Introduction
Vestibular schwannomas (VS) are benign nerve sheath
tumors of the vestibular nerve that arise from neoplastic Schwann
cells (1,2). These tumors usually appear
sporadically, but in rare cases (1:33,000) they are part of a
genetic disorder, called neurofibromatosis type 2. NF2 is
associated with the loss of the NF2 gene on chromosome 22, which
encodes for merlin, a tumor suppressor protein (3,4). NF2
patients develop a multitude of tumors like meningeomas,
ependymomas and as the hallmark tumors bilateral VS. These tumors
usually have a higher recurrence rate, grow faster, and are much
more adherent to the surrounding structures compared to their
sporadic counterparts (5).
Therefore, they are often associated with persistent cranial nerve
deficits and sole surgery is not a long-lasting solution in these
cases-in contrast to sporadic VS. Thus, an efficacious systemic
therapy is urgently needed.
The main known pathomechanism for vestibular
schwannoma is the loss of function by Merlin. Merlin is a 4.1
protein/ezrin/radixin/moesin protein (FERM) that connects the
cytoskeleton with the cell membrane. It is activated by the cells'
attachment to the extracellular matrix and by intercellular
adhesion (6). Merlin's loss of
function is the main known mechanism for the development of VS and
results in the activation of two signaling pathways. These are the
Ras/Raf/MEK pathway and the PI3K/Akt/mTOR pathway, which inhibit
apoptosis and result in higher cell survival or proliferation rates
(3,7,8).
Furthermore, the Hippo pathway and the VEGF-mediated signaling
pathway, are also activated by Merlin's loss of function (3,6). Indeed,
the VEGF-inhibitor Bevacizumab has been shown to effectively target
VEGF overexpression in individual cases of NF2 associated VS
(3), but only for a short period in
the majority of patients. To date, there is no effective systemic
treatment option available for VS in terms of maintaining a stable
disease state or even inducing tumor shrinkage. Therefore, there is
a huge necessity to identify useful molecular therapeutic targets,
especially for patients with NF2 (3,9).
Members of the A-Disintegrin and Metalloproteinase
protein (ADAM) family are therapeutic targets for many tumor
entities. The ADAM protein family consists of 21 functional
transmembrane proteins with 8 transmembrane domains. These include
a signal peptide, a propeptide, metalloproteinase activity, a
disintegrin sequence, a cysteine-rich region, an EGF-like domain, a
transmembrane part and a cytoplasmic tail (10,11). One
member of this protein family is ADAM9, which is encoded on
chromosome 8 and was first described in 1996 (12,13). It
plays a role in cell-adhesion and cell-signaling and is
overexpressed in several cancers like breast-, non-small cell
lung-, pancreas-, stomach-, hepatocellular-cancer, melanoma and
glioblastoma (10,14–17).
Two mature isoforms are produced by alternative mRNA
splicing, a shorter, secreted and a larger, transmembrane version.
The first one derives from a 68 kDa precursor and has a mature size
of 47 kDa while the latter is represented by a 114 kDa precursor
and matures as 84 kDa (11,18,19).
Functional differences of these isoforms are subject of recent
research. Especially the secreted isoform promotes cell migration
and is correlated with a higher tumor invasiveness by modulating
the interaction with integrins (10,11,14,18). One
further function of ADAM9 is protein-shedding of proHB-EGF by its
proteinase activity, which is induced by PKC. Furthermore, ADAM9 is
a ligand for integrins α2β1, α6β4 and others (10,14).
ADAM proteins contain a SH3-binding site, which can activate
SH3-domain-containing-signalling molecules like Src and Grb. In
contrast to other ADAM family members, ADAM9 cannot be specifically
inhibited (10,11,14,20).
Importantly, ADAM9 overexpression is observed under oxidative
stress with increased levels of reactive oxygen species, which are
regularly generated by cancer cells (21,22).
Recently, we demonstrated that CXCR4 is of relevance
for VS pathogenesis (23).
Therefore, we assumed that other proteins beside Merlin and CXCR4
also might be involved. To the best of our knowledge, no data on
ADAM9 expression in VS have been published so far. We hypothesized
that ADAM9 could be overexpressed in VS because of its role in
other solid cancers and its similarity to the function of Merlin.
Furthermore, ADAM9 could be a suitable therapeutic target for the
treatment of VS, as it is already therapeutically targeted in other
cancer types and in Alzheimer's disease (24). Therefore, we evaluated its potential
role as a prognostic marker for VS by examining its expression and
distribution in sporadic and NF2- associated VS tissue and its
correlation to clinical parameters of the patients, especially
their hearing-loss.
Materials and methods
Tissue samples
The study was approved by the local ethics committee
of the University Hospital Würzburg with the statement number
145/16 and written informed consent was obtained from the patients
for the use of their tissue. Tissue collection was from 2010–2015.
Directly after surgical excision, the tissue was divided in half:
one half was cryopreserved, and the other half was fixed in
formalin. All tumor samples were neuropathologically assessed
according to WHO-criteria (2).
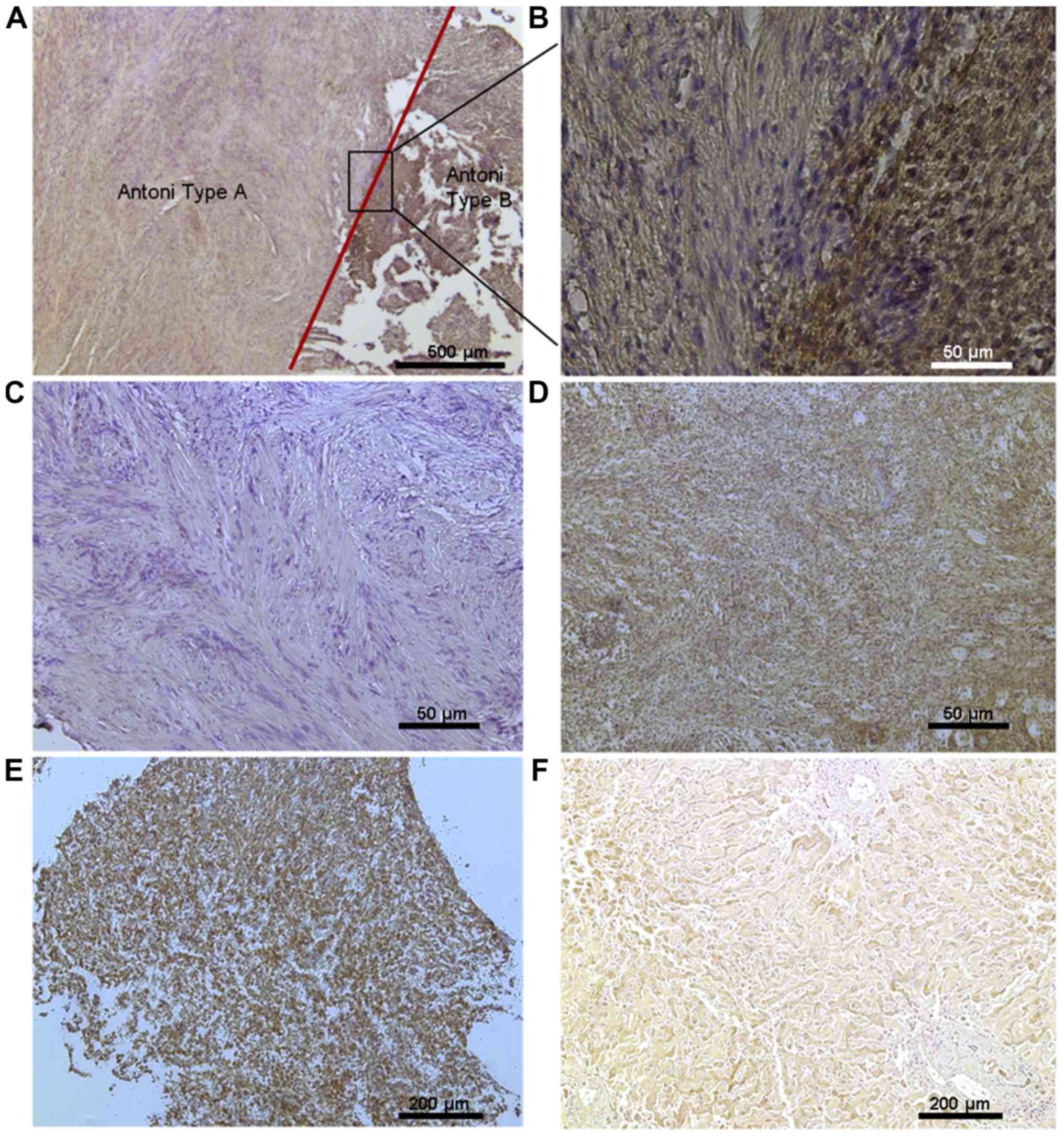
Typical for Antoni type A tissue is the high cell density in a
spindle-shaped arrangement, and for Antoni type B tissue a loose
meshwork of gelatinous and microcystic tissue is specific (25). Mixed types, called Antoni A/B, are
also common. As controls served 6 normal vestibular nerves that
were obtained from autopsies within the first 24 h after death and
4 sural nerves from biopsies.
Clinical parameters
Hearing function and tumor extension were
categorized using the Hannover Classification (26,27).
Tone and speech audiometry were measured several days before
surgery to estimate hearing function. Hearing deterioration was
categorized into 6 classes in 20 dB steps: H1 has a maximum of 20
dB hearing loss in tone audiometry and a similar good result in
speech audiometry, which is a nearly normal hearing function. H6
means deafness, with at least 100 dB hearing loss and no speech
discrimination (Table I). The tumor
extension into the cerebellopontine angle was estimated as follows:
T1 is an intrameatal tumor, T2 is an intra- and extrameatal tumor,
a T3A tumor fills the cerebellopontine cistern, a T3B tumor has
contact to the brainstem, a T4A tumor compresses the brainstem, and
a T4B tumor already dislocates the brain stem (Table II).
 | Table I.Hannover Classification of audiometry
results. |
Table I.
Hannover Classification of audiometry
results.
| Class | PTA result
(dB) | Speech
discrimination score (%) |
|---|
| H1 | 0–20 dB | 100-95 |
| H2 | 21–40 dB | 95–70 |
| H3 | 41–60 dB | 65-40 |
| H4 | 61–80 dB | 35–10 |
| H5 | 80–100 dB | 5-0 |
| H6 | >100 dB | 0 |
 | Table II.Hannover Classification of the tumor
extension. |
Table II.
Hannover Classification of the tumor
extension.
| Class | Tumor
extension |
|---|
| T1 | Purely
intrameatal |
| T2 | Intra- and
extrameatal |
| T3 | Filling the
cerebellopontine cistern |
|
T3A | Without brainstem
contact |
|
T3B | Reaching the
brainstem |
| T4 | Brainstem
compression |
|
T4A | Compressing the
brainstem |
|
T4B | Dislocating the
brainstem and compressing the fourth ventricle |
Tumor growth dynamics were categorized by magnetic
resonance imaging (MRI) performed during a ‘watch and wait’ period
before surgery. The histological proliferation rate was determined
according to Ki67 staining. Both groups comprised 15 slowly growing
VS (growth rate of less than 2 mm per year or <2% Ki67-positive
cells) and 15 tumors, which were faster growing (growth rate over 2
mm per year or >2% Ki67-positive cells) (28,29).
mRNA and protein extraction
NucleoSpin RNA/Protein kit (Macherey-Nagel) was used
to purify protein and mRNA from 30 mg tissue samples according to
the manufacturer's instructions. Utilizing the Qubit 2.0
Fluorometer (Thermo Fisher Scientific, Inc.) the concentrations of
the extracted mRNA and of the isolated protein were measured.
Reverse transcription of the mRNA to cDNA was performed by the
High-capacity RNA-to-cDNA kit (Applied Biosystems) and the T3000
Thermocycler (Biometra). The isoloated cDNA samples were stored at
−80°C.
Reverse transcription-quantitative PCR
(RT-qPCR)
The StepOnePlus Real-Time PCR System (Applied
Biosystems) was used for analysis of the ADAM9 mRNA expression in
VS and control nerve samples. The cDNA concentration was mixed with
TaqMan Universal Master Mix (Applied Biosystems) after adjustment
to the amount of sample with the lowest concentration. GAPDH-VIC PL
(HS99999905_m1; 5′3′ sequence: gggcgcctggtcaccagggctgctt) was used
as an internal control, and ADAM9_FAM (HS00177638_m1; 5′3′
sequence: gtgccactgggaatgctttgtgtgg) assays (Applied Biosystems)
were used to evaluate the relative ADAM9 expression in a duplex
setting. PCR was running for 10 min at 95°C followed by 50 cycles
of 15 sec at 95°C and 60 sec at 60°C. All samples were done in
triplicate. The data were analyzed utilizing the 2−∆∆Cq
method and GAPDH was used as loading control for normalization
(30).
Western blotting analysis
A total of 16 µl pure protein lysate of the sample
with the lowest concentration was used. A comparative amount of
protein lysate from the other samples was diluted in water (in all
16 µl volume) and used. These samples were mixed with sample buffer
(6.25 µl) and reducing agent (2.5 µl), incubated at 70°C for 10
min, and centrifuged for 1 min at 11,000 × g. 20 µl of each sample
were loaded onto a 4–12% polyacrylamide NuPage Bis-Tris gel
(Invitrogen). Electrophoresis was running for 1 h at 200 V and 120
mA using the XCell SureLock system (Invitrogen). The transferring
of the separated proteins to nitrocellulose membranes (Invitrogen)
was done with the iBlot kit and system (Invitrogen) following the
manufacturer´s instructions. Blocking of the membrane was carried
out with 5% nonfat milk powder (Roth) in TBST (0.1% Tween 20) at
room temperature for 1 h and probed with mouse polyclonal antibody
57934 against human ADAM9 (Abcam) at a dilution of 1:500 in TBST
(GE Healthcare UK Limited) and with γ-tubulin antibody T6557 in a
dilution of 1:5,000 in TBST (Sigma). The secondary antibody, sheep
anti-mouse IgG-HRP NA931V (Amersham), was diluted in TBST
(1:1,000). For protein detection the ECL Western Blotting Analysis
System (Amersham) was used. Visual analysis was performed by two
independent investigators, evaluating the presence or absence of
specific protein bands.
Immunohistochemistry
3 µm sections were cut from formalin-fixed
paraffin-embedded blocks of the VS tissue and stained with
anti-ADAM9 antibody (Abcam) using a 1:800 dilution in dilution
buffer (DCS). ADAM9 protein expression was visualized using a
poly-link secondary antibody and a peroxidase kit (Dako; DCS
Innovative Diagnostic Systems). Brown staining showed positive
signals, and for counterstaining hematoxylin was used.
Immunofluorescence analysis was also performed on 3
µm formalin-fixed paraffin sections. The slices were washed 2 × 10
min in xylol followed by an ethanol series with decreasing
concentrations for 5 min each in 100, 96, 70, and 50% ethanol.
Sections were blocked at a 1:2 dilution for 20 min with 10% goat
serum (Life Technologies) in antibody dilution buffer (DCS).
Double-staining was performed with anti-ADAM9 antibody (Abcam) at a
1:100 dilution in dilution buffer (DCS) and anti-S100 antibody
(Abcam) at a 1:100 dilution or anti-CD68 (Dianova) at 1:200 at 4°C
overnight. Protein expression was visualized with Cy3-anti-mouse
(red, Dianova) at a dilution of 1:100 and Cy2-anti-rabbit (green,
Dianova) at a dilution of 1:50 for 1 h at room temperature, both
used as secondary antibodies. Fluoroshield mounting medium (Abcam)
was used for the slides.
All immunohistochemically stained slides were
analyzed using a light microscope (Leica). Liver tissue served as
negative control and glioblastoma tissue as positive control for
the staining experiments.
Statistical analysis
StepOne software v2.3 and ExpressionSuite Software
v1.04 (Thermo Fisher Scientific, Inc.) was used for mRNA expression
analysis. GAPDH mRNA expression was analyzed to normalize the data.
Statistical analysis was performed with Microsoft Exel 2010 with
XLSTAT (Redmond). Normality was tested by Shapiro-Wilk test.
Statistical significance was determined using Mann-Whitney-U-test
and the Kruskal Wallis test. P<0.05 was considered to indicate a
statistically significant difference. Correlation was evaluated
using the Pearson correlation coefficient.
Results
Patient cohort
60 VS tumor samples from 58 patients (32 women, 26
men; mean age, 42 years) were analyzed in this study. Of the 60
samples, half were obtained from patients with NF2 (16 women, 14
men) and the other half from patients with sporadic VS (18 women,
12 men). The mean age of the control group was 57 years, the mean
age of the sporadic vestibular schwannoma group was 51 years. This
is not a significant difference. However, patients with
neurofibromatosis are much younger at tumor manifestation and time
of surgery compared to patients with sporadic vestibular
schwannoma.
mRNA expression of ADAM9 in VS
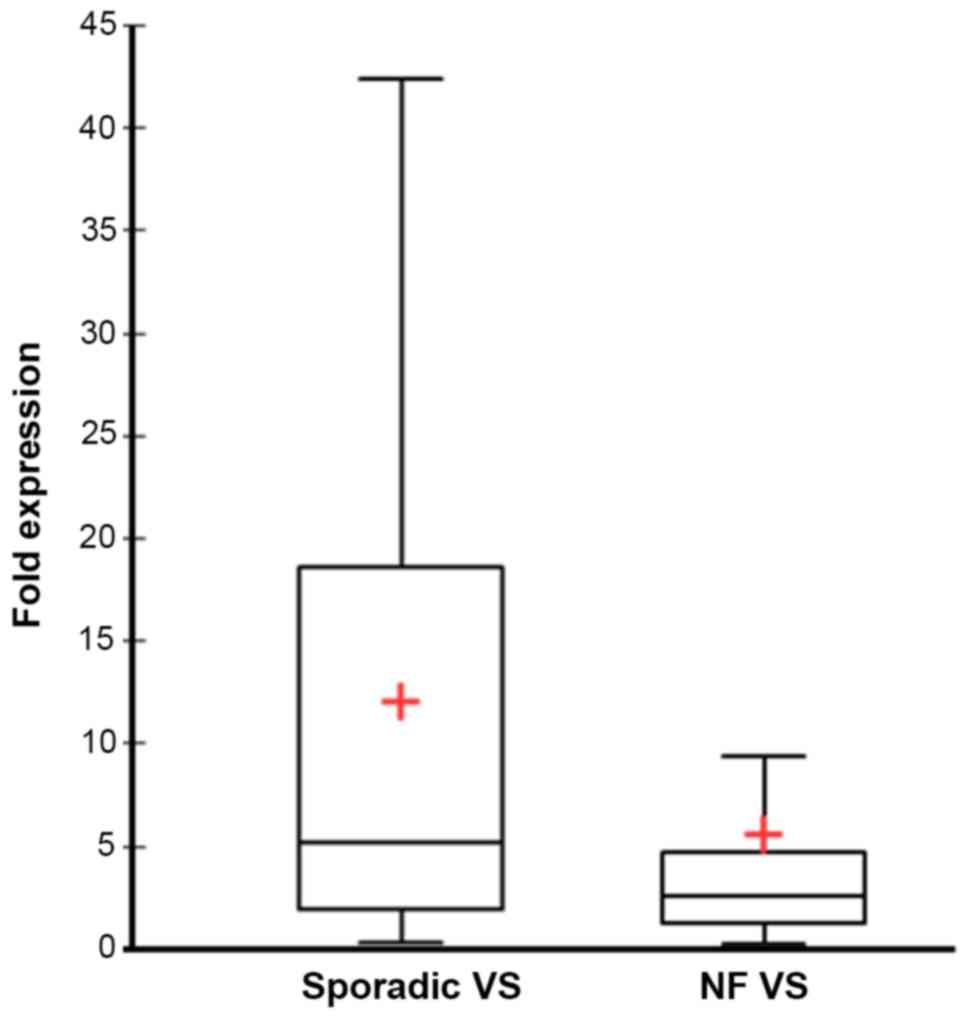
In comparison to the control group a mean 8.8 times
overexpression of ADAM9 mRNA (SD=13; median=3.4; 95% confidence
interval (CI)=5.5–12.1) was detected in all VS combined. The
subgroup of sporadic VS displayed a mean 12 times overexpression of
ADAM9 (SD = 13; median=5.2; 95% CI=7.4–16.7) (Fig. 1). VS with NF2 background had a mean
5.6 times overexpression of ADAM9 vs. the control group (SD=12.5;
median=2.2; 95% CI=1.1–10.0) (Fig.
1). The ADAM9 expression was significantly different between
the two subgroups (Mann-Whitney U test, P<0.019). The growth
velocity showed no correlation with ADAM9 expression levels, but
there was a trend (P=0.107): Rapidly progressive tumors
(Ki67>2%) had higher ADAM9 expression in NF2 cases, while the
opposite was observed in sporadic VS. In these cases, higher ADAM9
expression levels were found in the slowly progressive tumors
(Table III). Tumor extension
(Table IV) at surgery also did not
significantly correlate with ADAM9 mRNA-expression levels
(P=0.15).
 | Table III.Differences in 2−∆∆Cq
values for NF or sporadic growth and their different dynamics. |
Table III.
Differences in 2−∆∆Cq
values for NF or sporadic growth and their different dynamics.
| Tissue | Tumor growth
dynamic | 2−∆∆Cq
value |
|---|
| NF VS | s.p. | 3.28 |
|
| r.p. | 7.86 |
| Sporadic VS | s.p. | 15.45 |
|
| r.p. | 8.66 |
 | Table IV.Numbers of patients with NF VS and
sporadic VS according to their clinical features. |
Table IV.
Numbers of patients with NF VS and
sporadic VS according to their clinical features.
|
|
|
| Hearing
function | Histological/Antoni
typea |
|---|
|
|
|
|
|
|
|---|
| Tissue | Tumor growth
dynamic | Tumor extension
≤T3A | ≥T3B | H1/2 | H3/4 | H5/6 | A | B | A/B |
|---|
| NF VS | s.p. | 4 | 11 | 4 | 7 | 4 | 5 | 0 | 9 |
|
| r.p. | 1 | 14 | 6 | 1 | 8 | 4 | 0 | 11 |
| Sporadic VS | s.p. | 4 | 12 | 3 | 8 | 4 | 7 | 0 | 8 |
|
| r.p. | 6 | 8 | 5 | 6 | 4 | 6 | 2 | 7 |
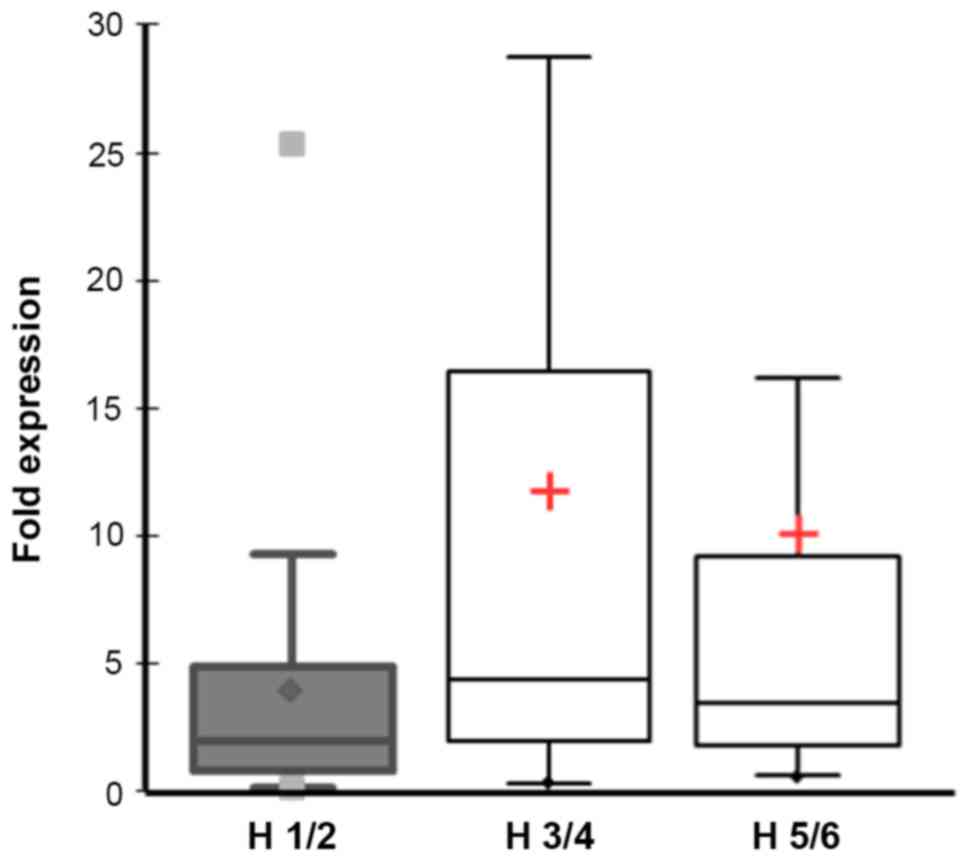
Importantly, the impairment of the hearing function
(18× H1/2, 22× H3/4 and 20× H5/6 according to Hannover
Classification) correlated strongly with ADAM9 expression (Pearson
correlation coefficient r~1). Patients with normal or good hearing
function had a low ADAM9 expression (mean 3.8 fold; SD=5.9;
median=2.1; 95% CI=1.2–6.7) versus patients with medium or high
hearing impairment, who had a much higher ADAM9 expression (mean
11.3 fold; SD=14.7; median=3.5; 95% CI=6.5–15.3). Differences were
statistically significant (Mann-Whitney U Test, p=0.027) (Fig. 2).
Protein expression of ADAM9 in VS
To confirm the ADAM9 overexpression in VS on protein
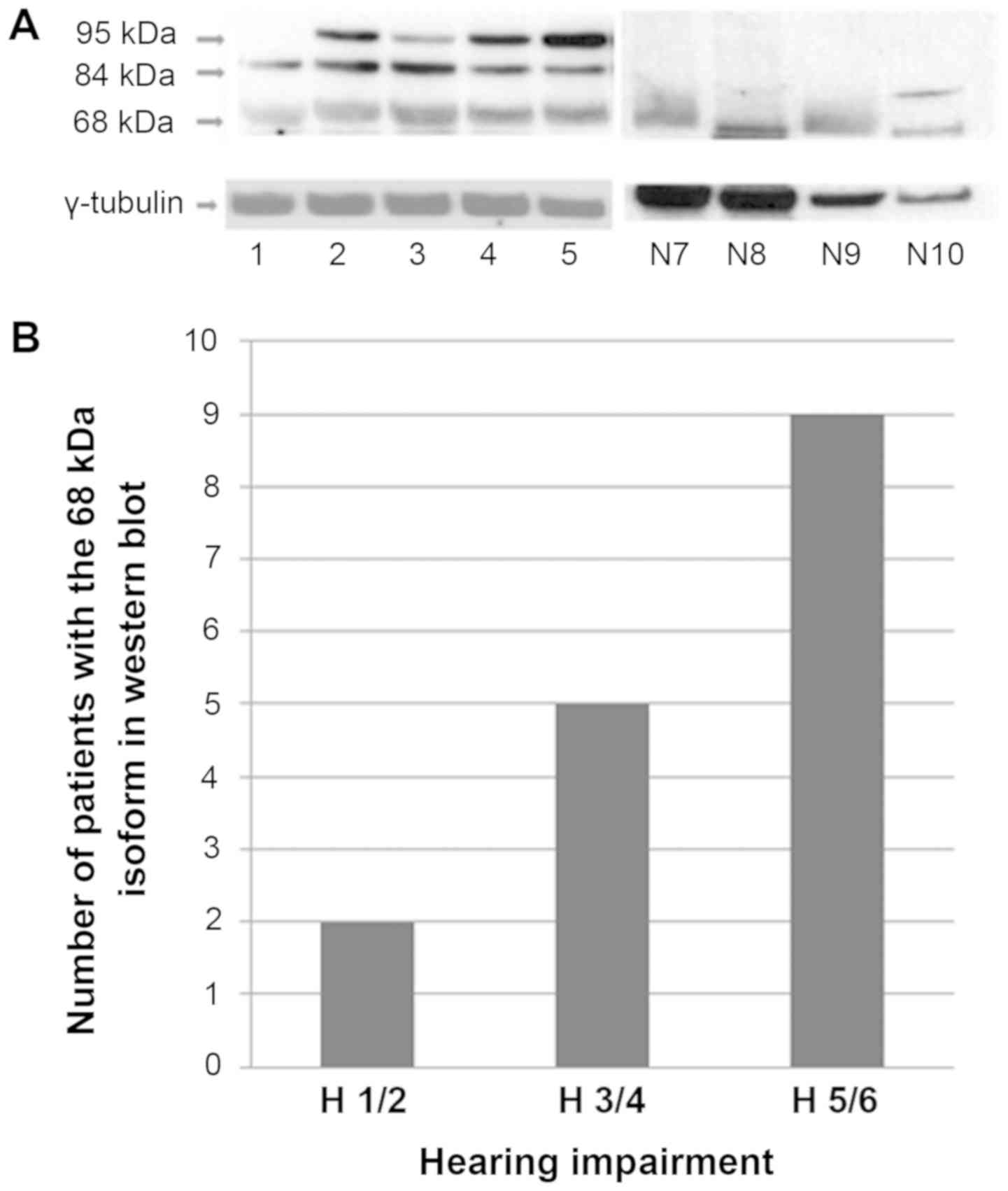
level Western blots were performed (Fig.
3A). Three ADAM9 isoforms of approximately 95, 84 and 68 kDa
were detectable (Fig. 3A),
corresponding to the two precursor proteins and the mature 84 kDa
transmembrane form. The 68 kDa isoform was detectable in fewer
cases than the 95 kDa isoform, but there was no significant
difference of distribution between cases with and without NF2
background (Table V). There was low
ADAM9 expression detectable in 50% of the control group and 50% had
no ADAM9 expression.
 | Table V.Numbers of patients according to
tumor growth dynamic and NF background as well as staining
intensity of the different isoforms in western blotting. |
Table V.
Numbers of patients according to
tumor growth dynamic and NF background as well as staining
intensity of the different isoforms in western blotting.
| Tissue | Tumor growth
dynamic | 68 kDa, n
(yes/no) | 95 kDa, n
(yes/no) |
|---|
| NF VS | s.p. | 2/13 | 10/5 |
|
| r.p. | 6/9 | 9/6 |
| Sporadic VS | s.p. | 5/10 | 7/8 |
|
| r.p. | 3/12 | 7/8 |
Importantly, there was a correlation of the secreted
ADAM9 isoform (68 kDa) expression detected by Western blot
(Fig. 3B) and the grade of hearing
impairment. Patients with good hearing function showed in two of 18
cases an expression of the 68 kDa isoform, patients with medium
hearing impairment in 5 of 22 cases and deaf patients in 9 of 20
cases.
ADAM9 protein expression could be detected by
immunohistochemistry of VS slices (Fig.
4). ADAM9 was localized predominantly membrane-bound and in the
cytoplasm of Schwann cells.
Antoni type A regions, with a high cell density in a
spindle shaped arrangement, showed weak or no ADAM9-staining in 42
of 46 cases. Antoni type B regions, which consist of a loose
meshwork of gelatinous and microcystic tissue, were strongly
immunopositive in 22 of 31 cases (Table
VI). This strong relationship between ADAM9 expression and the
histological architecture could be observed in sporadic as well as
in NF2 associated VS.
 | Table VI.ADAM9 staining results. Number of
patients with weak/no staining or strong staining for ADAM9
according to the histological type. Only a distribution in type A
and B regions were considered for analysis. Not for all
histological regions staining results were available. |
Table VI.
ADAM9 staining results. Number of
patients with weak/no staining or strong staining for ADAM9
according to the histological type. Only a distribution in type A
and B regions were considered for analysis. Not for all
histological regions staining results were available.
|
| NF VS, n | Sporadic VS, n |
|---|
|
|
|
|
|---|
| Histological
type | Weak/no
staining | Strong
staining | Weak/no
staining | Strong
staining |
|---|
| Type A region | 17 | 3 | 25 | 1 |
| Type B region | 3 | 11 | 6 | 11 |
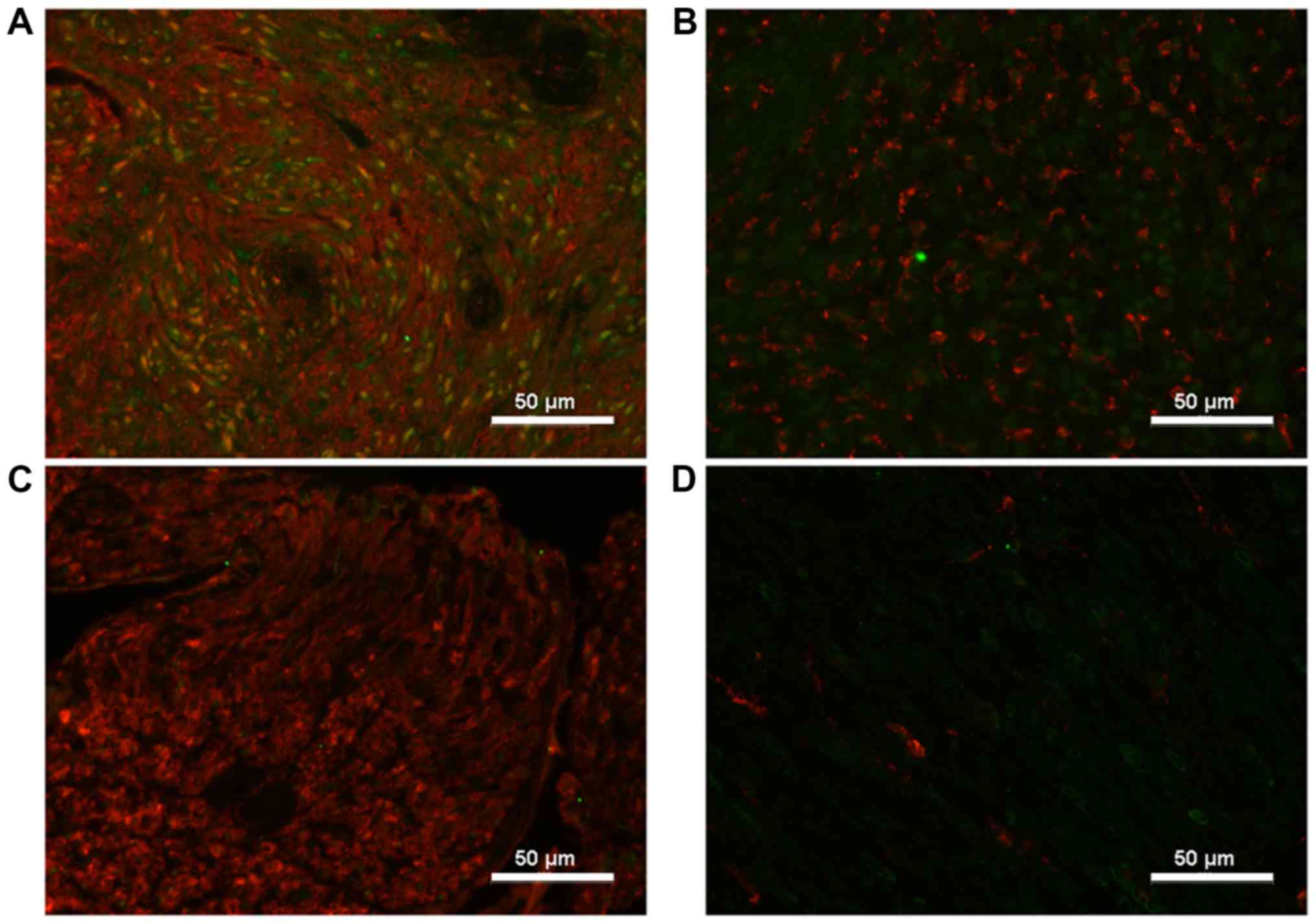
Immunofluorescent double-staining revealed that
ADAM9 was co-localized with the Schwann cell marker S100 in VS, but
not in healthy nerves (Fig. 5).
ADAM9 expression could only be detected within tumor cells while it
did not co-localize with the macrophage marker CD68 (Fig. 5). Vestibular nerves and sural nerves
of the control group displayed a very low expression of ADAM9, and
CD68 positive cells were only hardly found in these specimen.
Discussion
The present investigation demonstrates for the first
time that ADAM9 is expressed in VS by neoplastic Schwann cells, and
thus, could play a significant role in the pathogenesis of these
tumors.
ADAM9 is involved in cell-cell and cell-matrix
interaction by binding to several integrins and it promotes cell
migration in tumor cells (10,11).
However, its specific function in tumor pathogenesis has not been
elucidated at all so far (10,14). For
example, in breast cancer ADAM9 expression correlates with cancer
progression (31), in prostate
cancer it is a marker for relapse and in renal cancer it indicates
a poor prognosis (11). In prostate
cancer blocking ADAM9 expression induced apoptotic cell death in
vitro (11), and to treat pancreatic
cancer, it is targeted by the matrix-metalloproteinase-inhibitor
(CGS27023) (20).
ADAM protein family members are multifunctional
proteins and process other transmembrane proteins like the
TNF-α-receptor, L-Selectine, CD44 and Erb4/Her4 by their
proteolytic activity (11). This is
called protein shedding. Other examples are the cleavage of
EGF ligands and the FGF receptor, a key-mechanism during the
genesis of prostate cancer (14).
Protein shedding and cell migration enhance tumor invasion and are
essential steps in tumor pathogenesis.
In the current study, ADAM9 overexpression in VS
could be demonstrated by qPCR for the first time with a difference
between NF2 and sporadic cases. The two subgroups were not
completely comparable regarding the mean age. However, age as a
confounding factor between sporadic and NF2 vestibular schwannoma,
although possible, is unlikely in our opinion. ADAM9 showed its
highest expression in slow growing sporadic VS, medium expression
in rapidly growing tumors with and without NF2 and the lowest
expression in slow growing VS with NF2 (Table I). ADAM9 overexpression did not
correlate with the growth velocity or tumor extension, but with the
functional impairment and therefore could be a marker for tumor
invasiveness.
In daily clinical practice there is sometimes a
mismatch between tumor extension and functional impairment in VS.
Patients with a large tumor extension may have a good hearing
function while those with small tumors may have a high hearing
impairment (26). Although VS are
histologically benign tumors, invasiveness is sometimes observed
during surgery. Occasionally, the cranial nerves or even the
brainstem appear to be infiltrated by the tumor. Therefore,
invasive behavior could be an explanation for an over-proportional
hearing loss in some cases with small tumors. Although invasive
growth is more often seen in NF2-cases (5), growth velocity and tumor extension were
not significantly different between the NF2- and non-NF2-group
analyzed here. Evaluating invasiveness was out of the scope of our
study. Nevertheless, we reckon that the strong correlation between
ADAM9 expression and functional impairment could be due to
increased invasion of VS-cells in case of high ADAM9
expression.
This assumption is supported by the finding that
Antoni type A schwannomas, which show a higher cell density and a
more regular histological pattern, exhibited lower ADAM9 expression
levels while Antoni type B tumors with a higher grade of
degeneration (25) correlated with
higher levels of ADAM9. Therefore, ADAM9 might be relevant for
these histological differences and they are the correlate for tumor
invasiveness. How ADAM9 could contribute to these histological
changes is not known, perhaps its function as proteinase is
important.
Different ADAM9-isoforms were detected by
Western-blotting: The 95 kDa band could represent the
precursor-form of the mature 84 kDa transmembrane isoform even
though it was described with 114 kDa in the literature (11,18,19). The
68 kDa band might be the precursor-form of the mature secreted
protein. However, the latter was absent in our Western blot,
perhaps because it is dissoluble. In breast cancer cells the larger
114/84 kDa isoform of ADAM9 was identified as suppressor of
migration (19). On the other hand,
the secreted 68/47 kDa isoform, seems to be a promoter of migration
in liver metastasis (18). In our
cohort the larger 95 kDa isoform was expressed in more cases than
the secreted 68 kDa isoform. Higher levels of the secreted ADAM9
isoform correlated strongly with the functional impairment of the
patients. Thus, the secreted isoform, which was expressed in fewer
cases, appeared to be an indicator for invasiveness in VS, as has
been shown for migration in liver metastasis (18).
Further analysis of the isoform-expression depending
on growth dynamics in NF2 and non NF2 as well as the comparison
with the qPCR results were limited by the low number of only n=15
cases in each group. The growth velocities were not significantly
different and ADAM9 therefore probably is not relevant for the
different growth dynamics in cases with neurofibromatosis. The
level of ADAM9 expression exerts influence on the tumor
microenvironment. The disintegrin component of ADAM9 effectively
attracts neutrophils, which promote tumor growth by CXCR2
activation and the neutrophil elastase release for example in lung
cancer (32–34). Because of this effect and the other
described protein functions of ADAM9 like the cell-cell and
cell-matrix adhesion, it could be a promising target for a new
systemic therapeutic intervention. ADAM proteins can be ‘targeted’
by MMP inhibitors, specific inhibitors, antibodies, TIMPs and
prodomains of the protein (10,14,20). In
case of ADAM9 only antibody treatment and the use of prodomains is
feasible (14) because no elective
inhibitor exists so far and TIMPs do not work (10,14).
Specific inhibitors of other ADAM proteins (INCB3619) in
combination with different chemotherapeutics like gefitinib or
paclitaxel were effective in the treatment of several tumor types
like non-small cell lung cancer or breast cancer, without severe
side effects like musculoskeletal problems seen withMMP inhibitors
(10). Therefore, ADAM9 might be a
very promising new therapeutic target for the systemic treatment of
VS (10,11,14).
ADAM9 was overexpressed specifically by Schwann
cells of VS, but not of normal nerves. Its expression was higher in
sporadic cases compared to NF2-associated VS. ADAM9 expression
levels, especially of the secreted isoform, significantly
correlated with hearing loss of the patients and therefore could be
a marker for invasiveness of the tumor growth.
Acknowledgements
The authors would like to thank Mrs. Siglinde Kühnel
(technical assistant, University Hospital in Würzburg) and Mrs.
Elisabeth Karl (technical assistant, University Hospital in
Würzburg) for their technical assistance. The authors would also
like to thank Dr Jose M. Perez (Department of Neurosurgery,
University Hospital Würzburg) for providing samples and Professor
Ralf-Ingo Ernestus (Department of Neurosurgery, University Hospital
Würzburg) for supporting this work.
Funding
The present study was funded by the
Interdisciplinary Center of Clinical Research (IZKF) Würzburg
(Z-2/72) and the University of Würzburg in the funding program Open
Access Publishing.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MB, AFK, CM, ML and CH participated in the design of
the study. MB, AS and DDM performed the experiments and, together
with CMM, ML and CH, performed the data analysis and
interpretation. All authors contributed to data interpretation,
drafting or revising the article, gave final approval of the
version to be published, and agreed to be accountable for all
aspects of the work.
Ethics approval and consent to
participate
The study was approved by the local ethics committee
of the University Hospital Würzburg with the statement number
145/16 in July 2016 and written informed consent was obtained from
the patients for the use of their tissue and participation.
Patient consent for publication
All patients provided consent for publication.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ADAM
|
a disintegrin and
metalloproteinase
|
|
Akt
|
protein kinase B
|
|
bp
|
base pair
|
|
CCC
|
comprehensive cancer center
|
|
CD44
|
cell adhesion molecule 44
|
|
CD68
|
cell adhesion molecule 68
|
|
cDNA
|
complementary desoxyribonucleic
acid
|
|
Cy2/3
|
cyanine 2/3
|
|
dB
|
decibel
|
|
ECL
|
enhanced chemiluminescence
|
|
EGF
|
epidermal growth factor
|
|
Erb/Her4
|
erythroblastic oncogene
|
|
FAM
|
fluorescein amidite
|
|
FERM
|
4.1 ezrin/radixin/moesin protein
|
|
FGF-R
|
fibroblast growth factor receptor
|
|
Grb
|
growth factor receptor-bound
protein
|
|
HRP
|
horseradish peroxidase
|
|
Hx
|
hearing class according to the
Hannover Classification
|
|
IgG
|
immunoglobulin G
|
|
IHC
|
immunohistochemistry
|
|
IP3
|
inositol trisphosphate
|
|
MAP/MEK
|
mitogen-activated ERK kinase
|
|
MMP
|
matrix metalloproteinase
|
|
NF2
|
neurofibromatosis
|
|
PI3
|
phosphoinositide 3-kinase
|
|
proHB-EGF
|
pro-heparine binding epidermal growth
factor
|
|
PKC
|
protein kinase C
|
|
qPCR
|
quantitative polymerase chain
reaction
|
|
r.p.
|
rapidly progressive
|
|
S100
|
Schwann cell marker protein 100
|
|
SH3
|
src homology 3
|
|
Src
|
sarcoma
|
|
s.p.
|
slowly progressive
|
|
TIMP
|
tissue inhibitor of matrix
metalloproteinase
|
|
TNFα
|
tissue necrosis factor α
|
|
Tx
|
tumor extension according to the
Hannover Classification
|
|
VEGF
|
vascular endothelial growth factor
|
|
VIC
|
2′-chloro-7′phenyl-1,4-dichloro-6-carboxy-fluorescein
|
|
VS
|
Vestibular schwannoma
|
|
WB
|
western blotting
|
References
|
1
|
Stemmer-Rachamimov AO, Louis DN,
Gunnlaugur PN, Antonescu CR, Borowsky AD, Bronson RT, Burns DK,
Cervera P, McLaughlin ME, Reifenberger G, et al: Comparative
pathology of nerve sheath tumors in mouse models and humans. Cancer
Res. 64:3718–3724. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN, Ohgaki H, Wiestler OD and
Cavenee WK: WHO Classification of tumours of the central nervous
system. WHO/IARC Classification of tumours, 4th edition revised.
1:2016.
|
|
3
|
Lim SH, Ardern-Holmes S, McCowage G and de
Souza P: Systemic therapy in neurofibromatosis type 2. Cancer Treat
Rev. 40:857–861. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanemann CO: Magic but treatable? Tumours
due to loss of merlin. Bain. 131:606–615. 2008.
|
|
5
|
Hexter A, Jones A, Joe H, Heap L, Smith
MJ, Wallace AJ, Halliday D, Parry A, Taylor A, Raymond L, et al:
Clinical and molecular predictors of mortality in neurofibromatosis
2: A UK national analysis of 1192 patients. J Med Genet.
52:699–705. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cooper J and Giancotti FG: Molecular
insights into NF2/Merlin tumor suppressor function. FEBS Lett.
588:2743–2752. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Asthagiri AR, Parry DM, Butman JA, Kim HJ,
Tsilou ET, Zhuang Z and Lonser RR: Neurofibromatosis type 2.
Lancet. 373:1974–1986. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schulz A, Zoch A and Morrison H: A
neuronal function of the tumor suppressor protein merlin. Acta
Neuropathol Commun. 2:822014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schulz A, Büttner R, Hagel C, Baader SL,
Kluwe L, Salamon J, Mautner VF, Mindos T, Parkinson DB, Gehlhausen
JR, et al: The importance of nerve microenvironment for schwannoma
development. Acta Neuropathol. 132:289–307. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duffy MJ, McKiernan E, O´Donovan N and
McGowan PM: Role of ADAMs in cancer formation and progression. Clin
cancer Res. 15:1140–1144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klein T and Bischoff R: Active
metalloproteases of the A disintegrin and metalloprotease (ADAM)
family: Biological function and structure. J Proteome Res.
10:17–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weskamp G, Kratzschmar J, Reid MS and
Blobel CP: MDC9, a widely expressed cellular disintegrin containing
cytoplasmic SH3 ligand domains. J Cell Biol. 132:717–726. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McKie N, Dallas DJ, Edwards T, Apperley
JF, Russell RG and Croucher PI: Cloning of a novel membrane-linked
metalloproteinase from human myeloma cells. Biochem J. 318:459–462.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mochizuki S and Okada Y: ADAMs in cancer
cell proliferation and progression. Cancer Sci. 98:621–628. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fan X, Wang Y, Zhang C, Liu L, Yang S,
Wang Y, Xing L, Qian Z, Fang S, Qiao H and Jiang T: ADAM9
expression is associated with glioma tumor grade and histological
type, and acts as a prognostic factor in lower-grade gliomas. Int J
Mol Sci. 17(pii): E12762016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang CF, Yang SF, Chiou HL, Hsu WH, Hsu
JC, Liu CJ and Hsieh YH: Licochalcone A inhibits the invasive
potential of human glioma cells by targeting the MEK/ERK and ADAM9
signaling pathways. Food Funct. 9:6196–6204. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oria VO, Lopatta P, Schmitz T, Preca BT,
Nyström A, Conrad C, Bartsch JW, Kulemann B, Hoeppner J, Maurer J,
et al: ADAM9 contributes to vascular invasion in pancreatic ductal
adenocarcinoma. Mol Oncol. 13:456–479. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mazzocca A, Coppari R, De Franco R, Cho
JY, Libermann TA, Pinzani M and Toker A: A secreted form of ADAM9
promotes carcinoma invasion through tumor-stromal interactions.
Cancer Res. 65:4728–4738. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fry JL and Toker A: Secreted and
membrane-bound isoforms of protease ADAM9 have opposing effects on
breast cancer cell migration. Cancer Res. 70:8187–8198. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qian LW, Mizumoto K, Urashima T, Nagai E,
Maehara N, Sato N, Nakajima M and Tamaka M: Radiation-induced
increase in invasive potential of human pancreatic cancer cells and
its blockade by a matrix metalloproteinase inhibitor, CGS27023.
Clin Cancer Res. 8:1223–1227. 2002.PubMed/NCBI
|
|
21
|
Mongaret C, Alexandre J, Thomas-Schoemann
A, Bermudez E, Chéreau C, Nicco C, Goldwasser F, Weill B, Batteux F
and Lemare F: Tumor invasion induced by oxidative stress is
dependent on membrane ADAM 9 protein and its secreted form. Int J
Cancer. 129:791–798. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shigemura K, Sung SY, Kubo H, Arnold RS,
Fujisawa M, Gotoh A, Zhau HE and Chung LW: Reactive oxygen species
mediate androgen receptor- and serum starvation-elicited downstream
signaling of ADAM9 expression in human prostate cancer cells.
Prostate. 67:722–731. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Breun M, Schwerdtfeger A, Martellotta DD,
Kessler AF, Perez JM, Monoranu CM, Ernestus RI, Matthies C, Löhr M
and Hagemann C: CXCR4: A new player in vestibular schwannoma
pathogenesis. Oncotarget. 9:9940–9950. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hsia HE, Tüshaus J, Brummer T, Zheng Y,
Scilabra SD and Lichtenthaler SF: Functions of ‘A disintegrin and
metalloproteases (ADAMs)’ in the mammalian nervous system. Cell Mol
Life Sci. 76:3055–3081. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wippold FJ II, Lubner M, Perrin RJ, Lämmle
M and Perry A: Neuropathology for the neuroradiologist: Antoni A
and Antoni B tissue patterns. AJNR Am J Neuroradiol. 28:1633–1638.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Samii M and Matthies C: Management of 1000
vestibular schwannomas (acoustic neuromas): Hearing function in
1000 tumor resections. Neurosurgery. 40:248–262. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hummel M, Perez J, Hagen R, Gelbrich G,
Ernestus RI and Matthies C: When does hearing loss occur in
vestibular schwannoma surgery? Importance of auditory brainstem
response changes in early postoperative phase. World Neurosurg.
95:91–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moffat DA, Kasbekar A, Axon PR and Lloyd
SK: Growth characteristics of vestibular schwannoma. Otol Neurotol.
33:1053–1058. 2012.PubMed/NCBI
|
|
29
|
Sughrue ME, Kane AJ, Kaur R, Barry JJ,
Rutkowski MJ, Pitts LH, Cheung SW and Parsa AT: A prospective study
of hearing preservation in untreated vestibular schwannomas. J
Neurosurg. 114:381–385. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oria VO, Lopatta P and Schilling O: The
pleiotropic role of ADAM9 in the biology of solid tumors. Cell Mol
Life Sci. 75:2291–2301. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Heit B, Tavener S, Raharjo E and Kubes P:
An intracellular signaling hierarchy determines direction of
migration in opposing chemotactic gradients. J Cell Biol.
159:91–102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Amendola RS, Martin AC, Selistre-de-Araújo
HS, Paula-Neto HA, Saldanha-Gama R and Barja-Fidalgo C: ADAM9
disintegrin domain activates human neutrophils through an autocrine
circuit involving integrins and CXCR2. J Leukoc Biol. 97:951–962.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gong L, Cumpian AM, Caetano MS, Ochoa CE,
De la Garza MM, Lapid DJ, Mirabolfathinejad SG, Dickey BF, Zhou Q
and Moghaddam SJ: Promoting effect of neutrophils on lung
tumorigenesis is mediated by CXCR2 and neutrophil elastase. Mol
Cancer. 12:1542013. View Article : Google Scholar : PubMed/NCBI
|