%) metastases were dominant.
Metastases in the liver and peritoneum tended to exhibit higher
levels of MSLN expression than those in lymph nodes and other
organs.
 | Table III.MSLN expression in metastatic
lesions. |
Table III.
MSLN expression in metastatic
lesions.
|
|
| MSLN
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Metastatic
site | Total, n (%)
(n=44;) | Positive, n (%)
(n=30; 68%) | Negative, n (%)
(n=14; 32%) | Percentage of
positive cells, median (range) | P-value |
|---|
| Liver | 13 (30) | 8 (27) | 5 (36) | 75 (20–80) | 0.15 |
| Peritoneum | 7 (16) | 7 (53) | 0 (0) | 60 (5–100) |
|
| Lymph nodes | 17 (39) | 12 (40) | 5 (36) | 25 (5–100) |
|
| Othersa | 7 (16) | 3 (10) | 4 (29) | 10 (5–20) |
|
A weakly positive correlation was detected in MSLN
positivity (% positive cells) between primary sites and their
metastases (R=0.484, P<0.0001; Fig. S1).
Survival analysis of CRC patients
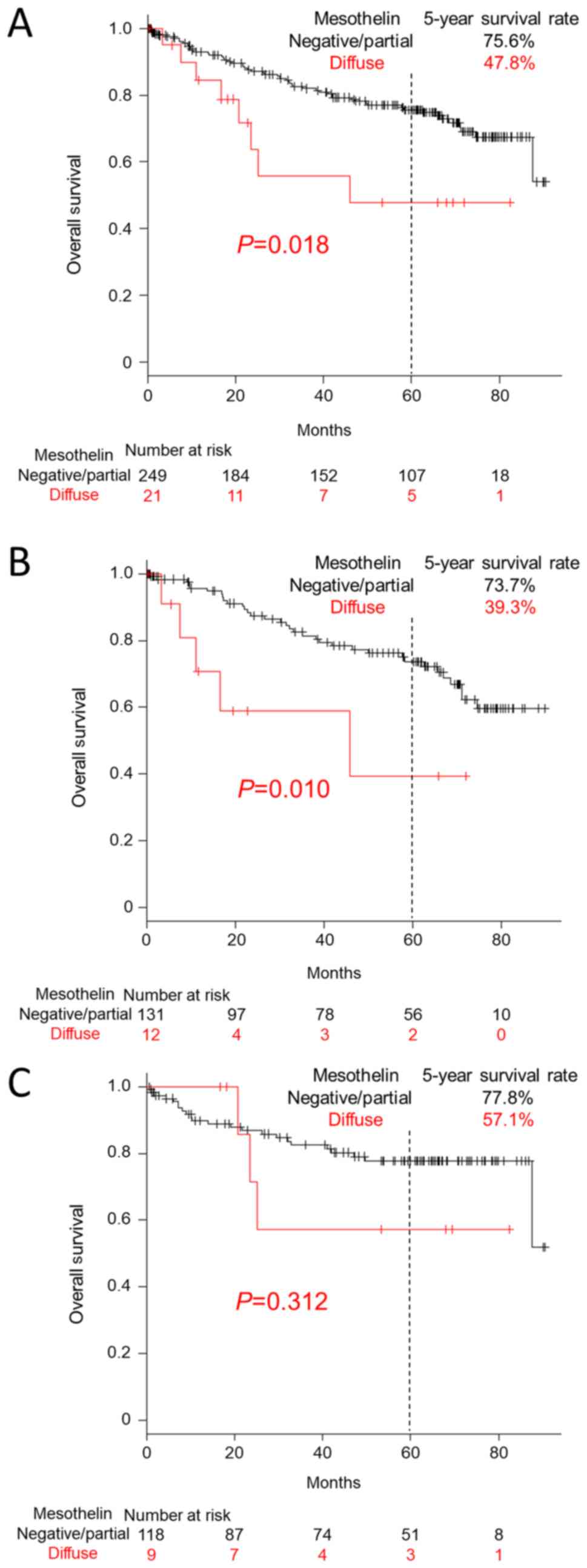
Survival was significantly shorter for patients with
diffuse expression of MSLN (100% positive cells) on the lumen/cell
membrane (47.8% vs. 75.6% in 5-year survival; P=0.018; Fig. 2A). Male CRC patients with diffuse
MSLN expression (P=0.010) but not female patients (P=0.312) showed
a significantly worse clinical outcome (Fig. 2B and C). Multivariate Cox hazards
regression analysis revealed younger age (<74 years) to be a
favorable prognostic factor (hazard ratio (HR), 0.44; 95%
confidence interval (CI), 0.25–0.76; P=0.0033). Poorly
differentiated histology (HR, 4.27; 95% CI, 2.30–7.92;
P<0.0001), peritoneal metastasis (HR, 2.34; 95% CI, 1.26–4.33;
P<0.0001), diffuse MSLN expression (HR, 2.26; 95% CI, 1.04–4.91;
P=0.039), and lymph node metastasis (HR, 2.21; 95% CI, 1.28–3.38;
P=0.0046) were identified as potential independent risk factors
(Table IV).
 | Table IV.Multivariate Cox hazards analysis of
patients with CRC. |
Table IV.
Multivariate Cox hazards analysis of
patients with CRC.
|
|
| 95% CI |
|
|---|
|
|
|
|
|
|---|
| Variables | Hazard ratio | Min | Max | P-value |
|---|
| Age (<74) | 0.44 | 0.25 | 0.76 | 0.0033 |
| Poorly
differentiated histology | 4.27 | 2.30 | 7.92 | <0.0001 |
| Peritoneal
metastasis | 2.34 | 1.26 | 4.33 | <0.0001 |
| Diffuse MSLN
expression | 2.26 | 1.04 | 4.91 | 0.039 |
| Lymph node
metastasis | 2.21 | 1.28 | 3.38 | 0.0046 |
MSLN expression in colon cancer cell
lines
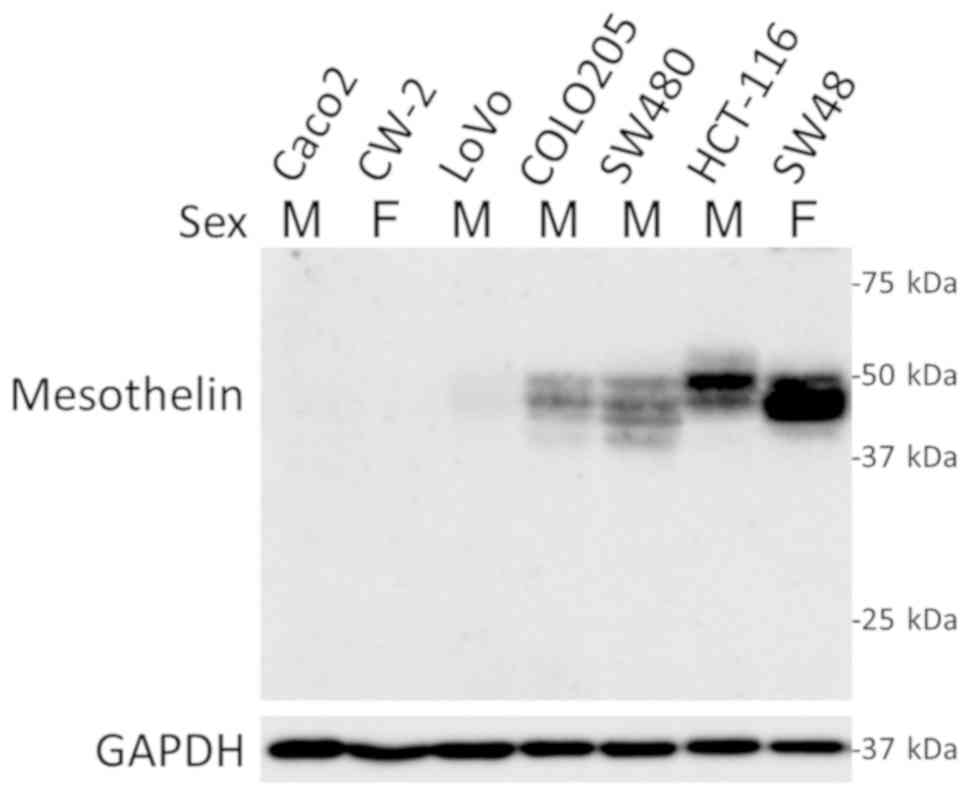
In cultured colon cancer cell lines, four out of
seven cell lines (57%) expressed MSLN with no association with sex
(Fig. 3). Further in vitro
studies showed no effect of β-estradiol, a major female sex
hormone, on MSLN expression in CW-2 and SW48 cells, both of which
were established from female patients (Fig. S2).
MSLN regulates colon cancer cell
proliferation but not migration and invasion
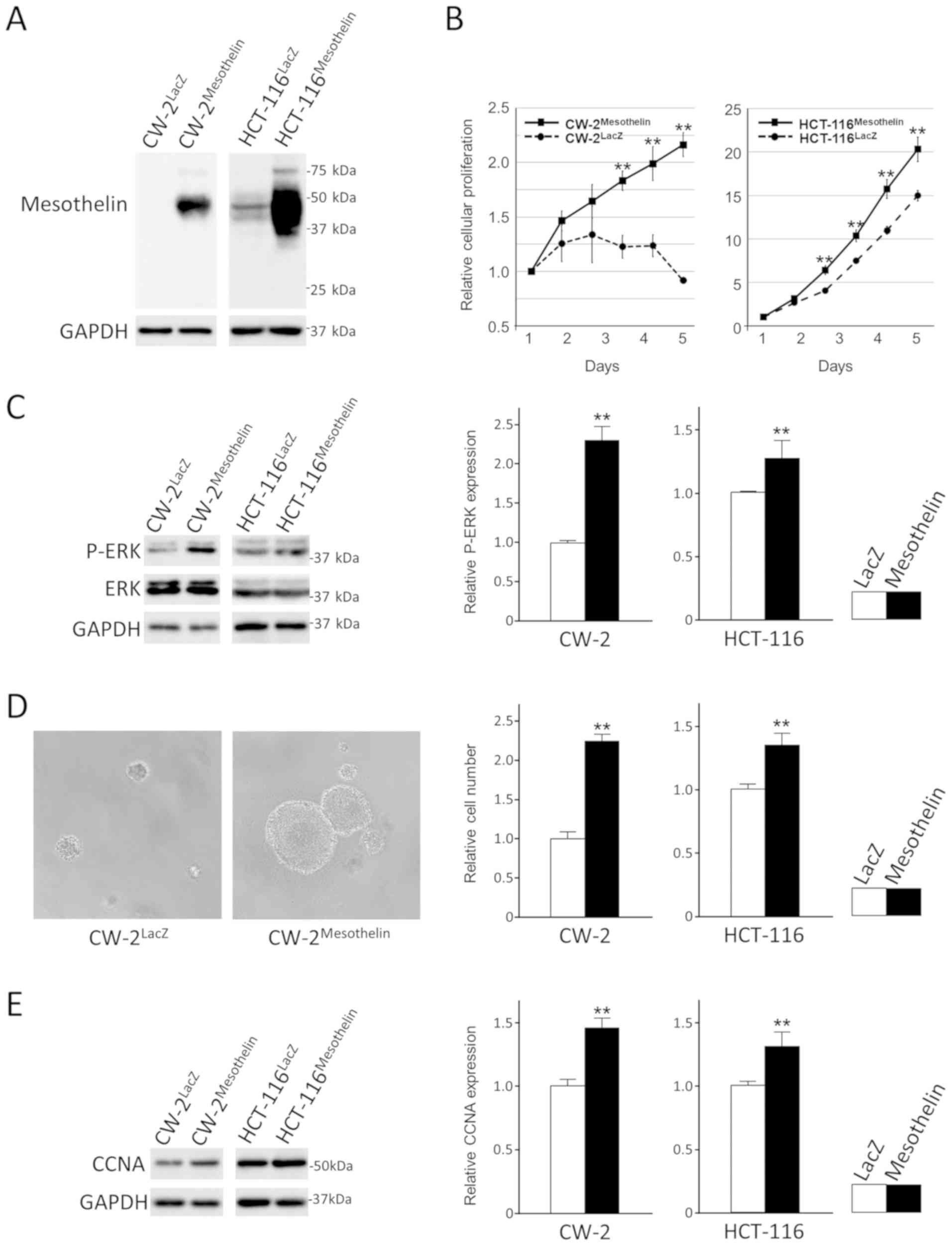
Additional experiments were performed to examine the
effects of MSLN expression in colon cancer cells. Forced expression
of MSLN in CW-2 and HCT-116 cells enhanced their proliferation or
survival significantly with phospho-ERK accumulation under
serum-reduced conditions (Fig.
4A-C). MSLN also enhanced anchorage-independent cell
proliferation of colon cancer cells (Fig. 4D). CCNA, one of markers for S phase,
were upregulated in MSLN-transfected cells (Fig. 4E). Furthermore, transient knock down
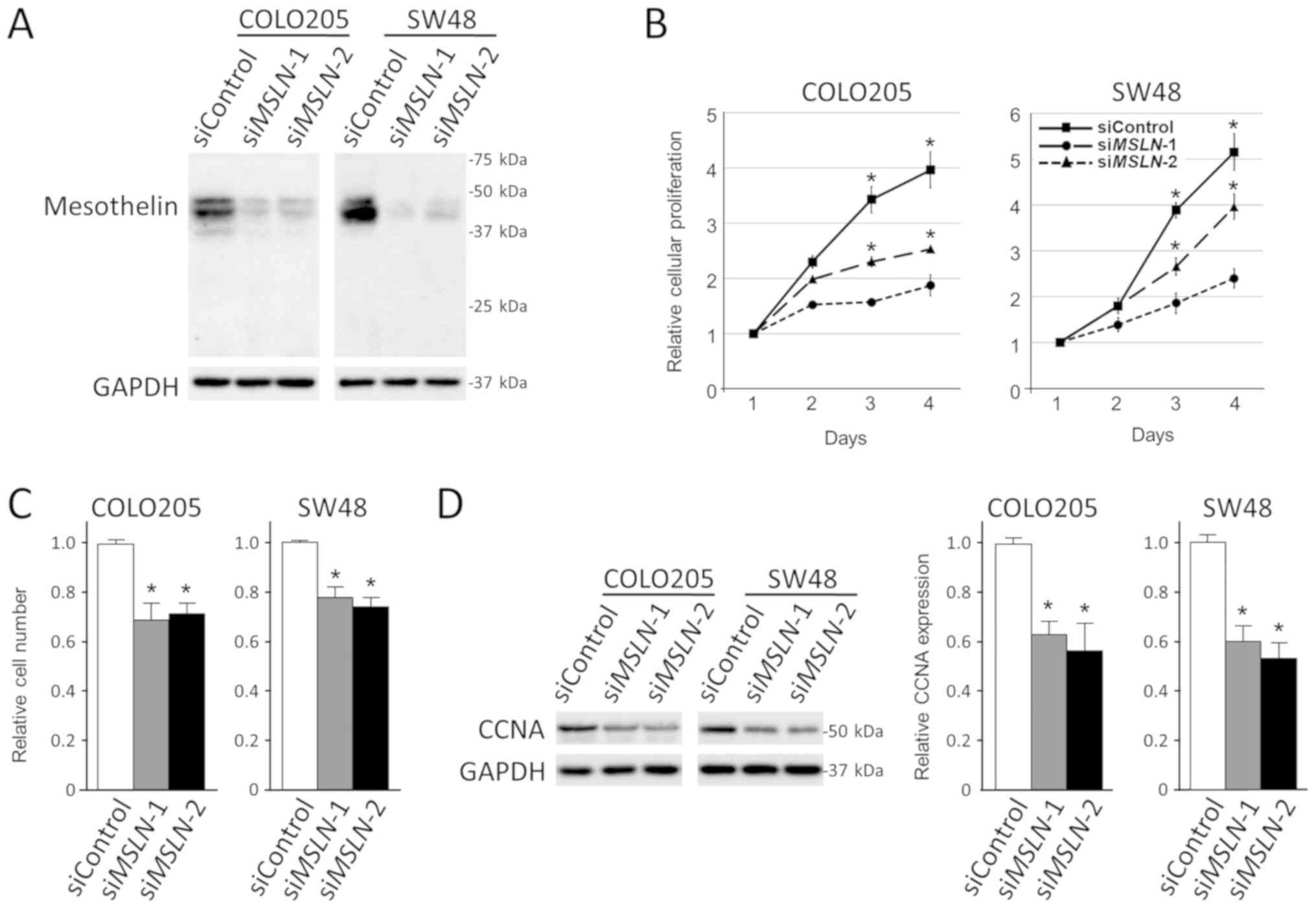
of MSLN significantly suppressed the proliferation of
COLO205 and SW48 cells in both adherent and anchorage-independent
conditions with downregulation of CCNA (Fig. 5). In contrast, MSLN did not alter the
migration and invasion of colon cancer cells under our experimental
conditions (Fig. S3). Based on
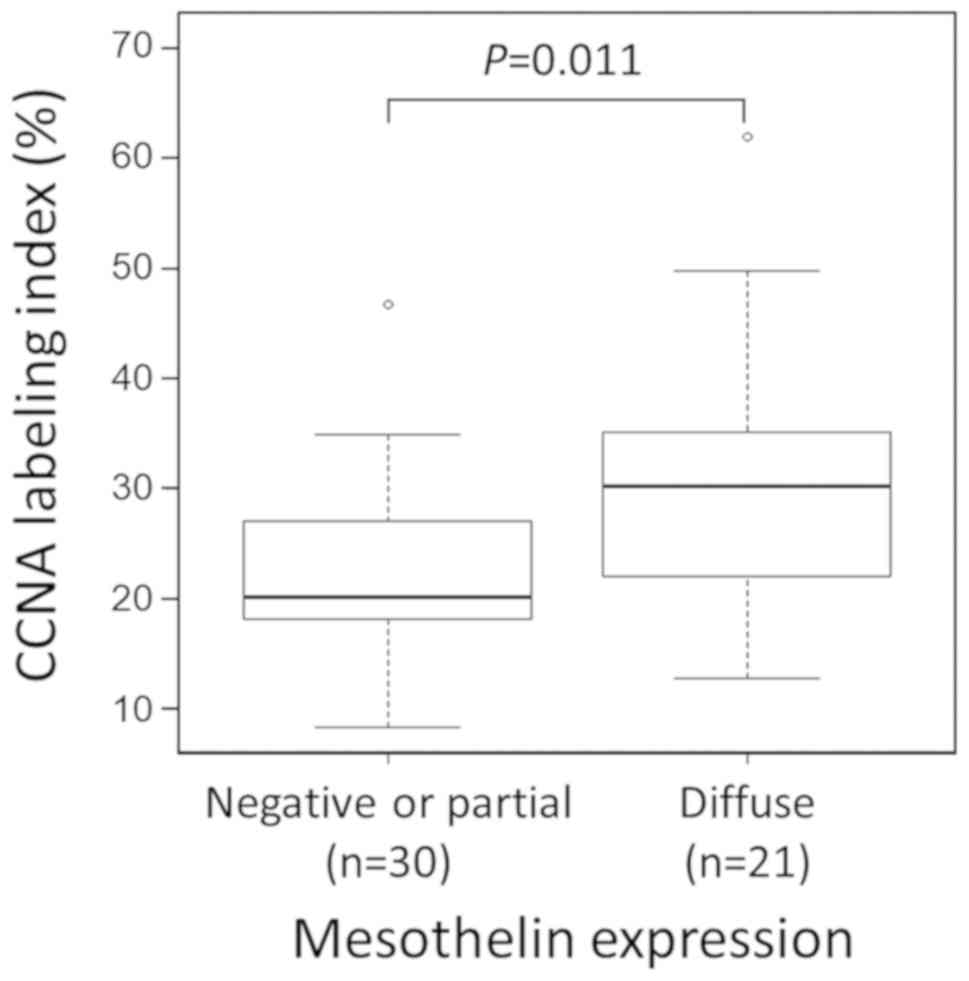
these observations, the proliferative activity of 21 diffusely
MSLN-expressing CRC cases and 30 arbitrary selected control cases
with negative or partial MSLN expression were compared by analyzing
CCNA labeling indices. Significantly higher rates of CCNA labeling
indices were observed in CRC with diffuse MSLN expression (P=0.011;
Fig. 6).
Discussion
MSLN is a cell surface protein that is highly
expressed in several types of malignant tumors and is associated
with clinical outcome. Recently, our research group identified a
significant number of MSLN-positive CRC cases in a comprehensive
immunohistochemical study using MN-1 monoclonal antibody (11). In the present study, the
clinicopathological profile and survival impact of MSLN were
analyzed by immunohistochemistry in 270 CRC cases. Furthermore,
molecular studies were performed to reveal the tumor biological
significance of MSLN in colon cancer cell lines.
In the present study, 53% (142/270 cases) of CRCs
were positive for luminal/membranous MSLN expression. This
positivity was slightly lower than that identified in our previous
study (61%, 115/188 cases) (11).
This might be due to the different autoimmunostaining systems used
(Ventana BenchMark XT and OptiView DAB universal kit vs. Leica
Bond-Max automation and Leica Refine detection kit). It is unclear
whether ethnicity (Japanese vs. Western populations) has an impact
on MSLN expression. From univariate analyses, a significant
correlation between MSLN expression and female sex (P=0.0042) was
identified (Table II). Overall
survival was significantly decreased in the cohort of patients with
diffuse MSLN expression (Fig. 2).
Furthermore, the multivariate Cox hazards regression analysis
identified diffuse MSLN expression (P=0.039) as a potential
independent risk factor (Table
IV).
Previous studies analyzed the impact of diffuse
(100% positive cells on the lumen/cell membrane) MSLN expression on
overall survival (11,14). The present study analyzed the impact
of diffuse MSLN expression on the survival of stage II to IV CRC
patients and determined its expression as a potential independent
risk factor (HR, 2.26; 95% CI, 1.04–4.91; P=0.039). Several studies
have analyzed the impact of tumor MSLN expression on the survival
of CRC patients (15–17). Shiraishi et al (15) found an adverse impact of MSLN
expression on the survival of stage II/III CRC patients with
statistical significance; however, Kawamata et al (16), found no significant difference in
stage I to IV patients. Both studies performed MSLN
immunohistochemistry using 5B2 anti-MSLN antibody. In contrast, the
present study used MN-1 antibody, which has a higher affinity and
positivity (% positive cells in immunohistochemistry) than 5B2
(11,24). Kim et al reported the
prognostic role of MSLN in microsatellite unstable CRCs using SP74
antibody (17); however, this type
of association was not confirmed in our cohort (data not shown).
The discrepancies in these studies might result from the patient
cohort (patient number and pathological stage) as well as the
anti-MSLN antibody used.
Prognosis prediction using MSLN immunohistochemistry
has also been reported in other tumor types. In cases of breast and
lung adenocarcinoma, aberrant high MSLN expression is reported to
be associated with poor prognosis (8,9,12). In contrast, prolonged survival in
patients with MSLN-expressing tumors was shown in ovarian serous
and thymic carcinomas (13,14). Our group also reported diffuse (100%
positive cells) MSLN expression as a favorable prognostic factor in
malignant pleural mesothelioma patients (11). Recently, prognostication by
comprehensive molecular profiling of malignant pleural mesothelioma
identified a poor prognosis cluster with an epithelial-mesenchymal
transition phenotype distinguished by high mRNA expression of
VIM, PECAM1, and TGFB1, and low miR-200 family
expression. Interestingly, these tumors also showed low MSLN
mRNA expression with MSLN promoter methylation (25). These results indicate that high MSLN
expression might be a favorable prognostic marker without tumor
biological significance in some tumor types including malignant
pleural mesothelioma. In the present study, we performed further
experiments to reveal the malignant potential of MSLN using colon
cancer cells and found that enhanced cellular proliferation was a
potential mechanism for the worse prognosis of MSLN-positive CRC
patients.
It has been reported that MSLN has pivotal roles in
tumor cell proliferation, invasion, and chemotherapy resistance
through the activation of oncogenic signaling such as PI3K/AKT and
ERK (26–28). However, the details of these
signaling events have not been fully identified. Our study
demonstrated the MSLN-dependent cellular proliferation of colon
cancer cells (Figs. 4 and 5). In our experimental conditions,
ectopically expressed MSLN enhanced the proliferation or survival
of colon cancer cells with an accumulation of phosphorylated-ERK
alone in serum-reduced conditions (Fig.
4B and C). This might be due to the enhanced basal activation
of ERK signaling by the serum components.
The regulatory mechanisms of MSLN are not fully
understood. Like CD274 (PD-L1) and PDCD1LG2 (PD-L2) (20,29),
female-dominant tumor MSLN expression was identified. In cultured
colon cancer cells, however, no clear association was identified
between sex and MSLN expression (Fig.
3). This might be due to the small number of colon cancer cell
lines analyzed in this study. Further analysis using β-estradiol, a
major female sex hormone, failed to modulate MSLN levels even in
MCF-7 cells with ESR1 expression (Fig.
S2). In the present study, the weakly positive correlation
between primary tumors and metastases for MSLN expression was
found. This may indicate, in part, some preserved characteristics
of CRC cells before and after metastasis. Thus, the regulatory
mechanisms of MSLN should be elucidated in the future.
Many anti-MSLN therapies such as a high-affinity
chimeric monoclonal antibody (MORAb-009), recombinant immunotoxins
(SS1P, RG7787/LMB-100), anti-MSLN antibody drug conjugates
(anetumab ravtansine, DMOT4039A, BMS-986148), or adoptive T-cell
immunotherapy using MSLN-specific CARs in autologous T lymphocytes
are currently being investigated in phase I and II studies
targeting advanced solid tumors including pancreatic cancer and
malignant mesothelioma with high MSLN expression (18). In the present study, over half of the
CRC patients showed tumor-specific MSLN expression. This
observation suggests that MSLN might be a good diagnostic and
therapeutic target in CRC patients. Furthermore, based on the
weakly positive correlation between MSLN expression in primary
tumors and their metastases (Fig.
S1), not only primary CRC tumors but also metastases might be
targeted by anti-MSLN therapeutics.
In the present study, a significant impact of MSLN
immunohistochemistry on the prognostication of CRC patients was
demonstrated. Additional molecular studies indicated the importance
of enhanced cellular proliferation induced by MSLN for worse
patient prognosis. Thus, MSLN-positive CRC patients with metastatic
lesions might be good candidates for MSLN-targeting
therapeutics.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Kazuko Tanimizu
and Mr. Naoki Igari (Aichi Medical University) for their assistance
with immunohistochemical staining. The authors would also like to
acknowledge Dr Yutaka Kondo (Nagoya University) for providing the
SW48 colon cancer cell line.
Funding
The current study was supported by the Scientific
Research (classification, C) from Japan Society for the Promotion
of Science (grant. no. 17K08706).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ShI and IP conceived the current study. ShI designed
and supervised the study. SaI and ShI performed molecular
experiments, histological and statistical analyses, constructed the
figures and tables, and wrote the manuscript. MR, HidI, TT, AI, HM,
KeK and HirI performed the immunohistochemical staining. SaI, ME,
NO and KuK collected and analyzed the clinical data. IP, KuK, KeK
and HirI critically reviewed the manuscript. All authors have read
and gave final approval to the submitted version.
Ethics approval and consent to
participate
The Institutional Ethical Review Board of Aichi
Medical University Hospital approved that the present study could
be performed without collecting patient consent by giving patients
the opportunity to opt-out.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
|
GPI
|
glycophosphatidylinositol
|
|
MPF
|
megakaryocyte potentiating factor
|
|
CAR
|
chimeric antigen receptors
|
|
DAB
|
3,3-diaminobenzidine
|
|
SDS
|
sodium sodecyl sulfate
|
References
|
1
|
Chang K and Pastan I: Molecular cloning of
mesothelin, a differentiation antigen present on mesothelium,
mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA.
93:136–140. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bera TK and Pastan I: Mesothelin is not
required for normal mouse development or reproduction. Mol Cell
Biol. 20:2902–2906. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang K, Pai LH, Pass H, Pogrebniak HW,
Tsao MS, Pastan I and Willingham MC: Monoclonal antibody K1 reacts
with epithelial mesothelioma but not with lung adenocarcinoma. Am J
Surg Pathol. 16:259–268. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hassan R, Kreitman RJ, Pastan I and
Willingham MC: Localization of mesothelin in epithelial ovarian
cancer. Appl Immunohistochem Mol Morphol. 13:243–247. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hassan R, Laszik ZG, Lerner M, Raffeld M,
Postier R and Brackett D: Mesothelin is overexpressed in
pancreaticobiliary adenocarcinomas but not in normal pancreas and
chronic pancreatitis. Am J Clin Pathol. 124:838–845. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miettinen M and Sarlomo-Rikala M:
Expression of calretinin, thrombomodulin, keratin 5, and mesothelin
in lung carcinomas of different types: An immunohistochemical
analysis of 596 tumors in comparison with epithelioid mesotheliomas
of the pleura. Am J Surg Pathol. 27:150–158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ordonez NG: Application of mesothelin
immunostaining in tumor diagnosis. Am J Surg Pathol. 27:1418–1428.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kachala SS, Bograd AJ, Villena-Vargas J,
Suzuki K, Servais EL, Kadota K, Chou J, Sima CS, Vertes E, Rusch
VW, et al: Mesothelin overexpression is a marker of tumor
aggressiveness and is associated with reduced recurrence-free and
overall survival in early-stage lung adenocarcinoma. Clin Cancer
Res. 20:1020–1028. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thomas A, Chen Y, Steinberg SM, Luo J,
Pack S, Raffeld M, Abdullaev Z, Alewine C, Rajan A, Giaccone G, et
al: High mesothelin expression in advanced lung adenocarcinoma is
associated with KRAS mutations and a poor prognosis. Oncotarget.
6:11694–11703. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Einama T, Homma S, Kamachi H, Kawamata F,
Takahashi K, Takahashi N, Taniguchi M, Kamiyama T, Furukawa H,
Matsuno Y, et al: Luminal membrane expression of mesothelin is a
prominent poor prognostic factor for gastric cancer. Br J Cancer.
107:137–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Inaguma S, Wang Z, Lasota J, Onda M,
Czapiewski P, Langfort R, Rys J, Szpor J, Waloszczyk P, Okoń K, et
al: Comprehensive immunohistochemical study of mesothelin (MSLN)
using different monoclonal antibodies 5B2 and MN-1 in 1562 tumors
with evaluation of its prognostic value in malignant pleural
mesothelioma. Oncotarget. 8:26744–26754. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li YR, Xian RR, Ziober A, Conejo-Garcia J,
Perales-Puchalt A, June CH, Zhang PJ and Tchou J: Mesothelin
expression is associated with poor outcomes in breast cancer.
Breast Cancer Res Treat. 147:675–684. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thomas A, Chen Y, Berman A, Schrump DS,
Giaccone G, Pastan I, Venzon DJ, Liewehr DJ, Steinberg SM,
Miettinen M, et al: Expression of mesothelin in thymic carcinoma
and its potential therapeutic significance. Lung Cancer.
101:104–110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yen MJ, Hsu CY, Mao TL, Wu TC, Roden R,
Wang TL and Shih IeM: Diffuse mesothelin expression correlates with
prolonged patient survival in ovarian serous carcinoma. Clin Cancer
Res. 12:827–831. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawamata F, Homma S, Kamachi H, Einama T,
Kato Y, Tsuda M, Tanaka S, Maeda M, Kajino K, Hino O, et al:
C-ERC/mesothelin provokes lymphatic invasion of colorectal
adenocarcinoma. J Gastroenterol. 49:81–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shiraishi T, Shinto E, Mochizuki S, Tsuda
H, Kajiwara Y, Okamoto K, Einama T, Hase K and Ueno H: Mesothelin
expression has prognostic value in stage II/III colorectal cancer.
Virchows Arch. 474:297–307. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim H, Chung Y, Paik SS, Jang K and Shin
SJ: Mesothelin expression and its prognostic role according to
microsatellite instability status in colorectal adenocarcinoma.
Medicine (Baltimore). 98:e162072019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lv J and Li P: Mesothelin as a biomarker
for targeted therapy. Biomark Res. 7:182019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Inaguma S, Lasota J, Felisiak-Golabek A,
Kowalik A, Wang Z, Zieba S, Kalisz J, Ikeda H and Miettinen M:
Histopathological and genotypic characterization of metastatic
colorectal carcinoma with PD-L1 (CD274)-expression: Possible roles
of tumour micro environmental factors for CD274 expression. J
Pathol Clin Res. 3:268–278. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inaguma S, Ito H, Riku M, Ikeda H and
Kasai K: Addiction of pancreatic cancer cells to zinc-finger
transcription factor ZIC2. Oncotarget. 6:28257–28268. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inaguma S, Kasai K and Ikeda H: GLI1
facilitates the migration and invasion of pancreatic cancer cells
through MUC5AC-mediated attenuation of E-cadherin. Oncogene.
30:714–723. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Inaguma S, Riku M, Hashimoto M, Murakami
H, Saga S, Ikeda H and Kasai K: GLI1 interferes with the DNA
mismatch repair system in pancreatic cancer through
BHLHE41-mediated suppression of MLH1. Cancer Res. 73:7313–7323.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Onda M, Willingham M, Nagata S, Bera TK,
Beers R, Ho M, Hassan R, Kreitman RJ and Pastan I: New monoclonal
antibodies to mesothelin useful for immunohistochemistry,
fluorescence-activated cell sorting, Western blotting, and ELISA.
Clin Cancer Res. 11:5840–5846. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hmeljak J, Sanchez-Vega F, Hoadley KA,
Shih J, Stewart C, Heiman D, Tarpey P, Danilova L, Drill E, Gibb
EA, et al: Integrative molecular characterization of malignant
pleural mesothelioma. Cancer Discov. 8:1548–1565. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bharadwaj U, Marin-Muller C, Li M, Chen C
and Yao Q: Mesothelin overexpression promotes autocrine IL-6/sIL-6R
trans-signaling to stimulate pancreatic cancer cell proliferation.
Carcinogenesis. 32:1013–1024. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang MC, Chen CA, Hsieh CY, Lee CN, Su
YN, Hu YH and Cheng WF: Mesothelin inhibits paclitaxel-induced
apoptosis through the PI3K pathway. Biochem J. 424:449–458. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Servais EL, Colovos C, Rodriguez L, Bograd
AJ, Nitadori J, Sima C, Rusch VW, Sadelain M and Adusumilli PS:
Mesothelin overexpression promotes mesothelioma cell invasion and
MMP-9 secretion in an orthotopic mouse model and in epithelioid
pleural mesothelioma patients. Clin Cancer Res. 18:2478–2489. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Masugi Y, Nishihara R, Hamada T, Song M,
da Silva A, Kosumi K, Gu M, Shi Y, Li W, Liu L, et al: Tumor
PDCD1LG2 (PD-L2) Expression and the lymphocytic reaction to
colorectal cancer. Cancer Immunol Res. 5:1046–1055. 2017.
View Article : Google Scholar : PubMed/NCBI
|