Introduction
Breast carcinoma is the most common malignant tumor
and leading cause of cancer mortality in women worldwide (1). Breast tissue biopsies remain the best
way to diagnose breast carcinoma. When malignant breast lumps are
localized to the breast tissue, the relative cure rate of radical
mastectomy or radiotherapy and chemotherapy is high. However, if
breast carcinoma is detected at an advanced stage, and the
carcinoma cells have spread outside the breast tissue, the
prognosis for survival is substantially decreased (2). Although the precise molecular mechanism
of breast carcinoma progression remains unclear, numerous studies
have revealed that transforming growth factor-β (TGF-β)/SMAD family
member (Smad) signaling pathways that regulate cell growth,
differentiation, proliferation and apoptosis serve an important
function in the progression of breast carcinoma (3–5).
The TGF-β signaling pathway is activated when TGF-β
directly binds to transmembrane TGF-β type II receptors (TβRIIs);
subsequently, TβRII recruits and activates TβRI. In turn, Smad2 or
Smad3 transiently bind to TβRI and become activated by TβRI-induced
phosphorylation in the cytoplasm. Phosphorylated (p-)Smad2 or
p-Smad3 form a heterologous complex with a co-Smad (Smad4), which
is translocated from the cytoplasm into the nucleus and binds to
specific DNA sequences to regulate particular gene transcription
(6).
Activated Smad2 or Smad3 exert different effects on
the biological function of carcinoma cells (7). In gastric carcinoma, Smad2 is
considered to protect the gastric mucosal epithelium from malignant
transformation, whereas Smad3 is not directly associated with the
initiation of gastric carcinoma, but is associated with the
epithelial-mesenchymal transition (EMT) in gastric epithelial cells
(8). In MDA-MB-231 breast carcinoma
cells, Smad2 and Smad3 have diametrically opposite effects, with
Smad3 knockdown resulting in a delayed bone metastasis of carcinoma
cells, and Smad2 knockdown resulting in an enhanced invasive
ability of MDA-MB-231 cells (9).
Smad4 has been identified as a tumor suppressor
gene, and its mutation inactivation or decreased expression is
often observed in tumor tissues, including colorectal and
pancreatic carcinomas (10,11). So far, information regarding the
function of Smad4 in breast carcinoma is very limited. A previous
study revealed that the expression of Smad4 in breast carcinoma
tissue was lower compared with that of surrounding normal adjacent
breast epithelial tissue, but the survival time of patients who
were Smad4-negative was longer (12). In addition, certain scholars believe
that Smad4 may inhibit the growth of breast carcinoma cells by
inducing apoptosis (13). However,
subsequent to studying the MCF10 cell series, corresponding to
different stages of breast cancer progression, it was revealed that
the expression level of the Smad4 protein increased from
non-malignant to highly malignant in highly invasive cells
(14). Above all else, the
aforementioned studies indicate that the function of Smad4 protein
in the progression of breast carcinoma is very complex.
In the present study, the ductal carcinoma subtype
with the highest incidence in breast cancer was selected as the
subject of the study (15).
Immunohistochemistry was used to examine the expression of Smad2,
p-Smad2 and Smad4 in 126 invasive breast ductal carcinoma tissues,
in order to investigate the correlation between the expression of
these proteins and various clinicopathological parameters, in
addition to the consistency of expression among them, analyze
combined markers and identify prognostic factors for patients with
breast ductal carcinoma.
Materials and methods
Patients and tissue specimens
A total of 126 breast ductal carcinoma specimens
were collected from the Department of General Surgery of Beihua
University Affiliated Hospital (Jilin, China) between January 2009
and December 2010. All specimens were confirmed by
hematoxylin-eosin staining for invasive breast ductal carcinoma. No
preoperative radiotherapy, chemotherapy or other antitumor
treatments were available. All patients were female, aged 28–84
years (median age, 48 years). Out of all cases, 17 were
well-differentiated, 79 moderately differentiated and 30 poorly
differentiated. All patients were followed up until December 2014.
The overall survival (OS) time was defined as the time between
initial surgery and mortality or last follow-up. The OS time was
8.5–69.5 months, and the median survival time 49 months. The
progression-free survival (PFS) time was defined as the time
between initial surgery and deterioration (relapse or metastasis)
or mortality. The PFS time was 5–69.5 months, and the median
progression time 34.5 months.
A portion of the samples were fixed in 10% buffered
formalin at 37°C for 3 h and embedded in paraffin. Specimens were
stained with hematoxylin for 5 min and eosin for 20–30 sec at room
temperature and examined histopathologically. Clinicopathological
parameters, including age, tumor size, lymph node metastasis,
distant metastasis, histological grade, estrogen (ER), progesterone
(PR) and human epidermal growth factor receptor 2 (HER2) were
available for all patients.
Immunohistochemical assay
Immunohistochemical assays were performed using the
conventional streptavidin-peroxidase method. In brief, 5 µm thick
paraffin-embedded tissues were dewaxed using xylene and dehydrated
using ethanol gradient (100, 95 and 80%). Antigen retrieval buffer
(citric acid and sodium citrate preparation) was used for
incubating tissue sections. Sections were boiled (95°C) for 20 min
and then cooled until they reached room temperature. Endogenous
peroxidase was blocked using 3% hydrogen peroxide (Fuzhou Maixin
Biotech Co., Ltd., Fuzhou, China) for 10 min at 37°C. To eliminate
non-specific staining, the sections were incubated with goat serum
(Fuzhou Maixin Biotech Co., Ltd.) for 20 min at room temperature.
They were then incubated with rabbit polyclonal anti-Smad2 (cat.
no. ab63576; Abcam, Cambridge, UK), rabbit polyclonal anti-Smad2
(phospho S467; cat. no. ab53100; Abcam) and rabbit monoclonal
anti-Smad4 (cat. no. ab40759; Abcam) antibodies at 4°C overnight.
These antibodies were diluted at a ratio of 1:100. Following 3
washes in PBS, biotinylated anti-rabbit immunoglobulin (cat. no.
KIT-9707; Fuzhou Maixin Biotech Co., Ltd.) were applied for 10 min
at room temperature. Processing was then conducted in a humidified
chamber at room temperature by the addition of
streptavidin-peroxidase (cat. no. KIT-9707; Fuzhou Maixin Biotech
Co., Ltd.) for 10 min. Next, the specimens were washed in PBS and
color-developed with 3,3′-diaminobenzidine (Fuzhou Maixin Biotech
Co., Ltd.) and hydrogen peroxide for 5 min. Subsequently, they were
counterstained with Mayer's hematoxylin (Fuzhou Maixin Biotech Co.,
Ltd.) for 30 sec-1 min at room temperature. Negative controls were
obtained by omission of the primary antibody and substitution of
the primary antibody with normal serum. The positive control
comprised a section of a breast ductal carcinoma block previously
demonstrated to be positive for the marker, which was incorporated
in each run. Stained specimens were imaged under a light microscope
(magnifications ×200 and ×400).
Immunohistochemistry evaluation
All immunohistochemistry-stained sections were
scored by at least 3 of the 4 independent experienced pathologists
involved in the present study (Dr Nannan Liu, Dr Chunyan Yu, Dr
Dongxue Qi and Dr Jihong Zhang) who had no prior knowledge of the
clinicopathologic parameters and clinical outcomes of the patients.
The distribution and intensity of Smad2, p-Smad2 and Smad4 staining
were observed using light microscopy (CX31; Olympus Corporation,
Tokyo, Japan), and at least 5 fields (×400) were analyzed for each
tissue section. Staining was evaluated using the Taubert scoring
system as previously described (16), according to the proportion of stained
cells and staining intensity. The number of immunopositive cells
was semi-quantitatively estimated as follows: i) The percentage of
positive cells was scored as 1 (1–10%), 2 (11–50%), 3 (51–75%) and
4 (>75%); ii) Staining intensity was scored as 0 (absent), 1
(weak), 2 (moderate) and 3 (intense). The immunoreactive scores
(IRS) were calculated by multiplying the scores of i) and ii)
(17), and were as follows: 0, no
staining; 1–4, weak staining; 5–8, moderate staining; and 9–12,
strong staining. An IRS of <1 was considered to indicate a
negative staining score for p-Smad2 and Smad4. The median score
(6.3) was selected as the cutoff point for the separation of ‘high
Smad2 expression’ (score > median) from ‘low Smad2 expression’
(score ≤ median) tissue sections. Results were confirmed by a
repeat of the staining experiment on sequential sections from the
same block.
Statistical analysis
Statistical analysis was conducted using SPSS
software version 25 for Windows (IBM Corp., Armonk, NY, USA). The
data were presented as the means of IRS values calculated for each
section. The Spearman's rank correlations coefficient was
calculated for analyzing the correlation between the expression of
Smad2, p-Smad2 and Smad4 and various clinicopathological
parameters. Cohen's κ coefficient was used to evaluate
inter-observer consistency in the quantification. The Kaplan-Meier
method (log-rank test) was used for OS and PFS curves. Cox
regression was used for univariate and multivariate analysis for OS
and PFS. P<0.05 was considered to indicate a statistically
significant difference.
Results
Association between Smad2, p-Smad2 and
Smad4 expression and clinicopathological parameters
Immunohistochemistry was performed to determine the
expression of Smad2, p-Smad2 and Smad4 in 126 primary breast ductal
carcinoma tissue specimens. As expected, immunohistochemistry
staining exhibited a predominantly cytoplasmic pattern of the Smad4
protein expression, while Smad2 and p-Smad2 were revealed in the
cytoplasm and nuclear compartments (Fig.
1). According to the IRS criteria, no cases of Smad2-negative
expression were observed among the evaluated specimens. The median
score of all cases was 5.7, with 60 (47.6%) carcinoma tissues being
Smad2-low (IRS<5.7) and 66 (52.4%) being Smad2-high (IRS≥5.7).
However, p-Smad2 and Smad4 were negatively expressed in a
proportion of cells. A total of 25 (19.8%) carcinoma tissues were
p-Smad2-negative (IRS<1), while 101 (80.2%) stained positive
(1≤IRS≤12). In addition, 21 (16.7%) carcinoma tissues were
Smad4-negative (IRS<1) and 105 (83.3%) stained positive
(1≤IRS≤12).
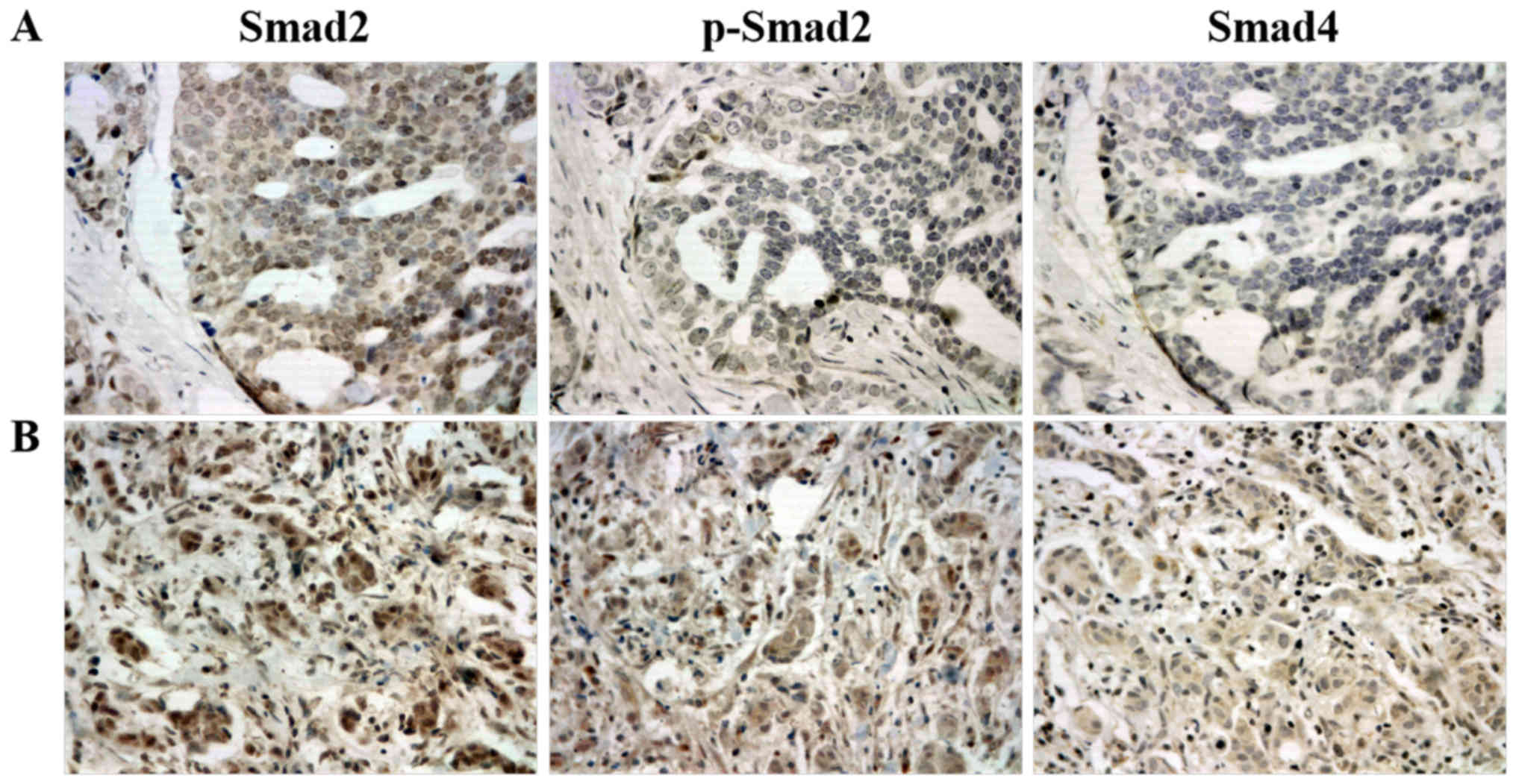 | Figure 1.Representative immunohistochemical
staining of Smad2, p-Smad2 and Smad4 in (A) and (B) two different
breast ductal carcinoma specimens. The expression of Smad2, p-Smad2
and Smad4 were strong, negative and negative in (A) specimens,
respectively. The expression of Smad2, p-Smad2 and Smad4 were
strong, moderate and weak in (B) specimens, respectively. Smad2,
SMAD family member 2; Smad4, SMAD family member 4; p-,
phosphorylated. |
The association between the expression of Smad2,
p-Smad2 and Smad4 and clinicopathological parameters was further
analyzed. Significant correlations were identified between Smad4
and HER2 expression (r=−0.179, P=0.044; Table I), but there was no statistically
significant correlation between Smad4 expression and any other
clinicopathological parameters, including age, tumor size, lymph
node metastasis, distant metastasis, histological grade, ER and PR
(P>0.05; Table I). Furthermore,
no statistically significant correlation was discovered between the
expression of p-Smad2 and Smad2 and any of the various
clinicopathological parameters (P>0.05; Table I).
 | Table I.Association of clinicopathological
parameters with Smad2, p-Smad2 and Smad4 expression in 126 patients
with breast ductal carcinoma. |
Table I.
Association of clinicopathological
parameters with Smad2, p-Smad2 and Smad4 expression in 126 patients
with breast ductal carcinoma.
|
|
| Smad2 |
|
| p-Smad2 |
|
| Smad4 |
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Variables | Cases | Low | High | r-value | P-value | Negative | Positive | r-value | P-value | Negative | Positive | r-value | P-value |
|---|
| Age (years) |
| 60 | 66 |
|
| 25 | 101 |
|
| 21 | 105 |
|
|
|
<48 | 62 | 31 | 31 | 0.047 | 0.602 | 11 | 51 | −0.052 | 0.564 | 10 | 52 | −0.014 | 0.875 |
| ≥48 | 64 | 29 | 35 |
|
| 14 | 50 |
|
| 11 | 53 |
|
|
| Histological
grade |
|
|
|
|
|
|
|
|
|
|
|
|
|
| I | 17 | 8 | 9 | −0.022 | 0.806 | 3 | 14 | −0.048 | 0.595 | 2 | 15 | −0.027 | 0.762 |
| II | 79 | 37 | 42 |
|
| 15 | 64 |
|
| 14 | 65 |
|
|
|
III | 30 | 15 | 15 |
|
| 7 | 23 |
|
| 5 | 25 |
|
|
| Tumor size |
|
|
|
|
|
|
|
|
|
|
|
|
|
| T2 | 99 | 51 | 48 | 0.149 | 0.095 | 19 | 80 | −0.031 | 0.729 | 16 | 83 | −0.026 | 0.773 |
| T3 | 27 | 9 | 18 |
|
| 6 | 21 |
|
| 5 | 22 |
|
|
| Lymph node
metastasis |
|
|
|
|
|
|
|
|
|
|
|
|
|
| N0 | 42 | 21 | 21 | 0.023 | 0.801 | 12 | 30 | 0.130 | 0.146 | 10 | 32 | 0.104 | 0.247 |
| N1 | 37 | 17 | 20 |
|
| 6 | 31 |
|
| 5 | 32 |
|
|
| N2 | 24 | 11 | 13 |
|
| 3 | 21 |
|
| 2 | 22 |
|
|
| N3 | 23 | 11 | 12 |
|
| 4 | 19 |
|
| 4 | 19 |
|
|
| Distant
metastasis |
|
|
|
|
|
|
|
|
|
|
|
|
|
| M0 | 118 | 57 | 61 | 0.053 | 0.557 | 24 | 94 | 0.048 | 0.594 | 20 | 98 | 0.029 | 0.746 |
| M1 | 8 | 3 | 5 |
|
| 1 | 7 |
|
| 1 | 7 |
|
|
| ER |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Negative | 92 | 43 | 49 | −0.029 | 0.747 | 20 | 72 | 0.078 | 0.384 | 18 | 72 | 0.141 | 0.114 |
|
Positive | 34 | 17 | 17 |
|
| 5 | 29 |
|
| 3 | 33 |
|
|
| PR |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Negative | 89 | 44 | 45 | 0.056 | 0.530 | 16 | 73 | −0.072 | 0.420 | 14 | 75 | −0.039 | 0.665 |
|
Positive | 37 | 16 | 21 |
|
| 9 | 28 |
|
| 7 | 30 |
|
|
| HER2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Negative | 94 | 46 | 48 | 0.045 | 0.615 | 15 | 79 | −0.167 | 0.062 | 12 | 82 | −0.179 | 0.044a |
|
Positive | 32 | 14 | 18 |
|
| 10 | 22 |
|
| 9 | 23 |
|
|
Consistent association among Smad4,
p-Smad2 and Smad2 expression
A p-Smad2/Smad4 co-positive expression was observed
in 100/126 cases. A p-Smad2/Smad4 co-negative expression was
observed in 20/126 cases. In addition, 5/126 cases had a
p-Smad2(−)/Smad4(+) and 1 had a p-Smad2(+)/Smad4(−) expression. Of
note, according to the κ consistency test results, a significant
agreement in the classification for Smad4 and p-Smad2 was revealed
in all tissue specimens (κ=0.841, P<0.001; Table II). However, the consistent
association was not presented in classification for between Smad2
and p-Smad2 (κ=0.024, P=0.394; data not shown) or Smad2 and Smad4
(κ=−0.013, P=0.632; data not shown).
 | Table II.Agreement between Smad4 and p-Smad2
expression. |
Table II.
Agreement between Smad4 and p-Smad2
expression.
|
|
| p-Smad2 |
|
|
|---|
|
|
|
|
|
|
|---|
| Smad4 | Cases | Negative | Positive | κ | P-value |
|---|
| Negative | 21 | 20 | 1 | 0.841 | 0.001a |
| Positive | 105 | 5 | 100 |
|
|
| Total | 126 | 25 | 101 |
|
|
Association between Smad2, p-Smad2 and
Smad4 expression and the survival of patients with breast ductal
carcinoma
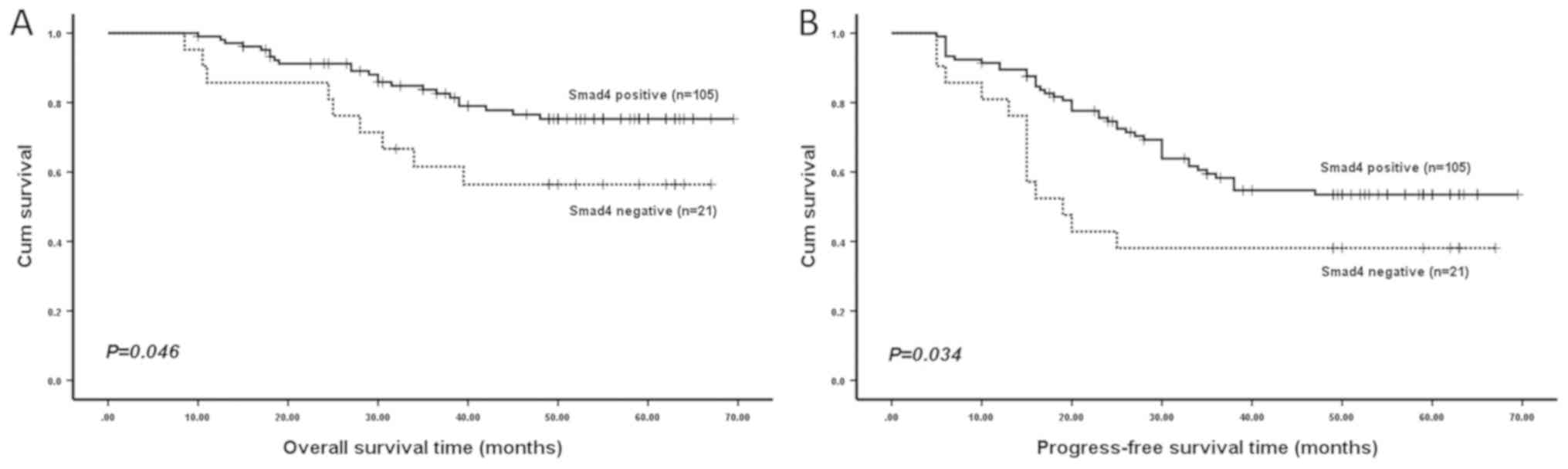
Kaplan-Meier analysis revealed that patients with an
Smad4-negative expression were likely to have a significantly
shorter OS time (P=0.046; Fig. 2)
and PFS time (P=0.034; Fig. 2) in
all 126 specimens compared with Smad4-positive patients. However,
no significant difference in OS time (P=0.181; data not shown) or
PFS time (P=0.063; data not shown) was detected between patients
with a positive and negative p-Smad2 expression. In addition, no
significant difference was identified in OS time (P=0.617; data not
shown) or PFS time (P=0.552; data not shown) between patients with
a positive and negative Smad2 expression.
Univariate and multivariate analysis
of prognostic variables in 126 patients with breast ductal
carcinoma
Univariate analysis revealed that distant metastasis
significantly predicted an increased risk of breast carcinoma
progression (P=0.017; Table III)
and poor OS time (P=0.010; Table
III). However, the risk of breast carcinoma progression was
significantly decreased in patients with Smad4-positive expression
(P=0.040; Table III), an effect
that was not observed for OS time (P=0.051; Table III). Other clinicopathological
parameters did not exhibit any statistically significant difference
in prognostic risk assessment (P>0.05; Table III). Multivariate Cox analysis
revealed that Smad4 expression did not remain a statistically
significant marker of deterioration [hazard ratio (HR), 0.539; 95%
confidence interval (CI), 0.290–1.002; P=0.051; data not shown]
once distant metastasis had been taken into account, whereas
distant metastasis remained a significant independent predictor
(HR, 2.945; 95% CI, 1.257–6.902; P=0.013; data not shown).
 | Table III.Univariate analysis of
clinicopathological parameters for overall and progression-free
survival in 126 breast ductal carcinoma patients. |
Table III.
Univariate analysis of
clinicopathological parameters for overall and progression-free
survival in 126 breast ductal carcinoma patients.
|
|
| Overall survival
time (months) | Progress-free
survival time (months) |
|---|
|
|
|
|
|
|---|
| Variable | Cases | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (year) | 126 |
| 0.502 |
| 0.982 |
|
<48 | 62 | 1 |
| 1 |
|
|
≥48 | 64 | 1.270
(0.632–2.554) |
| 0.994
(0.594–1.664) |
|
| Histological
grade |
|
| 0.093 |
| 0.130 |
| I | 17 | 1 |
| 1 |
|
| II | 79 | 2.211
(0.515–9.492) |
| 1.075
(0.475–2.430) |
|
|
III | 30 | 4.164
(0.922–18.809) |
| 1.881
(0.785–4.509) |
|
| Tumor size |
|
| 0.158 |
| 0.535 |
| T2 | 99 | 1 |
| 1 |
|
| T3 | 27 | 1.742
(0.806–3.768) |
| 1.216
(0.656–2.255) |
|
| Lymph node
metastasis |
|
| 0.964 |
| 0.702 |
| N0 | 42 | 1 |
| 1 |
|
| N1 | 37 | 0.842
(0.339–2.093) |
| 1.344
(0.710–2.543) |
|
| N2 | 24 | 1.018
(0.394–2.626) |
| 0.891
(0.411–1.932) |
|
| N3 | 23 | 1.099
(0.406–2.971) |
| 1.148
(0.530–2.489) |
|
| Distant
metastasis |
|
| 0.017a |
| 0.010a |
| M0 | 118 | 1 |
| 1 |
|
| M1 | 8 | 3.618
(1.262–10.369) |
| 3.072
(1.313–7.185) |
|
| ER |
|
| 0.819 |
| 0.970 |
|
Negative | 92 | 1 |
| 1 |
|
|
Positive | 34 | 1.094
(0.506–2.365) |
| 0.989
(0.549–1.780) |
|
| PR |
|
| 0.384 |
| 0.766 |
|
Negative | 89 | 1 |
| 1 |
|
|
Positive | 37 | 0.701
(0.315–1.560) |
| 1.087
(0.628–1.882) |
|
| HER2 |
|
| 0.098 |
| 0.563 |
|
Negative | 94 | 1 |
| 1 |
|
|
Positive | 32 | 1.832
(0.895–3.747) |
| 1.185
(0.666–2.109) |
|
| Smad2 |
|
| 0.618 |
| 0.558 |
|
Low | 60 | 1 |
| 1 |
|
|
High | 66 | 0.838
(0.419–1.617) |
| 1.168
(0.695–1.965) |
|
| p-Smad2 |
|
| 0.187 |
| 0.070 |
|
Negative | 25 | 1 |
| 1 |
|
|
Positive | 101 | 0.595
(0.275–1.286) |
| 0.581
(0.322–1.046) |
|
| Smad4 |
|
| 0.051 |
| 0.040a |
|
Negative | 21 | 1 |
| 1 |
|
|
Positive | 105 | 0.465
(0.215–1.005) |
| 0.523
(0.282–0.971) |
|
Association between co-negative and
co-positive p-Smad2/Smad4 expression and the survival of patients
with breast ductal carcinoma
Since Smad4 expression was significantly consistent
with p-Smad2 expression, as confirmed by Cohen's κ coefficient,
patients were divided into two subgroups, according to their
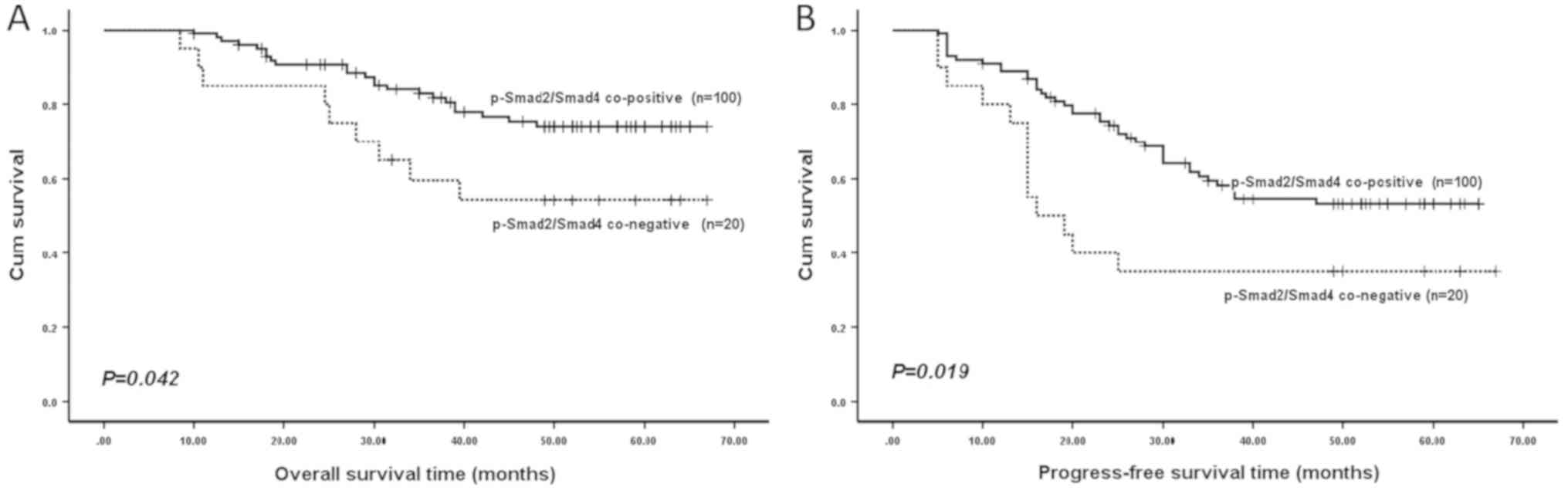
co-negative or co-positive p-Smad2/Smad4 expression. As revealed by
Kaplan-Meier analysis, patients in the p-Smad2/Smad4 co-negative
expression subgroups had a significantly shorter OS time (P=0.042;
Fig. 3) and PFS time (P=0.019;
Fig. 3) compared with those in the
co-positive expression subgroups.
Univariate and multivariate analysis
of prognostic variables in patients with breast ductal carcinoma
with a co-negative and co-positive p-Smad2/Smad4 expression
Univariate analysis results indicated that distant
metastasis and p-Smad2/Smad4 co-expression were significantly
associated with OS time (P=0.022 and P=0.047, respectively;
Table IV) and PFS time (P=0.012 and
P=0.023, respectively; Table IV).
Nevertheless, the other clinicopathological parameters evaluated,
including age, tumor size, lymph node metastasis, histological
type, ER, PR and HER2 expression did not significantly affect OS
and PFS time (Table IV).
Furthermore, multivariate Cox regression analysis indicated that
distant metastasis and co-negative or co-positive p-Smad2/Smad4
expression were also independent predictors for OS time (P=0.024
and P=0.049, respectively; Table V)
and PFS time (P=0.017 and P=0.030, respectively; Table V) of patients with breast ductal
carcinoma.
 | Table IV.Univariate analysis of
clinicopathological parameters for overall and progression-free
survival in patients with breast ductal carcinoma with a
co-negative and co-positive p-Smad2/Smad4 expression. |
Table IV.
Univariate analysis of
clinicopathological parameters for overall and progression-free
survival in patients with breast ductal carcinoma with a
co-negative and co-positive p-Smad2/Smad4 expression.
|
|
| Overall survival
time (months) | Progress-free
survival time (months) |
|---|
|
|
|
|
|
|---|
| Variable | Cases | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (year) | 120 |
| 0.516 |
| 0.999 |
|
<48 | 59 | 1 |
| 1 |
|
|
≥48 | 61 | 0.793
(0.395–1.596) |
| 1
(0.592–1.689) |
|
| Histological
grade |
|
| 0.084 |
| 0.284 |
| I | 16 | 1 |
| 1 |
|
| II | 76 | 2.163
(0.504–9.287) |
| 1.047
(0.463–2.367) |
|
|
III | 28 | 4.186
(0.927–18.904) |
| 1.660
(0.682–4.038) |
|
| Tumor size |
|
| 0.158 |
| 0.447 |
| T2 | 94 | 1 |
| 1 |
|
| T3 | 26 | 1.744
(0.806–3.772) |
| 1.272
(0.684–2.367) |
|
| Lymph node
metastasis |
|
| 0.917 |
| 0.683 |
| N0 | 40 | 1 |
| 1 |
|
| N1 | 36 | 0.806
(0.324–2.005) |
| 1.215
(0.637–2.316) |
|
| N2 | 23 | 0.998
(0.387–2.575) |
| 0.781
(0.351–1.740) |
|
| N3 | 21 | 1.176
(0.435–3.181) |
| 1.243
(0.573–2.694) |
|
| Distant
metastasis |
|
| 0.022a |
| 0.012a |
| M0 | 112 | 1 |
| 1 |
|
| M1 | 8 | 3.412
(1.190–9.777) |
| 2.986
(1.274–6.998) |
|
| ER |
|
| 0.770 |
| 0.919 |
|
Negative | 89 | 1 |
| 1 |
|
|
Positive | 31 | 1.122
(0.519–2.425) |
| 0.969
(0.529–1.774) |
|
| PR |
|
| 0.463 |
| 0.675 |
|
Negative | 85 | 1 |
| 1 |
|
|
Positive | 35 | 0.741
(0.333–1.650) |
| 1.127
(0.643–1.976) |
|
| HER2 |
|
| 0.125 |
| 0.796 |
|
Negative | 89 | 1 |
| 1 |
|
|
Positive | 31 | 1.751
(0.856–3.583) |
| 1.081
(0.598–1.954) |
|
| Smad2 |
|
| 0.750 |
| 0.543 |
|
Low | 59 | 1 |
| 1 |
|
|
High | 61 | 0.893
(0.446–1.788) |
| 1.178
(0.695–1.995) |
|
| p-Smad2 and
Smad4 |
|
| 0.047a |
| 0.023a |
|
p-Smad2/Smad4 co-negative | 20 | 1 |
| 1 |
|
|
p-Smad2/Smad4 co-positive | 100 | 0.458
(0.212–0.991) |
| 0.486
(0.261–0.905) |
|
 | Table V.Multivariate analysis of
clinicopathological parameters for overall and progression-free
survival in patients with breast ductal carcinoma with a
co-negative and co-positive p-Smad2/Smad4 expression. |
Table V.
Multivariate analysis of
clinicopathological parameters for overall and progression-free
survival in patients with breast ductal carcinoma with a
co-negative and co-positive p-Smad2/Smad4 expression.
| Variable | Comparison | HR | 95% CI | P-value |
|---|
| Overall survival
time (months) |
|
|
|
|
| Distant
metastasis | M0 vs. M1 | 3.383 | 1.178–9.713 | 0.024a |
| p-Smad2 and
Smad4 | p-Smad2/Smad4
co-negative vs. p-Smad2/Smad4 co-positive | 0.461 | 0.213–0.997 | 0.049a |
| Progression-free
survival time (months) |
|
|
|
|
| Distant
metastasis | M0 vs. M1 | 2.840 | 1.209–6.668 | 0.017a |
| p-Smad2 and
Smad4 | p-Smad2/Smad4
co-negative vs. p-Smad2/Smad4 co-positive | 0.502 | 0.269–0.937 | 0.030a |
Discussion
The results of the present study revealed that the
Smad4 expression was significantly negatively correlated with the
expression of HER2 in breast carcinoma tissues; as the positive
rate of Smad4 expression gradually decreased, the positive
expression rate of HER2 increased correspondingly, suggesting that
the expression characteristics of Smad4(−)/HER2(+) were present in
a portion of breast ductal carcinomas specimens. Although no direct
correlation has been reported between Smad4 and HER2, it has been
reported that the TGF-β/Smad and the HER2/Ras/extracellular
signal-regulated kinase (Erk) mitogen-activated protein kinase
(MAPK) pathway often directly interact and mutually regulate the
activities or expression of each other (18). One function of elevated HER2 activity
in breast carcinoma is to impede the growth inhibitory function of
TGF-β via the phosphorylation of R-Smad proteins by Erk MAPK
(19). It means that HER2-mediated
signal transformation may involve the shutdown of the TGF-β/Smad
tumor suppressive pathway. Subsequently, the inactivation of Smad4
may further interfere with TGF-β-induced growth inhibition.
Similarly, in human pancreatic ductal adenocarcinoma, the
activation of HER2 and inactivation of Smad4 were also the most
frequent gene alterations, which enabled human pancreatic ductal
epithelial cells to acquire sustaining proliferative signaling,
evade growth suppressors and finally result in tumorigenic
transformation (20). This may not
explain why patients with an Smad4-negative expression were more
likely to be HER2-positive in breast ductal carcinoma tissues, but
it may be important for understanding the expression
characteristics of Smad4(−)/HER2(+) in certain breast carcinoma
specimens. In addition, the study revealed that patients with
breast carcinoma with a high HER2 expression were generally
associated with a poor prognosis and high carcinoma cell
proliferation (21), suggesting that
HER2 has a positive regulatory effect on the proliferation of
cancer cells. By contrast, Smad4 functions as a tumor-suppressor
gene, which results in a negative regulatory effect on the
proliferation of cancer cells (22).
Therefore, there is expected to be a negative correlation between
the expression of Smad4 and HER2 in breast carcinoma tissues, but
the detailed underlying molecular mechanism and co-regulation
signal loop need to be further studied.
Although Smad2 is not so much regarded as a
tumor-suppressor gene, certain studies have demonstrated that the
functional inactivation of Smad2 is sufficient to inhibit the
physiological function of TGF-β (23,24).
Furthermore, one previous study tested Smad2-targeted knockout
mouse keratinocytes and revealed that Smad2−/− mice did
not naturally develop into skin tumor types, but that Smad2 may
accelerate tumor formation and malignant transformation in chemical
carcinogenesis experiments, and that Smad2−/− skin
cancer was poorly differentiated and exhibited an increase in EMT,
indicating that an Smad2-deficient epithelium was more likely to
form a tumor and malignant transformation (25). Accordingly, Smad2-deficiency in mice
did not cause intestinal tumor types, but it may accelerate the
malignant progression of late-stage invasive tumor types (26). In the present study, Smad2 was not
negatively expressed in any of the 126 breast ductal carcinoma
tissues, but 25 tissues were p-Smad2-negative. It was clear that
these 25 specimens were due to a lack of phosphorylation, not due
to a loss of Smad2 expression. More importantly, of the
p-Smad2-negative cases, 20 exhibited a p-Smad2/Smad4 co-negative
expression. Previous studies have revealed that the frequency of
Smad4 gene mutant inactivation was high in solid tumor types,
including breast carcinoma (27–29),
which may partially explain the loss of Smad4 expression in the
present study. Similar results have been discovered in the study of
prostate carcinoma cell lines with different invasive and
metastatic capacities: Smad2 was highly expressed in these cells,
but the expression levels of p-Smad2 and Smad4 were different from
each other, while a loss of expression was observed for each of
them (30). Based on this, it was
speculated that the progression of the malignant tumor types may be
due to the interruption of the Smad activation process in the TGF-β
signaling pathway.
In the present study, although neither p-Smad2 nor
Smad4 were independent predictors, a substantial agreement in
classification was observed between p-Smad2 and Smad4 expression.
In addition, a Kaplan-Meier curve and Cox proportional hazards
model revealed that p-Smad2/Smad4 co-positive patients had a longer
OS and PFS time, in addition to a better prognosis, compared with
p-Smad2/Smad4 co-negative patients. Based on the aforementioned
results, it was speculated that the progression of these
p-Smad2/Smad4 co-negative patients may have been due to the
inability of Smad2 to phosphorylate, and at the same time the loss
of Smad4 expression, which made Smad2 unable to form a heterologous
complex with Smad4, and thus unable to form a transcription complex
for the regulation of target gene transcription; as a result, the
TGF-β/Smad pathway was disrupted, resulting in cell growth
inhibition prevention and cell proliferation initiation (31). Another study revealed that the
expression of the interstitial marker proteins vimentin and
N-cadherin in breast carcinoma cells were increased following the
inhibition of Smad2 expression, while the expression of the
epithelial marker protein E-cadherin was decreased, suggesting the
occurrence of EMT. It was through EMT that epithelial cells
acquired an invasive ability, which promoted malignant tumor cell
metastasis (32). In turn, the
co-activation of p-Smad2 and Smad4 may serve a synergistic function
in tumor suppression by transmitting TGF-β signaling in the 100
p-Smad2/Smad4 co-positive expression breast carcinoma cases,
resulting in a longer survival time and better prognosis. In a
similar study, although the effect of p-Smad2 and Smad4
co-expression or co-inactivation on prognosis was not observed, the
OS time of patients with breast carcinoma which was
p-Smad2-positive was significantly longer compared with that of
p-Smad2-negative patients (33),
which may have been due to the distinct specimen groups, the
cut-offs used for the assessment or the antibodies used.
Furthermore, the close correlation between p-Smad2 and Smad4
expression was also discovered in other types of cancer, including
osteosarcoma; Smad4 expression was significantly associated with
p-Smad2 expression, and they each co-regulated the expression of
the cell cycle inhibitor p21/waf1 to inhibit tumor cell growth
(34).
In conclusion, the results of the present study
indicated that Smad4-positive patients had a better prognosis
compared with Smad4-negative patients, although Smad4 expression
could not be used as an independent predictor. Of note, at least in
a part of breast ductal carcinomas, patients with a p-Smad2/Smad4
co-negative expression had a poor prognosis, as compared with
p-Smad2/Smad4 co-positive patients. In addition, according to the
results of the present study, p-Smad2 and Smad4 co-expression or
co-inactivation may be seen as an independent predictor of
prognosis in patients with invasive breast ductal carcinoma. Since,
in most cases, the primary diagnosis of breast ductal carcinomas is
based on histopathological sections, such breast cancer samples are
therefore critical in guiding the clinical management of the
disease, potentially helping to avoid improper or excessive
treatment. Of course, it is also limited to detecting the
expression of proteins in paraffin samples only by
immunohistochemistry, and future research will attempt to confirm
the results of the present study by analyzing Smad2, p-Smad2 and
Smad4 mRNA and protein levels in fresh breast ductal carcinoma
samples.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Science and Technology projects in Jilin Province Department of
Education (grant no. JJKH20180356KJ), the Department of Science and
Technology of Jilin Province (grant no. 20180623018TC) and the
Jilin Province Health and Family Planning Commission (grant no.
2017J084).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
NL and CY contributed to the study design. DQ, CY,
JZ and NL contributed to the data analysis. DQ and JJ contributed
to the collection of the tissue samples and patient data. NL and CY
wrote the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was ethically approved by the
Ethics Committee of the Affiliated Hospital of Beihua University
(Jilin, China), and written informed consent was obtained from each
patient once the purpose and nature of the study had been fully
explained.
Patient consent for publication
Informed consent was obtained from all patients
regarding the publication of the data and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Akram M, Iqbal M, Daniyal M and Khan AU:
Awareness and current knowledge of breast cancer. Biol Res.
50:332017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leone BA, Vallejo CT, Romero AO,
Machiavelli MR, Pérez JE, Leone J and Leone JP: Prognostic impact
of metastatic pattern in stage IV breast cancer at initial
diagnosis. Breast Cancer Res Treat. 161:537–548. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen HS, Bai MH, Zhang T, Li GD and Liu M:
Ellagic acid induces cell cycle arrest and apoptosis through
TGF-β/Smad3 signaling pathway in human breast cancer MCF-7 cells.
Int J Oncol. 46:1730–1738. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lo PK, Zhang Y, Yao Y, Wolfson B, Yu J,
Han SY, Duru N and Zhou Q: Tumor-associated myoepithelial cells
promote the invasive progression of ductal carcinoma in situ
through activation of TGF β signaling. J Biol Chem.
292:11466–11484. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang KM, Kim W, Bae E, Gim J, Weist BM,
Jung Y, Hyun JS, Hernandez JB, Leem SH, Park T, et al: DRAK2
participates in a negative feedback loop to control TGF-β/Smads
signaling by binding to type I TGF-β receptor. Cell Rep.
2:1286–1299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Massagué J: TGF β signaling in context.
Nat Rev Mol Cell Biol. 13:616–630. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ungefroren H, Groth S, Sebens S, Lehnert
H, Gieseler F and Fändrich F: Differential roles of Smad2 and Smad3
in the regulation of TGF-β1-mediated growth inhibition and cell
migration in pancreatic ductal adenocarcinoma cells: Control by
Rac1. Mol Cancer. 10:672011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu Y, Li Q, Zhou X, Yu J, Mu Y, Munker S,
Xu C, Shen Z, Müllenbach R, Liu Y, et al: Decreased levels of
active SMAD2 correlate with poor prognosis in gastric cancer. PLoS
One. 7:e356842012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petersen M, Pardali E, van der Horst G,
Cheung H, van den Hoogen C, van der Pluijm G and Ten Dijke P: Smad2
and Smad3 have opposing roles in breast cancer bone metastasis by
differentially affecting tumor angiogenesis. Oncogene.
29:1351–1361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan P, Klingbiel D, Saridaki Z, Ceppa P,
Curto M, McKee TA, Roth A, Tejpar S, Delorenzi M, Bosman FT and
Fiocca R: Reduced expression of SMAD4 is associated with poor
survival in colon cancer. Clin Cancer Res. 22:3037–3047. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamada S, Fujii T, Shimoyama Y, Kanda M,
Nakayama G, Sugimoto H, Koike M, Nomoto S, Fujiwara M, Nakao A and
Kodera Y: SMAD4 expression predicts local spread and treatment
failure in resected pancreatic cancer. Pancreas. 44:660–664. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stuelten CH, Buck MB, Dippon J, Roberts
AB, Fritz P and Knabbe C: Smad4-Expression is decreased in breast
cancer tissues: A retrospective study. BMC Cancer. 6:252006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Q, Wu L, Oelschlager DK, Wan M,
Stockard CR, Grizzle WE, Wang N, Chen H, Sun Y and Cao X: Smad4
inhibits tumor growth by inducing apoptosis in estrogen
receptor-alpha-positive breast cancer cells. J Biol Chem.
280:27022–27028. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
So JY, Lee HJ, Kramata P, Minden A and Suh
N: Differential expression of key signaling proteins in MCF10 cell
lines, a human breast cancer progression model. Mol Cell Pharmacol.
4:31–40. 2012.PubMed/NCBI
|
|
15
|
Barroso-Sousa R and Metzger-Filho O:
Differences between invasive lobular and invasive ductal carcinoma
of the breast: Results and therapeutic implications. Ther Adv Med
Oncol. 8:261–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taubert H, Heidenreich C, Holzhausen HJ,
Schulz A, Bache M, Kappler M, Eckert AW, Würl P, Melcher I,
Hauptmann K, et al: Expression of survivin detected by
immunohistochemistry in the cytoplasm and in the nucleus is
associated with prognosis of leiomyosarcoma and synovial sarcoma
patients. BMC Cancer. 10:652010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo K: Signaling cross talk between
TGF-β/Smad and other signaling pathways. Cold Spring Harb Perspect
Biol. 9:a0221372017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kretzschmar M: Transforming growth
factor-beta and breast cancer: Transforming growth factor-beta/SMAD
signaling defects and cancer. Breast Cancer Res. 2:107–115. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang Z, Li Z, Wang X, Kang Y, Yuan Y, Niu
J, Wang H, Chatterjee D, Fleming JB, Li M, et al: Deciphering the
mechanisms of tumorigenesis in human pancreatic ductal epithelial
cells. Clin Cancer Res. 19:549–559. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Caldarella A, Crocetti E, Bianchi S,
Vezzosi V, Urso C, Biancalani M and Zappa M: Female breast cancer
status according to ER, PR and HER2 expression: A population based
analysis. Pathol Oncol Res. 17:753–758. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen L, Zhong J, Liu JH, Liao DF, Shen YY,
Zhong XL, Xiao X, Ding WJ, Peng XD, Xiong W and Zu XY: Pokemon
inhibits transforming growth factor β-Smad4-related cell
proliferation arrest in breast cancer through specificity protein
1. J Breast Cancer. 22:15–28. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ying Z, Tian H, Li Y, Lian R, Li W, Wu S,
Zhang HZ, Wu J, Liu L, Song J, et al: CCT6A suppresses SMAD2 and
promotes prometastatic TGF-β signaling. J Clin Invest.
127:1725–1740. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang J, Wahdan-Alaswad R and Danielpour D:
Critical role of Smad2 in tumor suppression and transforming growth
factor-beta-induced apoptosis of prostate epithelial cells. Cancer
Res. 69:2185–2190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hoot KE, Lighthall J, Han G, Lu SL, Li A,
Ju W, Kulesz-Martin M, Bottinger E and Wang XJ:
Keratinocyte-specific Smad2 ablation results in increased
epithelial-mesenchymal transition during skin cancer formation and
progression. J Clin Invest. 118:2722–2732. 2008.PubMed/NCBI
|
|
26
|
Hamamoto T, Beppu H, Okada H, Kawabata M,
Kitamura T, Miyazono K and Kato M: Compound disruption of smad2
accelerates malignant progression of intestinal tumors in apc
knockout mice. Cancer Res. 62:5955–5961. 2002.PubMed/NCBI
|
|
27
|
Zhao M, Mishra L and Deng CX: The role of
TGF-β/SMAD4 signaling in cancer. Int J Biol Sci. 14:111–123. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Voorneveld PW, Kodach LL, Jacobs RJ, Liv
N, Zonnevylle AC, Hoogenboom JP, Biemond I, Verspaget HW, Hommes
DW, de Rooij K, et al: Loss of SMAD4 alters BMP signaling to
promote colorectal cancer cell metastasis via activation of Rho and
ROCK. Gastroenterology. 147:196–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ding Z, Wu CJ, Chu GC, Xiao Y, Ho D, Zhang
J, Perry SR, Labrot ES, Wu X, Lis R, et al: SMAD4-Dependent barrier
constrains prostate cancer growth and metastatic progression.
Nature. 470:269–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu KJ, Zhu GF, Zhang D, Zeng J, Wang XY,
Xue Y, Zhang LL and He DL: Identification of TGF-beta/Smads pathway
in human prostate cancer cell lines and its significance. Zhonghua
Nan Ke Xue. 15:920–924. 2009.(In Chinese). PubMed/NCBI
|
|
31
|
Mhawech-Fauceglia P, Kesterson J, Wang D,
Akers S, DuPont NC, Clark K, Lele S and Liu S: Expression and
clinical significance of the transforming growth factor-β
signalling pathway in endometrial cancer. Histopathology. 59:63–72.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lv ZD, Kong B, Li JG, Qu HL, Wang XG, Cao
WH, Liu XY, Wang Y, Yang ZC, Xu HM and Wang HB: Transforming growth
factor-β 1 enhances the invasiveness of breast cancer cells by
inducing a Smad2-dependent epithelial-to-mesenchymal transition.
Oncol Rep. 29:219–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie W, Mertens JC, Reiss DJ, Rimm DL, Camp
RL, Haffty BG and Reiss M: Alterations of Smad signaling in human
breast carcinoma are associated with poor outcome: A tissue
microarray study. Cancer Res. 62:497–505. 2002.PubMed/NCBI
|
|
34
|
Won KY, Kim YW and Park YK: Expression of
smad and its signalling cascade in osteosarcoma. Pathology.
42:242–247. 2010. View Article : Google Scholar : PubMed/NCBI
|